Abstract
Zero-valent iron/peroxymonosulfate (Fe0/PMS) has been considered as a promising approach for wastewater treatment. Anions and cations are widely present in wastewater and have significant effects on the performance of the Fe0/PMS system for wastewater treatment. Thus, in the present study, tartrazine was selected as the target model; SO42−, NO3−, HCO3−, and Cl− were selected as representative anions and Ca2+, Cu2+, Mg2+, and Mn2+ were chosen as representative cations. The effect of these anions and cations on tartrazine removal and major radicals in the Fe0/PMS were systematically investigated. The presence of a certain concentration of SO42− and Cl− had positive, NO3− had negative, and HCO3− had negligible effects on tartrazine removal in the Fe0/PMS system. SO42− and HCO3− had a small effect on the contribution proportion of reduction, SO4•− and •OH; a certain concentration of Cl− could enhance the contribution proportion of •OH; and NO3− would decrease the contribution proportion of SO4•− and •OH. A certain concentration of each of Ca2+, Cu2+, Mg2+, and Mn2+ could enhance the tartrazine removal in the Fe0/PMS system. Ca2+, Cu2+, and Mg2+ had no effect of the contribution of reduction, SO4•− and •OH, while a certain concentration of Mn2+ could enhance the contribution proportion of SO4•−. These results can provide some references for the Fe0/PMS system to treat actual wastewater containing anions and cations.
1. Introduction
Recently, with the rapid development of the economy, water pollution has had a great adverse impact on human health and ecosystems [1]. The pollutants of water mainly include antibiotic pollution, phenol pollution, and dye pollution, among others [2,3,4]. Advanced oxidation processes (AOPs) have been declared as efficient techniques for wastewater treatment owing to the production of reactive species such as sulfate radical (SO4•−) and hydroxyl radical (•OH) [5,6,7,8,9,10].
Peroxymonosulfate (PMS) has been seen as the emerging oxidant because PMS’s O-O bond length is short, which is more easily broken [11]. PMS can be catalyzed by metal ions, ultraviolet, ultrasound, and carbon-based materials producing SO4•−, •OH, and other reactive species [12,13,14,15,16]. Among these catalytic methods, metal ions can be conducted simply and occurred in conventional environments. Among various mental ions (Co2+, Mn2+, Fe2+, and so on), Fe2+ is non-toxic, abundant, and cheap. Reactive species can be produced in the Fe2+/PMS system via Equations (1)–(3) [17,18,19,20]. However, the Fe2+/PMS process’s efficiency is limited by the low recycle efficiency of Fe2+.
Nowadays, zero-valent iron (Fe0) can be used to substitute Fe2+ because Fe2+ can be generated from the corrosion of Fe0 via Equation (4) [21]. Fe0 can also activate PMS directly via Equation (5), producing reactive species and Fe2+. Meanwhile, the produced Fe3+ via Equation (1) could be reduced to Fe2+ via Equation (6), which can further increase the efficiency [22]. Moreover, Fe0 loses electrons and undergoes a reduction reaction, which degrades pollutants [23].
Besides, anions and cations such as SO42−, HCO3−, Cl−, Ca2+, and Mg2+ are generally present in wastewater. These ions have a significantly effect on AOP’s efficiency for pollutants’ removal. Ions can enhance the corrosion rate of Fe0, but radicals could react with ions, forming less reactive species [24]. Therefore, the effect of both anions and cations on the Fe0/PMS process’s efficiency for pollutants’ removal and the role of various radicals (SO4•−, •OH, and so on) have not been systematically investigated and need further investigation. It is possible that these results might help to promote the application of the Fe0/PMS process.
In the present study, tartrazine was chosen as the model pollutant; SO42−, NO3−, Cl−, and HCO3− were chosen as anion representatives; and Ca2+, Cu2+, Mg2+, and Mn2+ were chosen as anion representatives. Then, the effect of these anions and cations on efficiency of Fe0/PMS process for tartrazine removal was investigated. Meanwhile, the effect of these anions and cations on the role of SO4•− and •OH and reduction in the Fe0/PMS system for tartrazine removal was also systematically studied.
2. Materials and Methods
2.1. Chemicals
Fe0 powder was bought from Shanghai Jinshan smelter (Shanghai, China). Tartrazine was obtained from Shanghai Macklin Biochemical Technology Co., Ltd., (Shanghai, China). Sodium sulfate (Na2SO4), sodium chloride (NaCl), sodium nitrate (NaNO3), and sodium bicarbonate (NaHCO3) were supplied by Aladdin China Company (Shanghai, China). Tert-butyl alcohol (TBA) was bought from Sinopharm Chemical Reagent Co., Ltd., (Shanghai, China).
2.2. Batch Experiments
All degradation experiments were performed in a 500 mL system using glass beakers with a 50 mg/L tartrazine solution under a constant stirring rate of 350 rpm. After adding 4 mM PMS and various ions, the initial pH would be adjusted to 3 using 1 M/0.1 M NaOH and HCl solutions. Then, 0.8 g/L Fe0 was added into the working solution and stirred with a mechanical stirrer to trigger the degradation reaction. Samples (1 mL) withdrawn at scheduled time intervals by a pipette were immediately quenched by rapidly adding 0.5 mL MeOH, and filtered through 0.22 µm membrane filters for further analysis. Quenching experiments were performed in order to distinguish the reactive species by adding 500 mM TBA into the solution before initiating the reaction.
2.3. Analytical Methods
Tartrazine concentration was detected by a spectrophotometer (VI-1501, Tianjin Gangdong Sci & Tech Development Co., Ltd., Tianjin, China) at 428 nm.
Tartrazine removal efficiency (η/%) and value of k were calculated by Equations (7) and (8), respectively.
where C0 is the initial tartrazine concentration and Ct is the tartrazine concentration at reaction time t.
3. Results and Discussion
3.1. Effect of SO42− on Tartrazine Removal and Major Radicals
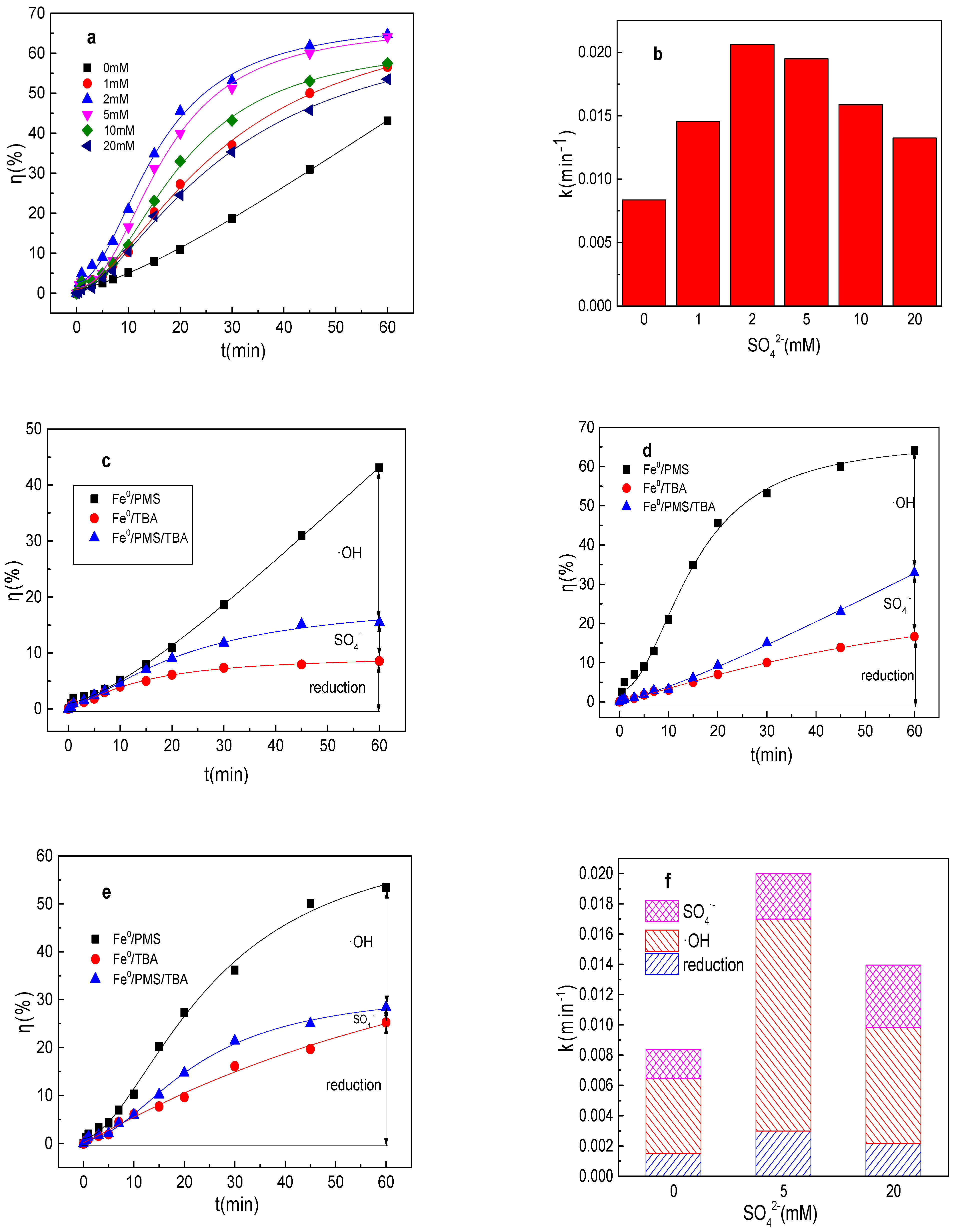
It can be seen from Figure 1a,b that the removal efficiency of tartrazine within 60 min by the Fe0/PMS process was 43.1% and the value of k was 0.0084 min−1 in the absence of any ion. The tartrazine removal efficiency and value of k were 56.5%, 0.0146 min−1; 64.7%, 0.0206 min−1; 64.1%, 0.0195 min−1; 57.5%, 0.0159 min−1; and 53.5%, 0.0132 min−1 in the presence of 1, 2, 5, 10, and 20 mM SO42−, respectively. The removal efficiency and value k increased with a low concentration of SO42−, but decreased with a large concentration of SO42−. To maintain the local charge balance, Fe2+ would move together with negatively charged anions, thus the presence of SO42− could also enhance the Fe0 corrosion rate, thereby enhancing the Fe2+ generation rate [24]. From this point, the tartrazine removal efficiency and value of k would increase in the presence of SO42−. However, the large concentration of SO42− induced the decrease in the tartrazine removal efficiency and value of k, which might be because the oxidation reduction potential (ORP) of SO4•−/SO42− would decrease in the presence of SO42−, thereby significantly decreasing the PMS activation efficiency [25]. The results were consistent with the results using Fe0/PMS treating Rhodamine B [25].
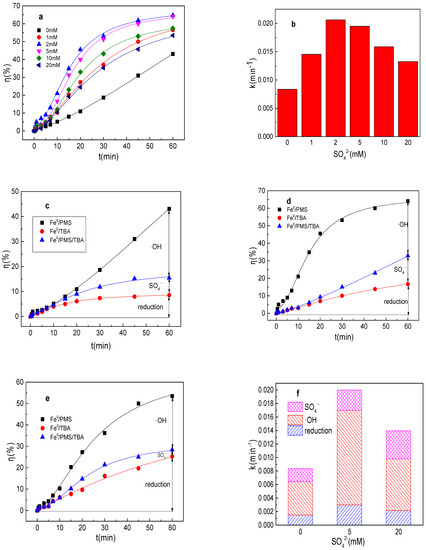
Figure 1.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different SO42− concentrations; (b) value of k in the presence of different SO42− concentrations; (c–e) tartrazine removal under different inhibitors in the presence of 0, 5, and 20 mM SO42−; and (f) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
Tartrazine could be removed by reduction and oxidized by SO4•− and •OH in the Fe0/PMS system. Therefore, the contributions of the reduction of SO4•− and •OH for tartrazine removal in the Fe0/PMS system in the absence and presence of SO42− were calculated by Equations (9)–(11), and the results are shown in Figure 1c–f. kreduction, k•OH, and kSO4•− for tartrazine removal in the Fe0/PMS system were all enhanced in the presence of SO42−, suggesting that the presence of SO42− could increase the generation of SO4•− and •OH. Reduction and oxidized by SO4•− and •OH exhibited 17.8%, 23.1%, and 59.1% contributions for tartrazine removal in the Fe0/PMS system in the absence of ion. Further, reduction and oxidization by SO4•− and •OH exhibited 15.0%, 20.1%, and 64.9% contribution for tartrazine removal in the Fe0/PMS system in the presence of 5 mM SO42−. In the presence of 20 mM SO42−, reduction and oxidization by SO4•− and •OH exhibited 15.4%, 29.6%, and 55.0% contribution for tartrazine removal in the Fe0/PMS system. Radical scavenging experiments suggested that the presence of SO42− had a negligible effect on the contribution role of reduction and oxidization by SO4•− and •OH in removing tartrazine, which might be because SO42− would increase the number of electrons lost from Fe0 and enhanced the reduction reaction. The increased Fe0 corrosion rate would also enhance the Fe2+ production rate, thereby enhancing PMS activation for SO4•− and •OH production.
3.2. Effect of NO3− on Tartrazine Removal and Major Radicals
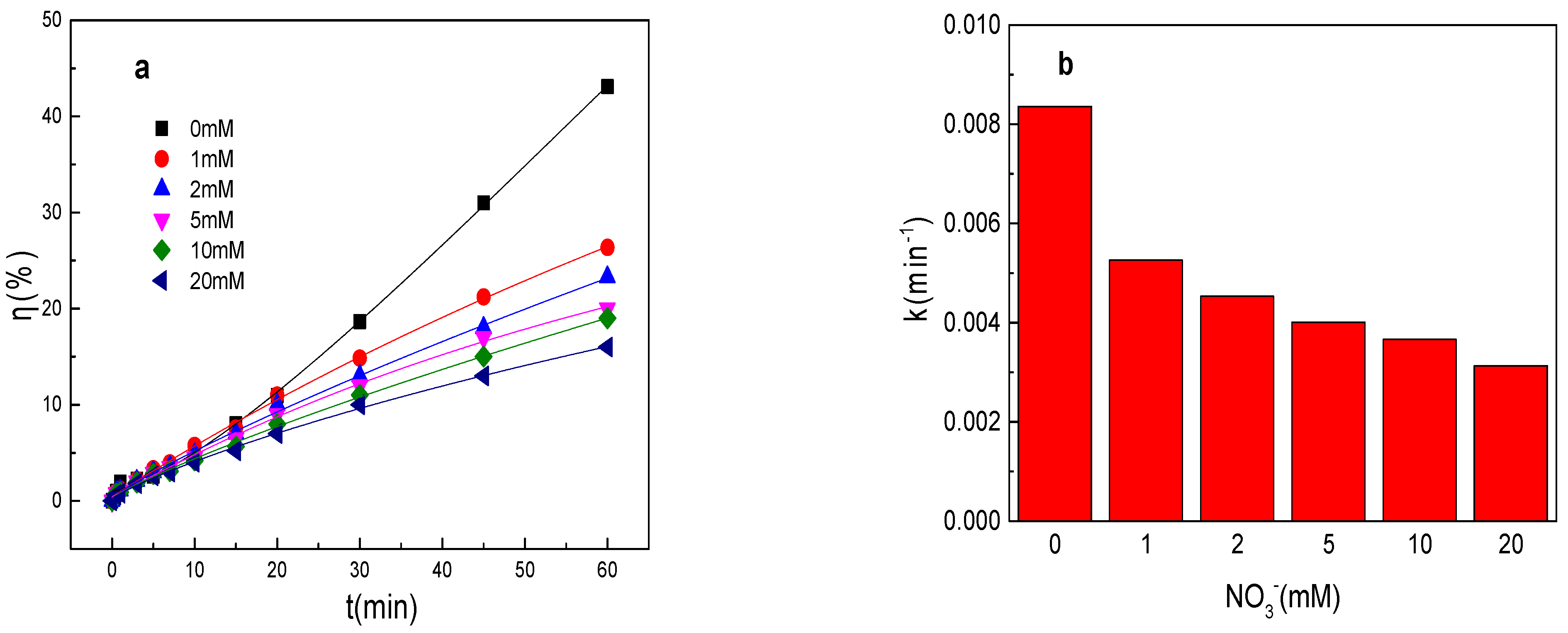
As shown in Figure 2a,b, the tartrazine removal efficiency and value of k were obviously decreased in the presence of NO3− in the Fe0/PMS system and the results were not in accordance with the results of SO42−. The tartrazine removal efficiency and value of k were only 26.4%, 0.0053 min−1; 23.3%, 0.0045 min−1; 20.3%, 0.0041 min−1; 19.3%, 0.0037 min−1; and 16.5%, 0.0032 min−1 in the presence of 1, 2, 5, 10, and 20 mM NO3−, respectively. NO3−, like SO42−, is also an anion that can improve the corrosion of Fe0. The tartrazine removal efficiency and value of k were obviously decreased in the presence of NO3− in the Fe0/PMS system, which might because of the following reasons: (1) Fe0 could directly react with NO3− to form iron oxides and affected the formation of Fe2+ via Equation (12) [26]; (2) NO3− could adsorb on Fe0 surface while iron powder eroded and many cavities were produced, which could also affect the Fe2+ generation [26]; and (3) SO4•− and •OH could react with NO3− to form less reactive species via Equations (13) and (14) [27]. According to literature reports, the effect of NO3− on the pollutant treatment efficiency of the PMS-based system was not obvious because of reason (3) [27]. Therefore, the effect of NO3− on the Fe0 corrosion rate was the main reason for the decrease in the treatment efficiency in the Fe0/PMS system.
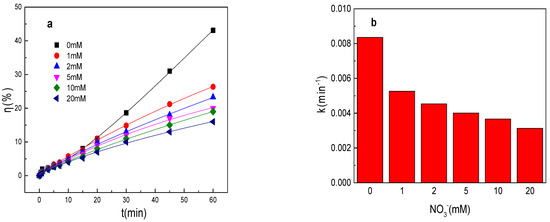
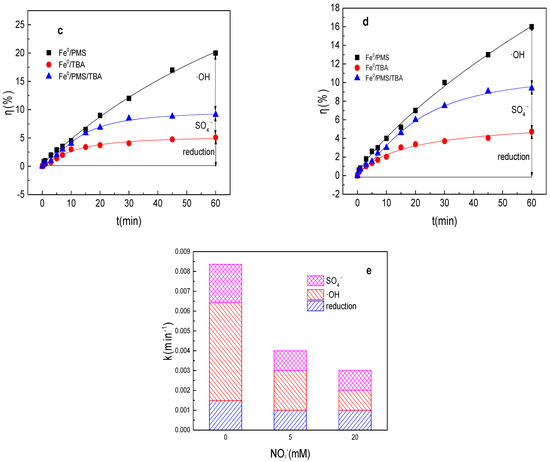
Figure 2.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different NO3− concentrations; (b) value of k in the presence of different NO3− concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM NO3−; and (e) contributions of SO4•− and •OH and reduction to tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
Radical scavenging experiments in the presence of NO3− in the Fe0/PMS system were also performed. As shown in Figure 2c–e, reduction of SO4•− and •OH also all contributed to tartrazine removal in the presence of NO3− in the Fe0/PMS system. kreduction, k•OH, and kSO4•− for tartrazine removal in the Fe0/PMS system were all decreased in the presence of NO3−, suggesting Fe2+ released by the reduction reaction was related to the PMS activation efficiency. Reduction and oxidized by SO4•− and •OH exhibited 26.2, 35.2%; 24.7%, 33.5%; and 49.1%, 31.3% contribution for tartrazine removal the Fe0/PMS system in the presence of 5 and 20 mM NO3−. Oxidation contribution for tartrazine removal decreased in the presence of NO3−, which might be because the presence of NO3− inhibited the generation of Fe2+.
3.3. Effect of Cl− on Tartrazine Removal and Major Radicals
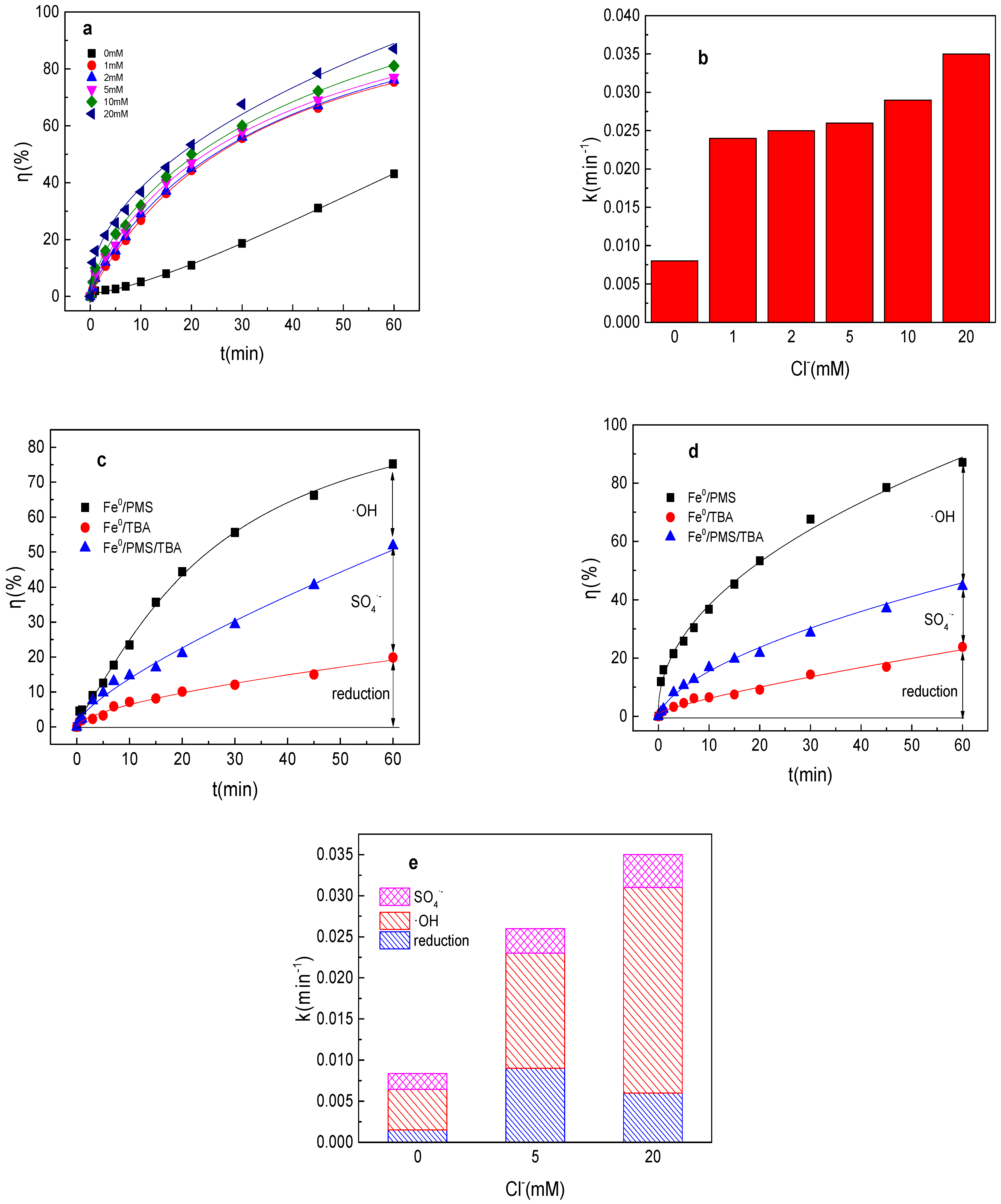
As shown in Figure 3a,b, the tartrazine removal efficiency and value of k were significantly increased in the presence of Cl− in the Fe0/PMS system. The tartrazine removal efficiency and value of k increased from to 43.1%, 0.0084 min−1 to 75.4%, 0.024 min−1; 76.1%, 0.025 min−1; 77.2%, 0.026 min−1; 81.4%, 0.029 min−1; and 87.0%, 0.035 min−1 in the presence of 1, 2, 5, 10, and 20 mM Cl−, respectively. Although Cl− could react with SO4•− and •OH to generate less reactive radicals via Equations (15) and (16) [27], the presence of Cl− could significantly enhance the Fe2+ generation, and then improved the production of SO4•− and •OH. Likewise, according to the literature reports, the reaction between Cl− and SO4•− and •OH had no obvious effect on pollutants’ removal in PMS-based systems [27]. Furthermore, the results also demonstrated that the positive effects of Cl− on tartrazine removal in the Fe0/PMS system were significantly stronger than the side effects.
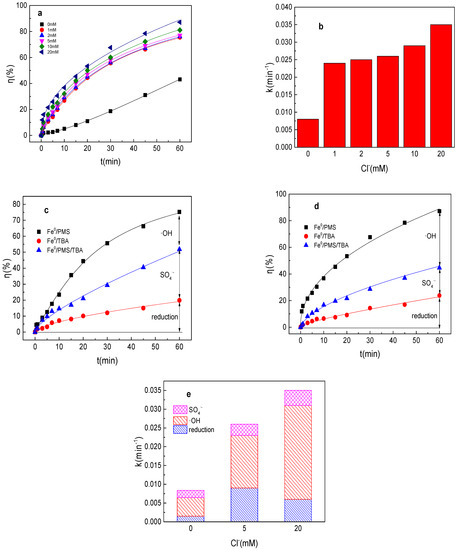
Figure 3.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different Cl− concentrations; (b) value of k in the presence of different Cl− concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM Cl−; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
According to the radical scavenging experiment results in Figure 3c–e, kreduction, k•OH, and kSO4•− for tartrazine removal in the Fe0/PMS system were all enhanced with the increase in the concentration of Cl−. Reduction and oxidization by SO4•− and •OH exhibited 34.6%, 17.1%; 11.6%, 11.4%; and 53.8%, 71.6% contribution to tartrazine removal in the Fe0/PMS system in the presence of 5 and 20 mM Cl−. The proportion of •OH contribution was significantly enhanced in the presence of Cl−, which might be because the oxidizing ability of •OH was stronger than that of SO4•− and the enhanced Fe2+ generation could improve both SO4•− and •OH, thus the contribution of •OH would be significantly enhanced.
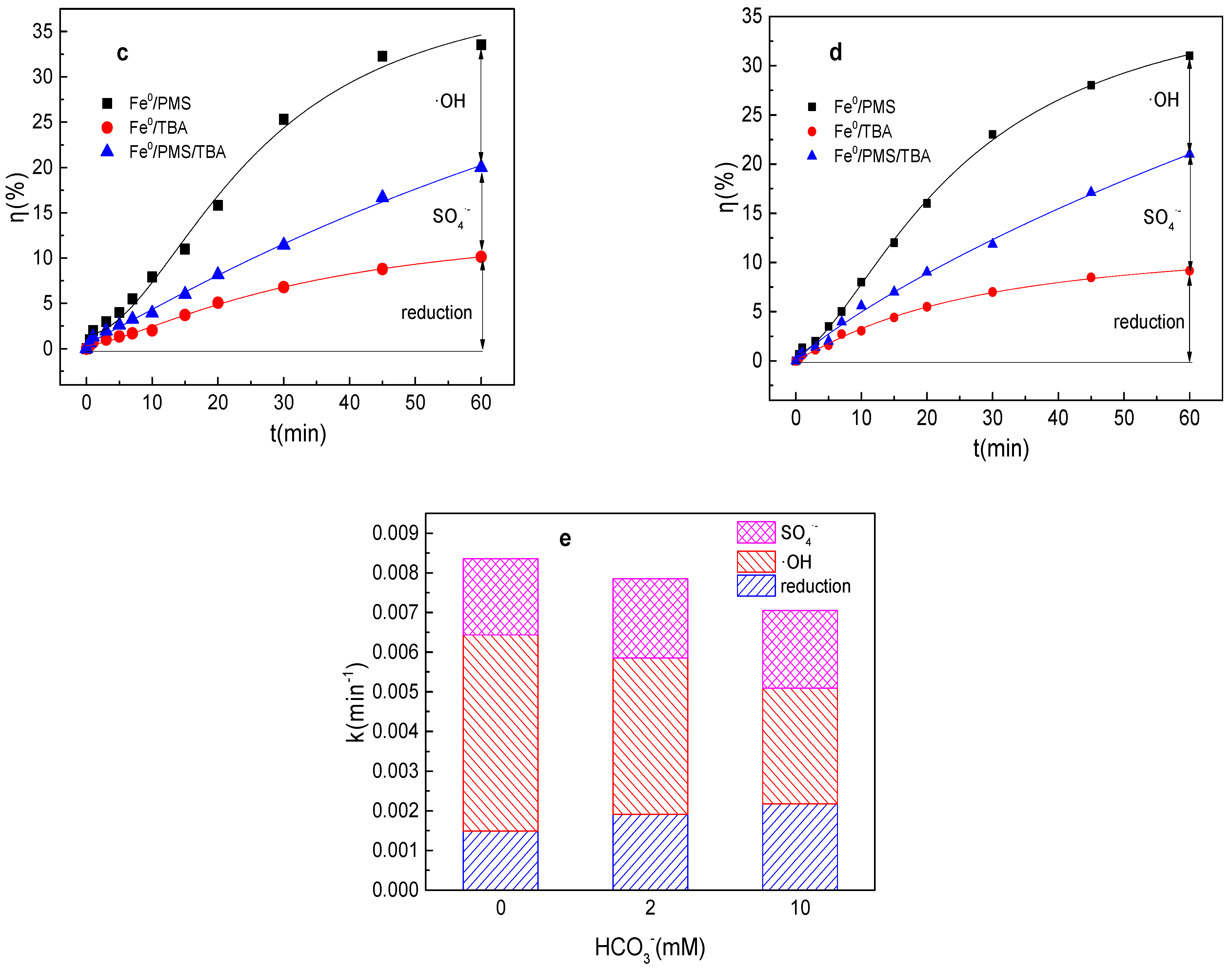
3.4. Effect of HCO3− on Tartrazine Removal and Major Radicals
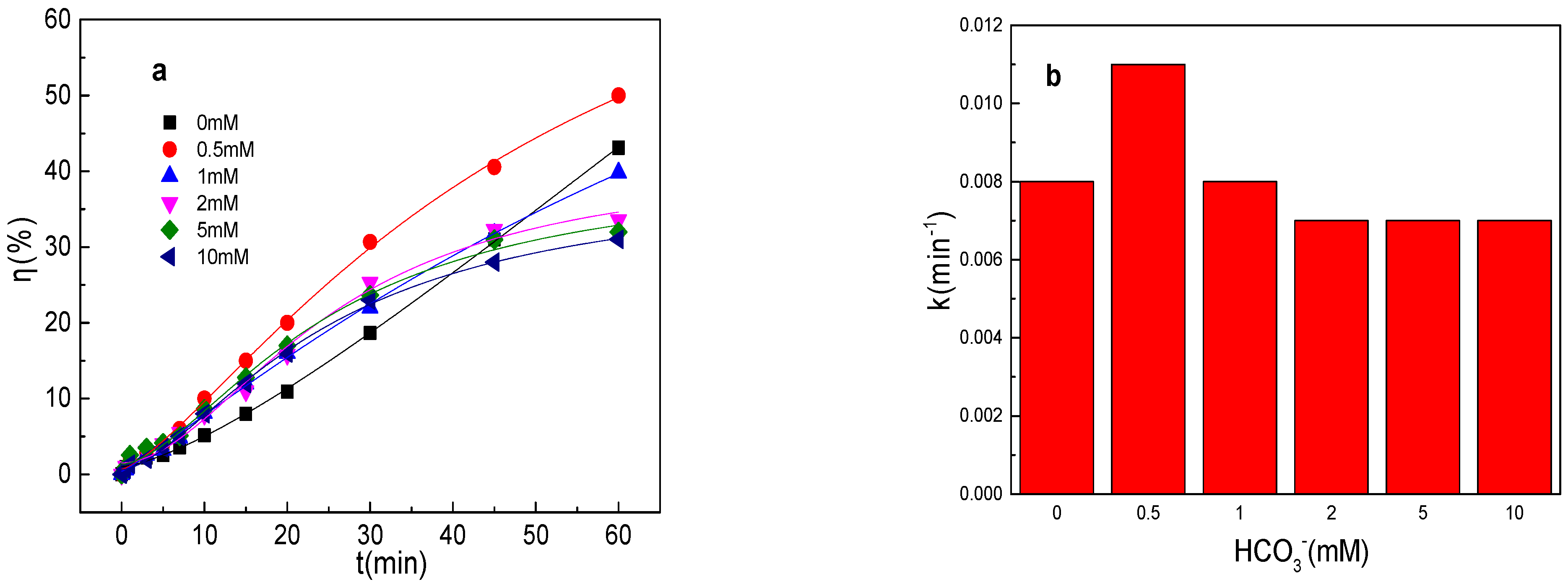
As depicted in Figure 4a,b, the tartrazine removal efficiency and value of k were almost unchanged in the presence of HCO3− in the Fe0/PMS system. HCO3− was also able to enhance the Fe2+ generation via Equation (17) [28]. Meanwhile, HCO3− might inhibit the release of Fe2+ from Fe0 owing to the generation of an insulating film and iron compounds on the Fe0 surface. Meanwhile, HCO3− could react with SO4•− and •OH to generate less reactive radicals via Equations (18) and (19). Moreover, HCO3− could consume both H+ and OH− by Equations (20)–(21), and thus maintain a stable pH during the reaction, which further inhibited Fe0 corrosion and PMS activation [28]. In general, HCO3− had a small effect on the removal of pollutants by the Fe0/PMS system. According to the literature reports, HCO3− had a significant effect on pollutants’ removal in PMS-based systems [27], thus the promotion of Fe0 corrosion by HCO3− had a very positive effect on pollutants’ removal efficiency in the Fe0/PMS system. According to the radical scavenging experiment results in Figure 4c–e, HCO3− also had a small effect on kreduction, k•OH, and kSO4•− and the contribution proportion of reduction of SO4•− and •OH for tartrazine removal in the Fe0/PMS system.
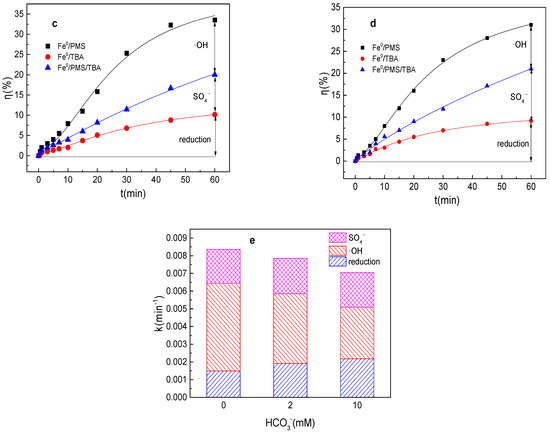
Figure 4.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different HCO3− concentrations; (b) value of k in the presence of different HCO3− concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM HCO3−; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
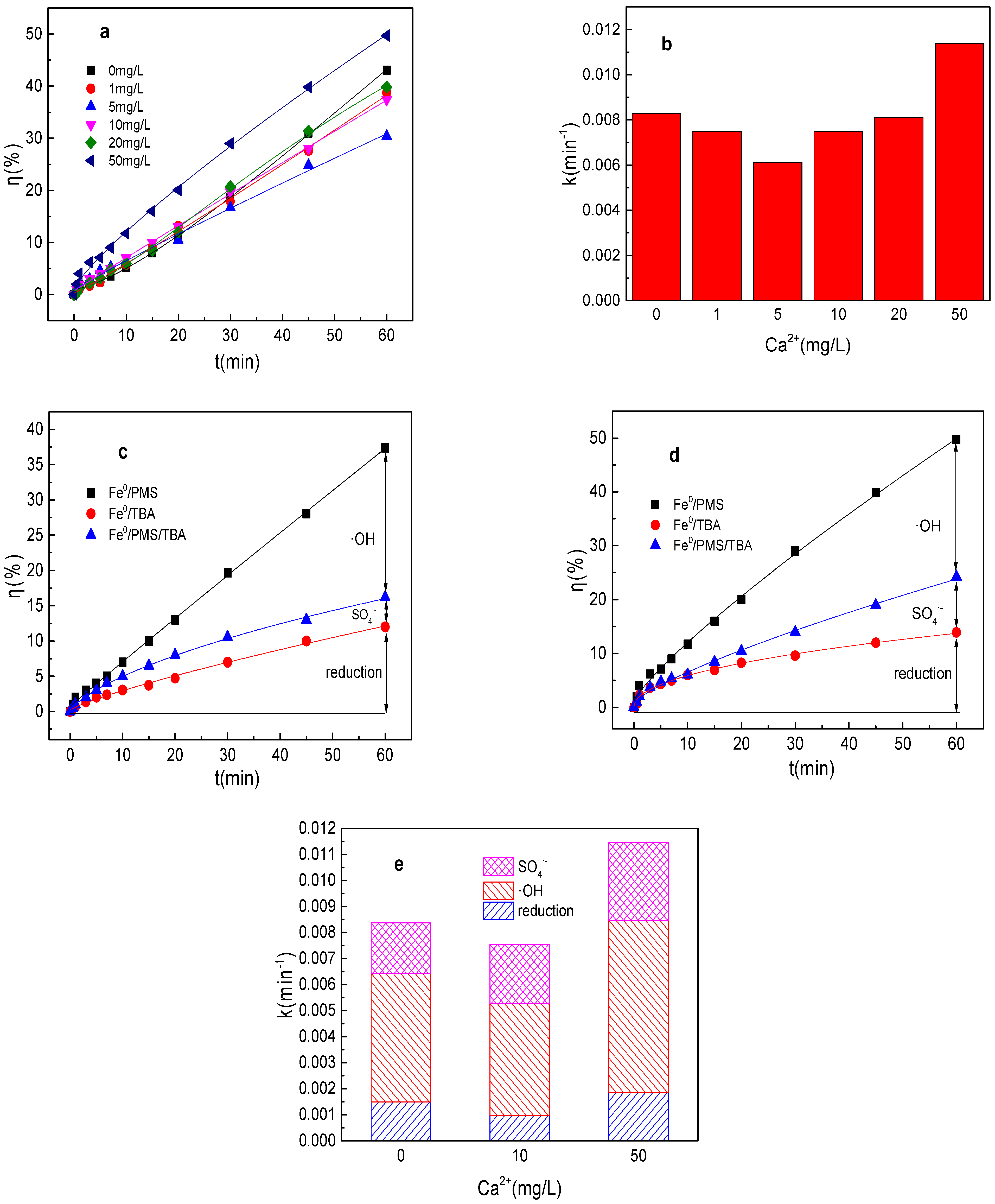
3.5. Effect of Ca2+ on Tartrazine Removal and Major Radicals
The effect of Ca2+ on tartrazine removal and major radicals in Fe0/PMS was also investigated and the results are shown in Figure 5a–e. Ca2+ had no obvious effect on tartrazine removal and value of k, and tartrazine removal and value of k could be enhanced from 43.1% and 0.0084 min−1 to 49.8% and 0.0114 min−1, respectively, while Ca2+ increased from 0 to 50 mg/L. According to the previous studies, Ca2+ could not efficiently activate PMS for pollutants’ removal. The adsorbance of Ca2+ on the Fe0′ surface was conducive to enhancing the PMS activation, and Ca2+′ bridging of negatively charged pollutants also favored degradation, possibly because of the enrichment of the catalyst by the substrate. Thus, a certain amount of Ca2+ could enhance the tartrazine removal in the Fe0/PMS system. Meanwhile, 50 mg/L Ca2+ also could enhance kreduction, k•OH, and kSO4•−, while it had no obvious effect on the contribution proportion of reduction of SO4•− and •OH for tartrazine removal in the Fe0/PMS system.
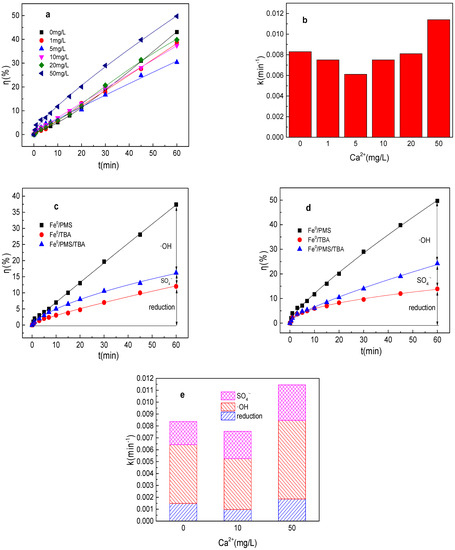
Figure 5.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different Ca2+ concentrations; (b) value of k in the presence of different Ca2+ concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM Ca2+; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
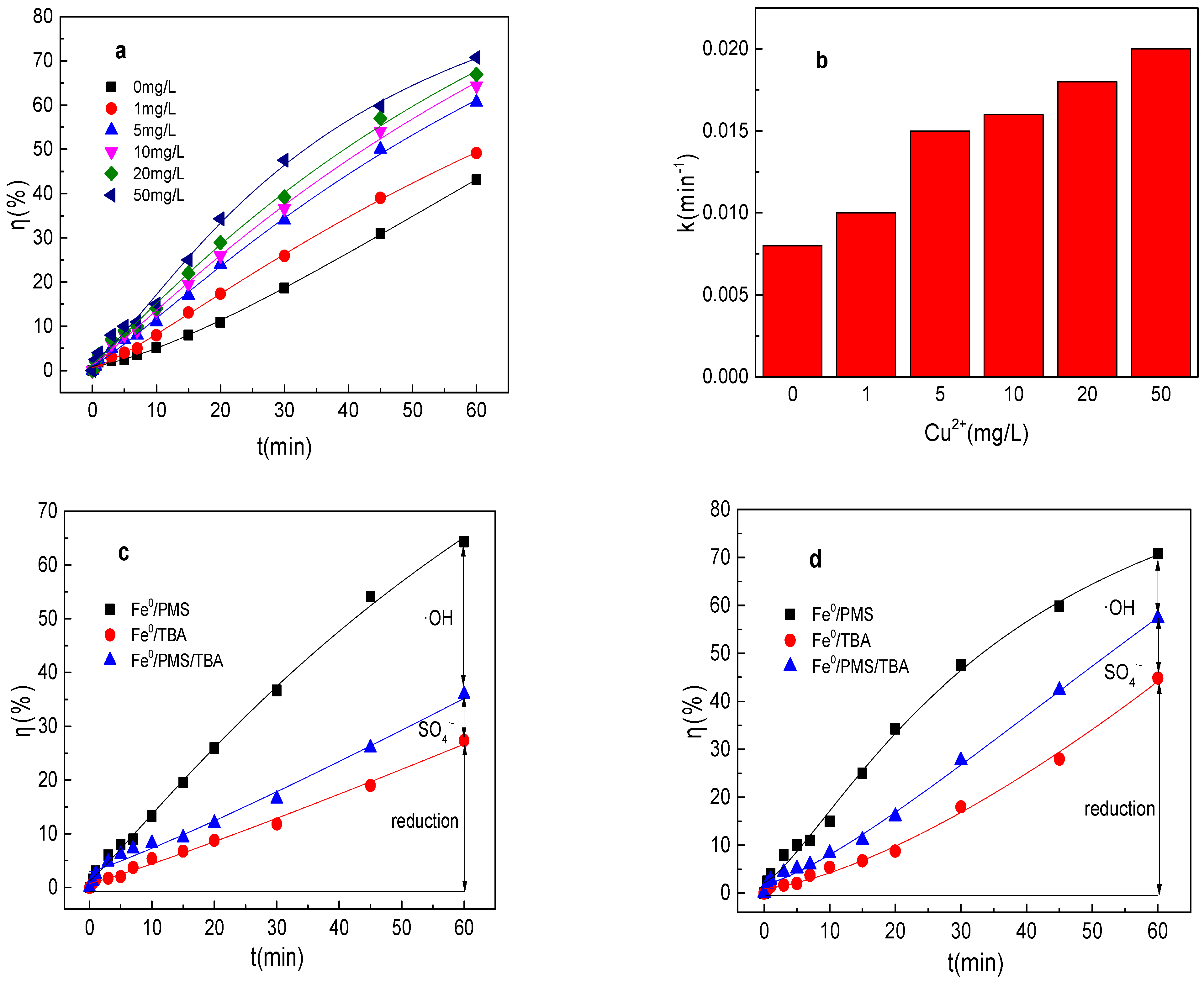
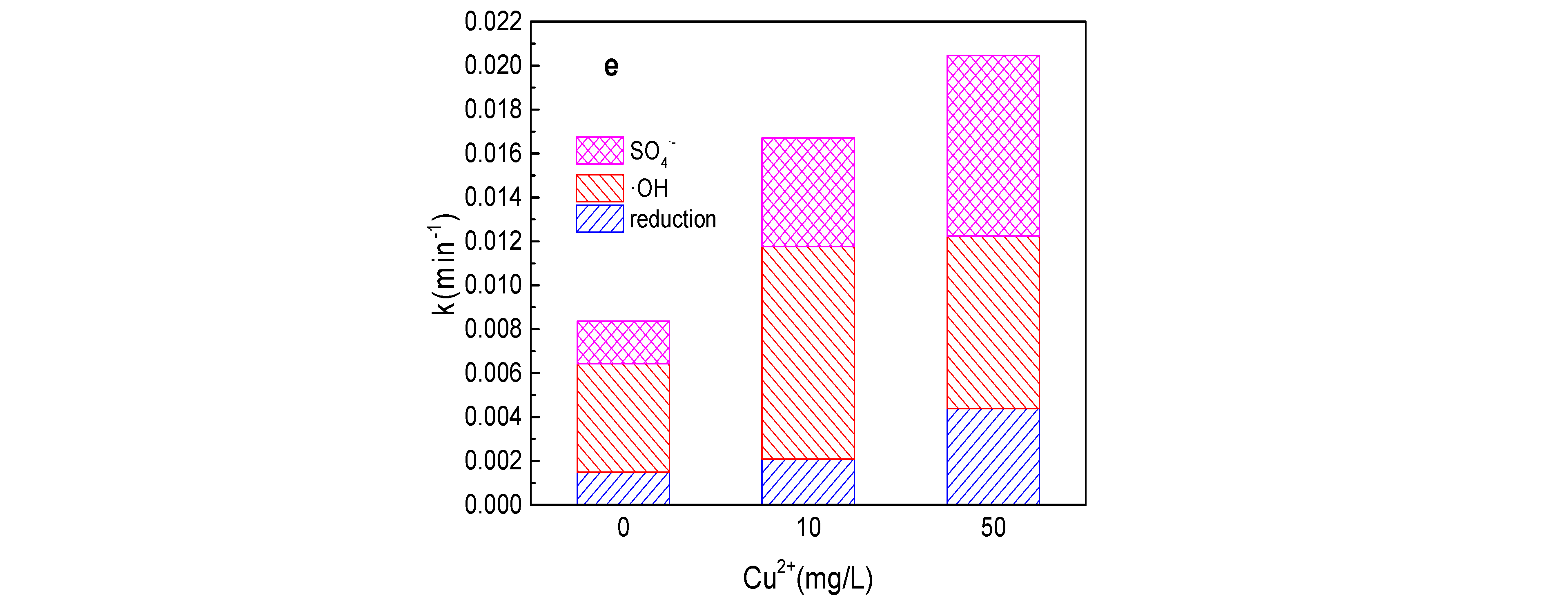
3.6. Effect of Cu2+ on Tartrazine Removal and Major Radicals
As shown in Figure 6a,b, the tartrazine removal and value of k increased from 43.1%, 0.0084 min−1 to 49.2%, 0.010 min−1, 60.6%, 0.015 min−1; 64.3%, 0.016 min−1; 66.9%, 0.018 min−1; and 70.8%, 0.020 min−1, respectively, while the Cu2+ concentration increased from 0 to 1, 5, 10, 20, and 50 mg/L in the Fe0/PMS system. The tartrazine removal efficiency increased with the addition of Cu2+, which might be mainly because PMS could be also activated by Cu2+ and Cu2+ could also promote the corrosion of Fe0. Meanwhile, Cu2+ could also inhibit SO4•− and •OH via Equations (22) and (23) [25]. In general, the presence of Cu2+ could enhance the tartrazine removal in the Fe0/PMS system. It could found from Figure 6c–e that kreduction, k•OH, and kSO4•− were all enhanced in the presence of Cu2+. Reduction and oxidization by SO4•− and •OH exhibited 12.4%, 21.4%; 29.7%, 38.4%; and 57.9%, 40.2% contributions for tartrazine removal in the Fe0/PMS system in the presence of 10 and 50 mg/L Cu2+. Cu2+ had no obvious effect on the contribution of reduction of SO4•− and •OH on tartrazine removal, which might be because Cu2+ could affect not only the PMS activation, but also the corrosion of Fe0.
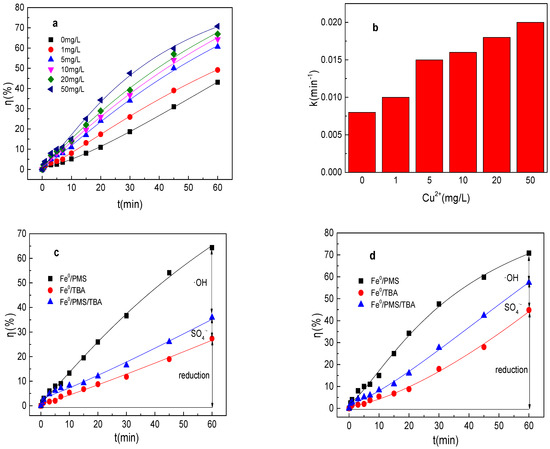
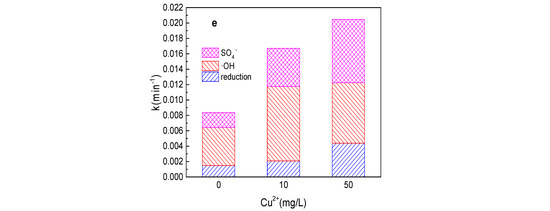
Figure 6.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different Cu2+ concentrations; (b) value of k in the presence of different Cu2+ concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM Cu2+; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
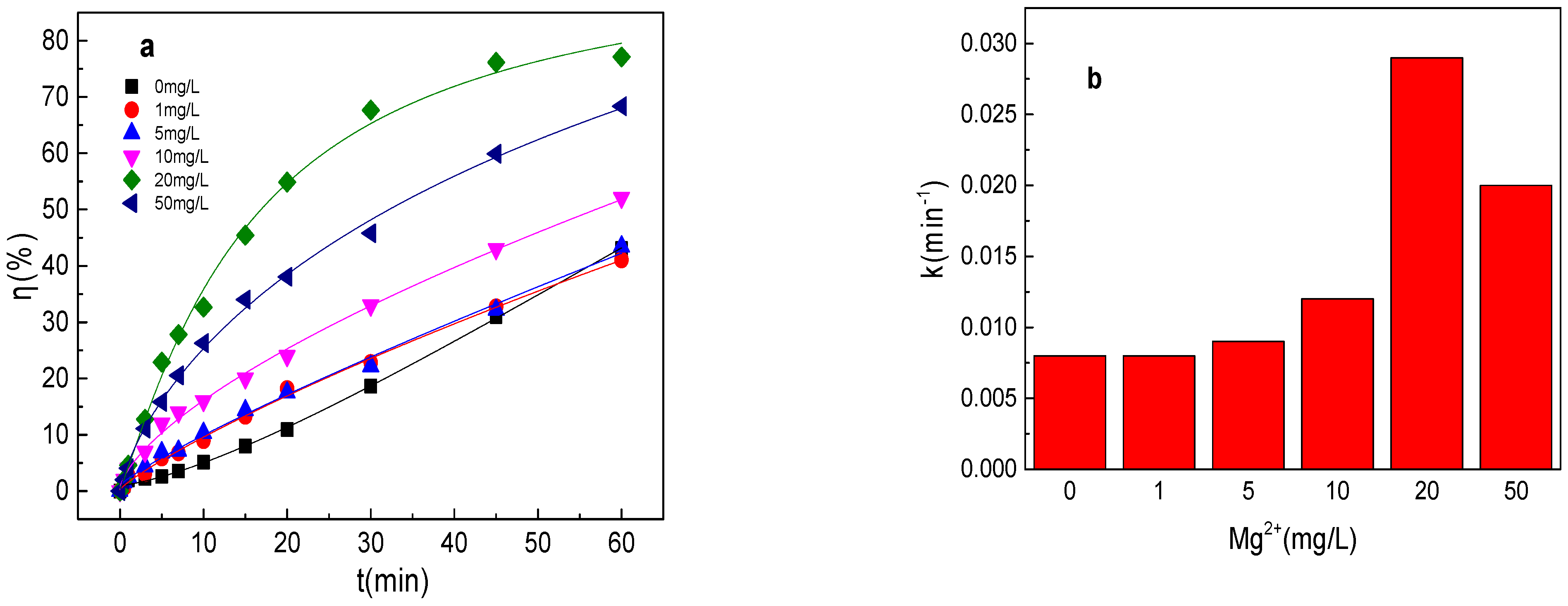
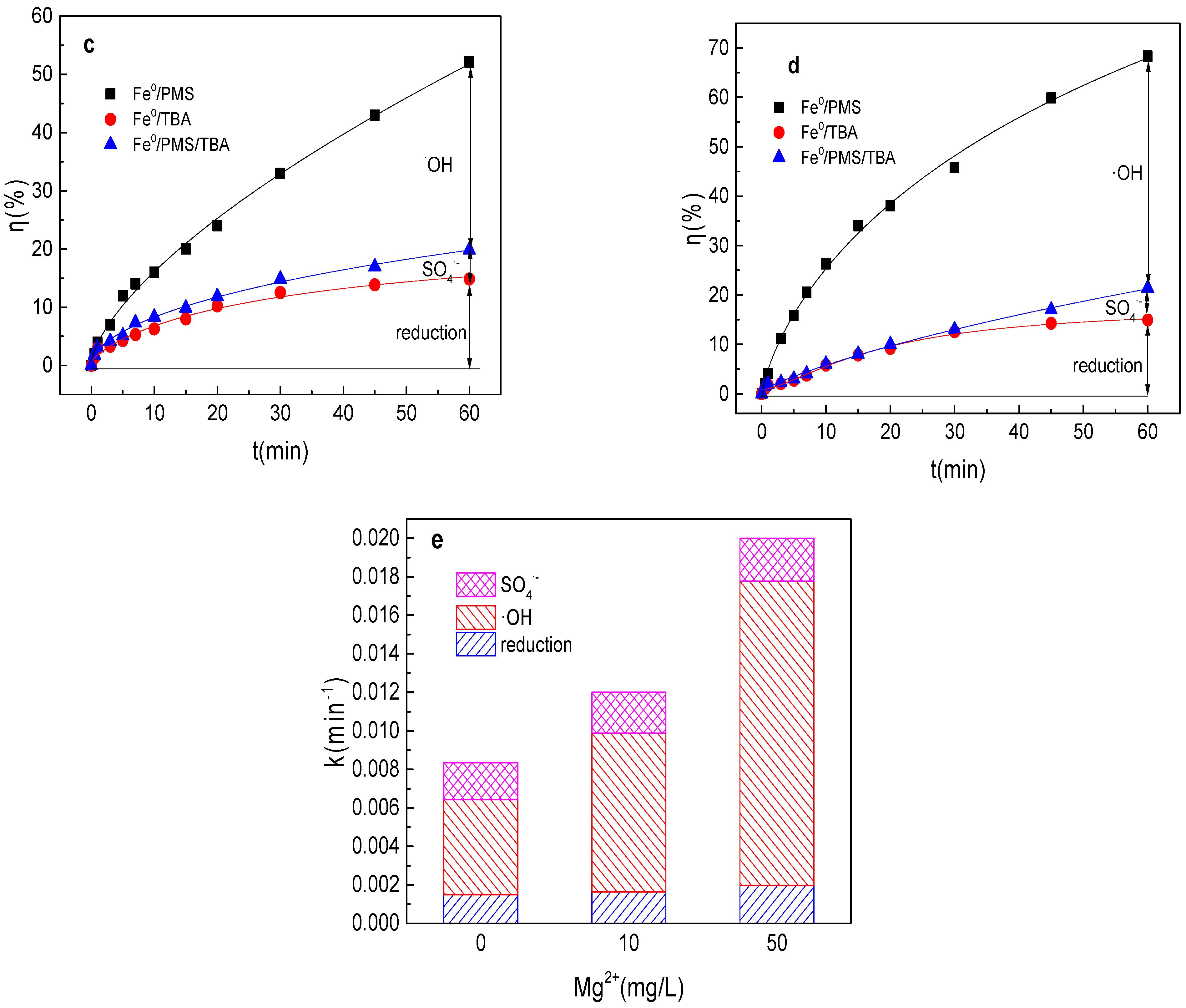
3.7. Effect of Mg2+ on Tartrazine Removal and Major Radicals
As shown in Figure 7a,b, tartrazine removal efficiency increased with the increase in Mg2+ concentration, and tartrazine removal and the value of k were 41.0%, 0.0086 min−1; 43.4%, 0.0091 min−1; 52.1%, 0.012 min−1; 77.1%, 0.029 min−1; and 68.4%, 0.020 min−1 in the presence of 1, 5, 10, 20, and 50 mg/L Mg2+ in the Fe0/PMS system, respectively. Mg2+ was similar to Cu2+, which could activate PMS and enhance the corrosion of Fe0, and thus could improve the Fe0/PMS system for tartrazine removal. The removal efficiency decreased at 50 mg/L Mg2+ in the Fe0/PMS system, which might be because of the reaction between Mg2+ and SO4•− and •OH via Equations (24) and (25) [25]. According to the radical scavenging experiment results from Figure 7c–e, kreduction, k•OH, and kSO4•− were all enhanced in the presence of Mg2+, which also confirmed that the presence of Mg2+ could enhance the corrosion of Fe0 and PMS activation. Moreover, Mg2+ also had no obvious effect of the contribution of reduction of SO4•− and •OH.
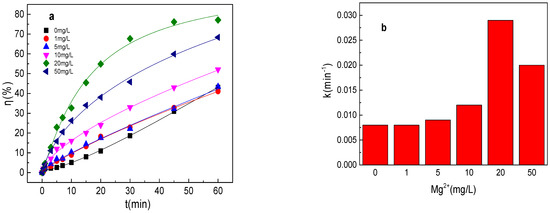
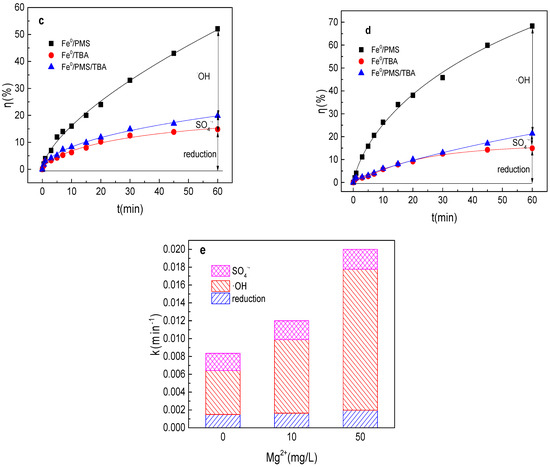
Figure 7.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different Mg2+ concentrations; (b) value of k in the presence of different Mg2+ concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM Mg2+; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
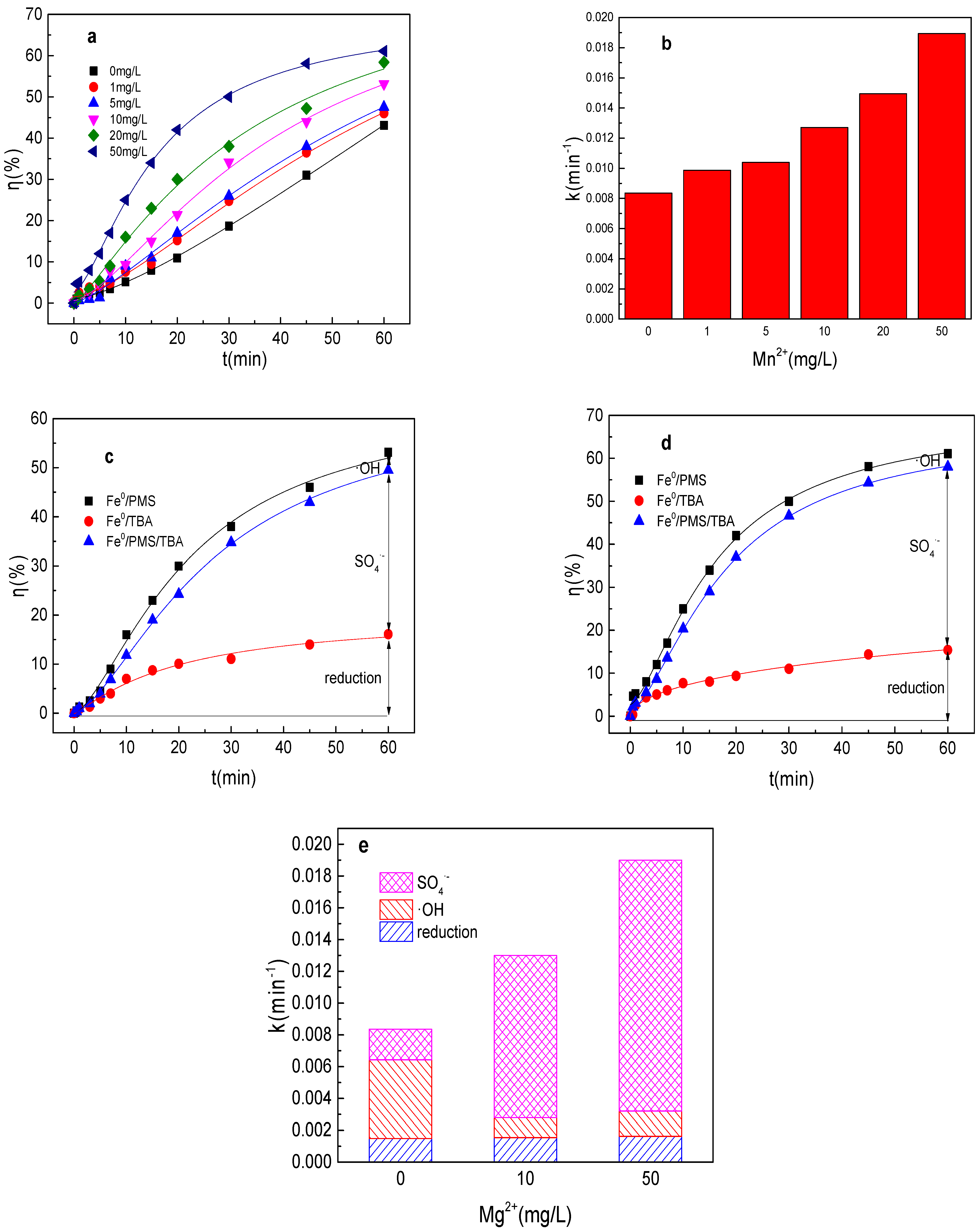
3.8. Effect of Mn2+ on Tartrazine Removal and Major Radicals
It could found from Figure 8a,b that Mn2+ could also significantly enhance the Fe0/PMS system for tartrazine removal. Tartrazine removal and value of k increased from 43.1%, 0.0084 min−1 to 46.1%, 0.0099 min−1; 47.6%, 0.010 min−1; 53.1%, 0.013 min−1; 58.4%, 0.015 min−1; and 61.2%, 0.019 min−1, while Mn2+ concentration increased from 0 to 1, 5, 10, 20, and 50 mg/L in the Fe0/PMS system, respectively. As previous literatures reported that Mn2+ was a good transition metal for PMS activation via Equations (26) and (27), it thus induced the increase in tartrazine removal efficiency in the Fe0/PMS system [29]. Meanwhile, the effect of Mn2+ on the Fe0/PMS system improvement for tartrazine removal was lower than that of Mg2+, which might be because Mn3+ and Mn2+ could react with Fe2+ via Equations (28) and (29), thus affecting its performance [29]. As depicted in Figure 8c–e, both kSO4•− and the contribution of SO4•− were significantly enhanced in the presence of Mn2+. The results also indicate that Mn2+ might affect the activation ability of Fe2+ and become the dominant activated metal.
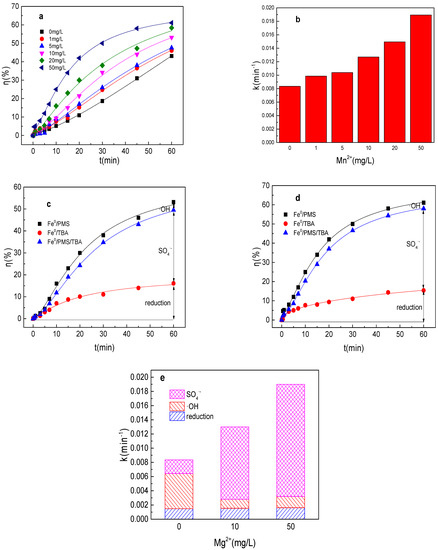
Figure 8.
(a) Tartrazine removal efficiency by the Fe0/PMS process in the presence of different Mn2+ concentrations; (b) value of k in the presence of different Mn2+ concentrations; (c,d) tartrazine removal under different inhibitors in the presence of 5 and 20 mM Mn2+; and (e) contributions of SO4•− and •OH and reduction for tartrazine removal. Condition: tartrazine 50 mg/L, Fe0 0.8 g/L, pH 3, PMS 4 mM, TBA 500 mM.
4. Conclusions
In this study, the effect of anions (SO42−, NO3−, HCO3−, and Cl−) and cations (Ca2+, Cu2+, Mg2+, and Mn2+) on tartrazine removal and major radicals in Fe0/PMS were systematically investigated. SO42−, Cl− Ca2+, Cu2+, Mg2+, and Mn2+ could enhance tartrazine removal; NO3− could inhibit tartrazine removal; and HCO3− had no obvious effect on tartrazine removal. SO42−, HCO3−, Ca2+, Cu2+, and Mg2+ had little effect on the contribution proportion of reduction of SO4•− and •OH; NO3− would decrease the contribution proportion of SO4•− and •OH, a certain concentration Cl− could enhance the contribution proportion of •OH; and a certain concentration of Mn2+ could enhance the contribution proportion of SO4•−. These dates can provide some references for the Fe0/PMS system to treat actual wastewater containing anions and cations.
Author Contributions
Conceptualization, Y.P.; data curation, W.Y.; formal analysis, T.J.; funding acquisition, L.F. and Y.P.; supervision, L.F. and Y.P.; writing—review and editing, W.Y., L.L. and J.X. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Student Innovation Training Program (2021NFUSPITP0758) and China Postdoctoral Science Foundation Project (2020M681552). This study was also financially supported by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Data Availability Statement
Data available on request due to restrictions e.g. privacy or ethical.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martinez, L.B.; Diza, C.B.; Morelos, C.S. Synergy of electrochemical and ozonation processes in industrial wastewater treatment. Chem. Eng. J. 2014, 165, 71–77. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Iftikhar, R.; Ikhlaq, A.; Riaz, I.; Jaleel, R.; Nusrat, R.; Javaid, R. Application of Fe RGO for the removal of dyes by catalytic ozonation process. Environ. Sci. Pollut. Res. 2022, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Con, Y.Q.; Li, Z.; Zhang, Y.; Wang, Q.; Xu, Q. Synthesis of α-Fe2O3/TiO2 nanotube arrays for photoelectro-Fenton degradation of phenol. Chem. Eng. J. 2012, 191, 356–363. [Google Scholar]
- Chang, M.-C.; Shu, H.-Y.; Yu, H.-H. An integrated technique using zero-valent iron and UV/H2O2 sequential process for complete decolorization and mineralization of C.I. Acid Black 24 wastewater. J. Hazard. Mater. 2006, 138, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Y.; Zhou, M.; Cai, J.; Tian, Y. Enhanced removal of emerging contaminants using persulfate activated by UV and pre-magnetized Fe0. Chem. Eng. J. 2019, 361, 908–918. [Google Scholar] [CrossRef]
- Masood, Z.; Ikhlaq, A.; Akram, A.; Qazi, U.Y.; Rizvi, O.S.; Javaid, R.; Alazmi, A.; Madkour, M.; Qi, F. Application of Nanocatalysts in Advanced Oxidation Processes for Wastewater Purification: Challenges and Future Prospects. Catalysts 2022, 12, 741. [Google Scholar] [CrossRef]
- Qazi, U.Y.; Javaid, R.; Ikhlaq, A.; Al-Sodani, K.A.A.; Rizvi, O.S.; Alazmi, A.; Asiri, A.M.; Ibn Shamsah, S.M. Synergistically Improved Catalytic Ozonation Process Using Iron-Loaded Activated Carbons for the Removal of Arsenic in Drinking Water. Water 2022, 14, 2406. [Google Scholar] [CrossRef]
- Fang, X.Y.; Wu, Y.; Xu, L.J.; Gan, L. Fast removal of bisphenol A by coconut shell biochar incorporated α-MnO2 composites via peroxymonosulfate activation. J. Water Process Eng. 2022, 49, 103071. [Google Scholar] [CrossRef]
- Qin, W.; Fang, G.; Wang, Y.; Zhou, D. Mechanistic understanding of polychlorinated biphenyls degradation by peroxymonosulfate activated with CuFe2O4 nanoparticles: Key role of superoxide radicals. Chem. Eng. J. 2018, 348, 526–534. [Google Scholar] [CrossRef]
- Segura, Y.; Martínez, F.; Melero, J.A.; Molina, R.; Chand, R.; Bremner, D.H. Enhancement of the advanced Fenton process (Fe0/H2O2) by ultrasound for the mineralization of phenol. Appl. Catal. B Environ. 2012, 113–114, 100–106. [Google Scholar] [CrossRef]
- Ling, C.; Wu, S.; Dong, T.; Dong, H.; Wang, Z.; Pan, Y.; Han, J. Sulfadiazine removal by peroxymonosulfate activation with sulfide-modified microscale zero-valent iron: Major radicals, the role of sulfur species, and particle size effect. J. Hazard. Mater. 2022, 423, 127082. [Google Scholar] [CrossRef]
- Wang, J.; Hasaer, B.; Yang, M.; Liu, R.; Hu, C.; Liu, H.; Qu, J. Anaerobically-digested sludge disintegration by transition metal ions-activated peroxymonosulfate (PMS): Comparison between Co2+, Cu2+, Fe2+ and Mn2+. Sci. Total Environ. 2020, 713, 136530. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Q.Q.; Lai, L.D.; Yao, G.; Lai, B. Degradation of p-nitrophenol (PNP) in aqueous solution by mFe/Cu-air-PS system. Chin. Chem. Lett. 2019, 30, 1129–1132. [Google Scholar] [CrossRef]
- Huang, W.; Xiao, S.; Zhong, H.; Yan, M.; Yang, X. Activation of persulfates by carbonaceous materials: A review. Chem. Eng. J. 2021, 418, 129297. [Google Scholar] [CrossRef]
- Giannakis, S.; Lin, K.-Y.A.; Ghanbari, F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs). Chem. Eng. J. 2021, 406, 127083. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Liu, Y.-L.; He, P.-N.; Zhang, X.; Wang, L.; Gu, H.-T.; Zhang, H.-C.; Ma, J. Further understanding the role of hydroxylamine in transformation of reactive species in Fe(II)/peroxydisulfate system. Chem. Eng. J. 2021, 418, 129464. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Lin, C.; Zhou, Z.; He, M.; Ouyang, W. Catalytic oxidation of contaminants by Fe0 activated peroxymonosulfate process: Fe(IV) involvement, degradation intermediates and toxicity evaluation. Chem. Eng. J. 2020, 382, 123013. [Google Scholar] [CrossRef]
- Cao, J.; Lai, L.; Lai, B.; Yao, G.; Chen, X.; Song, L. Degradation of tetracycline by peroxymonosulfate activated with zero-valent iron: Performance, intermediates, toxicity and mechanism. Chem. Eng. J. 2019, 364, 45–56. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Zhao, P.; Zhou, P.; Liu, Y.; Cheng, X.; Wang, J.; Yang, B.; Guo, H. Enhanced kinetic performance of peroxymonosulfate/ZVI system with the addition of copper ions: Reactivity, mechanism, and degradation pathways. J. Hazard. Mater. 2020, 393, 122399. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Q.; Zhou, M.; Cai, J.; Tian, Y.; Zhang, Y. Kinetic and mechanism study of UV/pre-magnetized-Fe0/oxalate for removing sulfamethazine. J. Hazard. Mater. 2020, 398, 122931. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhao, Q.; Du, J.; Wang, H.; Li, X.; Ren, N. Enhanced removal of sulfadiazine by sulfidated ZVI activated persulfate process: Performance, mechanisms and degradation pathways. Chem. Eng. J. 2020, 388, 124303. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhou, M.H.; Li, X.; Xu, L.T.; Tang, Z.X.; Liu, M.M. Novel Fenton-like process (pre-magnetized Fe0/H2O2) for efficient degradation of organic pollutants. Sep. Purif. Technol. 2016, 169, 83–92. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhou, M.H.; Cai, J.J.; Li, X.; Wang, W.; Li, B.; Sheng, X.J.; Tang, Z.X. Significant enhancement in treatment of salty wastewater by pre-magnetization Fe0/H2O2 process. Chem. Eng. J. 2018, 339, 411–423. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhou, M.H.; Zhang, Y.; Cai, J.J.; Li, B.; Sheng, X.J. Enhanced degradation of Rhodamine B by pre-magnetized Fe0/PS process: Parameters optimization, mechanism and interferences of ions. Sep. Purif. Technol. 2018, 203, 66–74. [Google Scholar] [CrossRef]
- Fan, X.; Guan, X.; Ma, J.; Ai, H. Kinetics and corrosion products of aqueous nitrate reduction by iron powder without reaction conditions control. J. Environ. Sci. 2009, 21, 1028–1035. [Google Scholar] [CrossRef]
- Long, X.; Xiong, Z.; Huang, R.; Yu, Y.; Zhou, P.; Zhang, H.; Yao, G.; Lai, B. Sustainable Fe(III)/Fe(II) cycles triggered by co-catalyst of weak electrical current in Fe(III)/peroxymonosulfate system: Collaboration of radical and non-radical mechanisms. Appl. Catal. B Environ. 2022, 327, 121716. [Google Scholar] [CrossRef]
- Pan, Y.W.; Zhang, Y.; Zhou, M.H.; Cai, J.J.; Tian, Y.S. Enhanced removal of antibiotics from secondary wastewater effluents by novel UV/pre-magnetized Fe0/H2O2 process. Water Res. 2019, 153, 144–159. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, X.; Yan, Y.; Sun, C.; Wu, H.; He, J.; Wang, D. Heterogeneous activation of peroxymonosulfate by different ferromanganese oxides for tetracycline degradation: Structure dependence and catalytic mechanism. Chem. Eng. J. 2018, 348, 263–270. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).