g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications
Abstract
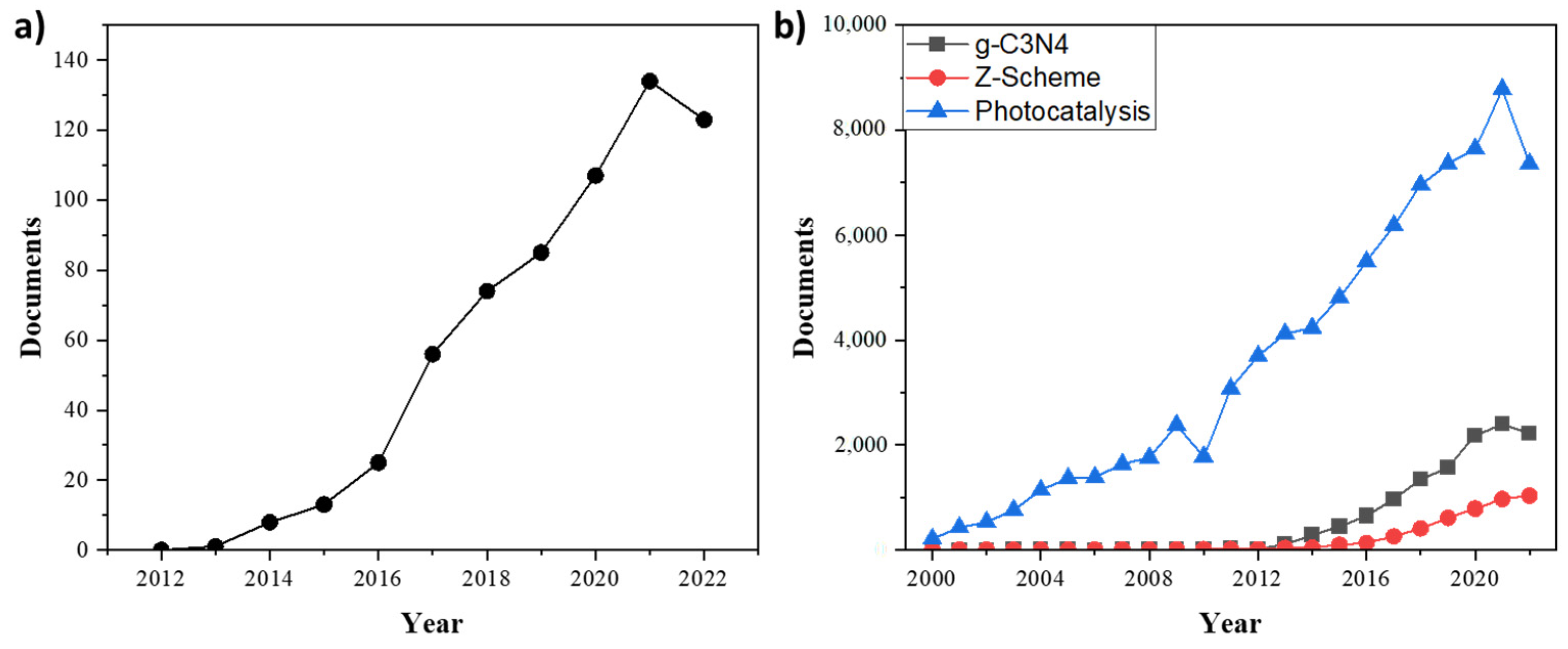
:1. Introduction
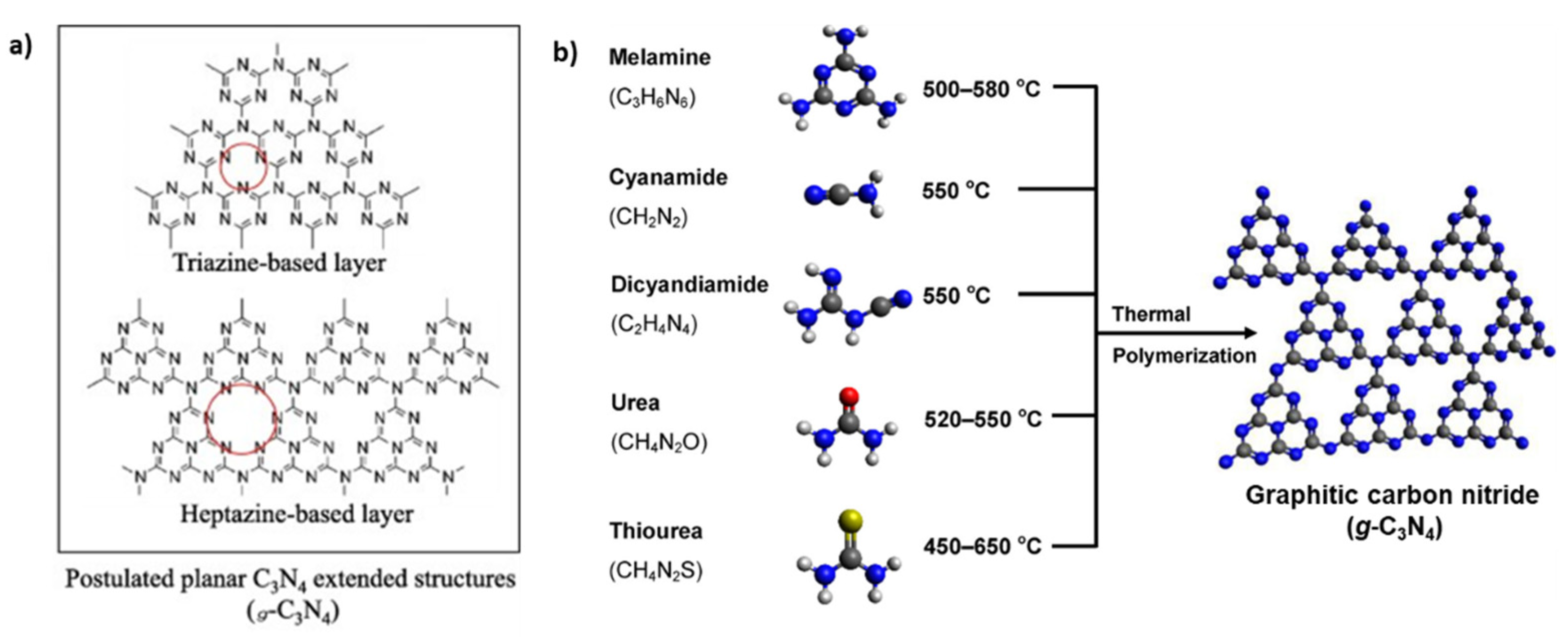
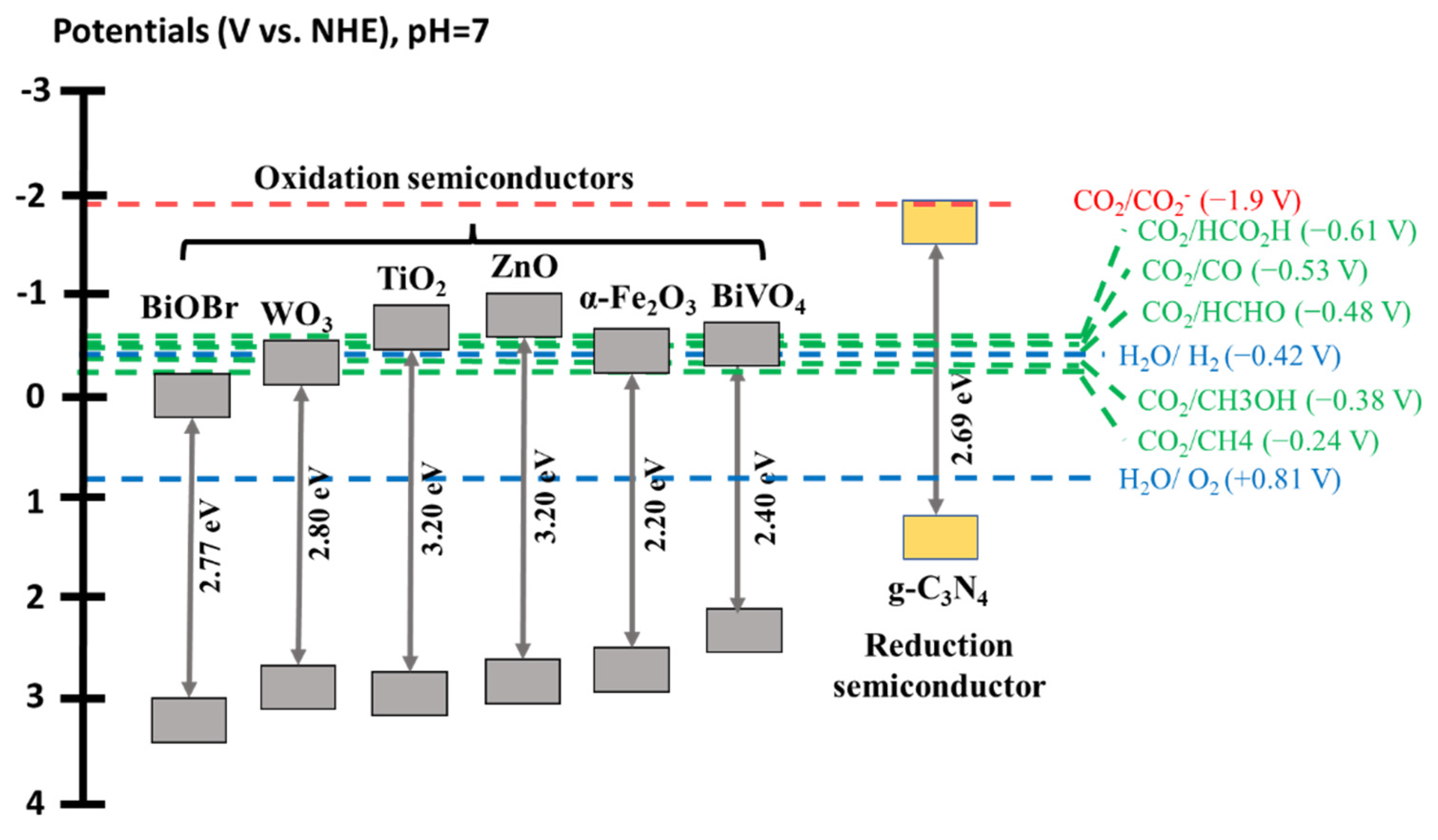
2. Pristine g-C3N4
3. Direct Z-Scheme Photocatalysts Based on g-C3N4
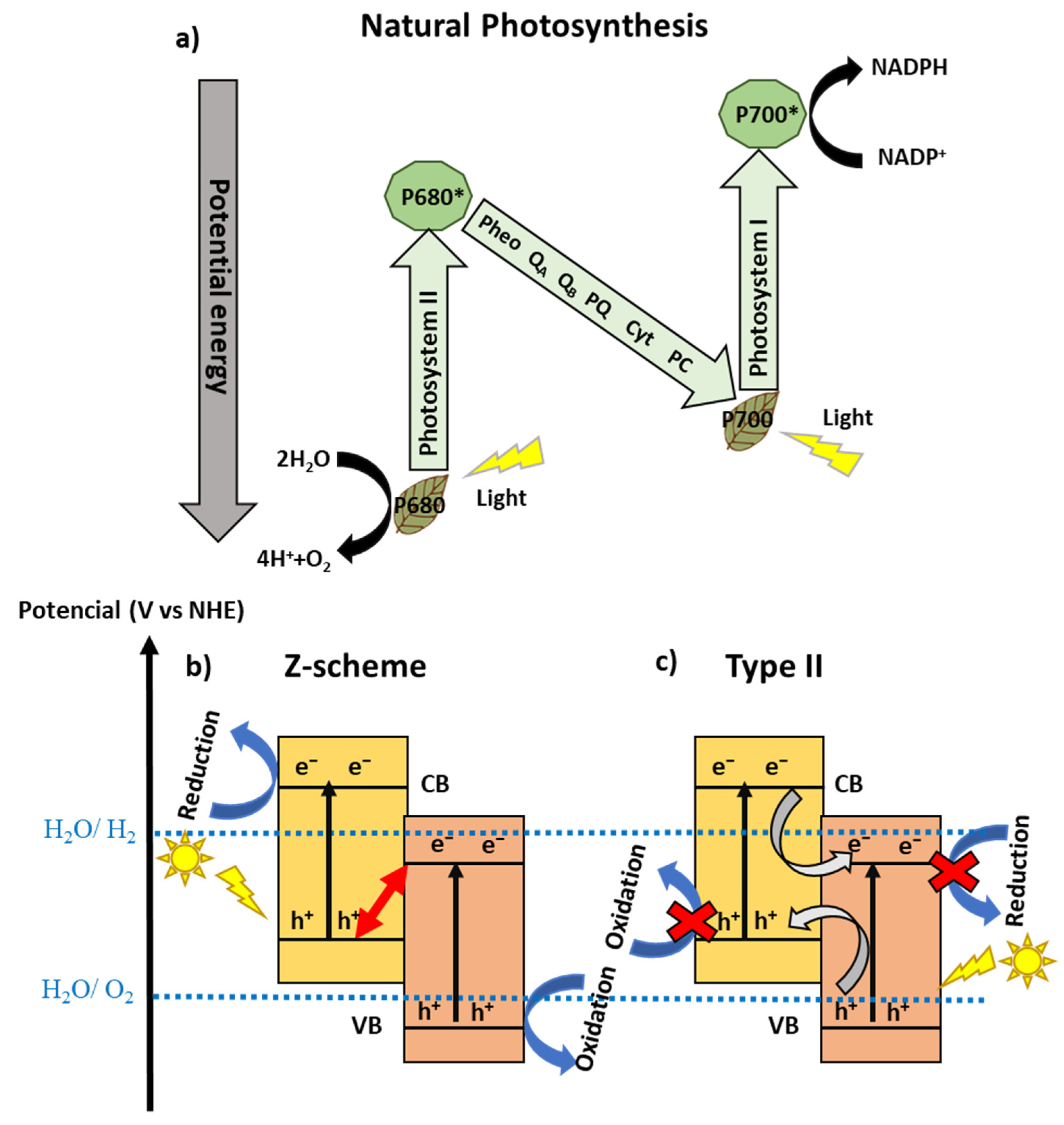
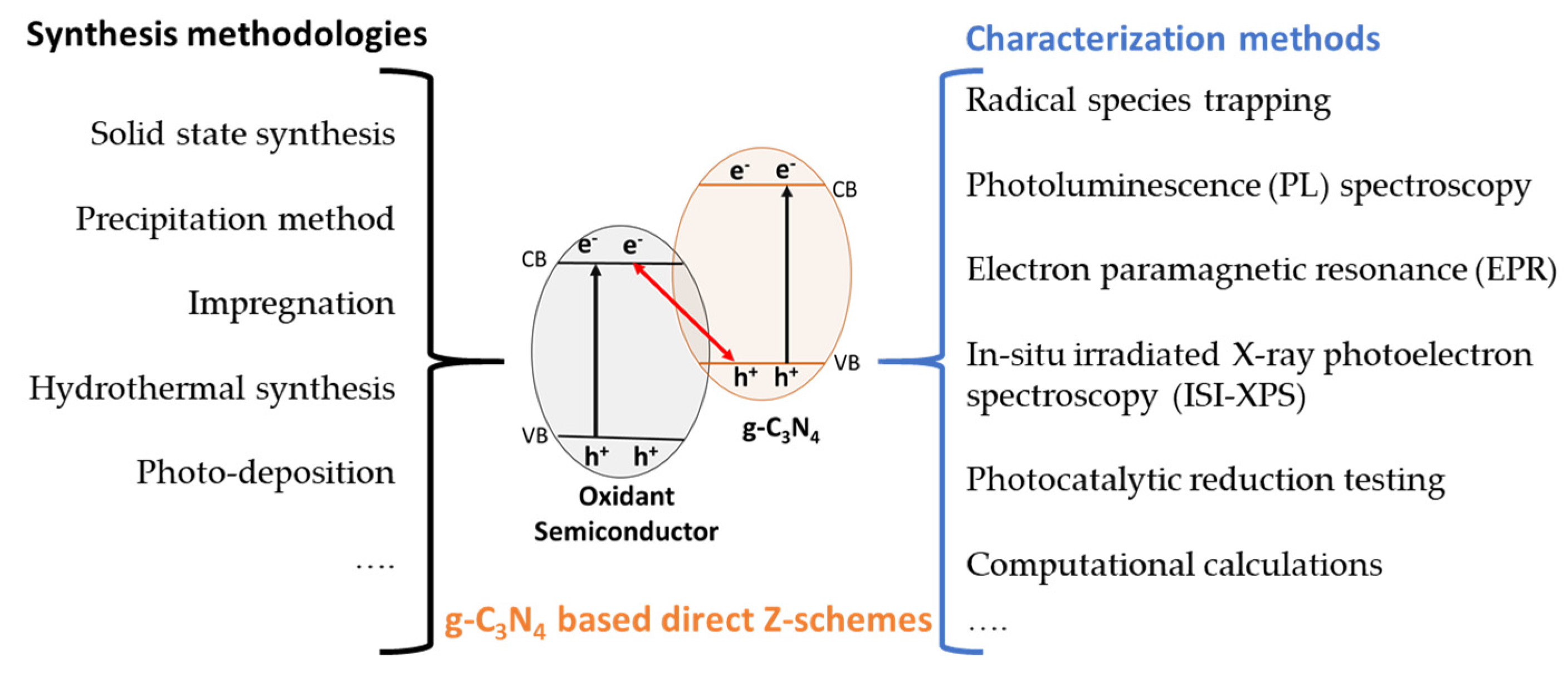
3.1. Direct Z-Scheme Photocatalysts
3.2. Synthesis and Characterization of Z-Scheme Photocatalysts Based on g-C3N4
4. Environmental Applications of Direct Z-Schemes Based on g-C3N4
4.1. Pollutant Remediation
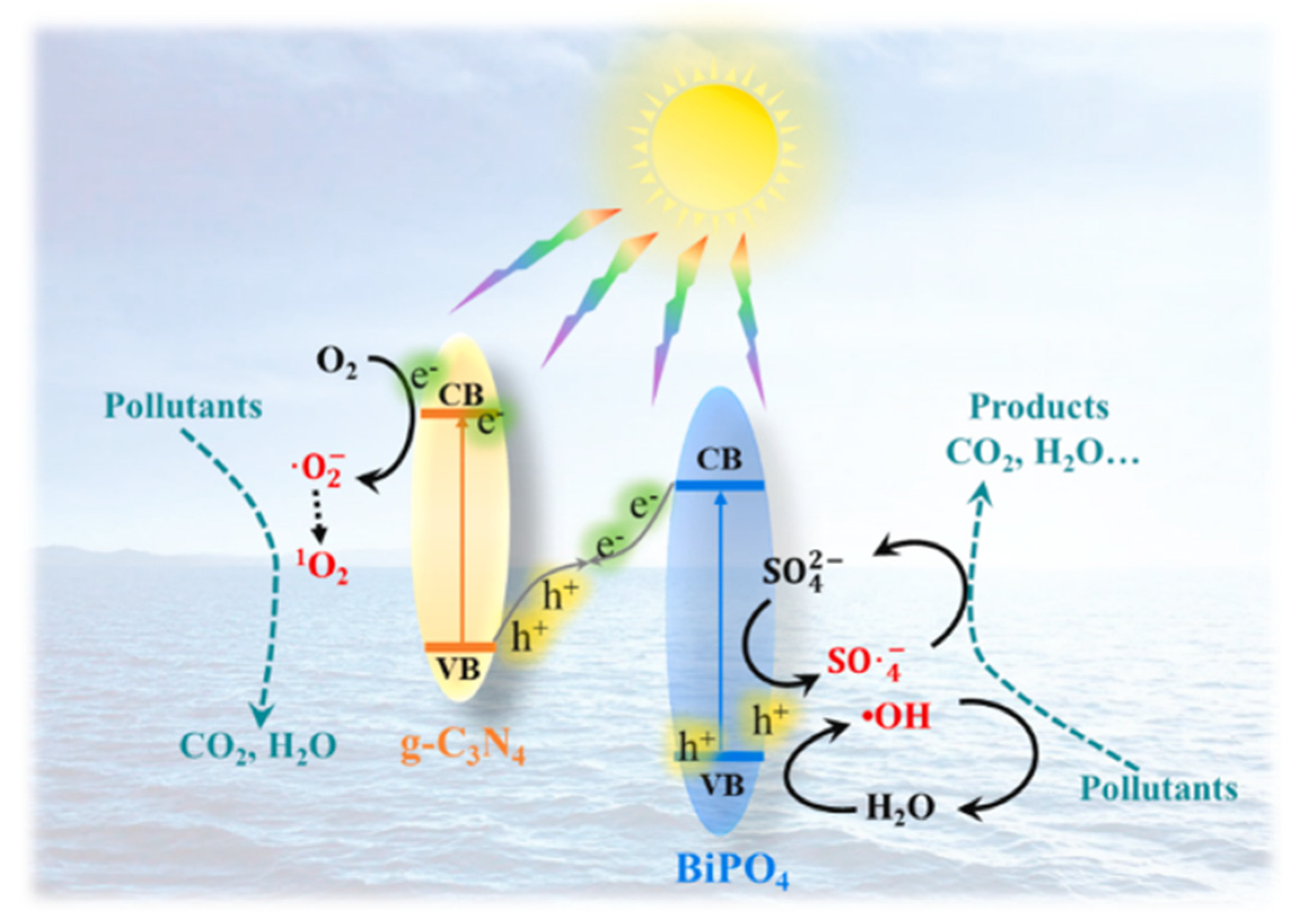
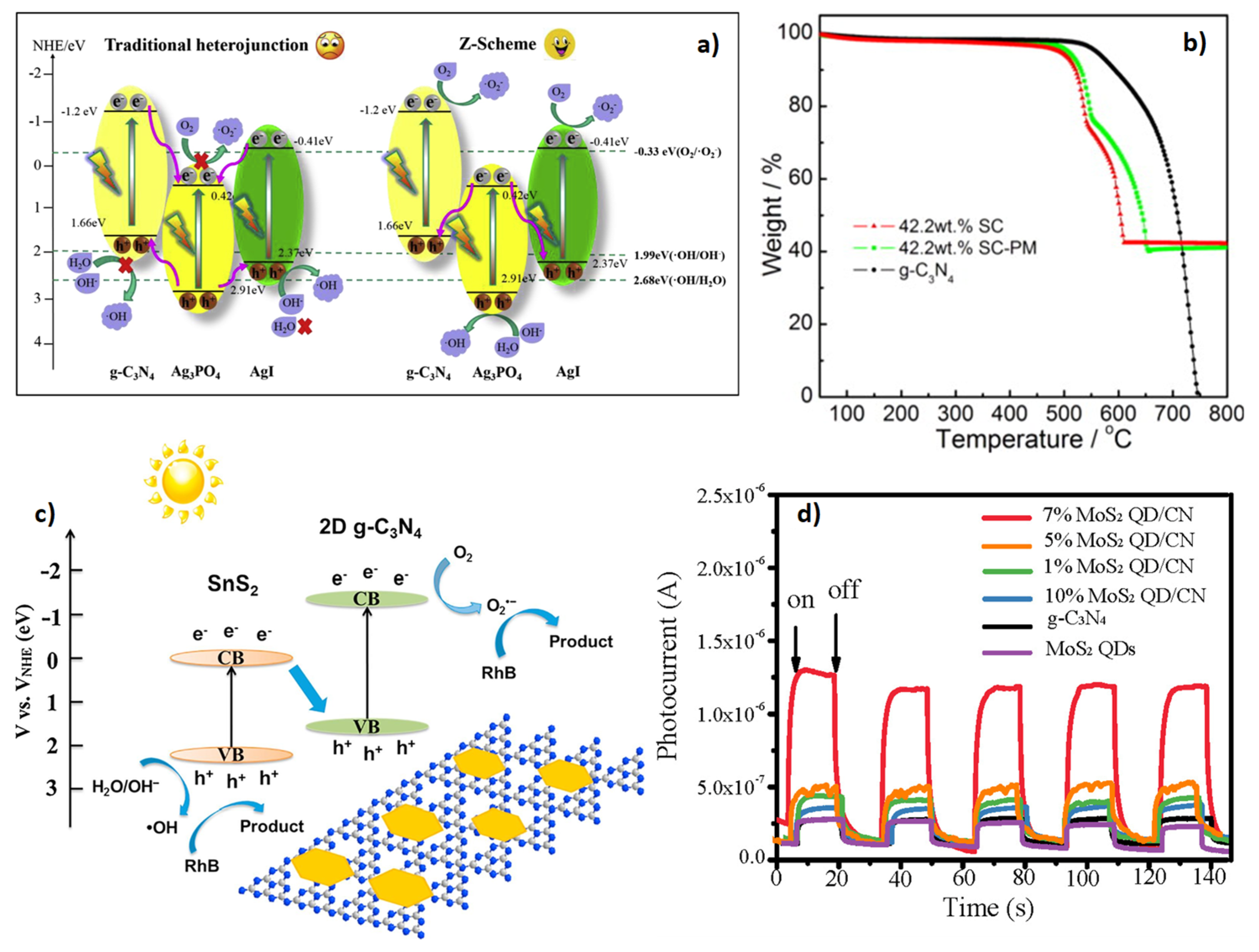
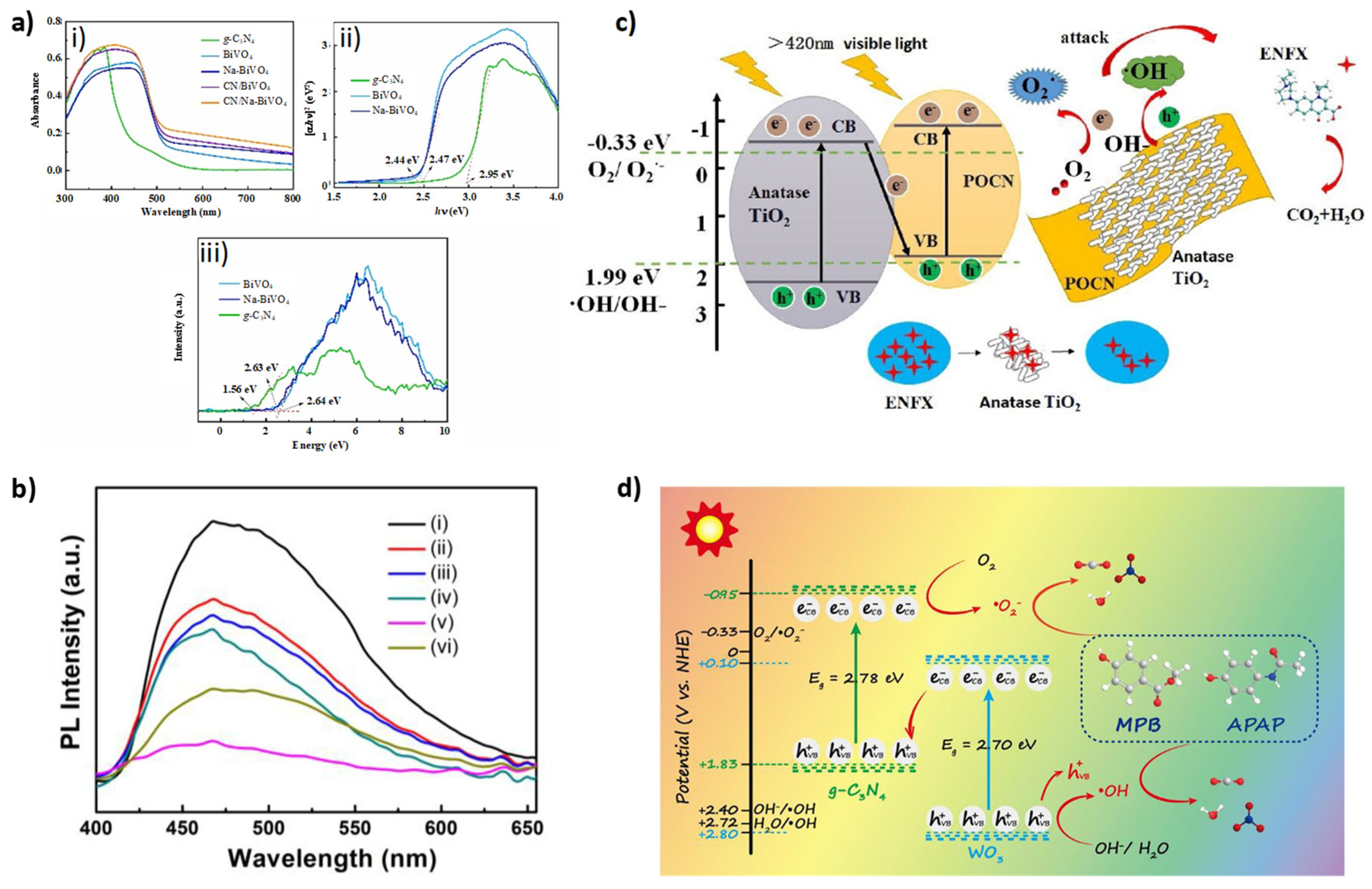
| Z-Scheme Photocatalyst | Fabrication | Irradiation Source | Pollutant/Photodegradation Performance/Reaction Time (min) | Reference |
|---|---|---|---|---|
| SnO2−x/g-C3N4 | solid state | 350W Xenon lamp (420 nm < λ < 800 nm) | RhB/100%/60 min | [116] |
| MoS2 QD/g-C3N4 | microemulsion method | 350W Xenon lamp (λ > 420 nm) | RhB/100%/9 min | [111] |
| Bi3O4Cl/g-C3N4 | solid phase calcination method | 250W Xenon lamp (λ > 420 nm) | RhB/98.3%/90 min | [125] |
| β-Bi2O3/g-C3N4 | sonication | 350W Xenon lamp (λ > 420 nm) | RhB/98%/80 min | [138] |
| g-C3N4/ZnO | thermal atomic layer deposition | 300W Xenon lamp | Cephalexin/92.7%/60 min | [139] |
| SnS2/g-C3N4 | solvothermal method | 300W Xenon lamp (λ > 400 nm) | RhB/94.8%/60 min | [126] |
| Fe3O4-OQs/Bi2O4/g-C3N4 | hydrothermal method | 250W Xenon lamp (λ > 420 nm) | RhB/92.7%/160 min | [140] |
| g-C3N4/TiO2 | solvent evaporation method | 500 W Xenon lamp | RhB/100%/20 min | [141] |
| α-Fe2O3/g-C3N4 | calcination | 100W LED lamp (λ = 420 nm) | Tetracycline/95.0%/60 min | [142] |
| ZnO/g-C3N4 | hydrothermal method | Visible light | Atrazine/90%/180 min | [143] |
| Co3O4/g-C3N4 | solid state | 300W Xenon lamp (λ > 420 nm) | Tetracycline/90.0%/60 min | [131] |
| AgI/Ag3PO4/g-C3N4 | in situ ion exchange method | 300W Xenon lamp (λ > 420 nm) | Nitenpyram/100%/4 min | [124] |
| g-C3N4/anatase TiO2 | calcination | 350W Xenon lamp (λ > 420 nm) | Enrofloxacin/98.5%/60 min | [135] |
| g-C3N4/Na-BiVO4 | hydrothermal method | 300W Xenon lamp (λ > 420 nm) | Tetracycline/98.2%/40 min | [130] |
| WO3/g-C3N4 | hydrothermal method | 300W Xenon lamp (λ > 400 nm) | MPB/98.2%/60 min | [136] |
| ZnO/g-C3N4 | hydrothermal method | Solar light | Rh B/98%/100 min | [144] |
| α-Fe2O3/g-C3N4 | sonication | 500 W Xenon lamp | Tetracycline/97.1%/80 min | [132] |
| CoCeOx/g-C3N4 | solid state | 300W Xenon lamp (λ > 420 nm) | Carbamazepine/90.1%/60 min | [134] |
4.2. H2 Production
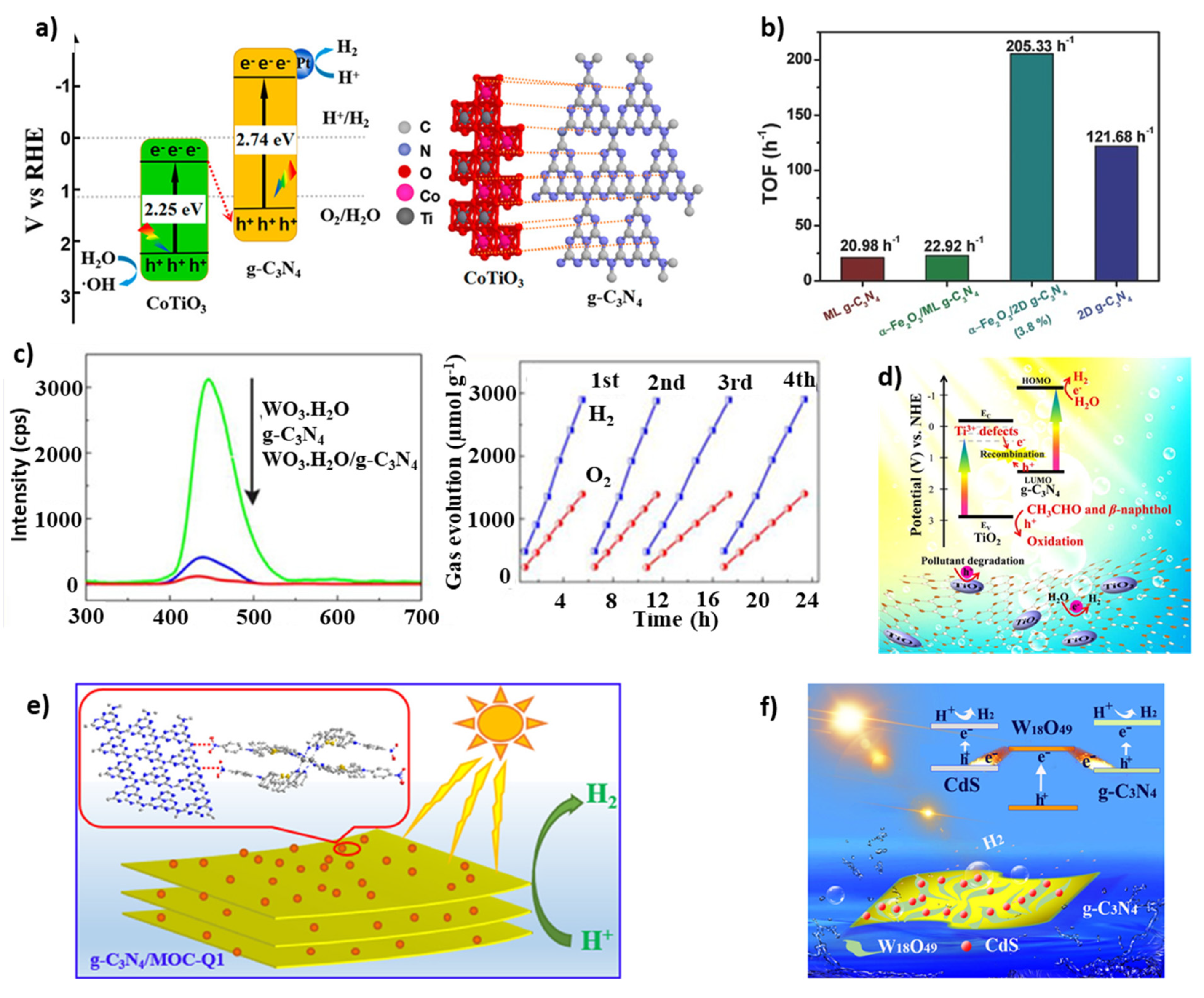
| Z-Scheme Photocatalyst | Fabrication Methodology | Irradiation Source | H2 Production Activity (µmol g−1 h−1) and AQE | Reference |
|---|---|---|---|---|
| CoTiO3/g-C3N4 | Solid-State | Xenon lamp (300 W, λ ≥ 420 nm) | 858 AQE: 38.4% (365 nm) | [151] |
| g-C3N4/ZnO | Deposition | Xenon lamp (300 W, λ ≥ 420 nm) | 322 | [156] |
| g-C3N4/PSi | Polycondensation reaction | Xenon lamp (300 W, λ ≥ 400 nm) | 870 | [157] |
| 2D α-Fe2O3/g-C3N4 | Solid-State | Xenon lamp (300 W, λ ≥ 420 nm) | 31400 AQE: 44.35% (420 nm) | [152] |
| WO3.H2O/g-C3N4 | Hydrothermal method | Xenon lamp (300 W, λ > 400 nm) | 482 AQE: 6.2% (420 nm) | [153] |
| g-C3N4/Ti3+-TiO2 | Solid-State | Xenon lamp (300 W, λ > 400 nm) | 1938 | [154] |
| Nb2O5/g-C3N4 | Hydrothermal | Xenon lamp (1000 W, 1.5G) | 110,000 | [115] |
| Bi2O2CO3/g-C3N4 | Heat treatment method | Xenon lamp (300 W, λ ≥ 400 nm) | 965 AQE: 7.14% (420 nm) | [158] |
| 2D/2D g-C3N4/Sn3O4 | Calcined in N2 | Xenon lamp (300 W, λ ≥ 400 nm) | 1960 | [159] |
| g-C3N4/MOC-Q1 | Deposition | Xenon lamp (300 W, λ ≥ 420 nm) | 4495 AQE: 0.50% (425 nm) | [155] |
| CdS/W18O49/g-C3N4 (CWOCN) | Chemical bath deposition | Xenon lamp (300 W, λ ≥ 420 nm) | 11,658 AQE: 26.73% (420 nm) | [116] |
| Cu2O/g-C3N4 | Solid-State | Xenon lamp (300 W, λ ≥ 420 nm) | 266.3 AQE: 13.40% (420 nm) | [160] |
4.3. CO2 Photoreduction
| Z-Scheme Photocatalyst | Fabrication | Irradiation Source | Products/Production (µmol g−1 h−1)/AQE | Reference |
|---|---|---|---|---|
| ZnO/g-C3N4 | Solid-state | 300 W simulated solar Xe arc lamp | CH3OH: 0.6 | [172] |
| SnO2-x/g-C3N4 | Solid-state | 500 W Xe lamp | CO: ~19 CH3OH: ~4 CH4: ~2 | [116] |
| g-C3N4/SnS2 | Hydrothermal method | 300 W Xenon lamp (λ ≥ 420 nm) | CH3OH: 2.24 CO: 0.64 | [91] |
| MoO3/g-C3N4 | impregnation method | 350 W Xenon lamp (800 nm > λ > 420 nm) | CO: ~18 CH3OH: ~7 CH4: ~1 | [85] |
| α-Fe2O3/g-C3N4 | Impregnation–hydrothermal method | Xenon lamp 0.21 Wcm−2 | CO: 27.2 AQE: 0.963% (420 nm) | [170] |
| AgCl/g-C3N4 | Deposition-precipitation method | 11 W fluorescent lamp | CH4: ~2 CH3COOH: ~0.75 HCOOH: ~0.31 AQE: 0.211% (475 nm) | [175] |
| Cu2V2O7/g-C3N4 | Calcination methodology | 20 W white bulbs (700 nm > λ > 400 nm) | CH4: 305 CO: 166 O2: 706 | [176] |
| g-C3N4/FeWO4 | Sonochemical method | 300 W Xenon lamp (100 mW/cm2) | CO: 6 AQE: ~0.3% (420 nm) | [83] |
| (Nb)TiO2/g-C3N4 | Calcination methodology | Two 30 W white bulbs | CH4: 562 CO:420 HCOOH:698 | [173] |
| 2D/2D g-C3N4/BiVO4 | Hydrothermal method | 300 W Xenon lamp (λ ≥ 420 nm) | CO: ~5.2 CH4: ~4.6 | [92] |
| NiMoO4/g-C3N4 | Calcined methodology | 30 W LED, (700 nm > λ > 400 nm) | CH4: 635 CO: 432 O2: 1853 HCOOH: 647 | [177] |
| α-Fe2O3/g-C3N4 | Hydrothermal method | 300 W xenon lamp | CO: 17.8 AQE: 0.31% (420 nm) | [178] |
| 3D/2D WO3/g-C3N4 | Hydrothermal method | 300 W Xenon lamp (100 mW cm−2) | CO: 145 CH4: 133 | [117] |
| La2Ti2O7/g-C3N4 | Ultrasonic-deposition method | Four blue LED (4 × 3 W, 420 nm) | CH3OH: ~4 CO: ~2.5 AQE: 3.61% (420 nm) | [179] |
| Bi2S3/g-C3N4 | Hydrothermal method | 300 W xenon lamp | CO: 6.84 CH4: 1.57 H2: 1.38 AQE: 2.31% (420 nm) | [180] |
| ZnO/ZnWO4/g-C3N4 | Calcination method | Xenon lamp (300 W, 0.95 mW/cm2) | CH4: 6.2 CH3OH: 3.8 CH3CH2OH: 2.1 CO: 1.3 | [181] |
| NiTiO3/g-C3N4 | Ultrasonic-calcination method | 300 W Xenon lamp (λ ≥ 420 nm) | CH3OH: 13.74 | [182] |
| Bi19S27Br3/g-C3N4 | Physical mixture (Strong grinding) | 300 W xenon lamp | CO: 12.87 | [174] |
5. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toman, M.A.; Morgenstern, R.D.; Anderson, J. The Economics of “When” Flexibility in the Design of Greenhouse Gas Abatement Policies. Annu. Rev. Environ. Resour. 1999, 24, 431–460. [Google Scholar]
- Vijayavenkataraman, S.; Iniyan, S.; Goic, R. A Review of Climate Change, Mitigation and Adaptation. Renew. Sustain. Energy Rev. 2012, 16, 878–897. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef]
- Paltsev, S. Energy Scenarios: The Value and Limits of Scenario Analysis. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, 1–19. [Google Scholar] [CrossRef]
- Solangi, K.H.; Islam, M.R.; Saidur, R.; Rahim, N.A.; Fayaz, H. A Review on Global Solar Energy Policy. Renew. Sustain. Energy Rev. 2011, 15, 2149–2163. [Google Scholar] [CrossRef]
- Kannan, N.; Vakeesan, D. Solar Energy for Future World—A Review. Renew. Sustain. Energy Rev. 2016, 62, 1092–1105. [Google Scholar] [CrossRef]
- Kabir, E.; Kumar, P.; Kumar, S.; Adelodun, A.A.; Kim, K.H. Solar Energy: Potential and Future Prospects. Renew. Sustain. Energy Rev. 2018, 82, 894–900. [Google Scholar] [CrossRef]
- Alharbi, N.S.; Hu, B.; Hayat, T.; Rabah, S.O.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient Elimination of Environmental Pollutants through Sorption-Reduction and Photocatalytic Degradation Using Nanomaterials. Front. Chem. Sci. Eng. 2020, 14, 1124–1135. [Google Scholar] [CrossRef]
- Saravanan, A.; Senthil Kumar, P.; Vo, D.V.N.; Jeevanantham, S.; Bhuvaneswari, V.; Anantha Narayanan, V.; Yaashikaa, P.R.; Swetha, S.; Reshma, B. A Comprehensive Review on Different Approaches for CO2 Utilization and Conversion Pathways. Chem. Eng. Sci. 2021, 236, 116515. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical Development and Prospects of Photocatalysts for Pollutant Removal in Water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Nishiyama, H.; Yamada, T.; Nakabayashi, M.; Maehara, Y.; Yamaguchi, M.; Kuromiya, Y.; Nagatsuma, Y.; Tokudome, H.; Akiyama, S.; Watanabe, T.; et al. Photocatalytic Solar Hydrogen Production from Water on a 100-M2 Scale. Nature 2021, 598, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Tuller, H.L. Solar to Fuels Conversion Technologies: A Perspective. Mater. Renew. Sustain. Energy 2017, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Wenderich, K.; Mul, G. Methods, Mechanism, and Applications of Photodeposition in Photocatalysis: A Review. Chem. Rev. 2016, 116, 14587–14619. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar]
- He, R.; Cao, S.; Zhou, P.; Yu, J. Recent Advances in Visible Light Bi-Based Photocatalysts. Chin. J. Catal. 2014, 35, 989–1007. [Google Scholar] [CrossRef]
- Han, G.; Sun, Y. Visible-Light-Driven Organic Transformations on Semiconductors. Mater. Today Phys. 2021, 16, 100297. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A Review on G-C3N4-Based Photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Hayat, A.; Al-Sehemi, A.G.; El-Nasser, K.S.; Taha, T.A.; Al-Ghamdi, A.A.; Shah Syed, J.A.; Amin, M.A.; Ali, T.; Bashir, T.; Palamanit, A.; et al. Graphitic Carbon Nitride (g–C3N4)–Based Semiconductor as a Beneficial Candidate in Photocatalysis Diversity. Int. J. Hydrogen Energy 2022, 47, 5142–5191. [Google Scholar] [CrossRef]
- Gan, X.; Lei, D.; Wong, K.Y. Two-Dimensional Layered Nanomaterials for Visible-Light-Driven Photocatalytic Water Splitting. Mater. Today Energy 2018, 10, 352–367. [Google Scholar] [CrossRef]
- Ismael, M. A Review on Graphitic Carbon Nitride (g-C3N4) Based Nanocomposites: Synthesis, Categories, and Their Application in Photocatalysis. J. Alloys Compd. 2020, 846, 156446. [Google Scholar] [CrossRef]
- Huang, D.; Li, Z.; Zeng, G.; Zhou, C.; Xue, W.; Gong, X.; Yan, X.; Chen, S.; Wang, W.; Cheng, M. Megamerger in Photocatalytic Field: 2D g-C3N4 Nanosheets Serve as Support of 0D Nanomaterials for Improving Photocatalytic Performance. Appl. Catal. B Environ. 2019, 240, 153–173. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. G-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in G-C3N4-Based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Li, X.; Fang, B. Emerging Polymeric Carbon Nitride Z-Scheme Systems for Photocatalysis. Cell Rep. Phys. Sci. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Belousov, A.S.; Fukina, D.G.; Koryagin, A.V. Metal–organic framework-basedheterojunction photocatalysts for organicpollutant degradation: Design, construction, and performances. J. Chem. Technol. Biotechnol. 2022, 97, 2675–2693. [Google Scholar] [CrossRef]
- Zhao, Y.; Linghu, X.; Shu, Y.; Zhang, J.; Chen, Z.; Wu, Y.; Shan, D.; Wang, B. Classification and Catalytic Mechanisms of Heterojunction Photocatalysts and the Application of Titanium Dioxide (TiO2)-Based Heterojunctions in Environmental Remediation. J. Environ. Chem. Eng. 2022, 10, 108077. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Emerging Frontiers of Z-Scheme Photocatalytic Systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Ghosh, U.; Pal, A. Graphitic Carbon Nitride Based Z Scheme Photocatalysts: Design Considerations, Synthesis, Characterization and Applications. J. Ind. Eng. Chem. 2019, 79, 383–408. [Google Scholar] [CrossRef]
- Zhang, W.; Mohamed, A.R.; Ong, W.J. Z-Scheme Photocatalytic Systems for Carbon Dioxide Reduction: Where Are We Now? Angew. Chem.-Int. Ed. 2020, 59, 22894–22915. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Guo, R.T.; Hong, L.F.; Ji, X.Y.; Lin, Z.D.; Li, Z.S.; Pan, W.G. A Review of Metal Oxide-Based Z-Scheme Heterojunction Photocatalysts: Actualities and Developments. Mater. Today Energy 2021, 21, 100829. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Li, X.; Garlisi, C.; Guan, Q.; Anwer, S.; Al-Ali, K.; Palmisano, G.; Zheng, L. A Review of Material Aspects in Developing Direct Z-Scheme Photocatalysts. Mater. Today 2021, 47, 75–107. [Google Scholar] [CrossRef]
- Zhang, H.; Tian, W.; Zhang, J.; Duan, X.; Liu, S.; Sun, H.; Wang, S. Carbon Nitride-Based Z-Scheme Photocatalysts for Non-Sacrificial Overall Water Splitting. Mater. Today Energy 2022, 23, 100915. [Google Scholar] [CrossRef]
- Lin, J.; Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Wang, S. Graphitic Carbon Nitride-Based Z-Scheme Structure for Photocatalytic CO2 Reduction. Energy Fuels 2021, 35, 7–24. [Google Scholar] [CrossRef]
- Kumar, Y.; Kumar, R.; Raizada, P.; Aslam, A.; Van Le, Q.; Singh, P.; Nguyen, V.-H. Novel Z-Scheme ZnIn2S4-based photocatalysts for solar-driven environmental and energy applications: Progress and perspectives. J. Mater. Sci. Technol. 2021, 87, 234–257. [Google Scholar] [CrossRef]
- Li, Y.; Pan, C.; Wang, G.; Leng, Y.; Jiang, P.; Dong, Y.; Zhu, Y. Improving the photocatalytic activity of benzyl alcohol oxidation by Z-scheme SnS/g-C3N4. New J. Chem. 2021, 45, 6611–6617. [Google Scholar] [CrossRef]
- Ma, X.; Huo, X.; Hao, K.; Song, L.; Yu, Q.; Liu, T.; Wang, Z. Visible Light Driven VO2/g-C3N4 Z-Scheme Composite Photocatalysts for Selective Oxidation of DL-1-Phenylethyl Alcohol under Vis-LEDs Irradiation and Aerobic Oxidation. Chem. Select 2021, 6, 2101–2211. [Google Scholar]
- Belousov, A.; Suleimanov, E.V. Application of metal–organic frameworks as an alternative to metal oxide-based photocatalysts for the production of industrially important organic chemicals. Green Chem. 2021, 23, 6172–6204. [Google Scholar] [CrossRef]
- Ma, J.; Yang, X.; Yao, S.; Guo, Y.; Sun, S. Photocatalytic Biorefinery to Lactic Acid: A Carbon Nitride Framework with O Atoms Replacing the Graphitic N Linkers Shows Fast Migration/Separation of Charge. ChemCatChem 2022, 14, e2022000. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A Review of the Current Status of Graphitic Carbon Nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A Critical Review on Graphitic Carbon Nitride (g-C3N4)-Based Materials: Preparation, Modification and Environmental Application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Kroke, E.; Schwarz, M. Novel Group 14 Nitrides. Coord. Chem. Rev. 2004, 248, 493–532. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef]
- Liu, N.; Li, T.; Zhao, Z.; Liu, J.; Luo, X.; Yuan, X.; Luo, K.; Luo, K.; He, J.; Yu, D.; et al. From Triazine to Heptazine: Origin of Graphitic Carbon Nitride as a Photocatalyst. ACS Omega 2020, 5, 12557–12567. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Ismael, M.; Wu, Y. A Mini-Review on the Synthesis and Structural Modification of g-C3N4-Based Materials, and Their Applications in Solar Energy Conversion and Environmental Remediation. Sustain. Energy Fuels 2019, 3, 2907–2925. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of Processing Temperature on Structure and Photocatalytic Properties of G-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.T.; Nguyen-Phu, H.; Shin, E.W. The Effect of Graphitic Carbon Nitride Precursors on the Photocatalytic Dye Degradation of Water-Dispersible Graphitic Carbon Nitride Photocatalysts. Appl. Surf. Sci. 2021, 537, 148027. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A Metal-Free Polymeric Photocatalyst for Hydrogen Production from Water under Visible Light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Aslam, I.; Hassan Farooq, M.; Ghani, U.; Rizwan, M.; Nabi, G.; Shahzad, W.; Boddula, R. Synthesis of Novel G-C3N4 Microrods: A Metal-Free Visible-Light-Driven Photocatalyst. Mater. Sci. Energy Technol. 2019, 2, 401–407. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Li, X.; Zhang, X.; Wang, C.; Liu, Y. Effect of Morphology on the Photocatalytic Activity of G-C3N4 Photocatalysts under Visible-Light Irradiation. Mater. Sci. Semicond. Process. 2015, 32, 76–81. [Google Scholar] [CrossRef]
- Ren, Y.; Zeng, D.; Ong, W.J. Interfacial Engineering of Graphitic Carbon Nitride (g-C3N4)-Based Metal Sulfide Heterojunction Photocatalysts for Energy Conversion: A Review. Chin. J. Catal. 2019, 40, 289–319. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, B.; Fan, J.; Ho, W.; Yu, J. G-C3N4-Based 2D/2D Composite Heterojunction Photocatalyst. Small Struct. 2021, 2, 2100086. [Google Scholar] [CrossRef]
- Wang, Y.; Suzuki, H.; Xie, J.; Tomita, O.; Martin, D.J.; Higashi, M.; Kong, D.; Abe, R.; Tang, J. Mimicking Natural Photosynthesis: Solar to Renewable H2 Fuel Synthesis by Z-Scheme Water Splitting Systems. Chem. Rev. 2018, 118, 5201–5241. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, S.; Zeng, G.; Gong, X.; Zhou, C.; Cheng, M.; Xue, W.; Yan, X.; Li, J. Artificial Z-Scheme Photocatalytic System: What Have Been Done and Where to Go? Coord. Chem. Rev. 2019, 385, 44–80. [Google Scholar] [CrossRef]
- Bard, A.J. Photoelectrochemistry and Heterogeneous Photo-Catalysis at Semiconductors. J. Photochem. 1979, 10, 59–75. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-Solid-State Z-Scheme in CdS-Au-TiO2 Three-Component Nanojunction System. Nat. Mater. 2006, 5, 782–786. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Dutta, V.; Raizada, P.; Khan, A.A.P.; Van Le, Q.; Thakur, V.K.; Biswas, J.K.; Selvasembian, R.; Singh, P. Controllable Functionalization of G-C3N4 Mediated All-Solid-State (ASS) Z-Scheme Photocatalysts towards Sustainable Energy and Environmental Applications. Environ. Technol. Innov. 2021, 24, 101972. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced Photocatalytic Performance of Direct Z-Scheme g-C3N4-TiO2 Photocatalysts for the Decomposition of Formaldehyde in Air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tian, W.; Zhang, H.; Duan, X.; Sun, H.; Wang, H.; Fang, Y.; Huang, Y.; Wang, S. Carbon Nitride-Based Z-Scheme Heterojunctions for Solar-Driven Advanced Oxidation Processes. J. Hazard. Mater. 2022, 434, 128866. [Google Scholar] [CrossRef]
- Zhao, D.; Guan, X.; Shen, S. Design of Polymeric Carbon Nitride-Based Heterojunctions for Photocatalytic Water Splitting: A Review. Environ. Chem. Lett. 2022, 7, 1–19. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-Scheme Photocatalysts: Principles, Synthesis, and Applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous Graphitic Carbon Nitride Synthesized via Direct Polymerization of Urea for Efficient Sunlight-Driven Photocatalytic Hydrogen Production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. G-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef]
- Wiley, J.B.; Kaner, R.B. Rapid Solid-State Precursor Synthesis of Materials. Science 1992, 255, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Keller, S.W.; Mallouk, T.E. Turning down the Heat: Design and Mechanism in Solid-State Synthesis. Science 1993, 259, 1558–1564. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, G.; Zou, M.; Wang, J.; Li, J. Effects of Calcining Temperature on Formation of Hierarchical TiO2/g-C 3N4 Hybrids as an Effective Z-Scheme Heterojunction Photocatalyst. Appl. Surf. Sci. 2018, 441, 1012–1023. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Shang, T.; Chen, L.; Gu, L.; Peng, T. Direct Z-Scheme g-C3N4/WO3 Photocatalyst with Atomically Defined Junction for H2 Production. Appl. Catal. B Environ. 2017, 219, 693–704. [Google Scholar] [CrossRef]
- Chrouda, A.; Mahmoud Ali Ahmed, S.; Babiker Elamin, M. Preparation of Nanocatalysts Using Deposition Precipitation with Urea: Mechanism, Advantages and Results. ChemBioEng Rev. 2022, 9, 248–264. [Google Scholar] [CrossRef]
- Wen, X.J.; Shen, C.H.; Fei, Z.H.; Fang, D.; Liu, Z.T.; Dai, J.T.; Niu, C.G. Recent Developments on AgI Based Heterojunction Photocatalytic Systems in Photocatalytic Application. Chem. Eng. J. 2020, 383, 123083. [Google Scholar] [CrossRef]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dash, S.; Tyagi, A.K. TiO2 Modification by Gold (Au) for Photocatalytic Hydrogen (H2) Production. Renew. Sustain. Energy Rev. 2016, 58, 1366–1375. [Google Scholar] [CrossRef]
- Olivares, F.; Peón, F.; Henríquez, R.; del Río, R.S. Strategies for Area-Selective Deposition of Metal Nanoparticles on Carbon Nanotubes and Their Applications: A Review. J. Mater. Sci. 2022, 57, 2362–2387. [Google Scholar] [CrossRef]
- Guo, W.; Fan, K.; Zhang, J.; Xu, C. 2D/2D Z-Scheme Bi2WO6/Porous-g-C3N4 with Synergy of Adsorption and Visible-Light-Driven Photodegradation. Appl. Surf. Sci. 2018, 447, 125–134. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Kiely, C.J. Strategies for the Synthesis of Supported Gold Palladium Nanoparticles with Controlled Morphology and Composition. Acc. Chem. Res. 2013, 46, 1759–1772. [Google Scholar] [CrossRef] [PubMed]
- Campanati, M.; Fornasari, G.; Vaccari, A. Fundamentals in the Preparation of Heterogeneous Catalysts. Catal. Today 2003, 77, 299–314. [Google Scholar] [CrossRef]
- Bhosale, R.; Jain, S.; Vinod, C.P.; Kumar, S.; Ogale, S. Direct Z-Scheme g-C3N4/FeWO4 Nanocomposite for Enhanced and Selective Photocatalytic CO2 Reduction under Visible Light. ACS Appl. Mater. Interfaces 2019, 11, 6174–6183. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, R.; Wang, H.; Hu, J.; Ren, T. One-Step Impregnation Method to Prepare Direct Z-Scheme LaCoO3/g-C3N4 Heterojunction Photocatalysts for Phenol Degradation under Visible Light. Appl. Surf. Sci. 2019, 491, 432–442. [Google Scholar] [CrossRef]
- Feng, Z.; Zeng, L.; Chen, Y.; Ma, Y.; Zhao, C.; Jin, R.; Lu, Y.; Wu, Y.; He, Y. In Situ Preparation of Z-Scheme MoO3/g-C3N4 Composite with High Performance in Photocatalytic CO2 Reduction and RhB Degradation. J. Mater. Res. 2017, 32, 3660–3668. [Google Scholar] [CrossRef]
- Zhou, D.; Yu, B.; Chen, Q.; Shi, H.; Zhang, Y.; Li, D.; Yang, X.; Zhao, W.; Liu, C.; Wei, G.; et al. Improved Visible Light Photocatalytic Activity on Z-Scheme g-C3N4 Decorated TiO2 Nanotube Arrays by a Simple Impregnation Method. Mater. Res. Bull. 2020, 124, 110757. [Google Scholar] [CrossRef]
- Darr, J.A.; Zhang, J.; Makwana, N.M.; Weng, X. Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions. Chem. Rev. 2017, 117, 11125–11238. [Google Scholar] [CrossRef]
- Kaya, C.; He, J.Y.; Gu, X.; Butler, E.G. Nanostructured Ceramic Powders by Hydrothermal Synthesis and Their Applications. Microporous Mesoporous Mater. 2002, 54, 37–49. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal Technology for Nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Jo, W.K.; Natarajan, T.S. Influence of TiO2 Morphology on the Photocatalytic Efficiency of Direct Z-Scheme g-C3N4/TiO2 Photocatalysts for Isoniazid Degradation. Chem. Eng. J. 2015, 281, 549–565. [Google Scholar] [CrossRef]
- Di, T.; Zhu, B.; Cheng, B.; Yu, J.; Xu, J. A Direct Z-Scheme g-C3N4/SnS2 Photocatalyst with Superior Visible-Light CO2 Reduction Performance. J. Catal. 2017, 352, 532–541. [Google Scholar] [CrossRef]
- Lu, M.; Li, Q.; Zhang, C.; Fan, X.; Li, L.; Dong, Y.; Chen, G.; Shi, H. Remarkable Photocatalytic Activity Enhancement of CO2 Conversion over 2D/2D g-C3N4/BiVO4 Z-Scheme Heterojunction Promoted by Efficient Interfacial Charge Transfer. Carbon 2020, 160, 342–352. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Huang, S.; Li, Y.; Zhang, X.; Zeng, G.; Niu, L.; Ling, Y.; Zhang, Y. Facile Construction of 2D G-C3N4 Supported Nanoflower-like NaBiO3 with Direct Z-Scheme Heterojunctions and Insight into Its Photocatalytic Degradation of Tetracycline. J. Hazard. Mater. 2021, 414, 125547. [Google Scholar] [CrossRef]
- Lakshmanareddy, N.; Navakoteswara Rao, V.; Cheralathan, K.K.; Subramaniam, E.P.; Shankar, M.V. Pt/TiO2 Nanotube Photocatalyst–Effect of Synthesis Methods on Valance State of Pt and Its Influence on Hydrogen Production and Dye Degradation. J. Colloid Interface Sci. 2019, 538, 83–98. [Google Scholar] [CrossRef]
- Magdziarz, A.; Colmenares, J.C. In Situ Coupling of Ultrasound to Electro-and Photo-Deposition Methods for Materials Synthesis. Molecules 2017, 22, 216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Qu, D.; An, L.; Gao, X.; Wen, Y.; Wang, X.; Sun, Z. Deliberate Construction of Direct Z -Scheme Photocatalysts through Photodeposition. J. Mater. Chem. A 2019, 7, 18348–18356. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Yen, D.T.K.; Ting, J.M. Enhanced Photocatalytic Performance of TiO2 through a Novel Direct Dual Z-Scheme Design. Appl. Surf. Sci. 2020, 533, 147506. [Google Scholar] [CrossRef]
- Rhimi, B.; Wang, C.; Bahnemann, D.W. Latest Progress in G-C3N4 Based Heterojunctions for Hydrogen Production via Photocatalytic Water Splitting: A Mini Review. J. Phys. Energy 2020, 2, 042003. [Google Scholar] [CrossRef]
- Pourshirband, N.; Nezamzadeh-Ejhieh, A. An Efficient Z-Scheme CdS/g-C3N4 Nano Catalyst in Methyl Orange Photodegradation: Focus on the Scavenging Agent and Mechanism. J. Mol. Liq. 2021, 335, 116543. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chang, T.F.M.; Chen, C.Y.; Sone, M.; Hsu, Y.J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.Q.; Liu, J.Y.; Liu, X.J. Enhanced Photocatalytic Performance of Direct Z-Scheme BiOCl-g-C3N4 Photocatalysts. RSC Adv. 2014, 4, 19456–19461. [Google Scholar] [CrossRef]
- Jiang, W.; Zong, X.; An, L.; Hua, S.; Miao, X.; Luan, S.; Wen, Y.; Tao, F.F.; Sun, Z. Consciously Constructing Heterojunction or Direct Z-Scheme Photocatalysts by Regulating Electron Flow Direction. ACS Catal. 2018, 8, 2209–2217. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Jiang, X.; Chen, S.; Meng, S.; Fu, X. Design of a Direct Z-Scheme Photocatalyst: Preparation and Characterization of Bi2O3/g-C3N4 with High Visible Light Activity. J. Hazard. Mater. 2014, 280, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Dai, B.; Tong, T.; Jiang, C.; Yu, J. In Situ Irradiated X-Ray Photoelectron Spectroscopy Investigation on a Direct Z-Scheme TiO2/CdS Composite Film Photocatalyst. Adv. Mater. 2019, 31, 1802981. [Google Scholar] [CrossRef]
- Han, Q.; Li, L.; Gao, W.; Shen, Y.; Wang, L.; Zhang, Y.; Wang, X.; Shen, Q.; Xiong, Y.; Zhou, Y.; et al. Elegant Construction of ZnIn2S4/BiVO4 Hierarchical Heterostructures as Direct Z-Scheme Photocatalysts for Efficient CO2 Photoreduction. ACS Appl. Mater. Interfaces 2021, 13, 15092–15100. [Google Scholar] [CrossRef]
- Zhou, Z.; Niu, X.; Zhang, Y.; Wang, J. Janus MoSSe/WSeTe Heterostructures: A Direct Z-Scheme Photocatalyst for Hydrogen Evolution. J. Mater. Chem. A 2019, 7, 21835–21842. [Google Scholar] [CrossRef]
- Abdul Nasir, J.; Munir, A.; Ahmad, N.; Haq, T.U.; Khan, Z.; Rehman, Z. Photocatalytic Z-Scheme Overall Water Splitting: Recent Advances in Theory and Experiments. Adv. Mater. 2021, 33, 105195. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W.; Abdullah, A.M.; Xu, G.; Varma, R.S. Engineering Graphitic Carbon Nitride (g-C3N4) for Catalytic Reduction of CO2 to Fuels and Chemicals: Strategy and Mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Vo, D.V.N.; Yaashikaa, P.R.; Karishma, S.; Jeevanantham, S.; Gayathri, B.; Bharathi, V.D. Photocatalysis for Removal of Environmental Pollutants and Fuel Production: A Review. Environ. Chem. Lett. 2021, 19, 441–463. [Google Scholar] [CrossRef]
- Yu, X.; Ng, S.F.; Putri, L.K.; Tan, L.L.; Mohamed, A.R.; Ong, W.J. Point-Defect Engineering: Leveraging Imperfections in Graphitic Carbon Nitride (g-C3N4) Photocatalysts toward Artificial Photosynthesis. Small 2021, 17, 2006851. [Google Scholar] [CrossRef]
- Fu, Y.; Li, Z.; Liu, Q.; Yang, X.; Tang, H. Construction of Carbon Nitride and MoS2 Quantum Dot 2D/0D Hybrid Photocatalyst: Direct Z-Scheme Mechanism for Improved Photocatalytic Activity. Chin. J. Catal. 2017, 38, 2160–2170. [Google Scholar] [CrossRef]
- Jing, L.; Xu, Y.; Liu, J.; Zhou, M.; Xu, H.; Xie, M.; Li, H.; Xie, J. Direct Z-Scheme Red Carbon Nitride/Rod-like Lanthanum Vanadate Composites with Enhanced Photodegradation of Antibiotic Contaminants. Appl. Catal. B Environ. 2020, 277, 119245. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, Z.; Yang, Q.; Shen, C.; Tang, G.; Zhao, S.; Zhang, J.; Chen, D.; Wei, Q.; Dong, X. Facile Construction of G-C3N4 Nanosheets/TiO2 Nanotube Arrays as Z-Scheme Photocatalyst with Enhanced Visible-Light Performance. ChemCatChem 2016, 8, 3064–3073. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Liu, C.; Sun, Z.; Qiu, M.; Yan, G.; Gao, F. Dual-Z-Scheme Heterojunction for Facilitating Spacial Charge Transport Toward Ultra-Efficient Photocatalytic H2 Production. Sol. RRL 2021, 5, 2100241. [Google Scholar] [CrossRef]
- Idrees, F.; Dillert, R.; Bahnemann, D.; Butt, F.K.; Tahir, M. In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination. Catalysts 2019, 9, 169. [Google Scholar] [CrossRef]
- He, Y.; Zhang, L.; Fan, M.; Wang, X.; Walbridge, M.L.; Nong, Q.; Wu, Y.; Zhao, L. Z-Scheme SnO2-x/g-C3N4 Composite as an Efficient Photocatalyst for Dye Degradation and Photocatalytic CO2 Reduction. Sol. Energy Mater. Sol. Cells 2015, 137, 175–184. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Mohd Nawawi, M.G. Highly STable 3D/2D WO3/g-C3N4 Z-Scheme Heterojunction for Stimulating Photocatalytic CO2 Reduction by H2O/H2 to CO and CH4 under Visible Light. J. CO2 Util. 2020, 41, 101270. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Reports 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Chen, X.; Wang, L.; Cai, W. Coupling of Heterogeneous Advanced Oxidation Processes and Photocatalysis in Efficient Degradation of Tetracycline Hydrochloride by Fe-Based MOFs: Synergistic Effect and Degradation Pathway. Chem. Eng. J. 2019, 369, 745–757. [Google Scholar] [CrossRef]
- Garza-Campos, B.; Brillas, E.; Hernández-Ramírez, A.; El-Ghenymy, A.; Guzmán-Mar, J.L.; Ruiz-Ruiz, E.J. Salicylic Acid Degradation by Advanced Oxidation Processes. Coupling of Solar Photoelectro-Fenton and Solar Heterogeneous Photocatalysis. J. Hazard. Mater. 2016, 319, 34–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, M.; Deng, J.; Deng, K.; Zhang, B.; Lv, K.; Sun, J.; Chen, L. Potocatalytic Oxidative Degradation of Organic Pollutant with Molecular Oxygen Activated by a Novel Biomimetic Catalyst ZnPz(Dtn-COOH)4. Appl. Catal. B Environ. 2013, 132–133, 90–97. [Google Scholar] [CrossRef]
- Xie, L.; Du, T.; Wang, J.; Ma, Y.; Ni, Y.; Liu, Z.; Zhang, L.; Yang, C.; Wang, J. Recent Advances on Heterojunction-Based Photocatalysts for the Degradation of Persistent Organic Pollutants. Chem. Eng. J. 2021, 426, 130617. [Google Scholar] [CrossRef]
- Al-Buriahi, A.K.; Al-Gheethi, A.A.; Senthil Kumar, P.; Radin Mohamed, R.M.S.; Yusof, H.; Alshalif, A.F.; Khalifa, N.A. Elimination of Rhodamine B from Textile Wastewater Using Nanoparticle Photocatalysts: A Review for Sustainable Approaches. Chemosphere 2022, 287, 132162. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Ao, Y.; Wang, C.; Wang, P. Facile Synthesis of Dual Z-Scheme g-C3N4/Ag3PO4/AgI Composite Photocatalysts with Enhanced Performance for the Degradation of a Typical Neonicotinoid Pesticide. Appl. Catal. B Environ. 2020, 268, 118395. [Google Scholar] [CrossRef]
- Che, H.; Che, G.; Dong, H.; Hu, W.; Hu, H.; Liu, C.; Li, C. Fabrication of Z-Scheme Bi3O4Cl/g-C3N4 2D/2D Heterojunctions with Enhanced Interfacial Charge Separation and Photocatalytic Degradation Various Organic Pollutants Activity. Appl. Surf. Sci. 2018, 455, 705–716. [Google Scholar] [CrossRef]
- Song, Y.; Gu, J.; Xia, K.; Yi, J.; Chen, H.; She, X.; Chen, Z.; Ding, C.; Li, H.; Xu, H. Construction of 2D SnS2/g-C3N4 Z-Scheme Composite with Superior Visible-Light Photocatalytic Performance. Appl. Surf. Sci. 2019, 467–468, 56–64. [Google Scholar] [CrossRef]
- López-Peñalver, J.J.; Sánchez-Polo, M.; Gómez-Pacheco, C.V.; Rivera-Utrilla, J. Photodegradation of Tetracyclines in Aqueous Solution by Using UV and UV/H2O2 Oxidation Processes. J. Chem. Technol. Biotechnol. 2010, 85, 1325–1333. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, C.; Qu, J.; Yang, M. Photodegradation of Tetracycline and Formation of Reactive Oxygen Species in Aqueous Tetracycline Solution under Simulated Sunlight Irradiation. J. Photochem. Photobiol. A Chem. 2008, 197, 81–87. [Google Scholar] [CrossRef]
- De Godos, I.; Muñoz, R.; Guieysse, B. Tetracycline Removal during Wastewater Treatment in High-Rate Algal Ponds. J. Hazard. Mater. 2012, 229–230, 446–449. [Google Scholar] [CrossRef]
- Kang, J.; Tang, Y.; Wang, M.; Jin, C.; Liu, J.; Li, S.; Li, Z.; Zhu, J. The Enhanced Peroxymonosulfate-Assisted Photocatalytic Degradation of Tetracycline under Visible Light by g-C3N4/Na-BiVO4 heterojunction Catalyst and Its Mechanism. J. Environ. Chem. Eng. 2021, 9, 105524. [Google Scholar] [CrossRef]
- Jin, C.; Wang, M.; Li, Z.; Kang, J.; Zhao, Y.; Han, J.; Wu, Z. Two Dimensional Co3O4/g-C3N4 Z-Scheme Heterojunction: Mechanism Insight into Enhanced Peroxymonosulfate-Mediated Visible Light Photocatalytic Performance. Chem. Eng. J. 2020, 398, 125569. [Google Scholar] [CrossRef]
- Wang, S.; Teng, Z.; Xu, Y.; Yuan, M.; Zhong, Y.; Liu, S.; Wang, C.; Wang, G.; Ohno, T. Defect as the Essential Factor in Engineering Carbon-Nitride-Based Visible-Light-Driven Z-Scheme Photocatalyst. Appl. Catal. B Environ. 2020, 260, 118145. [Google Scholar] [CrossRef]
- Aravind kumar, J.; Krithiga, T.; Sathish, S.; Renita, A.A.; Prabu, D.; Lokesh, S.; Geetha, R.; Namasivayam, S.K.R.; Sillanpaa, M. Persistent Organic Pollutants in Water Resources: Fate, Occurrence, Characterization and Risk Analysis. Sci. Total Environ. 2022, 831, 154808. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Ni, C.; He, E.; Yu, L.; Li, X. 3D/2D MOF-Derived CoCeOx/g-C3N4 Z-Scheme Heterojunction for Visible Light Photocatalysis: Hydrogen Production and Degradation of Carbamazepine. J. Alloys Compd. 2022, 890, 161786. [Google Scholar] [CrossRef]
- Huang, J.; Li, D.; Li, R.; Chen, P.; Zhang, Q.; Liu, H.; Lv, W.; Liu, G.; Feng, Y. One-Step Synthesis of Phosphorus/Oxygen Co-Doped g-C3N4/Anatase TiO2 Z-Scheme Photocatalyst for Significantly Enhanced Visible-Light Photocatalysis Degradation of Enrofloxacin. J. Hazard. Mater. 2020, 386, 121634. [Google Scholar] [CrossRef]
- Meng, J.; Wang, X.; Liu, Y.; Ren, M.; Zhang, X.; Ding, X.; Guo, Y.; Yang, Y. Acid-Induced Molecule Self-Assembly Synthesis of Z-Scheme WO3/g-C3N4 Heterojunctions for Robust Photocatalysis against Phenolic Pollutants. Chem. Eng. J. 2021, 403, 126354. [Google Scholar] [CrossRef]
- Liu, N.; Lu, N.; Yu, H.T.; Chen, S.; Quan, X. Enhanced Degradation of Organic Water Pollutants by Photocatalytic In-Situ Activation of Sulfate Based on Z-Scheme g-C3N4/BiPO4. Chem. Eng. J. 2022, 428, 132116. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Xiong, Z.; Tang, H.; Jiang, C. Fabrication of Flower-like Direct Z-Scheme β-Bi2O3/g-C3N4 Photocatalyst with Enhanced Visible Light Photoactivity for Rhodamine B Degradation. Appl. Surf. Sci. 2018, 436, 162–171. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-Scheme 2D/3D g-C3N4@ZnO with Enhanced Photocatalytic Activity for Cephalexin Oxidation under Solar Light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Ma, C.; Liu, X.; Meng, M.; Yan, Y. Fabrication of Magnetic Quantum Dots Modified Z-Scheme Bi2O4/g-C3N4 Photocatalysts with Superior Hydroxyl Radical Productivity for the Degradation of Rhodamine B. Appl. Surf. Sci. 2019, 493, 458–469. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Zeng, Y.; Liu, F.; Yuan, J.; Li, X.; Yu, Y.; Zhu, X.; Xiong, Z.; Yu, H.; et al. TiO2/Graphitic Carbon Nitride Nanosheets for the Photocatalytic Degradation of Rhodamine B under Simulated Sunlight. ACS Appl. Nano Mater. 2019, 2, 7255–7265. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A Novel α-Fe2O3@g-C3N4 Catalyst: Synthesis Derived from Fe-Based MOF and Its Superior Photo-Fenton Performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Truc, N.T.T.; Duc, D.S.; Van Thuan, D.; Tahtamouni, T.A.; Pham, T.D.; Hanh, N.T.; Tran, D.T.; Nguyen, M.V.; Dang, N.M.; Le Chi, N.T.P.; et al. The Advanced Photocatalytic Degradation of Atrazine by Direct Z-Scheme Cu Doped ZnO/g-C3N4. Appl. Surf. Sci. 2019, 489, 875–882. [Google Scholar] [CrossRef]
- Gayathri, K.; Teja, Y.N.; Prakash, R.M.; Hossain, M.S.; Alsalme, A.; Sundaravadivel, E.; Sakar, M. In Situ-Grown ZnO Particles on g-C3N4 Layers: A Direct Z-Scheme-Driven Photocatalyst for the Degradation of Dye and Pharmaceutical Pollutants under Solar Irradiation. J. Mater. Sci. Mater. Electron. 2022, 33, 9774–9784. [Google Scholar] [CrossRef]
- Jain, I.P. Hydrogen the Fuel for 21st Century. Int. J. Hydrogen Energy 2009, 34, 7368–7378. [Google Scholar] [CrossRef]
- Nazir, H.; Louis, C.; Jose, S.; Prakash, J.; Muthuswamy, N.; Buan, M.E.M.; Flox, C.; Chavan, S.; Shi, X.; Kauranen, P.; et al. Is the H2 Economy Realizable in the Foreseeable Future? Part I: H2 Production Methods. Int. J. Hydrogen Energy 2020, 45, 13777–13788. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic Water Splitting-The Untamed Dream: A Review of Recent Advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Fajrina, N.; Tahir, M. A Critical Review in Strategies to Improve Photocatalytic Water Splitting towards Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 540–577. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Chen, X.; Shi, R.; Chen, Q.; Zhang, Z.; Jiang, W.; Zhu, Y.; Zhang, T. Three-Dimensional Porous g-C3N4 for Highly Efficient Photocatalytic Overall Water Splitting. Nano Energy 2019, 59, 644–650. [Google Scholar] [CrossRef]
- Ye, R.; Fang, H.; Zheng, Y.Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D A-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, M.; Li, L.; Pi, Y.; Yan, G.; Yang, L. A Direct Z-Scheme Van Der Waals Heterojunction (WO3·H2O/g-C3N4) for High Efficient Overall Water Splitting under Visible-Light. Sol. RRL 2018, 2, 1800148. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Wang, C.; Xu, J.; Du, X.; Li, L. Ti3+ Defect Mediated G-C3N4 /TiO2 Z-Scheme System for Enhanced Photocatalytic Redox Performance. Appl. Surf. Sci. 2018, 448, 288–296. [Google Scholar] [CrossRef]
- Qin, S.; Lei, Y.; Guo, J.; Huang, J.F.; Hou, C.P.; Liu, J.M. Constructing Heterogeneous Direct Z-Scheme Photocatalysts Based on Metal-Organic Cages and Graphitic-C3N4 for High-Efficiency Photocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 25960–25971. [Google Scholar] [CrossRef]
- Wang, J.; Xia, Y.; Zhao, H.; Wang, G.; Xiang, L.; Xu, J.; Komarneni, S. Oxygen Defects-Mediated Z-Scheme Charge Separation in g-C3N4/ZnO Photocatalysts for Enhanced Visible-Light Degradation of 4-Chlorophenol and Hydrogen Evolution. Appl. Catal. B Environ. 2017, 206, 406–416. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Mao, Z.; Fahlman, B.D.; Wang, D. Construction of Z-Scheme Heterostructure with Enhanced Photocatalytic H2 Evolution for g-C3N4 Nanosheets via Loading Porous Silicon. J. Catal. 2017, 356, 22–31. [Google Scholar] [CrossRef]
- Yang, C.; Xue, Z.; Qin, J.; Sawangphruk, M.; Rajendran, S.; Zhang, X.; Liu, R. Visible Light-Driven Photocatalytic H2 Generation and Mechanism Insights into Bi2O2CO3 /G-C3N4 Z-Scheme Photocatalyst. J. Phys. Chem. C 2019, 123, 4795–4804. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, H.; Yang, C.; Li, M.; Zhao, Y.; Chen, F. Post-Activation of in Situ B–F Codoped g-C3N4 for Enhanced Photocatalytic H2 Evolution. Appl. Surf. Sci. 2018, 441, 621–630. [Google Scholar] [CrossRef]
- Xu, B.; Wang, B.; Zhang, H.; Yang, P. Z-Scheme Cu2O Nanoparticle/Graphite Carbon Nitride Nanosheet Heterojunctions for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2022, 5, 8475–8483. [Google Scholar] [CrossRef]
- Olabi, A.G.; Abdelkareem, M.A. Renewable Energy and Climate Change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Kuc, T.; Rozanski, K.; Zimnoch, M.; Necki, J.M.; Korus, A. Anthropogenic Emissions of CO2 and CH4 in an Urban Environment. Appl. Energy 2003, 75, 193–203. [Google Scholar] [CrossRef]
- Sullivan, I.; Goryachev, A.; Digdaya, I.A.; Li, X.; Atwater, H.A.; Vermaas, D.A.; Xiang, C. Coupling Electrochemical CO2 Conversion with CO2 Capture. Nat. Catal. 2021, 4, 952–958. [Google Scholar] [CrossRef]
- Lewis, N.S. Developing a Scalable Artificial Photosynthesis Technology through Nanomaterials by Design. Nat. Nanotechnol. 2016, 11, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 2005. [Google Scholar]
- Khalil, M.; Gunlazuardi, J.; Ivandini, T.A.; Umar, A. Photocatalytic Conversion of CO2 Using Earth-Abundant Catalysts: A Review on Mechanism and Catalytic Performance. Renew. Sustain. Energy Rev. 2019, 113, 109246. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.; Gong, J. CO2 Photo-Reduction: Insights into CO2 Activation and Reaction on Surfaces of Photocatalysts. Energy Environ. Sci. 2016, 9, 2177–2196. [Google Scholar] [CrossRef]
- Collado, L.; Reynal, A.; Coronado, J.M.; Serrano, D.P.; Durrant, J.R.; De la Peña O’Shea, V.A. Effect of Au Surface Plasmon Nanoparticles on the Selective CO2 Photoreduction to CH4. Appl. Catal. B Environ. 2015, 178, 177–185. [Google Scholar] [CrossRef]
- Dong, G.; Zhang, L. Porous Structure Dependent Photoreactivity of Graphitic Carbon Nitride under Visible Light. J. Mater. Chem. 2012, 22, 1160–1166. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef]
- Liu, T.; Hao, L.; Bai, L.; Liu, J.; Zhang, Y.; Tian, N.; Huang, H. Z-Scheme Junction Bi2O2(NO3)(OH)/g-C3N4 for Promoting CO2 Photoreduction. Chem. Eng. J. 2022, 429, 132268. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced Photocatalytic Activity of G-C3N4 for Selective CO2 Reduction to CH3OH via Facile Coupling of ZnO: A Direct Z-Scheme Mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Giang Bach, L.; Thi Hanh, N.; Pham, T.D.; Thi Phuong Le Chi, N.; Tran, D.T.; Nguyen, M.V.; Nguyen, V.N. The Superior Photocatalytic Activity of Nb Doped TiO2/g-C3N4 Direct Z-Scheme System for Efficient Conversion of CO2 into Valuable Fuels. J. Colloid Interface Sci. 2019, 540, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ji, M.; Chen, H.; Weng, Y.X.; Zhong, J.; Li, Y.; Wang, S.; Chen, Z.; Xia, J.; Li, H. Interfacial Chemical Bond Modulated Bi19S27Br3/g-C3N4 Z-Scheme Heterojunction for Enhanced Photocatalytic CO2 Conversion. Appl. Catal. B Environ. 2022, 307, 121162. [Google Scholar] [CrossRef]
- Murugesan, P.; Narayanan, S.; Manickam, M.; Murugesan, P.K.; Subbiah, R. A Direct Z-Scheme Plasmonic AgCl@g-C3N4 Heterojunction Photocatalyst with Superior Visible Light CO2 Reduction in Aqueous Medium. Appl. Surf. Sci. 2018, 450, 516–526. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Hanh, N.T.; Nguyen, M.V.; Le Chi, N.T.P.; Van Noi, N.; Tran, D.T.; Ha, M.N.; Trung, D.Q.; Pham, T.D. Novel Direct Z-Scheme Cu2V2O7/g-C3N4 for Visible Light Photocatalytic Conversion of CO2 into Valuable Fuels. Appl. Surf. Sci. 2018, 457, 968–974. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Pham, T.D.; Nguyen, M.V.; Van Thuan, D.; Trung, D.Q.; Thao, P.; Trang, H.T.; Nguyen, V.N.; Tran, D.T.; Minh, D.N.; et al. Advanced NiMoO4/g-C3N4 Z-Scheme Heterojunction Photocatalyst for Efficient Conversion of CO2 to Valuable Products. J. Alloys Compd. 2020, 842, 155860. [Google Scholar] [CrossRef]
- Shen, Y.; Han, Q.; Hu, J.; Gao, W.; Wang, L.; Yang, L.; Gao, C.; Shen, Q.; Wu, C.; Wang, X.; et al. Artificial Trees for Artificial Photosynthesis: Construction of Dendrite-Structured α-Fe2O3/g-C3N4Z-Scheme System for Efficient CO2 Reduction into Solar Fuels. ACS Appl. Energy Mater. 2020, 3, 6561–6572. [Google Scholar] [CrossRef]
- Wang, K.; Jiang, L.; Wu, X.; Zhang, G. Vacancy Mediated Z-Scheme Charge Transfer in a 2D/2D La2Ti2O7/g-C3N4 nanojunction as a Bifunctional Photocatalyst for Solar-to-Energy Conversion. J. Mater. Chem. A 2020, 8, 13241–13247. [Google Scholar] [CrossRef]
- Guo, R.T.; Liu, X.Y.; Qin, H.; Wang, Z.Y.; Shi, X.; Pan, W.G.; Fu, Z.G.; Tang, J.Y.; Jia, P.Y.; Miao, Y.F.; et al. Photocatalytic Reduction of CO2 into CO over Nanostructure Bi2S3 Quantum Dots/g-C3N4 Composites with Z-Scheme Mechanism. Appl. Surf. Sci. 2020, 500, 144059. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Xu, Q.; Xiong, D.; Xia, P. High-Efficient Separation of Photoinduced Carriers on Double Z-Scheme Heterojunction for Superior Photocatalytic CO2 Reduction. J. Colloid Interface Sci. 2020, 564, 303–312. [Google Scholar] [CrossRef]
- Guo, H.; Wan, S.; Wang, Y.; Ma, W.; Zhong, Q.; Ding, J. Enhanced Photocatalytic CO2 Reduction over Direct Z-Scheme NiTiO3/g-C3N4 Nanocomposite Promoted by Efficient Interfacial Charge Transfer. Chem. Eng. J. 2021, 412, 128646. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Catalá, J.; Greco, R.; Navlani-García, M.; Cao, W.; Berenguer-Murcia, Á.; Cazorla-Amorós, D. g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications. Catalysts 2022, 12, 1137. https://doi.org/10.3390/catal12101137
Fernández-Catalá J, Greco R, Navlani-García M, Cao W, Berenguer-Murcia Á, Cazorla-Amorós D. g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications. Catalysts. 2022; 12(10):1137. https://doi.org/10.3390/catal12101137
Chicago/Turabian StyleFernández-Catalá, Javier, Rossella Greco, Miriam Navlani-García, Wei Cao, Ángel Berenguer-Murcia, and Diego Cazorla-Amorós. 2022. "g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications" Catalysts 12, no. 10: 1137. https://doi.org/10.3390/catal12101137
APA StyleFernández-Catalá, J., Greco, R., Navlani-García, M., Cao, W., Berenguer-Murcia, Á., & Cazorla-Amorós, D. (2022). g-C3N4-Based Direct Z-Scheme Photocatalysts for Environmental Applications. Catalysts, 12(10), 1137. https://doi.org/10.3390/catal12101137









