Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides
Abstract
:1. Introduction
2. Experimental Section
2.1. General Information
2.2. Synthesis of BP-CN Catalyst
2.3. Characterizations
2.4. Catalytic Activity Test
3. Results and Discussion
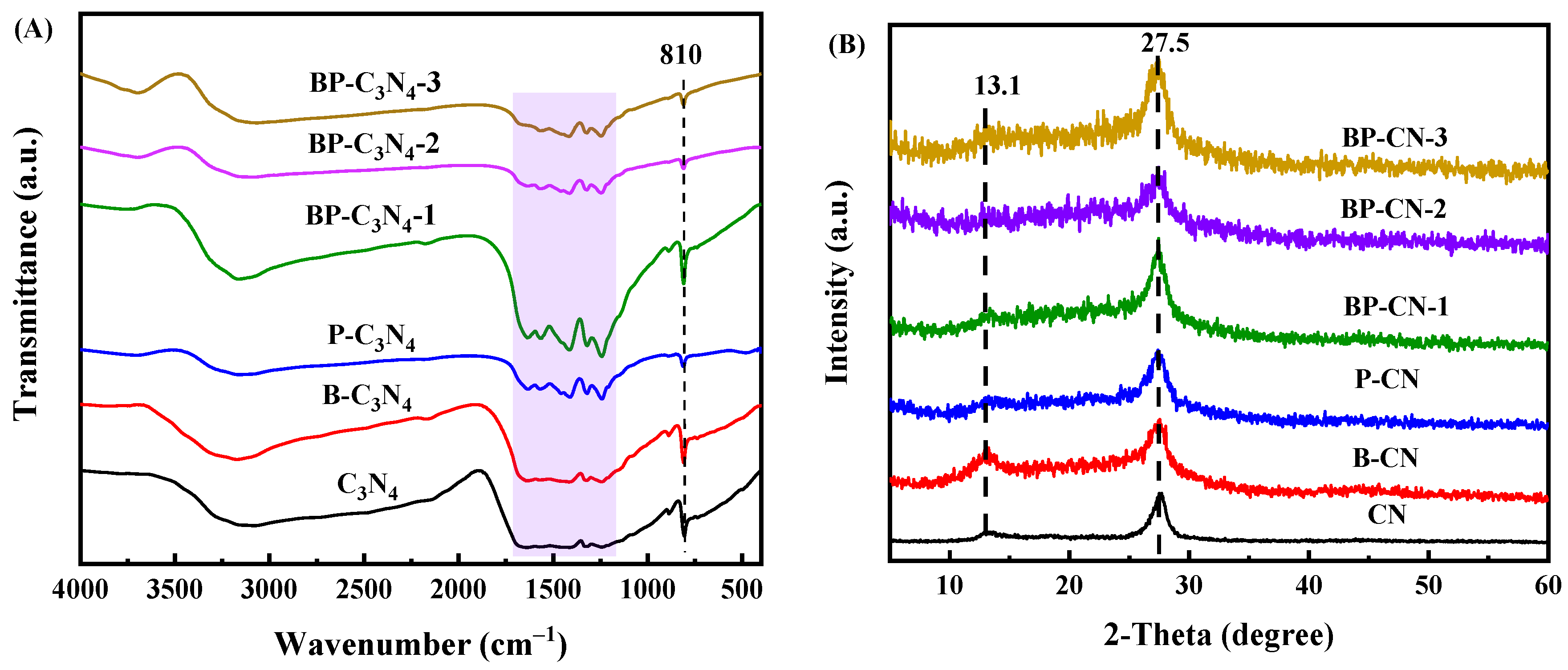
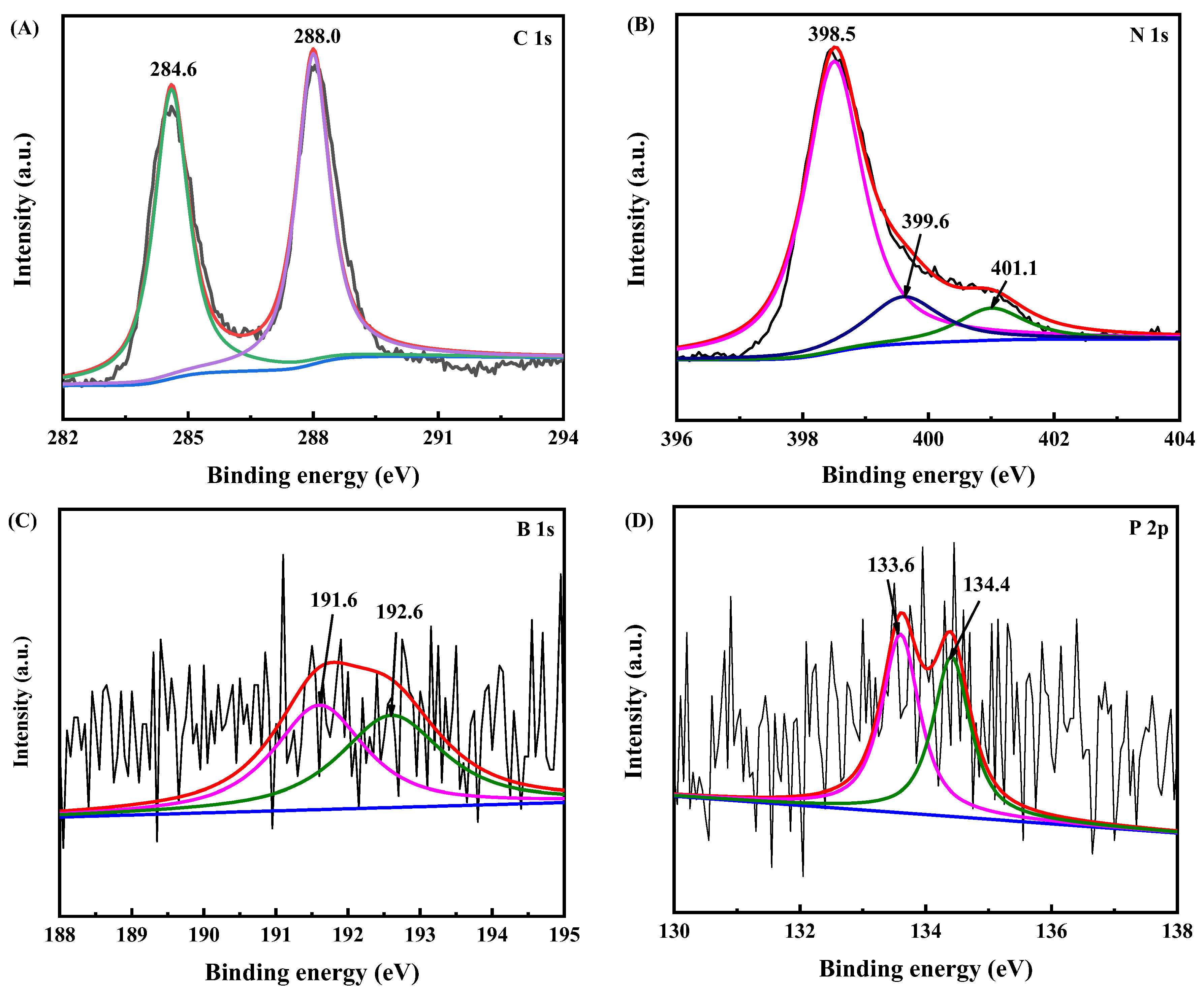
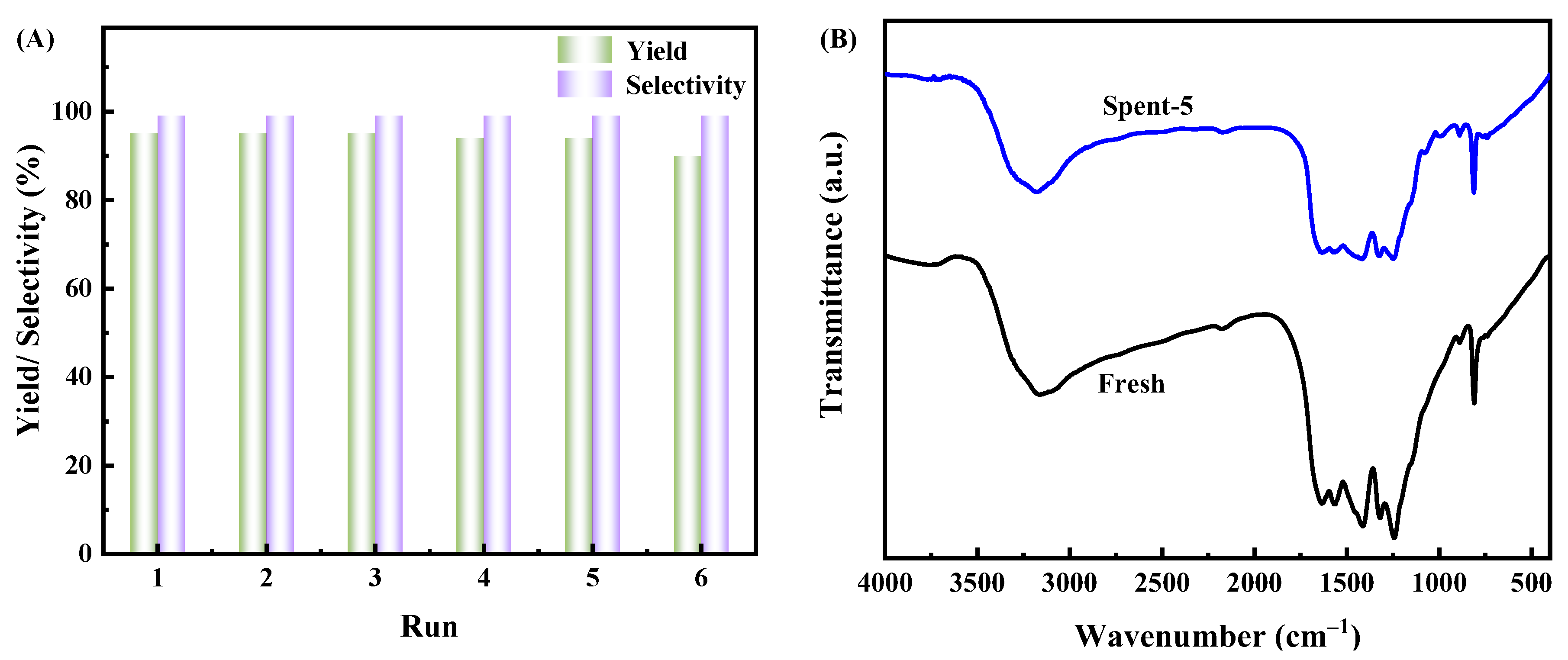
3.1. Catalyst Characterization
3.2. Catalyst SCREENING
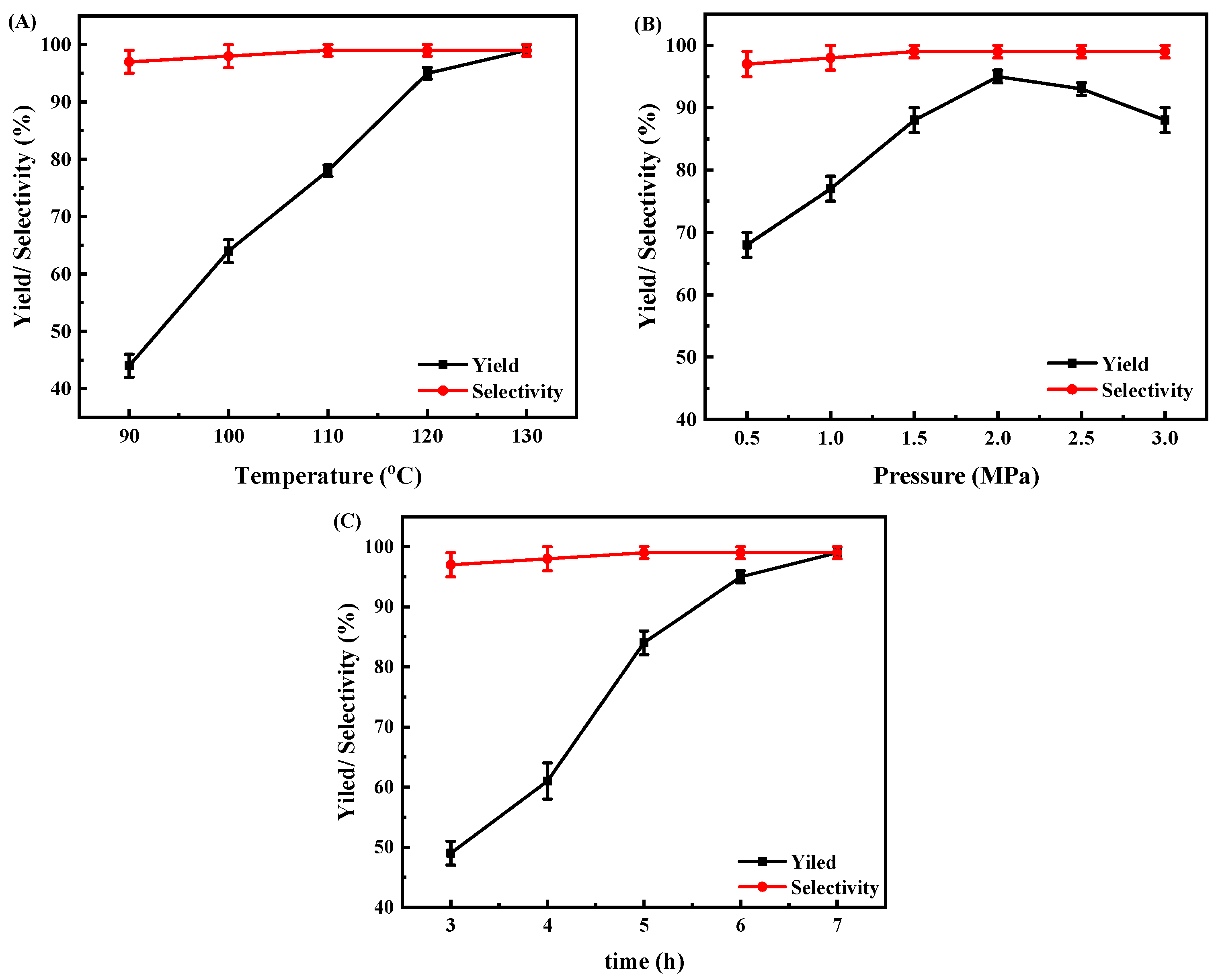
3.3. Effects of Reaction Conditions
3.4. Catalyst Recyclability
3.5. Catalyst Universality
3.6. Comparisons of CN-Based Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhi, Y.; Shao, P.; Feng, X.; Xia, H.; Zhang, Y.; Shi, Z.; Mu, Y.; Liu, X. Covalent organic frameworks: Efficient, metal-free, heterogeneous organocatalysts for chemical fixation of CO2 under mild conditions. J. Mater. Chem. A 2018, 6, 374–382. [Google Scholar] [CrossRef]
- Chakraborty, J.; Nath, I.; Song, S.; Kao, C.; Verpoort, F. Synergistic performance of a sub-nanoscopic cobalt and imidazole grafted porous organic polymer for CO2 fixation to cyclic carbonates under ambient pressure without a co-catalyst. J. Mater. Chem. A 2020, 8, 13916–13920. [Google Scholar] [CrossRef]
- Li, W.; Qi, K.; Lu, X.; Qi, Y.; Zhang, J.; Zhang, B.; Qi, W. Electrochemically assisted cycloaddition of carbon dioxide to styrene oxide on copper/carbon hybrid electrodes: Active species and reaction mechanism. Chem. Eur. J. 2022, 28, e202200622. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Zhang, T.; Schulman, E.; Liu, D.; Meng, J. Hierarchical porous metallized poly-melamine-formaldehyde (PMF) as a low-cost and high-efficiency catalyst for cyclic carbonate synthesis from CO2 and epoxides. J. Mater. Chem. A 2018, 6, 8441–8448. [Google Scholar] [CrossRef]
- Lyubimov, S.; Zvinchuk, A.; Chowdhury, B. Iodine as an efficient and available activator of sodium and potassium halides in carbon dioxide addition to epoxides. Russian Chem. Bull. 2021, 70, 1324–1327. [Google Scholar] [CrossRef]
- Mujmule, R.; Chung, W.; Kim, H. Chemical fixation of carbon dioxide catalyzed via hydroxyl and carboxyl-rich glucose carbonaceous material as a heterogeneous catalyst. Chem. Eng. J. 2020, 395, 125164. [Google Scholar] [CrossRef]
- Qing, Y.; Liu, T.; Zhao, B.; Bao, X.; Yuan, D.; Yao, Y. Cycloaddition of di-substituted epoxides and CO2 under ambient conditions catalysed by rare-earth poly(phenolate) complexes. Inorg. Chem. Front. 2022, 9, 2969–2979. [Google Scholar] [CrossRef]
- Emelyanov, M.; Lisov, A.; Medvedev, M.; Maleev, V.; Larionov, V. Cobalt(III) complexes as bifunctional hydrogen-bond donor catalysts featuring halide anions for cyclic carbonate synthesis at ambient temperature and pressure: A mechanistic insight. Asian J. Org. Chem. 2022, 11, 202100811. [Google Scholar] [CrossRef]
- Emelyanov, M.; Stoletova, N.; Lisov, A.; Medvedev, M.; Smol’yakov, A.; Maleev, V.; Larionov, V. An octahedral cobalt(iii) complex based on cheap 1,2-phenylenediamine as a bifunctional metal-templated hydrogen bond donor catalyst for fixation of CO2 with epoxides under ambient conditions. Inorg. Chem. Front. 2021, 8, 3871–3884. [Google Scholar] [CrossRef]
- Campisciano, V.; Valentino, L.; Morena, A.; Santiago-Portillo, A.; Saladino, N.; Gruttadauria, M.; Aprile, C.; Giacalone, F. Carbon nanotube supported aluminum porphyrin-imidazolium bromide crosslinked copolymer: A synergistic bifunctional catalyst for CO2 conversion. J. CO2 Util. 2022, 57, 101884. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Lan, J.; Xu, P.; Sun, J. Porous Zn(Bmic)(AT) MOF with Abundant Amino Groups and Open Metal Sites for Efficient Capture and Transformation of CO2. Inorg. Chem. 2019, 58, 13917–13926. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.; Tariq, S.; Luque, R.; Verpoort, F. Self-sacrifice MOFs for heterogeneous catalysis: Synthesis mechanisms and future perspectives. Mater. Today 2022, 55, 137–169. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Wu, L.; Zhong, H.; Luo, N.; Zhu, Y.; Tong, M.; Long, Z.; Chen, G. Silanol-enriched viologen-based ionic porous hybrid polymers for efficient eatalytic CO2 fixation into cyclic carbonates under mild conditions. Chem. Eng. 2019, 7, 16907–16916. [Google Scholar]
- Biswas, T.; Mahalingam, V. g-C3N4 and tetrabutylammonium bromide catalyzed efficient conversion of epoxide to cyclic carbonate under ambient conditions. New J. Chem. 2017, 41, 14839–14842. [Google Scholar] [CrossRef]
- Ong, W.; Tan, L.; Ng, Y.; Yong, S.; Chai, S. Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental Remediation: Are we a step closer to achieving sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Kong, X.; Liang, Y.; Li, Z.; Wu, S.; Chang, C.; Luo, S.; Cui, Z. In situ synthesis of a novel Mn3O4/g-C3N4 p-n heterostructure photocatalyst for water splitting. J. Colloid Interf. Sci. 2021, 586, 778–784. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, J.; Jin, B.; Wu, Z.; Yang, S.; Zhang, S.; Tian, Y.; Fang, Y.; Hou, Y.; Zhou, X. Sustainable synthesis of low-cost nitrogen-doped-carbon coated Co3W3C@g-C3N4 composite photocatalyst for efficient hydrogen evolution. Chem. Eng. J. 2021, 426, 131208. [Google Scholar] [CrossRef]
- Liang, H.; Wang, J.; Li, Q.; Liang, C.; Feng, Y.; Kang, M. Supported ZnBr2 and carbon nitride bifunctional complex catalysts for the efficient cycloaddition of CO2 with diglycidyl ethers. New J. Chem. 2018, 42, 16127–16137. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Appl. Catal. B-Environ. 2013, 136, 269–277. [Google Scholar] [CrossRef]
- Xu, J.; Gan, Y.; Hu, P.; Zheng, H.; Xue, B. Comprehensive insight into the support effect of graphitic carbon nitride for zinc halides on the catalytic transformation of CO2 into cyclic carbonates. Catal. Sci. Technol. 2018, 8, 5582–5593. [Google Scholar] [CrossRef]
- Su, Q.; Yao, X.; Cheng, W.; Zhang, S. Boron-doped melamine-derived carbon nitrides tailored by ionic liquids for catalytic conversion of CO2 into cyclic carbonates. Green Chem. 2017, 19, 2957–2965. [Google Scholar] [CrossRef]
- Zhu, J.; Diao, T.; Wang, W.; Xu, X.; Sun, X.; Carabineiro, S.; Zhao, Z. Boron doped graphitic carbon nitride with acid-base duality for cycloaddition of carbon dioxide to epoxide under solvent-free condition. Appl. Catal. B-Environ. 2017, 219, 92–100. [Google Scholar] [CrossRef]
- Lan, D.; Wang, H.; Chen, L.; Au, C.; Yin, S. Phosphorous-modified bulk graphitic carbon nitride: Facile preparation and application as an acid-base bifunctional and efficient catalyst for CO2 cycloaddition with epoxides. Carbon 2016, 100, 81–89. [Google Scholar] [CrossRef]
- Guo, S.; Deng, Z.; Li, M.; Jiang, B.; Tian, C.; Pan, Q.; Fu, H. Phosphorus-doped carbon nitride tubes with a layered micro-nanostructure for enhanced visible-light photocatalytic hydrogen evolution. Angew. Chem. Int. Edit. 2016, 55, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Hou, L.; Guo, X.; Fu, R.; Sun, J. Construction of covalent bonding oxygen-doped carbon nitride/graphitic carbon nitride Z-scheme heterojunction for enhanced visible-light-driven H2 evolution. Chem. Eng. J. 2020, 383, 123132. [Google Scholar] [CrossRef]
- Raza, A.; Haidry, A.; Saddique, J. In-situ synthesis of Cu2ZnSnS4/g-C3N4 heterojunction for superior visible light-driven CO2 reduction. J. Phys. Chem. Solids 2022, 165, 110694. [Google Scholar] [CrossRef]
- Samanta, S.; Srivastava, R. A novel method to introduce acidic and basic bi-functional sites in graphitic carbon nitride for sustainable catalysis: Cycloaddition, esterification, and transesterification reactions. Sustain. Energ. Fuels 2017, 1, 1390–1404. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Liu, Y.; Chen, Z.; Yang, H.; Yue, Z.; Fang, Q.; Zhi, Y.; Shan, S. Zn and N co-doped porous carbon nanosheets for photothermally-driven CO2 cycloaddition. J. Catal. 2022, 407, 65–76. [Google Scholar] [CrossRef]
- Sakuna, P.; Ketwong, P.; Ohtani, B.; Trakulmututa, J.; Kobkeatthawin, T.; Luengnaruemitchai, A.; Smith, S. The influence of metal-doped graphitic carbon nitride on photocatalytic conversion of acetic acid to carbon dioxide. Front. Chem. 2022, 10, 825786. [Google Scholar] [CrossRef]
- Zhu, T.; Song, Y.; Ji, H.; Xu, Y.; Song, Y.; Xia, J.; Yin, S.; Li, Y.; Xu, H.; Zhang, Q.; et al. Synthesis of g-C3N4/Ag3VO4 composites with enhanced photocatalytic activity under visible light irradiation. Chem. Eng. J. 2015, 271, 96–105. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Fu, G.; Chen, Y.; Yang, C.; Sun, J. Experimental and theoretical investigation for the cycloaddition of carbon dioxide to epoxides catalyzed by potassium and boron co-doped carbon nitride. J. Colloid Interf. Sci. 2022, 609, 523–534. [Google Scholar] [CrossRef]
- Ma, X.; Tian, J.; Wang, M.; Shen, M.; Zhang, L. Polymeric carbon nitride supported Bi nanoparticles as highly efficient CO2 reduction electrocatalyst in a wide potential range. J. Colloid Interf. Sci. 2022, 608, 1676–1684. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, G.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. Synergistic effect between boron containing metal-organic frameworks and light leading to enhanced CO2 cycloaddition with epoxides. Chem. Eng. J. 2022, 437, 135363. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Zheng, Y. Insights into photocatalytic CO2 reduction on C3N4: Strategy of simultaneous B, K co-doping and enhancement by N vacancies. Appl. Catal. B Environ. 2019, 254, 270–282. [Google Scholar] [CrossRef]
- Liu, J.; Yang, G.; Liu, Y.; Zhang, D.; Hu, X.; Zhang, Z. Efficient conversion of CO2 into cyclic carbonates at room temperature catalyzed by Al-salen and imidazolium hydrogen carbonate ionic liquid. Green Chem. 2020, 22, 4509–4515. [Google Scholar] [CrossRef]
- Ge, S.; Cheng, G.; Ke, H. Triethanolamine borate as bifunctional Lewis pair catalyst for the cycloaddition of CO2 with epoxides. J. CO2 Util. 2022, 57, 101873. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Wang, X.; Sun, J. Polymeric ionic liquid with carboxyl anchored on mesoporous silica for efficient fixation of carbon dioxide. J. Colloid Interf. Sci. 2022, 618, 44–55. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, P.; Zhang, M.; Wang, S.; Liu, M.; Liu, F. Fabrication of robust and bifunctional cyclotriphosphazene-based periodic mesoporous organosilicas for efficient CO2 adsorption and catalytic conversion. Chem. Eng. J. 2021, 418, 129360. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, Y.; Sun, J. Conversion of CO2 with epoxides to cyclic carbonates catalyzed by amino acid ionic liquids at room temperature. J. CO2 Util. 2022, 56, 101840. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, Q.; Fu, Y.; Song, L.; Liang, J.; Xu, X.; Wang, H.; Li, J.; Tu, S.; Lu, X.; et al. Sponge-like quaternary ammonium-based poly(ionic liquid)s for high CO2 capture and efficient cycloaddition under mild conditions. J. Mater. Chem. A 2017, 5, 25594–25600. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Xu, P.; Yang, L.; Zhang, J.; Sun, J. Facile synthesis of zinc halide-based ionic liquid for efficient conversion of carbon dioxide to cyclic carbonates. Mol. Catal. 2020, 480, 110637. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.; Duan, P.; Zhang, W.; Hu, Q. Conversion of CO2 into organic carbonates over a fiber-supported ionic liquid catalyst in impellers of the agitation system. ACS Sustain. Chem. Eng. 2018, 6, 7119–7127. [Google Scholar] [CrossRef]
- Zhong, H.; Gao, J.; Sa, R.; Yang, S.; Wu, Z.; Wang, R. Carbon dioxide conversion upgraded by host-guest cooperation between nitrogen-rich covalent organic framework and imidazolium-based ionic polymer. ChemSusChem 2020, 13, 6323–6329. [Google Scholar]
- Song, H.; Wang, Y.; Xiao, M.; Liu, L.; Liu, Y.; Liu, X.; Gai, H. Design of novel poly(ionic liquids) for the conversion of CO2 to cyclic carbonates under mild conditions without solvent. ACS Sustain. Chem. Eng. 2019, 7, 9489–9497. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, W.; Yue, C.; Wu, W.; Xiao, L. Multilayered supported ionic liquids bearing a carboxyl group: Highly efficient catalysts for chemical fixation of carbon dioxide. J. Environ. Chem. Eng. 2016, 4, 2565–2572. [Google Scholar] [CrossRef]
- Yang, C.; Chen, Y.; Qu, Y.; Zhang, J.; Sun, J. Phase-controllable polymerized ionic liquids for CO2 fixation into cyclic carbonates. Sustain. Energ. Fuels 2021, 5, 1026–1033. [Google Scholar] [CrossRef]
- Xu, J.; Gan, Y.; Pei, J.; Xue, B. Metal-free catalytic conversion of CO2 into cyclic carbonate by hydroxylfunctionalized graphitic carbon nitride materials. Mol. Catal. 2020, 491, 110979. [Google Scholar] [CrossRef]
- Xu, J.; Wu, F.; Jiang, Q.; Shang, J.; Li, Y. Metal halides supported on mesoporous carbon nitride as efficient heterogeneous catalysts for the cycloaddition of CO2. J. Mol. Catal. A-Chem. 2015, 403, 77–83. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, T.; Li, C.; Xu, W.; Wang, J.; Bai, J. Synthesis of carbon fibers support graphitic carbon nitride immobilize ZnBr2 catalyst in the catalytic reaction between styrene oxide and CO2. J. CO2 Util. 2019, 34, 716–724. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Chen, Y.; Yang, C.; Yang, L.; Sun, J. Facile synthesis of carboxyl- and hydroxyl-functional carbon nitride catalyst for efficient CO2 cycloaddition. Mol. Catal. 2021, 515, 111924. [Google Scholar] [CrossRef]



| Samples | SBET (m2 g−1) | Average Pore Diameter (nm) | B (wt%) | P (wt%) |
|---|---|---|---|---|
| CN | 2.2 | 0.01 | — | — |
| B-CN | 13.8 | 24.5 | 1.21 | — |
| P-CN | 12.5 | 14.1 | — | 1.27 |
| BP-CN-1 | 11.9 | 12.1 | 0.10 | 1.37 |
| BP-CN-2 | 9.3 | 13.7 | 0.48 | 3.56 |
| BP-CN-3 | 5.5 | 20.7 | 0.96 | 5.00 |
| Entry | Catalyst | CoCatalyst | Yield b (%) | Selectivity b (%) |
|---|---|---|---|---|
| 1 | — | — | 0 | — |
| 2 | CN | — | 2 | — |
| 3 | — | Bu4NBr | 25 | 98 |
| 4 | CN | Bu4NBr | 26 | 99 |
| 5 | B-CN | Bu4NBr | 50 | 99 |
| 6 | P-CN | Bu4NBr | 44 | 98 |
| 7 | BP-CN-1 | Bu4NBr | 64 | 98 |
| 8 | BP-CN-2 | Bu4NBr | 58 | 98 |
| 9 | BP-CN-3 | Bu4NBr | 53 | 98 |
| 10 c | — | Bu4NBr | 55 | 98 |
| 11 c | — | Bu4NI | 59 | 98 |
| 12 c | BP-CN-1 | Bu4NI | 99 | 98 |
| Entry | Epoxides | Products | Results a | ||
|---|---|---|---|---|---|
| Conversion b (%) | Yield b (%) | Selectivity b (%) | |||
| 1 |  | 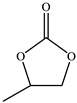 | 96 | 95 | 99 |
| 2 | 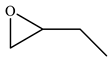 |  | 87 | 86 | 99 |
| 3 | 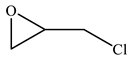 |  | 99 | 99 | 99 |
| 4 | 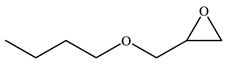 | 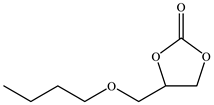 | 74 | 73 | 98 |
| 5 c | 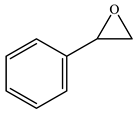 |  | 85 | 82 | 97 |
| 6 c |  |  | 66 | 63 | 96 |
| Entry | Catalysts | Solvent | Reaction Condition | Yield (%) | TOF | TON | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| Tem. (°C) | Press (MPa) | T(h) | |||||||
| 1 | eg-CN-OH | DMF | 140 | 2.0 | 6.0 | 60 | 5.8 | 35.0 | [47] |
| 2 | mpg-CN/ZnCl2(10) | DMF | 140 | 2.5 | 6.0 | 72 | 7.0 | 42.0 | [48] |
| 3 | Zn-CN/C | DMF | 140 | 2.0 | 8.0 | 74 | 5.4 | 43.3 | [49] |
| 4 b | B0.1CN/SBA-15 | – | 130 | 3.0 | 24 | 95 | 1.2 | 28.5 | [22] |
| 5 | MCNB(0.01) | – | 130 | 2.8 | 6.0 | 31 | 1.5 | 9.1 | [21] |
| 6 | K,B-CN-4(Bu4NBr) | – | 110 | 2.0 | 6.0 | 87 | 5.1 | 30.7 | [31] |
| 7 | HCN-FD(Bu4NI) | – | 110 | 2.0 | 6.0 | 92 | 5.4 | 32.4 | [50] |
| 8 | P-CN-2 (Bu4NBr) | – | 100 | 2.0 | 4.0 | 99 | 4.8 | 19.3 | [23] |
| 9 | B,P-CN-1(Bu4NBr) | – | 120 | 2.0 | 6.0 | 95 | 5.5 | 33.1 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zhao, X.; Yang, T. Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides. Catalysts 2022, 12, 1196. https://doi.org/10.3390/catal12101196
Yang C, Zhao X, Yang T. Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides. Catalysts. 2022; 12(10):1196. https://doi.org/10.3390/catal12101196
Chicago/Turabian StyleYang, Chaokun, Xin Zhao, and Tuantuan Yang. 2022. "Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides" Catalysts 12, no. 10: 1196. https://doi.org/10.3390/catal12101196
APA StyleYang, C., Zhao, X., & Yang, T. (2022). Boron and Phosphorus Co-Doped Graphitic Carbon Nitride Cooperate with Bu4NBr as Binary Heterogeneous Catalysts for the Cycloaddition of CO2 to Epoxides. Catalysts, 12(10), 1196. https://doi.org/10.3390/catal12101196