Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose
Abstract
:1. Introduction
2. Ethanol Feedstock
2.1. First-Generation Feedstocks
2.2. Second-Generation Feedstocks
2.3. Third- and Fourth-Generation Feedstocks
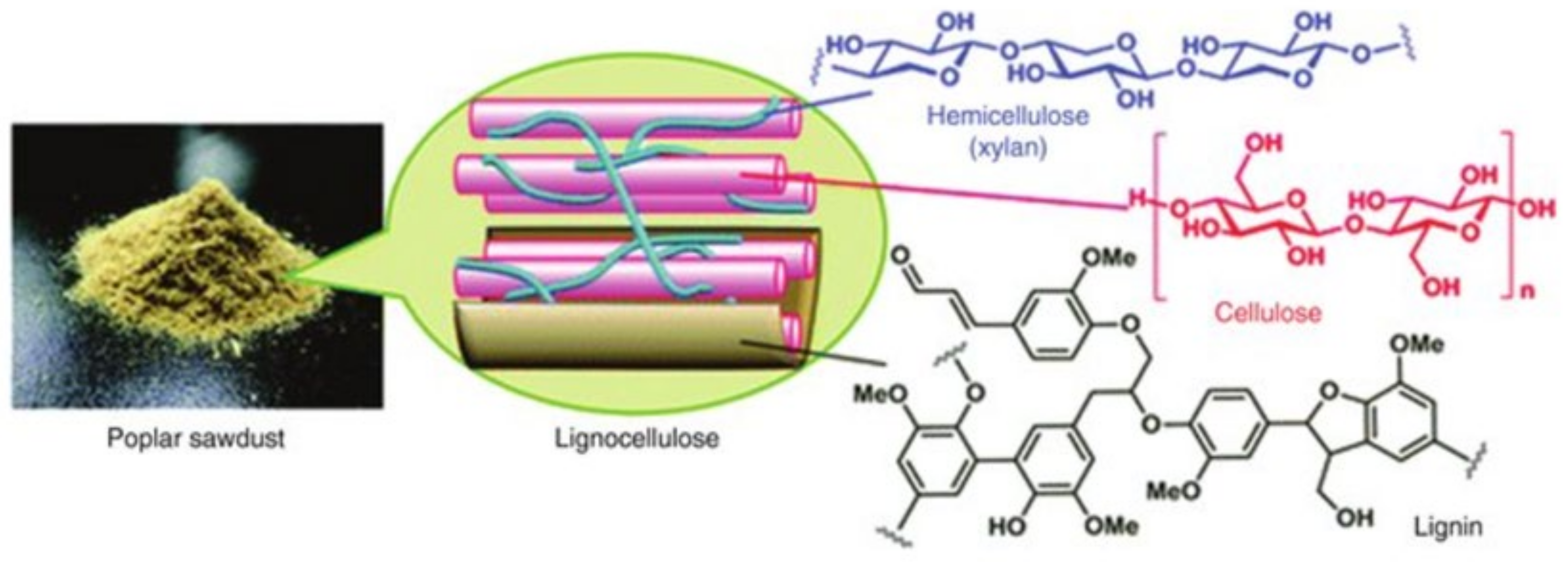
3. Lignocellulose
Structure
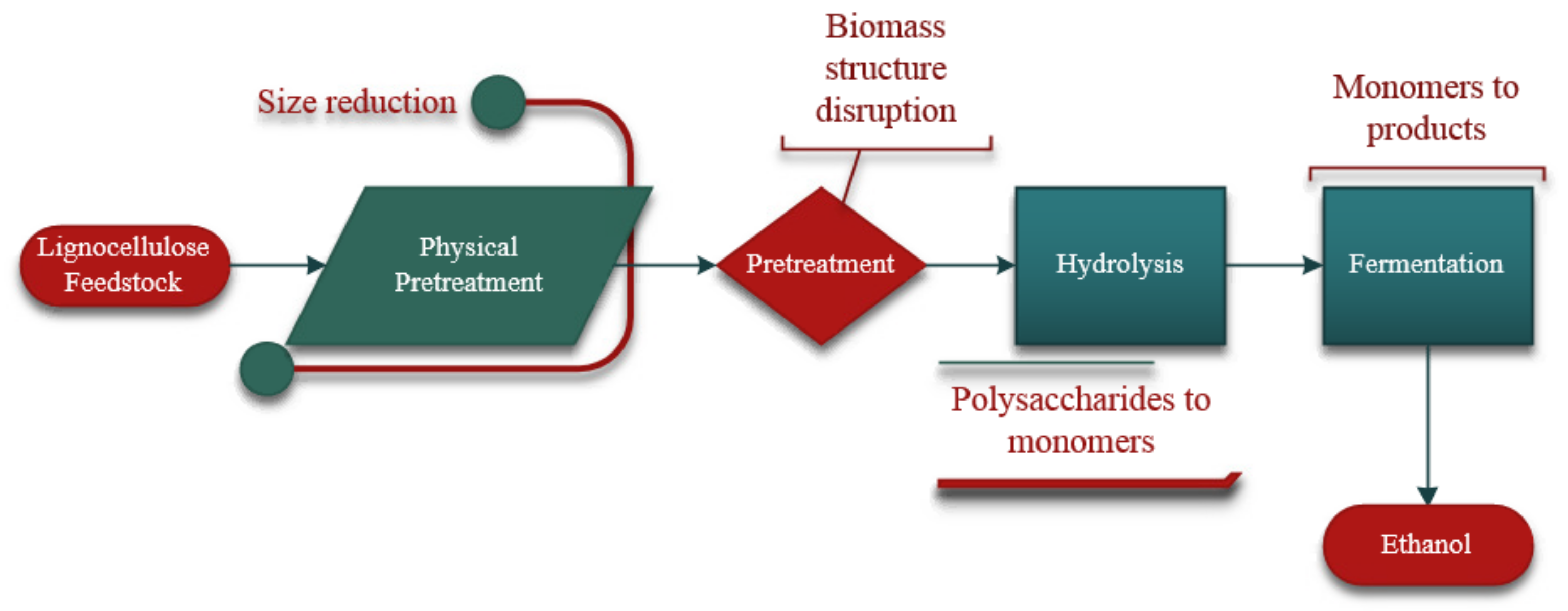
4. Conversion of Lignocellulose to Bioethanol
4.1. Pretreatment
4.1.1. Physical Pretreatment
4.1.2. Chemical Pretreatment
Acidic Pretreatment
Alkaline Pretreatment
Oxidative Pretreatment
4.1.3. Physicochemical Pretreatment
Solvent Fractionation
Steam Explosion
Hydrothermal Pretreatment
4.1.4. Biological Pretreatment
4.2. Hydrolysis
4.2.1. Chemical Hydrolysis
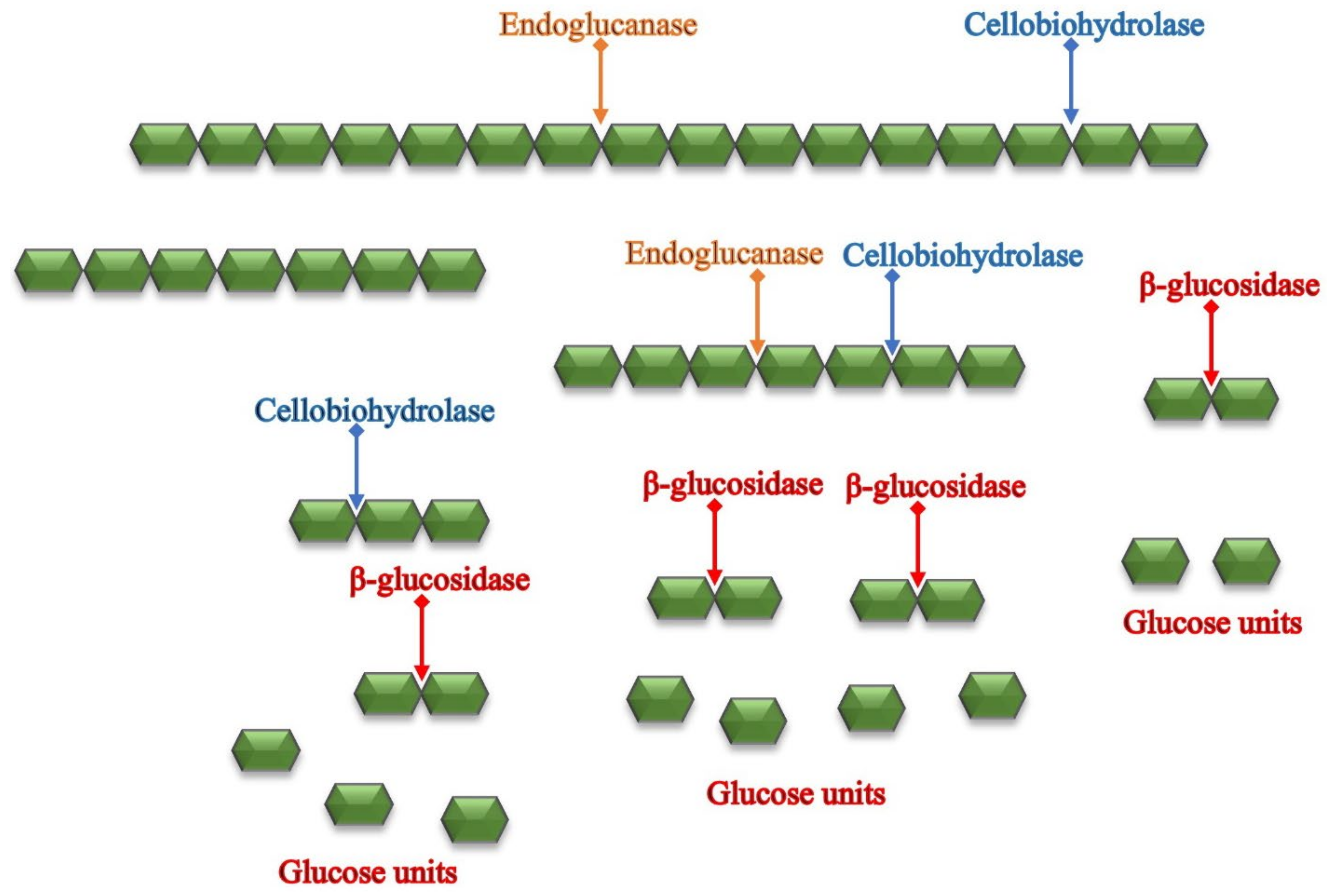
4.2.2. Enzymatic Hydrolysis
4.3. Enzyme Kinetics and Modeling
5. Lignocellulose Enzymatic Hydrolysis Challenges and Potential Solutions
5.1. Heterogeneous Mixture
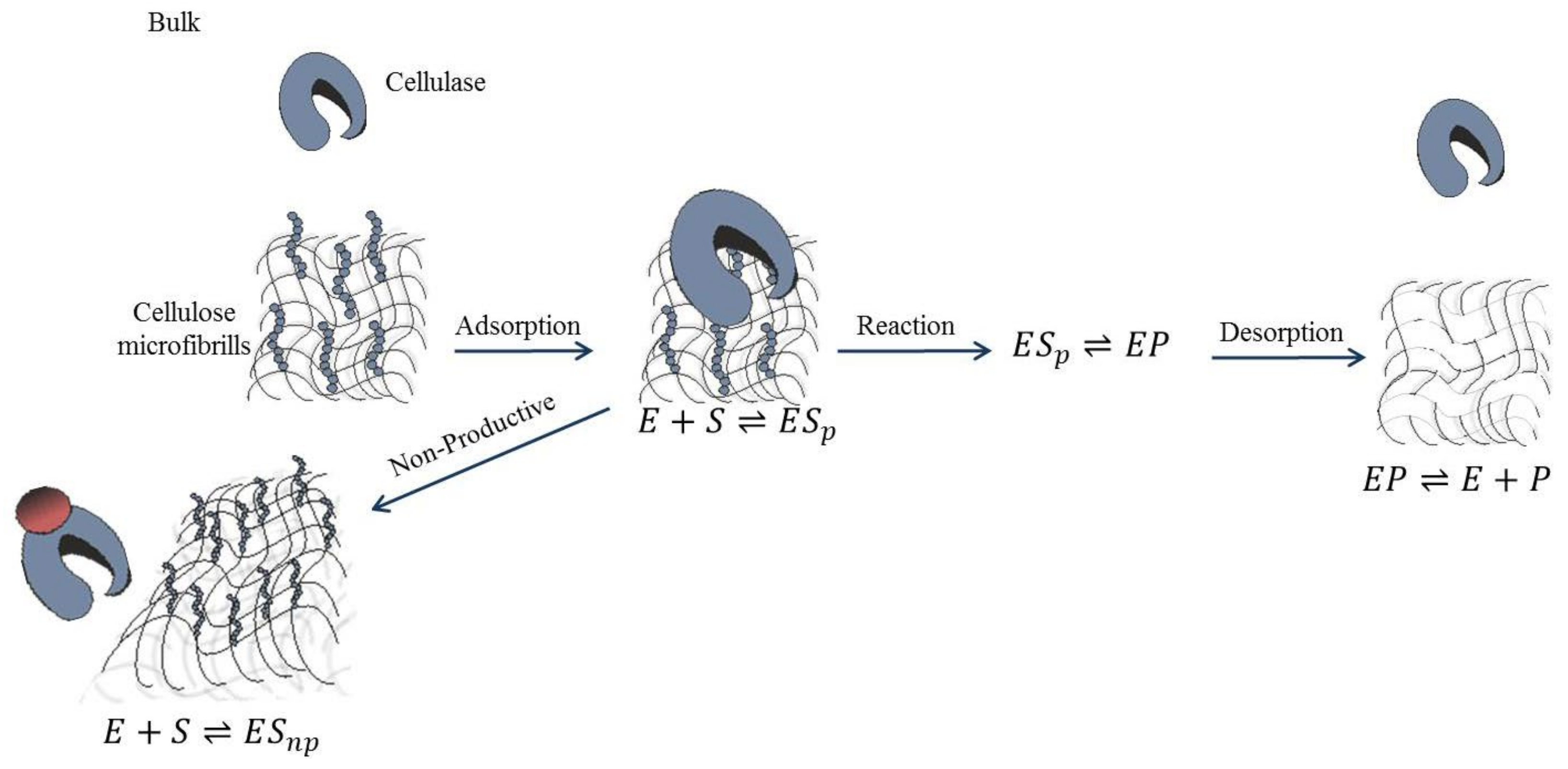
5.2. Enzyme Inhibition
5.3. Immobilization: A Solution to the Challenges of Heterogeneous Mixtures
5.4. Membrane Technology: A Solution to the Challenges of Product Inhibition
5.4.1. MBRs Configurations
5.4.2. Membrane Selection
5.4.3. Key Factors Affecting the Performance of MBRs
6. MBRs Prospects
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hanif, I. Impact of fossil fuels energy consumption, energy policies, and urban sprawl on carbon emissions in East Asia and the Pacific: A panel investigation. Energy Strat. Rev. 2018, 21, 16–24. [Google Scholar] [CrossRef]
- Solarin, S.A.; Al-Mulali, U.; Gan, G.G.G.; Shahbaz, M. The impact of biomass energy consumption on pollution: Evidence from 80 developed and developing countries. Environ Sci Pollut Res. 2018, 25, 22641–22657. [Google Scholar] [CrossRef] [PubMed]
- García-Olivares, A.; Solé, J.; Osychenko, O. Transportation in a 100% renewable energy system. Energy Convers. Manag. 2018, 158, 266–285. [Google Scholar] [CrossRef]
- Mączyńska, J.; Krzywonos, M.; Kupczyk, A.; Tucki, K.; Sikora, M.; Pińkowska, H.; Bączyk, A.; Wielewska, I. Production and use of biofuels for transport in Poland and Brazil—The case of bioethanol. Fuel 2019, 241, 989–996. [Google Scholar] [CrossRef]
- Wang, Z. Does biomass energy consumption help to control environmental pollution? Evidence from BRICS countries. Sci. Total Environ. 2019, 670, 1075–1083. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A.; Ankaram, S.; Duan, Y.; Awasthi, M.K. Chapter 5—Biofuel Production From Biomass: Toward Sustainable Development. In Current Developments in Biotechnology and Bioengineering; Kumar, S., Kumar, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 79–92. [Google Scholar]
- Ong, H.C.; Chen, W.-H.; Singh, Y.; Gan, Y.Y.; Chen, C.-Y.; Show, P.L. A state-of-the-art review on thermochemical conversion of biomass for biofuel production: A TG-FTIR approach. Energy Convers. Manag. 2020, 209, 112634. [Google Scholar] [CrossRef]
- Hadar, Y. Sources for Lignocellulosic Raw Materials for the Production of Ethanol. In Lignocellulose Conversion: Enzymatic and Microbial Tools for Bioethanol Production; Faraco, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 21–38. [Google Scholar]
- Yang, H.; Zhang, X.; Luo, H.; Liu, B.; Shiga, T.M.; Li, X.; Kim, J.I.; Rubinelli, P.; Overton, J.C.; Subramanyam, V.; et al. Overcoming cellulose recalcitrance in woody biomass for the lignin-first biorefinery. Biotechnol. Biofuels 2019, 12, 171. [Google Scholar] [CrossRef]
- Sathitsuksanoh, N.; George, A.; Zhang, Y.-H.P. New lignocellulose pretreatments using cellulose solvents: A review. J. Chem. Technol. Biotechnol. 2013, 88, 169–180. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Wertz, J.-L.; Bédué, O. Lignocellulosic Biorefineries; EPFL Press: New York, NY, USA, 2013. [Google Scholar]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Kumar, R.; Dhurandhar, R.; Chakrabortty, S.; Ghosh, A.K. Chapter 12—Downstream process: Toward cost/energy effectiveness. In Handbook of Biofuels; Sahay, S., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 249–260. [Google Scholar]
- Mussatto, S.I.; Teixeira, J.A. Lignocellulose as raw material in fermentation processes. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Formatex Research Center: Badajoz, Spain, 2010; p. 11. [Google Scholar]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Chang, R.; Gross, A.S.; Chu, J.-W. Degree of Polymerization of Glucan Chains Shapes the Structure Fluctuations and Melting Thermodynamics of a Cellulose Microfibril. J. Phys. Chem. B. 2012, 116, 8074–8083. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. BioResources 2007, 2, 472–499. [Google Scholar]
- Schläfle, S.; Tervahartiala, T.; Senn, T.; Kölling-Paternoga, R. Quantitative and visual analysis of enzymatic lignocellulose degradation. Biocatal. Agric. Biotechnol. 2017, 11, 42–49. [Google Scholar] [CrossRef]
- Yu, J.; Paterson, N.; Blamey, J.; Millan, M. Cellulose, xylan and lignin interactions during pyrolysis of lignocellulosic biomass. Fuel 2017, 191, 140–149. [Google Scholar] [CrossRef]
- Gall, D.L.; Ralph, J.; Donohue, T.J.; Noguera, D.R. Biochemical transformation of lignin for deriving valued commodities from lignocellulose. Curr. Opin. Biotechnol. 2017, 45, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- da Costa Sousa, L.; Chundawat, S.P.; Balan, V.; Dale, B.E. ‘Cradle-to-grave’assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 2009, 20, 339–347. [Google Scholar] [CrossRef]
- Gaikwad, A. Effect of Particle Size on the Kinetics of Enzymatic Hydrolysis of Microcrystalline Cotton Cellulose: A Modeling and Simulation Study. Appl. Biochem. Biotechnol. 2019, 187, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef]
- Lloyd, T.A.; Wyman, C.E. Combined sugar yields for dilute sulfuric acid pretreatment of corn stover followed by enzymatic hydrolysis of the remaining solids. Bioresour. Technol. 2005, 96, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.P.; Bura, R.; Mabee, W.E.; Berlin, A.; Pan, X.; Saddler, J.N. Substrate Pretreatment: The Key to Effective Enzymatic Hydrolysis of Lignocellulosics. In Biofuels; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 67–93. [Google Scholar]
- Hsu, T.-C.; Guo, G.-L.; Chen, W.-H.; Hwang, W.-S. Effect of dilute acid pretreatment of rice straw on structural properties and enzymatic hydrolysis. Bioresour. Technol. 2010, 101, 4907–4913. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Zhang, X.; Tan, T. The correlation between cellulose allomorphs (I and II) and conversion after removal of hemicellulose and lignin of lignocellulose. Bioresour. Technol. 2015, 193, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dai, X.; Zhou, S.-L.; Gan, Y.-Y.; Xiong, Z.-Y.; Qin, Y.-H.; Ma, J.; Yang, L.; Wu, Z.-K.; Wang, T.-L. Ultrasound-assisted alkaline pretreatment for enhancing the enzymatic hydrolysis of rice straw by using the heat energy dissipated from ultrasonication. Bioresour. Technol. 2017, 241, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Raj, K.; Krishnan, C. High sugar yields from sugarcane (Saccharum officinarum) bagasse using low-temperature aqueous ammonia pretreatment and laccase-mediator assisted enzymatic hydrolysis. Ind. Crops Prod. 2018, 111, 673–683. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- An, S.; Li, W.; Liu, Q.; Xia, Y.; Zhang, T.; Huang, F.; Lin, Q.; Chen, L. Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour. Technol. 2019, 271, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Aaltonen, O.; Ylinen, P. Organosolv pulping—Methods and pulp properties. Biomass 1987, 13, 45–65. [Google Scholar] [CrossRef]
- Sun, F.; Chen, H. Organosolv pretreatment by crude glycerol from oleochemicals industry for enzymatic hydrolysis of wheat straw. Bioresour. Technol. 2008, 99, 5474–5479. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Um, B.H.; Im, D.J.; Lee, J.H.; Oh, K.K. Combined Ball Milling and Ethanol Organosolv Pretreatment to Improve the Enzymatic Digestibility of Three Types of Herbaceous Biomass. Energies 2018, 11, 2457. [Google Scholar] [CrossRef]
- Tang, C.; Shan, J.; Chen, Y.; Zhong, L.; Shen, T.; Zhu, C.; Ying, H. Organic amine catalytic organosolv pretreatment of corn stover for enzymatic saccharification and high-quality lignin. Bioresour. Technol. 2017, 232, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Zhu, Z.; Sathitsuksanoh, N.; Vinzant, T.; Schell, D.J.; McMillan, J.D.; Zhang, Y.-H.P. Comparative study of corn stover pretreated by dilute acid and cellulose solvent-based lignocellulose fractionation: Enzymatic hydrolysis, supramolecular structure, and substrate accessibility. Biotechnol. Bioeng. 2009, 103, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Rollin, J.A.; Zhu, Z.; Sathitsuksanoh, N.; Zhang, Y.-H.P. Increasing cellulose accessibility is more important than removing lignin: A comparison of cellulose solvent-based lignocellulose fractionation and soaking in aqueous ammonia. Biotechnol. Bioeng. 2011, 108, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, R.; Cheng, T.; Guo, J.; Xian, M.; Liu, H. Imidazolium-based ionic liquids for cellulose pretreatment: Recent progresses and future perspectives. Appl. Microbiol. Biotechnol. 2017, 101, 521–532. [Google Scholar] [CrossRef]
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714. [Google Scholar] [CrossRef]
- Grewal, J.; Ahmad, R.; Khare, S.K. Development of cellulase-nanoconjugates with enhanced ionic liquid and thermal stability for in situ lignocellulose saccharification. Bioresour. Technol. 2017, 242, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Husson, E.; Auxenfans, T.; Herbaut, M.; Baralle, M.; Lambertyn, V.; Rakotoarivonina, H.; Rémond, C.; Sarazin, C. Sequential and simultaneous strategies for biorefining of wheat straw using room temperature ionic liquids, xylanases and cellulases. Bioresour. Technol. 2018, 251, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Mol, A.; Schulten, E.; Happel, A.; Mussatto, S.I. Bench scale steam explosion pretreatment of acid impregnated elephant grass biomass and its impacts on biomass composition, structure and hydrolysis. Ind. Crops Prod. 2017, 106, 48–58. [Google Scholar] [CrossRef]
- Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I.; Chamorro, M.Á.; Sáez, F.; Ballesteros, M.; Moreno, A.D. A Sequential Steam Explosion and Reactive Extrusion Pretreatment for Lignocellulosic Biomass Conversion within a Fermentation-Based Biorefinery Perspective. Fermentation 2017, 3, 15. [Google Scholar] [CrossRef]
- Scholl, J.L.; Menegol, D.; Pitarelo, A.P.; Fontana, R.C.; Filho, A.Z.; Ramos, L.P.; Dillon, A.J.P.; Camassola, M. Ethanol production from sugars obtained during enzymatic hydrolysis of elephant grass (Pennisetum purpureum, Schum.) pretreated by steam explosion. Bioresour. Technol. 2015, 192, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Stanley, H.; Ezeife, C.; Onwukwe, C. Bioethanol Production from Elephant Grass (Pennisetum purpureum). Niger. J. Biotechnol. 2017, 32, 1–6. [Google Scholar] [CrossRef]
- Montipó, S.; Ballesteros, I.; Fontana, R.C.; Liu, S.; Martins, A.F.; Ballesteros, M.; Camassola, M. Integrated production of second generation ethanol and lactic acid from steam-exploded elephant grass. Bioresour. Technol. 2018, 249, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Zhang, H.; Zhao, J.; Lei, M.; Huang, H. Direct and simultaneous determination of representative byproducts in a lignocellulosic hydrolysate of corn stover via gas chromatography–mass spectrometry with a Deans switch. J. Chromatogr. A 2011, 1218, 5319–5327. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Li, Y.; Zhang, H.; Zheng, H.; Huang, H. Factors to decrease the cellulose conversion of enzymatic hydrolysis of lignocellulose at high solid concentrations. Cellulose 2014, 21, 2409–2417. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Sun, S.; Cao, X.; Sun, R. Effect of hydrothermal pretreatment on the structural changes of alkaline ethanol lignin from wheat straw. Sci. Rep. 2016, 6, 39354. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.K.; Xu, C.; Qin, W. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valor. 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Maibam, P.D.; Maiti, S.K. A Strategy for Simultaneous Xylose Utilization and Enhancement of Cellulase Enzyme Production by Trichoderma reesei Cultivated on Liquid Hydrolysate Followed by Induction with Feeding of Solid Sugarcane Bagasse. Waste Biomass Valor. 2020, 11, 3151–3160. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Supriya, R.D.; Sindhu, R.; Binod, P.; Nair, R.B.; Pandey, A.; Gnansounou, E. Chapter 7—Biological pretreatment of lignocellulosic biomass—Current trends and future perspectives. In Second and Third Generation of Feedstocks; Basile, A., Dalena, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 197–212. [Google Scholar]
- Machado, A.d.S.; Ferraz, A. Biological pretreatment of sugarcane bagasse with basidiomycetes producing varied patterns of biodegradation. Bioresour. Technol. 2017, 225, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Wu, Y.; Ge, S.; Xia, C.; Mei, C.; Kim, K.-H.; Cai, L.; Smith, L.M.; Lee, J.; Shi, S.Q. Application of intermittent ball milling to enzymatic hydrolysis for efficient conversion of lignocellulosic biomass into glucose. Renew. Sustain. Energy Rev. 2021, 136, 110442. [Google Scholar] [CrossRef]
- Fei, X.; Jia, W.; Wang, J.; Chen, T.; Ling, Y. Study on enzymatic hydrolysis efficiency and physicochemical properties of cellulose and lignocellulose after pretreatment with electron beam irradiation. Int. J. Biol. Macromol. 2020, 145, 733–739. [Google Scholar] [CrossRef]
- Gu, H.; An, R.; Bao, J. Pretreatment refining leads to constant particle size distribution of lignocellulose biomass in enzymatic hydrolysis. Chem. Eng. J. 2018, 352, 198–205. [Google Scholar] [CrossRef]
- Manmai, N.; Unpaprom, Y.; Ponnusamy, V.K.; Ramaraj, R. Bioethanol production from the comparison between optimization of sorghum stalk and sugarcane leaf for sugar production by chemical pretreatment and enzymatic degradation. Fuel 2020, 278, 118262. [Google Scholar] [CrossRef]
- Sołowski, G.; Konkol, I.; Cenian, A. Production of hydrogen and methane from lignocellulose waste by fermentation. A review of chemical pretreatment for enhancing the efficiency of the digestion process. J. Clean. Prod. 2020, 267, 121721. [Google Scholar] [CrossRef]
- Jørgensen, H.; Kristensen, J.B.; Felby, C. Enzymatic conversion of lignocellulose into fermentable sugars: Challenges and opportunities. Biofuels Bioprod. Biorefining 2007, 1, 119–134. [Google Scholar] [CrossRef]
- Srivastava, N.; Srivastava, M.; Mishra, P.; Gupta, V.K.; Molina, G.; Rodriguez-Couto, S.; Manikanta, A.; Ramteke, P. Applications of fungal cellulases in biofuel production: Advances and limitations. Renew. Sustain. Energy Rev. 2018, 82, 2379–2386. [Google Scholar] [CrossRef]
- Tiwari, R.; Nain, L.; Labrou, N.E.; Shukla, P. Bioprospecting of functional cellulases from metagenome for second generation biofuel production: A review. Crit. Rev. Microbiol. 2018, 44, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Rosgaard, L.; Pedersen, S.; Cherry, J.R.; Harris, P.; Meyer, A.S. Efficiency of new fungal cellulase systems in boosting enzymatic degradation of barley straw lignocellulose. Biotechnol. Prog. 2006, 22, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, C.A.; Rocha, V.A.; Groposo, C.; de Castro, A.M.; Pereira, N.P., Jr. Enzymes and accessory proteins involved in the hydrolysis of lignocellulosic biomass for bioethanol production. Mycol. Curr. Future Dev. 2016, 1, 23–56. [Google Scholar]
- Jeoh, T.; Cardona, M.J.; Karuna, N.; Mudinoor, A.R.; Nill, J. Mechanistic kinetic models of enzymatic cellulose hydrolysis—A review. Biotechnol. Bioeng. 2017, 114, 1369–1385. [Google Scholar] [CrossRef]
- Al-Zuhair, S. The effect of crystallinity of cellulose on the rate of reducing sugars production by heterogeneous enzymatic hydrolysis. Bioresour. Technol. 2008, 99, 4078–4085. [Google Scholar] [CrossRef]
- Petrášek, Z.; Eibinger, M.; Nidetzky, B. Modeling the activity burst in the initial phase of cellulose hydrolysis by the processive cellobiohydrolase Cel7A. Biotechnol. Bioeng. 2019, 116, 515–525. [Google Scholar] [CrossRef]
- Praestgaard, E.; Elmerdahl, J.; Murphy, L.; Nymand, S.; McFarland, K.C.; Borch, K.; Westh, P. A kinetic model for the burst phase of processive cellulases. FEBS J. 2011, 278, 1547–1560. [Google Scholar] [CrossRef]
- Bansal, P.; Hall, M.; Realff, M.J.; Lee, J.H.; Bommarius, A.S. Modeling cellulase kinetics on lignocellulosic substrates. Biotechnol. Adv. 2009, 27, 833–848. [Google Scholar] [CrossRef]
- Warden, A.C.; Little, B.A.; Haritos, V.S. A cellular automaton model of crystalline cellulose hydrolysis by cellulases. Biotechnol. Biofuels 2011, 4, 39. [Google Scholar] [CrossRef]
- Yang, B.; Willies, D.M.; Wyman, C.E. Changes in the enzymatic hydrolysis rate of Avicel cellulose with conversion. Biotechnol. Bioeng. 2006, 94, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.A. Kinetic studies on insoluble cellulose–cellulase system. Biotechnol. Bioeng. 1975, 17, 1421–1433. [Google Scholar] [CrossRef] [PubMed]
- Peitersen, N.; Ross, E.W. Mathematical model for enzymatic hydrolysis and fermentation of cellulose by Trichoderma. Biotechnol. Bioeng. 1979, 21, 997–1017. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Analysis of process integration and intensification of enzymatic cellulose hydrolysis in a membrane bioreactor. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 688–698. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Kinetic dynamics in heterogeneous enzymatic hydrolysis of cellulose: An overview, an experimental study and mathematical modelling. Process Biochem. 2003, 38, 1003–1018. [Google Scholar] [CrossRef]
- van Zyl, J.M.; van Rensburg, E.; van Zyl, W.H.; Harms, T.M.; Lynd, L.R. A Kinetic Model for Simultaneous Saccharification and Fermentation of Avicel With Saccharomyces cerevisiae. Biotechnol. Bioeng. 2011, 108, 924–933. [Google Scholar] [CrossRef]
- Zhang, Y.-H.P.; Lynd, L.R. A functionally based model for hydrolysis of cellulose by fungal cellulase. Biotechnol. Bioeng. 2006, 94, 888–898. [Google Scholar] [CrossRef]
- Valentine, J.; Clifton-Brown, J.; Hastings, A.; Robson, P.; Allison, G.; Smith, P. Food vs. fuel: The use of land for lignocellulosic ‘next generation’ energy crops that minimize competition with primary food production. GCB Bioenergy 2012, 4, 1–19. [Google Scholar] [CrossRef]
- Vasile, E.; Bran, Ş.D. Lignocellulose bio resources and renewable energy. Intern. Audit. Risk Manag. 2017, 47, 23–30. [Google Scholar]
- Zhao, X.; Qi, F.; Liu, D. Hierarchy Nano- and Ultrastructure of Lignocellulose and Its Impact on the Bioconversion of Cellulose. In Nanotechnology for Bioenergy and Biofuel Production; Rai, M., da Silva, S.S., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 117–151. [Google Scholar]
- Londo, M.; van Stralen, J.; Uslu, A.; Mozaffarian, H.; Kraan, C. Lignocellulosic biomass for chemicals and energy: An integrated assessment of future EU market sizes, feedstock availability impacts, synergy and competition effects, and path dependencies. Biofuels Bioprod. Biorefining 2018, 12, 1065–1081. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Yang, X.-L.; Dubale, A.A.; Li, R.-F.; Ma, Y.-M.; Wang, L.-M.; Hou, G.-H.; Guan, R.-F.; Xie, M.-H. Hydrolysis of cellulose using cellulase physically immobilized on highly stable zirconium based metal-organic frameworks. Bioresour. Technol. 2018, 270, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Luo, J.; Wan, Y. Immobilization of cellulase on a core-shell structured metal-organic framework composites: Better inhibitors tolerance and easier recycling. Bioresour. Technol. 2018, 268, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases immobilization on chitosan-coated magnetic nanoparticles: Application for Agave Atrovirens lignocellulosic biomass hydrolysis. Bioprocess. Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tan, Z.; Feng, H.; Qiu, J. Cellulose as a template to fabricate a cellulase-immobilized composite with high bioactivity and reusability. New J. Chem. 2018, 42, 1665–1672. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Northrop, D.B. On the meaning of Km and V/K in enzyme kinetics. J. Chem. Educ. 1998, 75, 1153. [Google Scholar] [CrossRef]
- Shi, J.; Wu, D.; Zhag, L.; Simmons, B.A.; Singh, S.; Yang, B.; Wyman, C.E. Dynamic changes of substrate reactivity and enzyme adsorption on partially hydrolyzed cellulose. Biotechnol. Bioeng. 2017, 114, 503–515. [Google Scholar] [CrossRef]
- Haldar, D.; Gayen, K.; Sen, D. Enumeration of monosugars’ inhibition characteristics on the kinetics of enzymatic hydrolysis of cellulose. Process Biochem. 2018, 72, 130–136. [Google Scholar] [CrossRef]
- Gan, Q.; Allen, S.; Taylor, G. Design and operation of an integrated membrane reactor for enzymatic cellulose hydrolysis. Biochem. Eng. J. 2002, 12, 223–229. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Wang, G.; Zhao, C.; Ma, Y.; Yang, W. Immobilization of cellulase on styrene/maleic anhydride copolymer nanoparticles with improved stability against pH changes. Chem. Eng. J. 2018, 336, pp. 152–159. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.P.; da Silva, S.S.; Rai, M. Immobilized Nanoparticles-Mediated Enzymatic Hydrolysis of Cellulose for Clean Sugar Production: A Novel Approach. Curr. Nanosci. 2019, 15, 296–303. [Google Scholar] [CrossRef]
- Guo, R.; Zheng, X.; Wang, Y.; Yang, Y.; Ma, Y.; Zou, D.; Liu, Y. Optimization of Cellulase Immobilization with Sodium Alginate-Polyethylene for Enhancement of Enzymatic Hydrolysis of Microcrystalline Cellulose Using Response Surface Methodology. Appl. Biochem. Biotechnol. 2021, 193, 2043–2060. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.P.; Pawar, K.D. Immobilization of cellulase on iron tolerant Pseudomonas stutzeri biosynthesized photocatalytically active magnetic nanoparticles for increased thermal stability. Mater. Sci. Eng. C 2020, 106, 110169. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Chang, R.; Tsai, W.-B. Poly(acrylic acid) nanogel as a substrate for cellulase immobilization for hydrolysis of cellulose. Colloids Surf. B Biointerfaces 2017, 152, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kang, J.; Yang, B.; Zhao, L.; Hou, Z.; Tang, B. Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob. Chin. J. Catal. 2016, 37, 389–397. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, J.; Feng, H.; Zang, L.; Sakai, E. Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J. Magn. Magn. Mater. 2015, 375, 117–123. [Google Scholar] [CrossRef]
- Ladole, M.R.; Mevada, J.S.; Pandit, A.B. Ultrasonic hyperactivation of cellulase immobilized on magnetic nanoparticles. Bioresour. Technol. 2017, 239, 117–126. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.-K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Liao, H.; Chen, D.; Yuan, L.; Zheng, M.; Zhu, Y.; Liu, X. Immobilized cellulase by polyvinyl alcohol/Fe2O3 magnetic nanoparticle to degrade microcrystalline cellulose. Carbohydr. Polym. 2010, 82, 600–604. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Z.; Su, C.; Feng, Z.; Wang, H.; Yu, J.; Su, W. High yielding, one-step mechano-enzymatic hydrolysis of cellulose to cellulose nanocrystals without bulk solvent. Bioresour. Technol. 2021, 331, 125015. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chang, P.; Zhu, X.; Zhang, S. Anemone-inspired enzymatic film for cellulose heterogeneous catalysis. Carbohydr. Polym. 2021, 260, 117795. [Google Scholar] [CrossRef] [PubMed]
- Rajnish, K.N.; Samuel, M.S.; John J, A.; Datta, S.; Chandrasekar, N.; Balaji, R.; Jose, S.; Selvarajan, E. Immobilization of cellulase enzymes on nano and micro-materials for breakdown of cellulose for biofuel production-a narrative review. Int. J. Biol. Macromol. 2021, 182, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Bhagia, S.; Wyman, C.E.; Kumar, R. Impacts of cellulase deactivation at the moving air–liquid interface on cellulose conversions at low enzyme loadings. Biotechnol. Biofuels 2019, 12, 96. [Google Scholar] [CrossRef] [Green Version]
- Fenila, F.; Shastri, Y. Optimization of cellulose hydrolysis in a non-ideally mixed reactors. Comput. Chem. Eng. 2019, 128, 340–351. [Google Scholar] [CrossRef]
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304. [Google Scholar] [CrossRef]
- Acosta-Fernández, R.; Poerio, T.; Nabarlatz, D.; Giorno, L.; Mazzei, R. Enzymatic Hydrolysis of Xylan from Coffee Parchment in Membrane Bioreactors. Ind. Eng. Chem. Res. 2020, 59, 7346–7354. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef]
- Mameri, N.; Hamdache, F.; Abdi, N.; Belhocine, D.; Grib, H.; Lounici, H.; Piron, D. Enzymatic saccharification of olive mill solid residue in a membrane reactor. J. Membr. Sci. 2000, 178, 121–130. [Google Scholar] [CrossRef]
- Knutsen, J.S.; Davis, R.H. Cellulase Retention and Sugar Removal by Membrane Ultrafiltration During Lignocellulosic Biomass Hydrolysis. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; pp. 585–599. [Google Scholar]
- Abels, C.; Thimm, K.; Wulfhorst, H.; Spiess, A.C.; Wessling, M. Membrane-based recovery of glucose from enzymatic hydrolysis of ionic liquid pretreated cellulose. Bioresour. Technol. 2013, 149, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Mazzei, R.; Gebreyohannes, A.Y.; Papaioannou, E.; Nunes, S.P.; Vankelecom, I.F.J.; Giorno, L. Enzyme catalysis coupled with artificial membranes towards process intensification in biorefinery—A review. Bioresour. Technol. 2021, 335, 125248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, R.; Li, Q.; Qi, W.; He, Z. Enzymatic saccharification of pretreated corn stover in a fed-batch membrane bioreactor. Bioenerg. Res. 2011, 4, 134–140. [Google Scholar] [CrossRef]
- Su, Z.; Luo, J.; Li, X.; Pinelo, M. Enzyme membrane reactors for production of oligosaccharides: A review on the interdependence between enzyme reaction and membrane separation. Sep. Purif. Technol. 2020, 243, 116840. [Google Scholar] [CrossRef]
- Nguyenhuynh, T.; Nithyanandam, R.; Chong, C.H.; Krishnaiah, D. Configuration modification of a submerged membrane reactor for enzymatic hydrolysis of cellulose. Biocatal. Agric. Biotechnol. 2017, 12, 50–58. [Google Scholar] [CrossRef]
- Zain, M.M.; Mohammad, A.W.; Hairom, N.H.H. Flux and permeation behaviour of ultrafiltration in sugaring out cellulose hydrolysate solution: A membrane screening. J. Phys. Sci. 2017, 28, 25. [Google Scholar] [CrossRef]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Al-Zuhair, S. Dynamic model of simultaneous enzymatic cellulose hydrolysis and product separation in a membrane bioreactor. Biochem. Eng. J. 2021, 174, 108107. [Google Scholar] [CrossRef]
- Al-Zuhair, S.; Al-Hosany, M.; Zooba, Y.; Al-Hammadi, A.; Al-Kaabi, S. Development of a membrane bioreactor for enzymatic hydrolysis of cellulose. Renew. Energy 2013, 56, 85–89. [Google Scholar] [CrossRef]
- Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; van der Bruggen, B.; Al-Zuhair, S. Simultaneous Enzymatic Cellulose Hydrolysis and Product Separation in a Radial-Flow Membrane Bioreactor. Molecules 2022, 27, 288. [Google Scholar] [CrossRef] [PubMed]
- Andrić, P.; Meyer, A.S.; Jensen, P.A.; Dam-Johansen, K. Effect and modeling of glucose inhibition and in situ glucose removal during enzymatic hydrolysis of pretreated wheat straw. Appl. Biochem. Biotechnol. 2010, 160, 280. [Google Scholar] [CrossRef] [PubMed]
- Bélafi-Bakó, K.; Koutinas, A.; Nemestóthy, N.; Gubicza, L.; Webb, C. Continuous enzymatic cellulose hydrolysis in a tubular membrane bioreactor. Enzym. Microb. Technol. 2006, 38, 155–161. [Google Scholar] [CrossRef]
- Na’aman, W.W.; Saufi, S.M.; Seman, M.A.; Yussof, H.W.; Mohammad, A. Fabrication of Asymmetric Nanofiltration Flatsheet Membrane for the Separation of Acetic Acid from Xylose and Glucose. Chem. Eng. Trans. 2017, 56, 1201–1206. [Google Scholar]
- Qi, B.; Luo, J.; Chen, G.; Chen, X.; Wan, Y. Application of ultrafiltration and nanofiltration for recycling cellulase and concentrating glucose from enzymatic hydrolyzate of steam exploded wheat straw. Bioresour. Technol. 2012, 104, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Maheswari R, U.; Sikder, J.; Chakraborty, S.; da Silva, S.S.; Santos, J.C.d. Membranes as a tool to support biorefineries: Applications in enzymatic hydrolysis, fermentation and dehydration for bioethanol production. Renew. Sustain. Energy Rev. 2017, 74, 873–890. [Google Scholar] [CrossRef]
- Ran, F.; Li, J.; Lu, Y.; Wang, L.; Nie, S.; Song, H.; Zhao, L.; Sun, S.; Zhao, C. A simple method to prepare modified polyethersulfone membrane with improved hydrophilic surface by one-pot: The effect of hydrophobic segment length and molecular weight of copolymers. Mater. Sci. Eng. C 2014, 37, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, A.; Madaeni, S.S.; Mansourpanah, Y. Fabrication of polyethersulfone (PES) membranes with nano-porous surface using potassium perchlorate (KClO4) as an additive in the casting solution. Desalination 2010, 258, 79–86. [Google Scholar] [CrossRef]
- Thy, N.H.T.; Nithyanandam, R. Fractionation of hydrolyzed microcrystalline cellulose by ultrafiltration membrane. J. Eng. Sci. Technol. 2016, 11, 136–148. [Google Scholar]
- Lozano, P.; Bernal, B.; Jara, A.G.; Belleville, M.-P. Enzymatic membrane reactor for full saccharification of ionic liquid-pretreated microcrystalline cellulose. Bioresour. Technol. 2014, 151, 159–165. [Google Scholar] [CrossRef]
- Amit, K.; Nakachew, M.; Yilkal, B.; Mukesh, Y. A review of factors affecting enzymatic hydrolysis of pretreated lignocellulosic biomass. Res. J. Chem. Environ. 2018, 22, 62–67. [Google Scholar]
- Liu, J.; Lu, J.; Cui, Z. Enzymatic hydrolysis of cellulose in a membrane bioreactor: Assessment of operating conditions. Bioprocess. Biosyst. Eng. 2011, 34, 525–532. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefining Innov. A Sustain. Econ. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Koullas, D.; Christakopoulos, P.; Kekos, D.; Macris, B.J.; Koukios, E.G. Correlating the effect of pretreatment on the enzymatic hydrolysis of straw. Biotechnol. Bioeng. 1992, 39, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Afedzi, A.E.K.; Rattanaporn, K.; Parakulsuksatid, P. Impeller selection for mixing high-solids lignocellulosic biomass in stirred tank bioreactor for ethanol production. Bioresour. Technol. Rep. 2022, 17, 100935. [Google Scholar] [CrossRef]
- Mahboubi, A.; Uwineza, C.; Doyen, W.; de Wever, H.; Taherzadeh, M.J. Intensification of lignocellulosic bioethanol production process using continuous double-staged immersed membrane bioreactors. Bioresour. Technol. 2020, 296, 122314. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Ramarao, B.V.; Ramaswamy, S. Separation and Purification Technologies in Biorefineries; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Le-Clech, P.; Jefferson, B.; Judd, S.J. A comparison of submerged and sidestream tubular membrane bioreactor configurations. Desalination 2005, 173, 113–122. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Tang, B.; Ding, J.; Zhang, Z. Membrane fouling mechanism of biofilm-membrane bioreactor (BF-MBR): Pore blocking model and membrane cleaning. Bioresour. Technol. 2018, 250, 398–405. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F. Membrane fouling in aerobic granular sludge (AGS)-membrane bioreactor (MBR): Effect of AGS size. Water Res. 2019, 157, 445–453. [Google Scholar] [CrossRef]
- Wang, Y.X.; Dong, M.J.; Zhuang, W.C. Enzymatic Saccharification of Cellulose Pretreated from Lignocellulosic Biomass: Status and Prospect. Adv. Mater. Res. 2012, 446, 2809–2814. [Google Scholar] [CrossRef]
- Wu, D.; Wei, Z.; Mohamed, T.A.; Zheng, G.; Qu, F.; Wang, F.; Zhao, Y.; Song, C. Lignocellulose biomass bioconversion during composting: Mechanism of action of lignocellulase, pretreatment methods and future perspectives. Chemosphere 2022, 286, 131635. [Google Scholar] [CrossRef]
- Edeh, I. Bioethanol Production: An Overview; IntechOpen: London, UK, 2020. [Google Scholar]


| MBRs Configurations | Membrane | Substrate | Enzyme | Operational Conditions | Conversion | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Composite | MWCO | Type | Pretreatment | Flux | Substrate (g/L) | Enzyme (g/L) | T (oC) | pH | Press (bar) | t (h) | Mixing (rpm) | ||||
| Dead-end filtration | UF | Polysulfone | 10 kDa | Alpha-cellulose fiber | - | C8546 T. reesei | 7–9 L/m2 h | 25 | 0.1 | 40 | 4.7 | 0.7 | 48 | - a | 53% | [98] |
| Cellulose acetate | 10 kDa | Xylan extracted from coffee parchment | - | Xylanase, A. niger | nd | 1 | 0.11 | 40 | 4.6 | nd | 3 | 200 | 97% | [115] | ||
| PES | 10 kDa | Microcrystalline Cellulose | NaOH | Cellic CTec2-with high level of β-glucosidase | 10 mL/min | 100 | 2.4 | 50 | 5 | nd | 8 | 200 | 7.6% | [123] | ||
| Polysulfone | 10 kDa | Corn Stover | Aquas ammonia (SAA) | (A) Spezyme CP, T. reesei (B) Novozyme 188 | - | 5 | (A) 60 FPU/g (B) 30 CBU/g | 45 | 4.8 | 0.6 | 20 | 120 | 82% | [121] | ||
| Dilute sulfuric acid-sodium hydroxide | 10 | 94% | ||||||||||||||
| Submerged filtration | Dialysis | Spectra/Pro6 | 1 kDa | Wheat straw | Heat | (A) Celluclast 1.5 L T. reesei (B) Novozyme 188 A. niger | - | 1 | (A) 4.1, and (B) 1.08 | 50 | 5 | - | 72 | 350 | 28% | [128] |
| Tubular filtration | UF | Non-woven textile-polyethylene (PE) | nd | Solka Floc powder | - | Celluclast T. reesei | 80 mL/min | 25 | 3 | 50 | 4.8 | - | 25 | - | 50% | [129] |
| Mavicell cellulose pellets | Heat | 10 | 70% | |||||||||||||
| Enzymatic hydrolysis | Enzyme-related factors |
|
| Substrate-related factors |
| |
| Membrane performance | Membrane-related factors |
|
| MBR Design | Advantages | Disadvantages | |
|---|---|---|---|
| Hybrid MBRs | Reaction and filtration are separated |
|
|
| Integrated MBRs Reaction and filtration combined | Dead-end filtration MBR |
|
|
| Inverted dead-end filtration MBR |
|
| |
| Tubular MBR |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mardeai, S.; Elnajjar, E.; Hashaikeh, R.; Kruczek, B.; Van der Bruggen, B.; Al-Zuhair, S. Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose. Catalysts 2022, 12, 1121. https://doi.org/10.3390/catal12101121
Al-Mardeai S, Elnajjar E, Hashaikeh R, Kruczek B, Van der Bruggen B, Al-Zuhair S. Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose. Catalysts. 2022; 12(10):1121. https://doi.org/10.3390/catal12101121
Chicago/Turabian StyleAl-Mardeai, Saleha, Emad Elnajjar, Raed Hashaikeh, Boguslaw Kruczek, Bart Van der Bruggen, and Sulaiman Al-Zuhair. 2022. "Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose" Catalysts 12, no. 10: 1121. https://doi.org/10.3390/catal12101121
APA StyleAl-Mardeai, S., Elnajjar, E., Hashaikeh, R., Kruczek, B., Van der Bruggen, B., & Al-Zuhair, S. (2022). Membrane Bioreactors: A Promising Approach to Enhanced Enzymatic Hydrolysis of Cellulose. Catalysts, 12(10), 1121. https://doi.org/10.3390/catal12101121











