Fluoride-Doped TiO2 Photocatalyst with Enhanced Activity for Stable Pollutant Degradation
Abstract
:1. Introduction
2. Results
2.1. Preliminary Tests
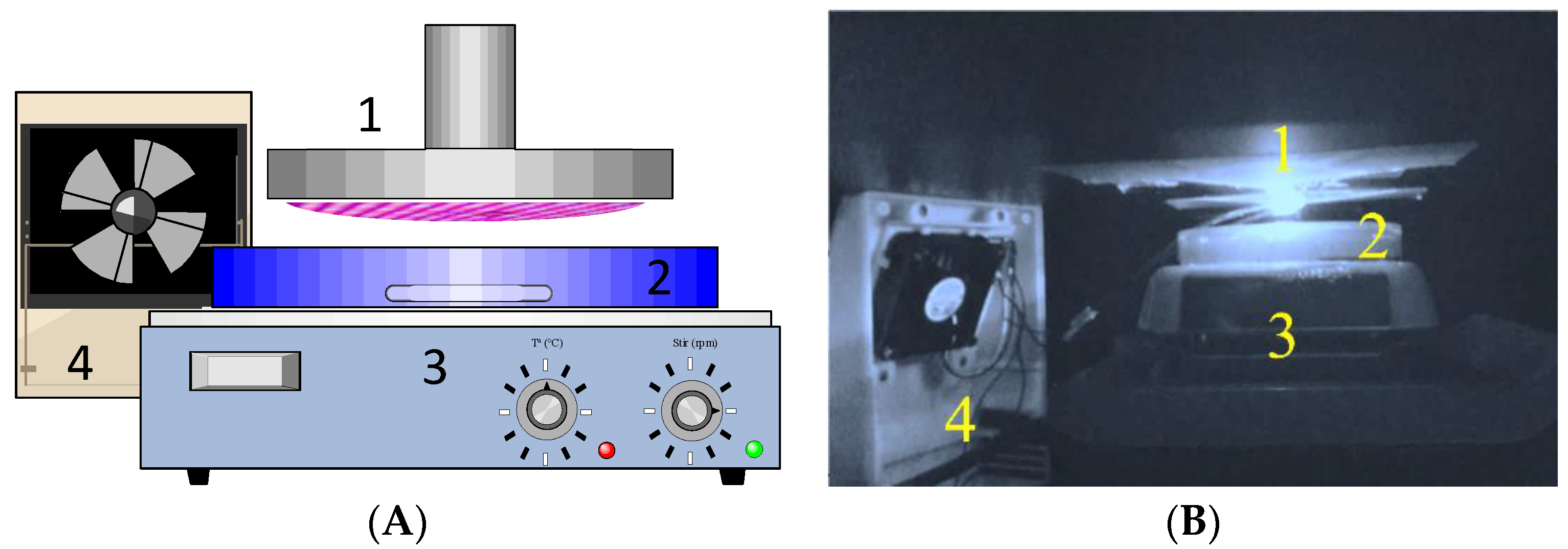
2.1.1. Rector Set-up
2.1.2. Catalyst Doping Dosage
2.1.3. Catalyst Concentration
2.2. Characterization
2.2.1. XRD
2.2.2. Raman
2.2.3. SEM, EDS and ICP
2.2.4. UV-Vis Spectra
2.2.5. Electrochemical Measurements
EIS
ECSA
2.3. Pollutant Abatement
2.3.1. Catalyst Evaluation
2.3.2. Long-Pulse Radiation Procedure
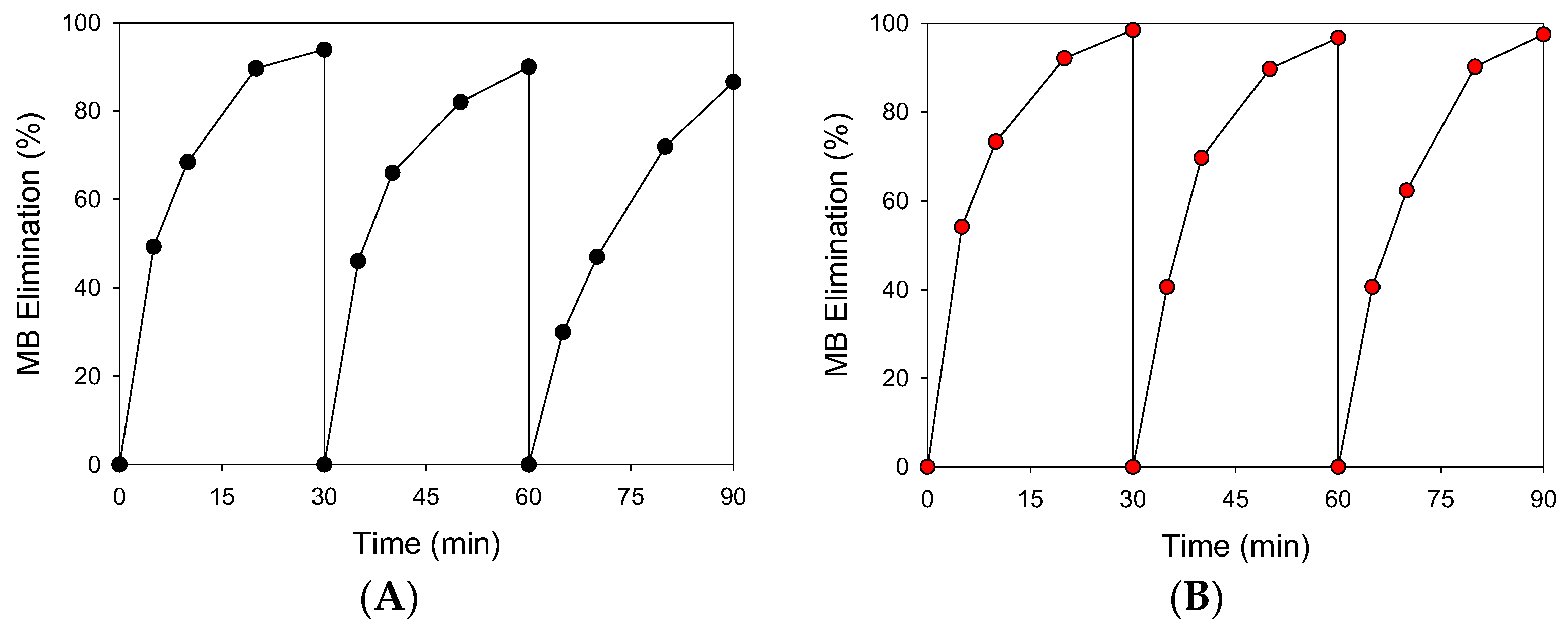
2.3.3. Reusability
2.3.4. Pollutant Concentration
2.3.5. Treatment of More Complex Effluents
3. Discussion
3.1. Preliminary Tests
3.1.1. Reactor Set-up
3.1.2. Catalyst Doping Dosage
3.1.3. Catalyst Concentration
3.2. Characterization
3.2.1. XRD
3.2.2. Raman
3.2.3. SEM and EDS
3.2.4. UV-Vis Spectra
3.2.5. Electrochemical Measurements
EIS
ECSA
3.3. Pollutant Abatement
3.3.1. Catalyst Evaluation
3.3.2. Long-Pulse Radiation Procedure
3.3.3. Reusability
3.3.4. Pollutant Concentration
3.3.5. Treatment of More Complex Effluents
3.3.6. Comparison with Previous Studies
4. Materials and Methods
4.1. Reagents
4.2. Catalyst Synthesis
4.3. Reactor Set-Up
4.4. Characterization
4.4.1. X-ray Diffraction (XRD)
4.4.2. Raman
4.4.3. SEM, EDS, and ICP
4.4.4. UV-Vis
4.4.5. Electrochemical Measurements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kurniawan, T.A.; Mengting, Z.; Fu, D.; Yeap, S.K.; Othman, M.H.D.; Avtar, R.; Ouyang, T. Functionalizing TiO2 with graphene oxide for enhancing photocatalytic degradation of methylene blue (MB) in contaminated wastewater. J. Environ. Manag. 2020, 270, 110871–110879. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Bahmanrokh, G.; Kumar, V.; Ho, N.; Koshy, P.; Sorrell, C.C. Synthesis of V- and Mo-doped/codoped TiO2 powders for photocatalytic degradation of methylene blue. Nano-Struct. Nano-Objects 2020, 24, 100557–100568. [Google Scholar] [CrossRef]
- D’Souza, L.P.; Prakash, R.M.; Balakrishna, R.G. Chapter 3 Anion-Modified Photocatalysts. In Photocatalytic Systems by Design; Sakar, M., Balakrishna, R.G., Do, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 55–83. [Google Scholar]
- Jafari, A.J.; Kalantary, R.R.; Esrafili, A.; Moslemzadeh, M. Photo-catalytic degradation of bisphenol-a from aqueous solutions using GF/Fe-TiO2-CQD hybrid composite. J. Environ. Health Sci. Eng. 2021, 19, 837–849. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Chakinala, N.; Gogate, R.R.; Chakinala, G. Highly efficient bi-metallic bismuth-silver doped TiO2 photocatalyst for dye degradation. Korean J. Chem. Eng. 2021, 38, 2468–2478. [Google Scholar] [CrossRef]
- Zheng, X.; Han, H.; Ye, X.; Meng, S.; Zhao, S.; Wang, X.; Chen, S. Fabrication of Z-scheme WO3/KNbO3 photocatalyst with enhanced separation of charge carriers. Chem. Res. Chin. Univ. 2020, 36, 901–907. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic degradation of bisphenol A using titanium dioxide@ nanodiamond composites under UV light illumination. J. Colloid. Interface Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef]
- Acosta-Esparza, M.A.; Rivera, L.P.; Pérez-Centeno, A.; Zamudio-Ojeda, A.; González, D.R.; Chávez-Chávez, A.; Santana-Aranda, M.A.; Santos-Cruz, J.; Quiñones-Galván, J. UV and Visible light photodegradation of methylene blue with graphene decorated titanium dioxide. Mater. Res. Express. 2020, 7, 35504–35512. [Google Scholar] [CrossRef]
- Bibi, S.; Ahmad, A.; Anjum, M.A.R.; Haleem, A.; Siddiq, M.; Shah, S.S.; Kahtani, A.A. Photocatalytic degradation of malachite green and methylene blue over reduced graphene oxide (rGO) based metal oxides (rGO-Fe3O4/TiO2) nanocomposite under UV-visible light irradiation. J. Environ. Chem. Eng. 2021, 9, 105580–105591. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean Prod. 2021, 280, 124518–124531. [Google Scholar] [CrossRef]
- Niu, L.; Zhao, X.; Tang, Z.; Lv, H.; Wu, F.; Wang, X.; Zhao, T.; Wang, J.; Wu, A.; Giesy, J. Difference in performance and mechanism for methylene blue when TiO2 nanoparticles are converted to nanotubes. J. Clean Prod. 2021, 297, 126498–126509. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.; Haghighat, F. Effect of surface fluorination of P25-TiO2 coated on nickel substrate for photocatalytic oxidation of methyl ethyl ketone in indoor environments. J. Environ. Chem. Eng. 2019, 7, 103390–103401. [Google Scholar] [CrossRef]
- Gao, Q.; Si, F.; Zhang, S.; Fang, Y.; Chen, X.; Yang, S. Hydrogenated F-doped TiO2 for photocatalytic hydrogen evolution and pollutant degradation. Int. J. Hydrogen Energy 2019, 44, 8011–8019. [Google Scholar] [CrossRef]
- Rani, M.; Keshu Shanker, U. Efficient degradation of organic pollutants by novel titanium dioxide coupled bismuth oxide nanocomposite: Green synthesis, kinetics and photoactivity. J. Environ. Manag. 2021, 300, 113777–113791. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, F.; Ueno, M.; Chand, R.; Shibata, Y.; Luitel, H.N. Effect of silanization of titanium dioxide on photocatalytic decomposition of 2,4-dinitropheonol under irradiation with artificial UV light and sunlight. J. Chem. Technol. Biotechnol. 2014, 89, 81–87. [Google Scholar] [CrossRef]
- Habib, I.Y.; Burhan, J.; Jaladi, F.; Lim, C.M.; Usman, A.; Kumara, N.T.R.N.; Tsang, S.C.E.; Mahadi, A.H. Effect of Cr doping in CeO2 nanostructures on photocatalysis and H2O2 assisted methylene blue dye degradation. Catal. Today 2020, 375, 506–513. [Google Scholar] [CrossRef]
- Diao, W.; Xu, J.; Rao, X.; Zhang, Y. Facile Synthesis of Fluorine Doped Rutile TiO2 Nanorod Arrays for Photocatalytic Removal of Formaldehyde. Catal. Lett. 2022, 152, 1029–1039. [Google Scholar] [CrossRef]
- Ahmad, N.; Sultana, S.; Sabir, S.; Khan, M.Z. Exploring the visible light driven photocatalysis by reduced graphene oxide supported Ppy/CdS nanocomposites for the degradation of organic pollutants. J. Photochem. Photobiol. A 2020, 386, 112129–112142. [Google Scholar] [CrossRef]
- Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A. Characterization of TiO2-based semiconductors for photocatalysis by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2016, 387, 183–189. [Google Scholar] [CrossRef]
- Díez, A.M.; Valencia, H.E.; Meledina, M.; Mayer, J.; Kolen’ko, Y.V. Photocatalytic-fenton process under simulated solar radiation promoted by a suitable catalyst selection. Catalysts 2021, 11, 885. [Google Scholar] [CrossRef]
- Lian, X.; Chen, S.; He, F.; Dong, S.; Liu, E.; Li, H.; Xu, K. Photocatalytic degradation of ammonium dinitramide over novel S-scheme g-C3N4/BiOBr heterostructure nanosheets. Sep. Purif. Technol. 2022, 286, 120449–120459. [Google Scholar] [CrossRef]
- Garg, R.; Gupta, R.; Bansal, A. Degradation mechanism, reaction pathways and kinetics for the mineralization of Bisphenol A using hybrid ZnO/graphene oxide nano-catalysts. Korean J. Chem. Eng. 2021, 38, 485–497. [Google Scholar] [CrossRef]
- Arias, M.C.; Aguilar, C.; Piza, M.; Zarazua, E.; Anguebes, F.; Cordova, V. Removal of the Methylene Blue Dye (MB) with Catalysts of Au-TiO2: Kinetic and Degradation Pathway. Mod. Res. Catal. 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Brahmi, C.; Benltifa, M.; Ghali, M.; Dumur, F.; Simonnet-Jégat, C.; Monnier, V.; Morlet-Savary, F.; Bousselmi, L.; Lalevée, J. Polyoxometalates/polymer composites for the photodegradation of bisphenol-A. J. Appl. Polym. Sci. 2021, 138, 50864–50876. [Google Scholar] [CrossRef]
- Pupo Nogueira, R.F.; Trovó, A.G.; Modé, D.F. Solar photodegradation of dichloroacetic acid and 2,4-dichlorophenol using an enhanced photo-Fenton process. Chemosphere 2002, 48, 385–391. [Google Scholar] [CrossRef]
- Bangun, J.; Adesina, A.A. The photodegradation kinetics of aqueous sodium oxalate solution using TiO2 catalyst. Appl. Catal. A Gen. 1998, 175, 221–235. [Google Scholar] [CrossRef]
- Sun, X.; Xu, K.; Chatzitakis, A.; Norby, T. Photocatalytic generation of gas phase reactive oxygen species from adsorbed water: Remote action and electrochemical detection. J. Environ. Chem. Eng. 2021, 9, 104809–104818. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Samoila, P.; Airinei, A.; Olaru, N.; Rusu, D.; Rosca, I.; Suchea, M. Photocatalytic and antimicrobial activity of electrospun ZnO:Ag nanostructures. J. Alloy. Compd. 2020, 834, 155144–155155. [Google Scholar] [CrossRef]
- Arunagiri, C. Enhanced Visible Light Photocatalytic Degradation of Fe-Doped ZnO Nanoparticles For Organic Dyes; Research Square: Newcastle, UK, 2021. [Google Scholar]
- Peng, H.; Xu, L.; Zhang, W.; Liu, F.; Lu, X.; Lu, W.; Danish, M.; Lin, K. Different kinds of persulfate activation with base for the oxidation and mechanism of BDE209 in a spiked soil system. Sci. Total Environ. 2017, 574, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Subalakshmi, K.; Senthilselvan, J. Effect of fluorine-doped TiO2 photoanode on electron transport, recombination dynamics and improved DSSC efficiency. Sol. Energy 2018, 171, 914–928. [Google Scholar] [CrossRef]
- Noh, K.; Oh, H.; Bo-Ra, K.; Jung, S.; Kang, W.; Sun-Jae, K. Photoelectrochemical Properties of Fe2O3 Supported on TiO2-Based Thin Films Converted from Self-Assembled Hydrogen Titanate Nanotube Powders. J. Nanomater. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mahanta, U.; Khandelwal, M.; Deshpande, A.S. TiO2@SiO2 nanoparticles for methylene blue removal and photocatalytic degradation under natural sunlight and low-power UV light. Appl. Surf. Sci. 2022, 576, 151745–151756. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kuk, Y.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef] [PubMed]
- Mahanthappa, M.; Kottam, N.; Yellappa, S. Enhanced photocatalytic degradation of methylene blue dye using CuSCdS nanocomposite under visible light irradiation. Appl. Surf. Sci. 2019, 475, 828–838. [Google Scholar] [CrossRef]
- Liu, J.; Shi, H.; Sans, C.; Sun, L.; Yuan, X.; Pan, F.; Xia, D. Insights into the photocatalytic ozonation over Ag2O-ZnO@g-C3N4 composite: Cooperative structure, degradation performance, and synergistic mechanisms. J. Environ. Chem. Eng. 2022, 10, 107285–107298. [Google Scholar] [CrossRef]
- Sultana, S.; Ahmad, N.; Ahmad, E.; Sabir, S.; Khan, M.Z. Electrochemical synthesis of novel aluminium oxyhydroxide-decorated MnO2/chitosan nanocomposite with efficient photocatalytic and antibacterial activity. Nanotechnol. Environ. Eng. 2020, 5, 20. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Liu, Y. Fabrication of a novel high photocatalytic Ag/Ag3PO4/P25 (TiO2) heterojunction catalyst for reducing electron-hole pair recombination and improving photo-corrosion. Mater. Res. Express. 2019, 6, 65515–65525. [Google Scholar] [CrossRef]
- Arifin, M.N.; Rezaul Karim, K.M.; Abdullah, H.; Khan, M.R. Synthesis of titania doped copper ferrite photocatalyst and its photoactivity towards methylene blue degradation under visible light irradiation. Bull. Chem. React. Eng. Catal. 2019, 14, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Luo, S.; Lei, X.; Liu, H.; Liu, Y.; Xu, X.; Jiang, Z.; Li, X. Synthesis and photocatalytic performance of nano-CeO2 by a PVP-assisted microwave interface method for organic dye degradation. Ionics 2020, 26, 5829–5839. [Google Scholar] [CrossRef]
- Murcia, J.J.; Cely, Á.C.; Rojas, H.A.; Hidalgo, M.C.; Navío, J.A. Fluorinated and platinized titania as effective materials in the photocatalytic treatment of dyestuffs and stained wastewater coming from handicrafts factories. Catalysts 2019, 9, 179. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. Removal of organic pollutants from wastewater by advanced oxidation processes and its combination with membrane processes. Chem. Eng. Process.-Process Intensif. 2021, 169, 108631–108653. [Google Scholar] [CrossRef]
- Pham, D.C.; Cao, T.M.D.; Nguyen, M.C.; Nguyen, T.D.; Nguyen, V.H.; Bui, V.H.; Nguyen, T.T.T. Integrating Photocatalysis and Microfiltration for Methylene Blue Degradation: Kinetics and Cost Estimation. Chem. Eng. Technol. 2022, 45, 1748–1758. [Google Scholar] [CrossRef]
- Coleman, N.; Lovander, M.D.; Leddy, J.; Gillan, E.G. Phosphorus-Rich Metal Phosphides: Direct and Tin Flux-Assisted Synthesis and Evaluation as Hydrogen Evolution Electrocatalysts. Inorg. Chem. 2019, 58, 5013–5024. [Google Scholar] [CrossRef] [PubMed]





| MB (mg/L) | Catalyst (mg/L) | Lamp (nm, w) | Time (min) | Degradation (%) | EC (W·h/mg) | Reference |
|---|---|---|---|---|---|---|
| 20 | F-TiO2 (800) | 360–365 nm, 4.8 W | 15 | 78.84 | 0.070 | This study |
| 20 | F-TiO2 (800) | 360–365 nm, 4.8 W | 30 | 96.24 | 0.121 | This study |
| 73.57 | Graphene-TiO2 (50) | 360 nm, 17 W | 480 | 87 | 2.12 | [9] |
| 10 | F-TiO2 (200) | 300 W | 20 | 88 | 11.25 | [14] |
| 5 | GO-TiO2 (200) | 500 W | 60 | 92 | 108.7 | [1] |
| 3.2 | V/Mo-TiO2 (1000) | 365 nm, 8 W | 60 | 86.7 | 2.88 | [2] |
| 10 | Zeolite/Ni-TiO2 (50) | UV, 16 W | 120 | 100 | 3.20 | [11] |
| 10 | TiO2 nanoparticles (60) | UV, 300 W, >420 nm | 24 | 99 | 12.12 | [12] |
| 10 | WO3/KNbO3 (3000) | 365 nm, 375 W | 40 | 98 | 24.3 | [7] |
| 3 | rGO-Fe3O4/TiO2 (150) | UV, 500 W | 70 | 99 | 196.4 | [10] |
| BPA (mg/L) | Catalyst (mg/L) | Lamp (nm, w) | Time (min) | Degradation (%) | EC (W·h/mg) | Reference |
| 20 | F-TiO2 (800) | 360–365 nm, 4.8 W | 30 | 98 | 0.122 | This study |
| 10 | WO3/KNbO3 (3000) | 365 nm, 375 W | 240 | 49 | 300 | [7] |
| 10 | Nanodiamond-TiO2 (80) | UV, 20 W | 100 | 96 | 3.47 | [8] |
| 20 | Glass Fibers Fe-TiO2-carbon quantum dots (n.r.) | λ < 472 nm, 55 W | 60 | 100 | 2.75 | [4] |
| 20 | ZnO-Graphene oxide | 254 nm, 15 W | 60 | 99.5 | 0.75 | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díez, A.M.; Núñez, I.; Pazos, M.; Sanromán, M.Á.; Kolen’ko, Y.V. Fluoride-Doped TiO2 Photocatalyst with Enhanced Activity for Stable Pollutant Degradation. Catalysts 2022, 12, 1190. https://doi.org/10.3390/catal12101190
Díez AM, Núñez I, Pazos M, Sanromán MÁ, Kolen’ko YV. Fluoride-Doped TiO2 Photocatalyst with Enhanced Activity for Stable Pollutant Degradation. Catalysts. 2022; 12(10):1190. https://doi.org/10.3390/catal12101190
Chicago/Turabian StyleDíez, Aida M., Iván Núñez, Marta Pazos, M. Ángeles Sanromán, and Yury V. Kolen’ko. 2022. "Fluoride-Doped TiO2 Photocatalyst with Enhanced Activity for Stable Pollutant Degradation" Catalysts 12, no. 10: 1190. https://doi.org/10.3390/catal12101190
APA StyleDíez, A. M., Núñez, I., Pazos, M., Sanromán, M. Á., & Kolen’ko, Y. V. (2022). Fluoride-Doped TiO2 Photocatalyst with Enhanced Activity for Stable Pollutant Degradation. Catalysts, 12(10), 1190. https://doi.org/10.3390/catal12101190










