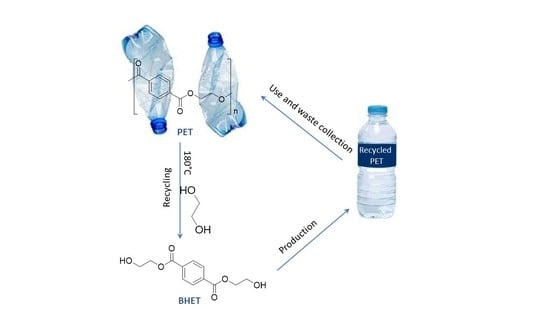
The Contribution of Commercial Metal Amides to the Chemical Recycling of Waste Polyesters
Abstract
1. Introduction
2. Results and Discussion
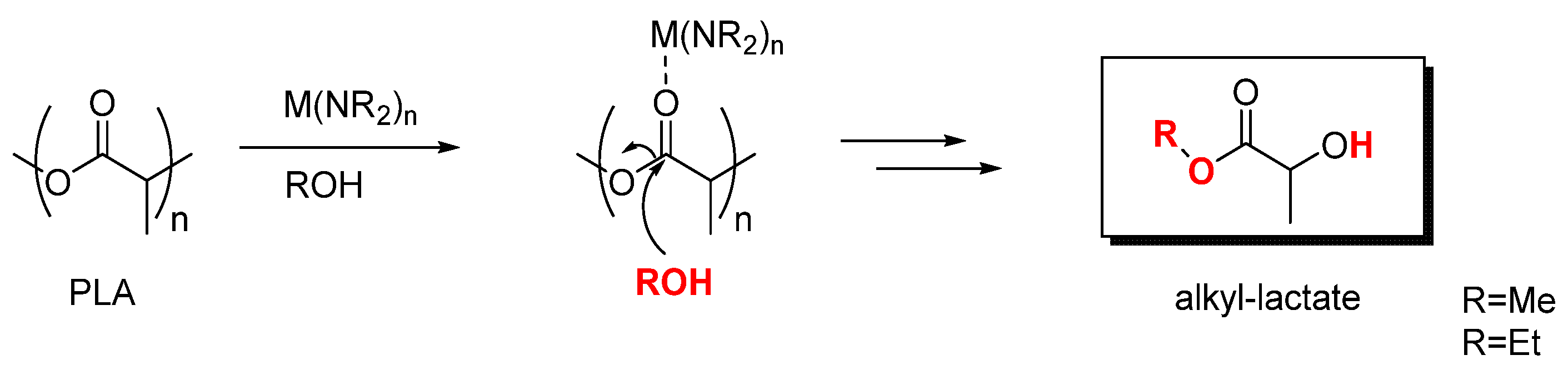
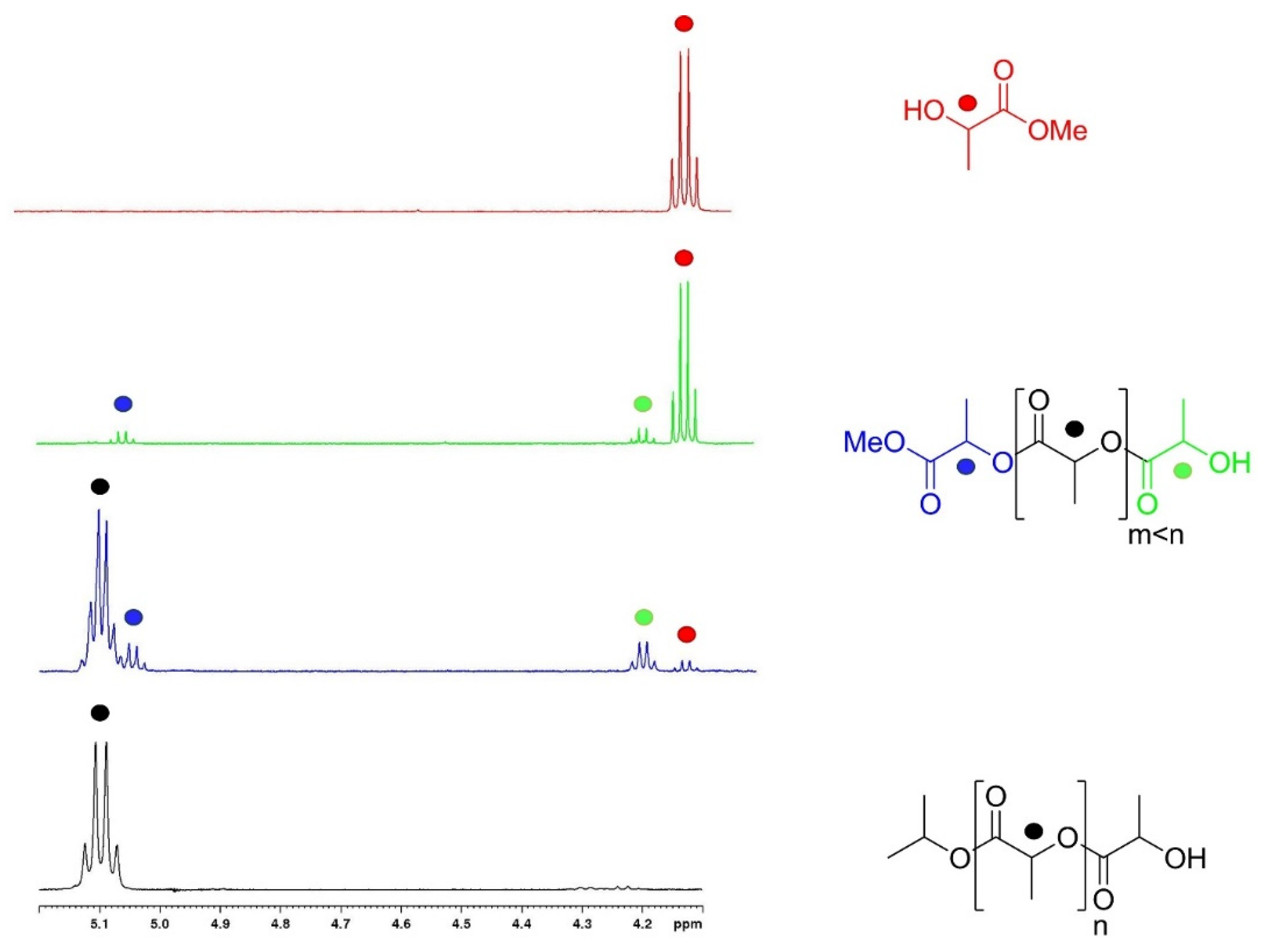
2.1. Degradation of PLA via Alcoholysis
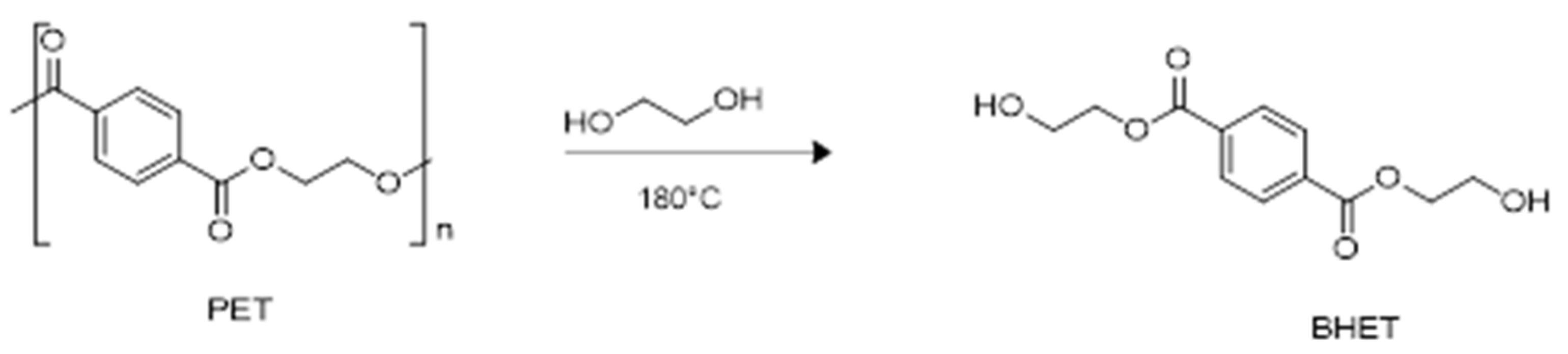
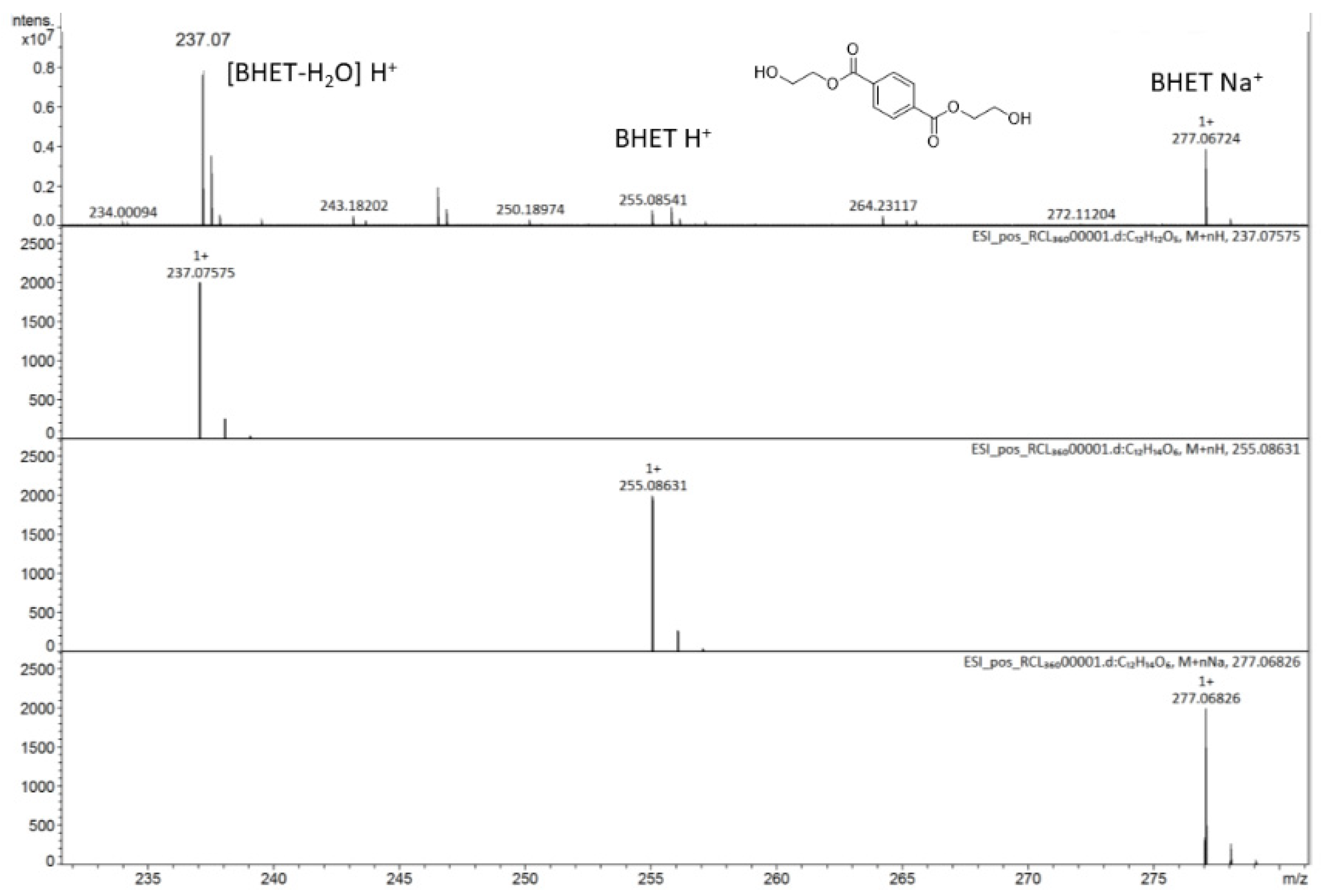
2.2. Glycolysis of PET
3. Experimental Section
3.1. Materials and Methods
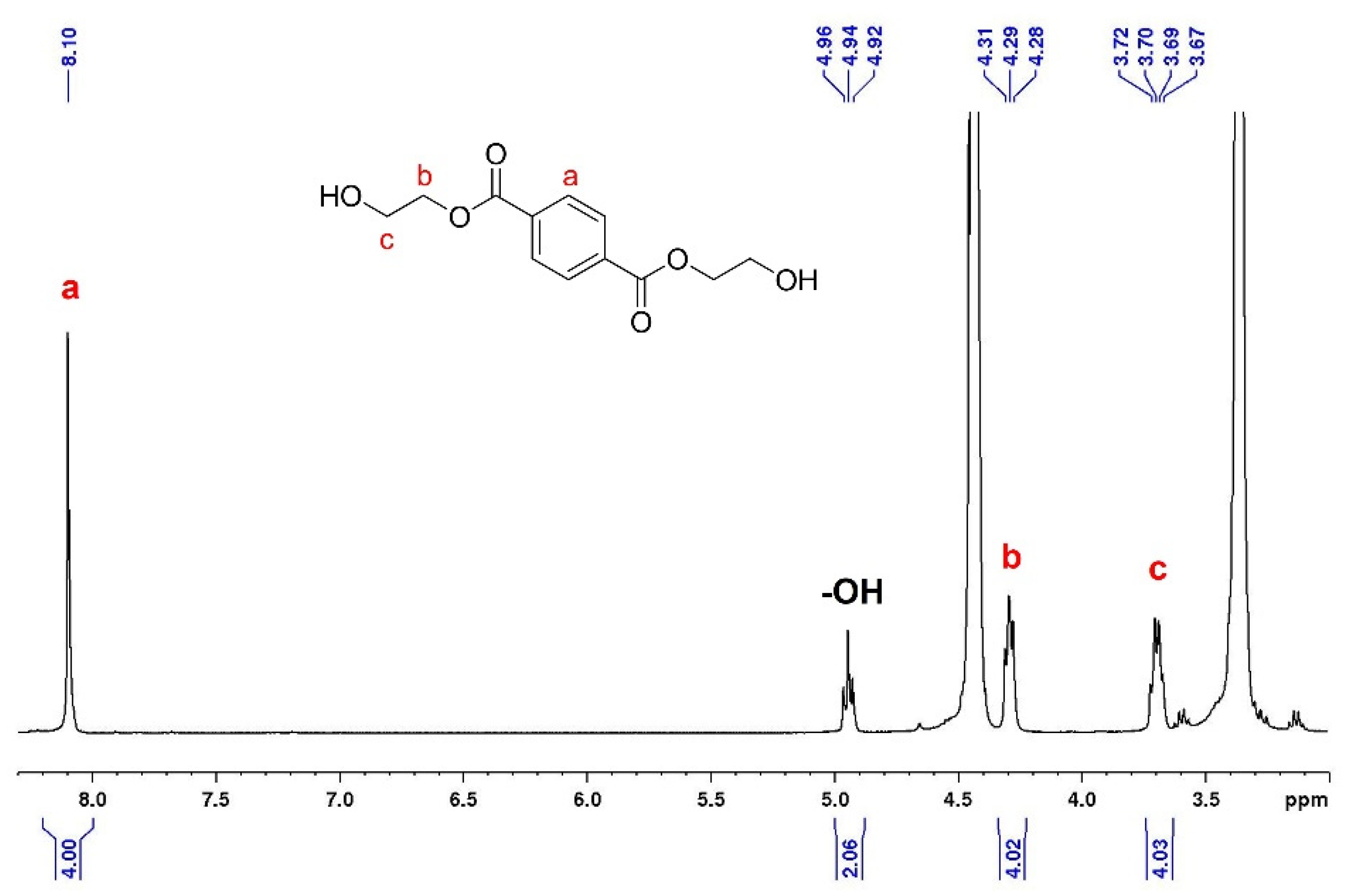
3.2. NMR Analysis
3.3. GPC Size Exclusion Chromatography
3.4. General Procedure for the Depolymerization of Polylactide
3.5. General Procedure of PET Glycolysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Millican, J.M.; Agarwal, S. Plastic Pollution: A Material Problem? Macromolecules 2021, 54, 4455–4469. [Google Scholar] [CrossRef]
- MacLeod, M.A.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; da Costa, J.P.; Mouneyrac, C.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. Solutions and Integrated Strategies for the Control and Mitigation of Plastic and Microplastic Pollution. Int. J. Environ. Res. Public Health 2019, 16, 2411. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Simon, N.; Raubenheimer, K.; Urho, N.; Unger, S.; Azoulay, D.; Farrelly, T.; Sousa, J.; van Asselt, H.; Carlini, G.; Sekomo, C.; et al. A binding global agreement to address the life cycle of plastics. Science 2021, 373, 43–47. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th Anniversary Perspective: There Is a Great Future in Sustainable Polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354. [Google Scholar] [CrossRef]
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. ChemSusChem 2021, 14, 56–72. [Google Scholar] [CrossRef]
- Kosloski-Oh, S.C.; Wood, Z.A.; Manjarrez, Y.; Pablo de los Rios, J.; Fieser, M.E. Catalytic methods for chemical recycling or upcycling of commercial polymers. Mater. Horiz. 2021, 8, 1084. [Google Scholar] [CrossRef] [PubMed]
- De Hoe, G.X.; Şucu, T.; Shaver, M.P. Sustainability and Polyesters: Beyond Metals and Monomers to Function and Fate. Acc. Chem. Res. 2022, 55, 1514–1523. [Google Scholar] [CrossRef]
- Zhang, X.; Fevre, M.; Jones, G.O.; Waymouth, R.M. Catalysis as an Enabling Science for Sustainable Polymers. Chem. Rev. 2018, 118, 839. [Google Scholar] [CrossRef]
- Schwach, G.; Coudane, J.; Engel, R.; Vert, M. Zn lactate as initiator of dl-lactide ring opening polymerization and comparison with Sn octoate. Polym. Bull. 1996, 37, 771–776. [Google Scholar] [CrossRef]
- Nijenhuis, A.J.; Grijpma, D.W.; Pennings, A.J. Lewis acid catalyzed polymerization of L-lactide. Kinetics and mechanism of the bulk polymerization. Macromolecules 1992, 25, 6419–6424. [Google Scholar] [CrossRef]
- Wang, X.; Liao, K.; Quan, D.; Wu, Q. Bulk Ring-Opening Polymerization of Lactides Initiated by Ferric Alkoxides. Macromolecules 2005, 38, 4611–4617. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Berl, M.; Scharnagl, N. Poly(lactones). 9. Polymerization mechanism of metal alkoxide initiated polymerizations of lactide and various lactones. Macromolecules 1988, 21, 286–293. [Google Scholar] [CrossRef]
- Stevels, W.M.; Ankoné, M.J.K.; Dijkstra, P.J.; Feijen, J. Kinetics and Mechanism of l-Lactide Polymerization Using Two Different Yttrium Alkoxides as Initiators. Macromolecules 1996, 29, 6132–6138. [Google Scholar] [CrossRef]
- Ruelas-Leyva, P.J.; Picos-Corrales, A.L. Lactides and Lactones Yielding Eco-Friendly and Biocompatible Polymers by Metal-Catalyzed Ring-Opening Polymerization. Mini-Rev. Org. Chem. 2021, 18, 680–689. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides-Methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef]
- Pilone, A.; Press, K.; Goldberg, I.; Kol, M.; Mazzeo, M.; Lamberti, M. Gradient Isotactic Multiblock Polylactides from Aluminum Complexes of Chiral Salalen Ligands. J. Am. Chem. Soc. 2014, 136, 2940–2943. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereochemistry of Lactide Polymerization with Chiral Catalysts: New Opportunities for Stereocontrol Using Polymer Exchange Mechanisms. J. Am. Chem. Soc. 2002, 124, 1316. [Google Scholar] [CrossRef]
- Osten, K.M.; Aluthge, D.C.; Mehrkhodavandi, P. Ligand Design in Enantioselective Ring-Opening Polymerization of Lactide. In Ligand Design in Metal Chemistry: Reactivity and Catalysis; Wiley: Hoboken, NJ, USA, 2016; pp. 270–307. [Google Scholar]
- Tschan, M.J.L.; Gauvin, R.M.; Thomas, C.M. Controlling polymer stereochemistry in ring-opening polymerization: A decade of advances shaping the future of biodegradable polyesters. Chem. Soc. Rev. 2021, 50, 13587–13608. [Google Scholar] [CrossRef] [PubMed]
- Bucknall, D.G. Plastics as a Materials System in a Circular Economy: Plastics in the Circular Economy. Philos. Trans. R. Soc. A 2020, 378, 20190268. [Google Scholar] [CrossRef] [PubMed]
- Coates, G.W.; Getzler, Y.D.Y.L. Chemical Recycling to Monomer for an Ideal, Circular Polymer Economy. Nat. Rev. Mater. 2020, 5, 501. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Jones, M.D. A circular economy approach to plastic waste. Polym. Degrad. Stab. 2019, 165, 170–181. [Google Scholar] [CrossRef]
- Payne, J.; Jones, M.D. The Chemical Recycling of Polyesters for a Circular Plastics Economy: Challenges and Emerging Opportunities. ChemSusChem 2021, 14, 4041–4070. [Google Scholar] [CrossRef]
- Vogt, B.D.; Stokes, K.K.; Kumar, S.K. Why Is Recycling of Postconsumer Plastics so Challenging? ACS Appl. Polym. Mater. 2021, 3, 4325. [Google Scholar] [CrossRef]
- Available online: https://www.morganstanley.com.au/ideas/the-circular-economy-of-plastics (accessed on 5 September 2022).
- Santulli, F.; Lamberti, M.; Mazzeo, M. A Single Catalyst for Promoting Reverse Processes: Synthesis and Chemical Degradation of Polylactide. ChemSusChem 2021, 14, 5470–5475. [Google Scholar] [CrossRef]
- Santulli, F.; Gravina, G.; Lamberti, M.; Tedesco, C.; Mazzeo, M. Zinc and magnesium catalysts for the synthesis for PLA and its degradation: Clues for catalyst design. Mol. Catal. 2022, 528, 112480. [Google Scholar] [CrossRef]
- Payne, J.; McKeown, P.; Driscoll, O.; Kociok-Kohn, G.; Emanuelsson, E.A.C.; Jones, M.D. Make or break: Mg(II)- and Zn(II)-catalen complexes for PLA production and recycling of commodity polyesters. Polym. Chem. 2021, 12, 1086–1096. [Google Scholar] [CrossRef]
- Payne, J.M.; Kociok-Köhn, G.; Emanuelsson, E.A.C.; Jones, M.D. Zn(II)- and Mg(II)-Complexes of a Tridentate {ONN} Ligand: Application to Poly(lactic acid) Production and Chemical Upcycling of Polyesters. Macromolecules 2021, 54, 8453–8469. [Google Scholar] [CrossRef]
- Payne, J.M.; Kamran, M.; Davidson, M.G.; Jones, M.D. Versatile Chemical Recycling Strategies: Value-Added Chemicals from Polyester and Polycarbonate Waste. ChemSusChem 2022, 15, e202200255. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, G.; Lv, C.; Dong, B.; Zhou, L.; Wang, Q. Zn(HMDS)2 as a Versatile Transesterification Catalyst for Polyesters Synthesis and Degradation toward a Circular Materials Economy Approach. ACS Sustain. Chem. Eng. 2020, 8, 18347–18353. [Google Scholar] [CrossRef]
- Yang, R.; Xu, G.; Dong, B.; Guo, X.; Wang, Q. Selective, Sequential, and “One-Pot” Depolymerization Strategies for Chemical Recycling of Commercial Plastics and Mixed Plastics. ACS Sustain. Chem. Eng. 2022, 10, 9860–9871. [Google Scholar] [CrossRef]
- Dutt, K.; Soni, R.K. A review on synthesis of value added products from polyethylene terephthalate (PET) waste. Polym. Sci. Ser. B 2013, 55, 430–452. [Google Scholar] [CrossRef]
- Fliedel, C.; Vila-Vicosa, D.; Calhorda, M.J.; Dagorne, S.; Aviles, T. Dinuclear Zinc-N-Heterocyclic Carbene Complexes for Either the Controlled Ring-Opening Polymerization of Lactide or the Controlled Degradation of Polylactide Under Mild Conditions. ChemCatChem 2014, 6, 1357–1367. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes—A review. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- Román-Ramírez, L.A.; Powders, M.; McKeown, P.; Jones, M.D.; Wood, J. Ethyl Lactate Production from the Catalytic Depolymerisation of Post-consumer Poly(lactic acid). J. Polym. Environ. 2020, 28, 2956–2964. [Google Scholar] [CrossRef]
- Barnard, E.; Arias, J.J.R.; Thielemans, W. Chemolytic depolymerisation of PET: A review. Green Chem. 2021, 23, 3765. [Google Scholar] [CrossRef]
- Damayanti, N.; Wu, H.S. Strategic possibility routes of recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Fukushima, K.; Coady, D.J.; Jones, G.O.; Almegren, H.A.; Alabdulrahman, A.M.; Alsewailem, F.D.; Horn, H.W.; Rice, J.E.; Hedrick, J.L. Unexpected efficiency of cyclic amidine catalysts in depolymerizing poly(ethylene terephthalate). J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1606–1611. [Google Scholar] [CrossRef]
- Jehanno, C.; Demarteau, J.; Mantione, D.; Arno, M.C.; Ruipérez, F.; Hedrick, J.L.; Dove, A.P.; Sardon, H. Selective Chemical Upcycling of Mixed Plastics Guided by a Thermally Stable Organocatalyst. Angew. Chem. Int. Ed. 2021, 133, 6784. [Google Scholar] [CrossRef]
- Jehanno, C.; Perez-Madrigal, M.M.; Demarteau, J.; Sardon, H.; Dove, A.P. Organocatalysis for depolymerisation. Polym. Chem. 2019, 10, 172–186. [Google Scholar] [CrossRef]
- Delle Chiaie, K.R.; McMahon, F.R.; Williams, E.J.; Price, M.J.; Dove, A.P. Dual-catalytic depolymerization of polyethylene terephthalate (PET). Polym. Chem. 2020, 11, 1450–1453. [Google Scholar] [CrossRef]
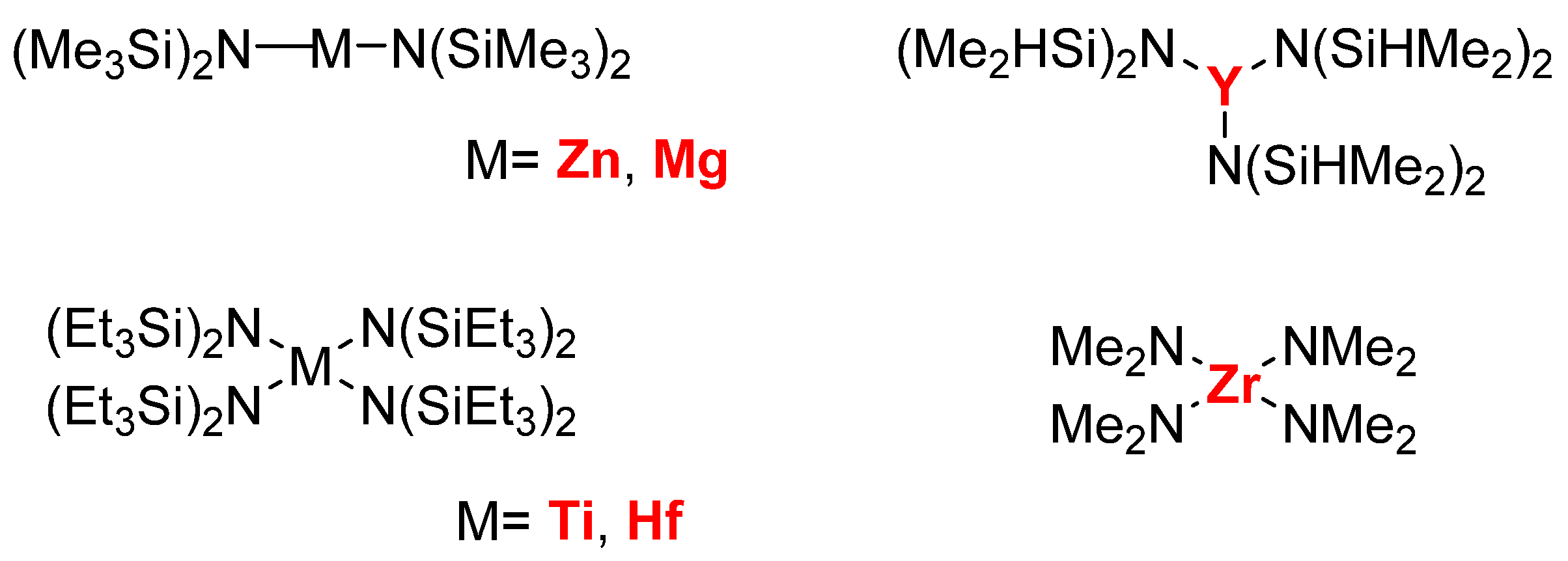
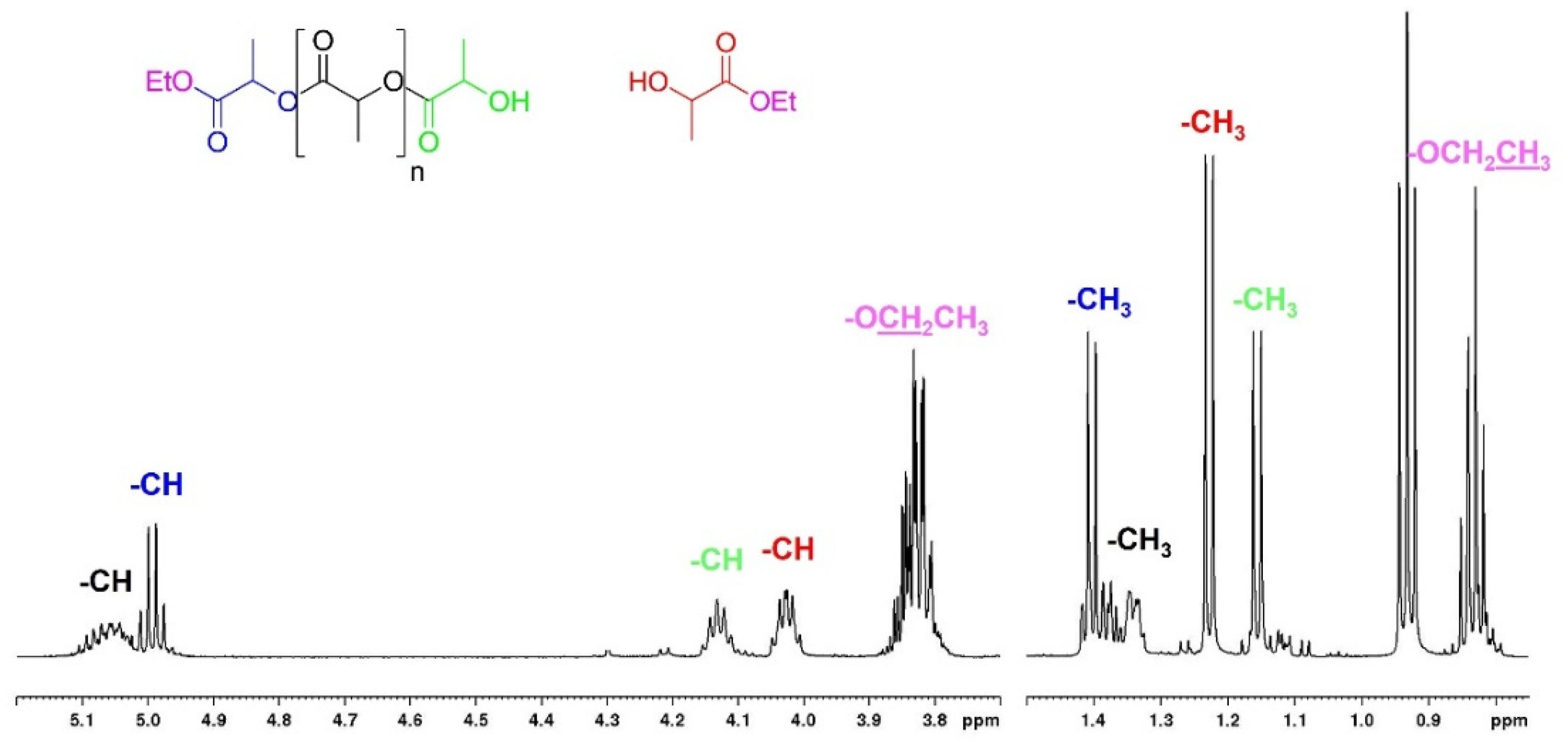
| Run [a] | Cat | Time (h) | XInt [b] (%) | SMe-La [b] (%) | YMe-La [b] (%) |
|---|---|---|---|---|---|
| 1 | Zn | 2 | 39 | 100 | 39 |
| 2 | Mg | 2 | 90 | 100 | 90 |
| 3 | Y | 19 | 98 | 100 | 98 |
| 4 | Ti | 24 | 100 | 89 | 89 |
| 5 | Zr | 8 | 11 | 69 | 8 |
| 6 | Hf | 2 | 10 | 52 | 5 |
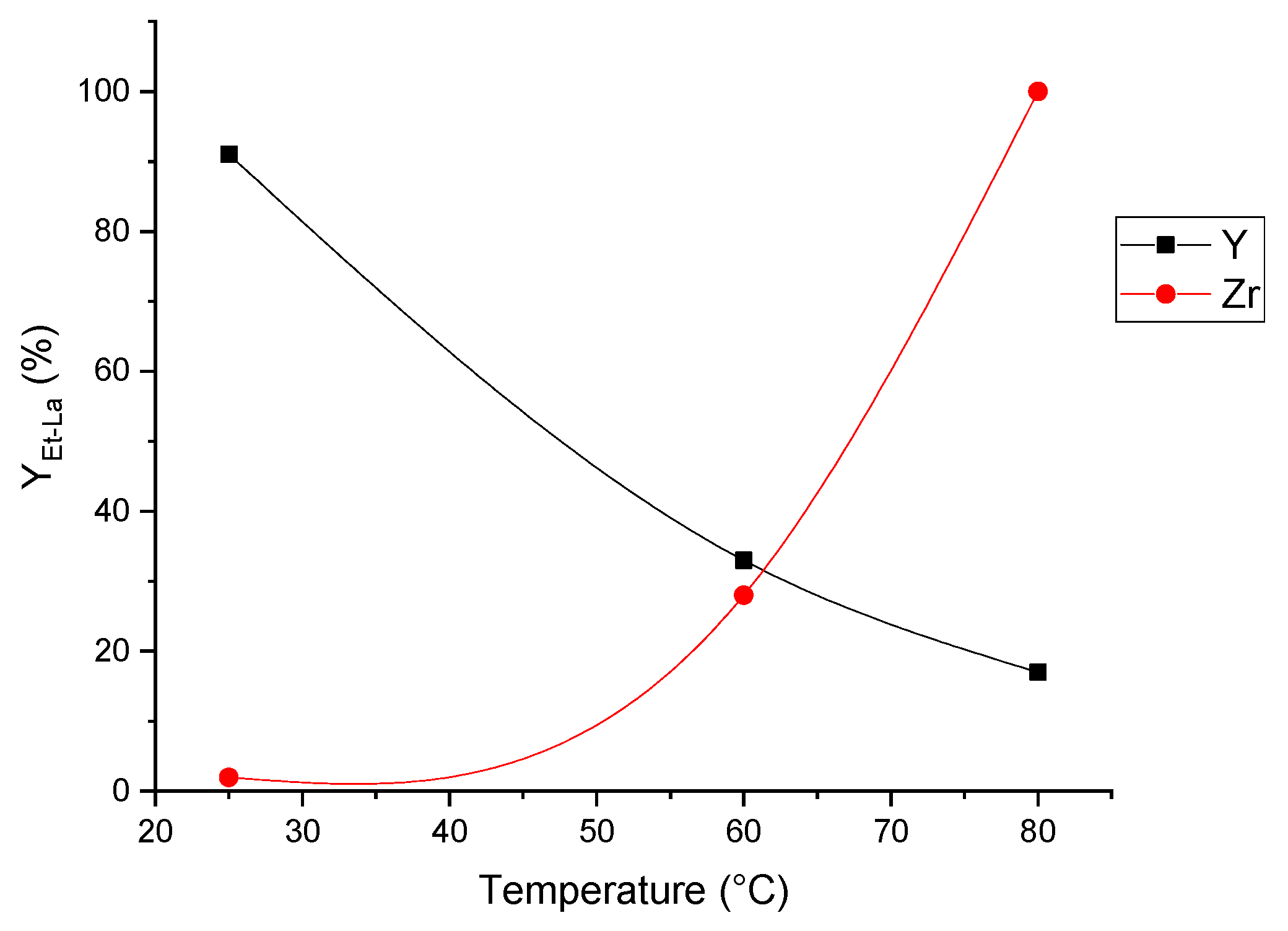
| Run [a] | Cat | T (°C) | XInt [b] (%) | SEt-La [b] (%) | YEt-La [b] (%) |
|---|---|---|---|---|---|
| 1 | Zn | 25 | 100 | 83 | 83 |
| 2 | Mg | 25 | 93 | 98 | 91 |
| 3 | Y | 25 | 100 | 91 | 91 |
| 4 | Ti | 25 | 75 | 39 | 29 |
| 5 | Zr | 25 | 3 | 51 | 2 |
| 6 | Hf | 25 | 5 | 0.2 | 1 |
| 7 | Zn | 80 | 100 | 100 | 100 |
| 8 | Mg | 80 | 57 | 50 | 29 |
| 9 | Y | 80 | 17 | 100 | 17 |
| 10 | Ti | 80 | 100 | 100 | 100 |
| 11 | Zr | 80 | 100 | 100 | 100 |
| 12 | Hf | 80 | 89 | 54 | 48 |
| 13 | Y | 60 | 57 | 58 | 33 |
| 14 | Zr | 60 | 41 | 68 | 28 |
| Run [a] | Cat | Conv (%) | Sel BHET (%) | Yield BHET (%) |
|---|---|---|---|---|
| 1 | Zn | 92 | 26 | 24 |
| 2 | Mg | 96 | 24 | 22 |
| 3 | Y | 96 | 58 | 26 |
| 4 | Ti | 52 | 15 | 8 |
| 5 | Zr | 86 | 78 | 65 |
| 6 | Hf | 73 | 28 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santulli, F.; Lamberti, M.; Annunziata, A.; Lastra, R.C.; Mazzeo, M. The Contribution of Commercial Metal Amides to the Chemical Recycling of Waste Polyesters. Catalysts 2022, 12, 1193. https://doi.org/10.3390/catal12101193
Santulli F, Lamberti M, Annunziata A, Lastra RC, Mazzeo M. The Contribution of Commercial Metal Amides to the Chemical Recycling of Waste Polyesters. Catalysts. 2022; 12(10):1193. https://doi.org/10.3390/catal12101193
Chicago/Turabian StyleSantulli, Federica, Marina Lamberti, Andrea Annunziata, Rita Chiara Lastra, and Mina Mazzeo. 2022. "The Contribution of Commercial Metal Amides to the Chemical Recycling of Waste Polyesters" Catalysts 12, no. 10: 1193. https://doi.org/10.3390/catal12101193
APA StyleSantulli, F., Lamberti, M., Annunziata, A., Lastra, R. C., & Mazzeo, M. (2022). The Contribution of Commercial Metal Amides to the Chemical Recycling of Waste Polyesters. Catalysts, 12(10), 1193. https://doi.org/10.3390/catal12101193