Screening of Fusarium moniliforme as Potential Fungus for Integrated Biodelignification and Consolidated Bioprocessing of Napier Grass for Bioethanol Production
Abstract
:1. Introduction
2. Results and Discussion
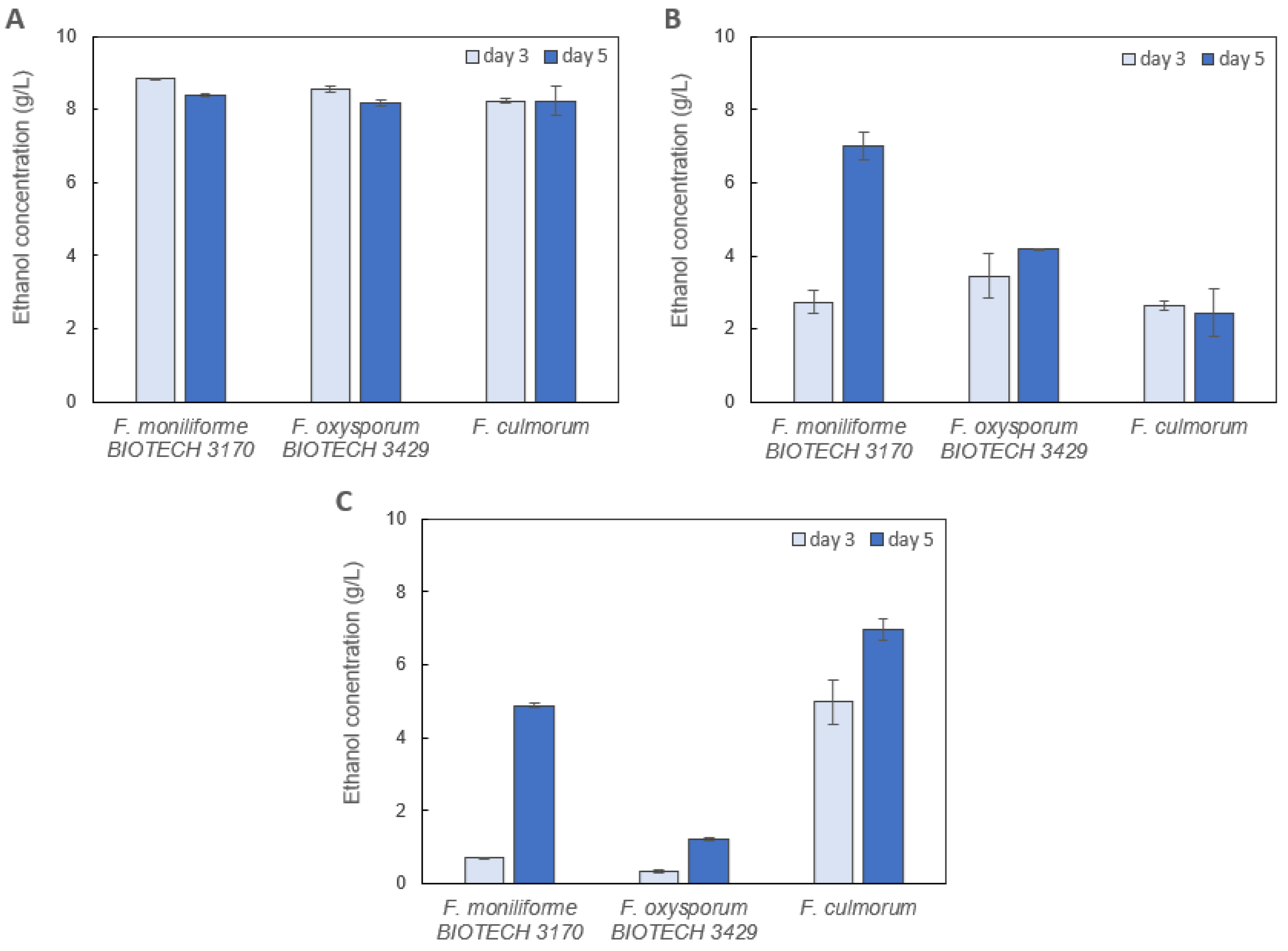
2.1. Screening of Ethanol-Producing Fungi
2.1.1. Preliminary Screening
2.1.2. Final Screening
2.2. Cofermentation of Glucose and Xylose
2.3. Integrated Biodelignification and CBP of Napier Grass
2.4. Maximum Ethanol Production
3. Materials and Methods
3.1. Fungal Strains and Inoculation
3.2. Carbon Substrate and Culture Medium
3.3. Screening of Ethanol-Producing Fungi
3.3.1. Preliminary Screening
3.3.2. Final Screening
3.4. Cofermentation of Glucose and Xylose
3.5. Integrated Biodelignification and CBP of Napier Grass
3.6. Maximum Ethanol Production
3.7. Analytical Methods
3.8. Calculations
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haghighi Mood, S.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic Biomass to Bioethanol, a Comprehensive Review with a Focus on Pretreatment. Renew. Sustain. Energy Rev. 2013, 27, 77–93. [Google Scholar] [CrossRef]
- Amore, A.; Faraco, V. Potential of Fungi as Category I Consolidated BioProcessing Organisms for Cellulosic Ethanol Production. Renew. Sustain. Energy Rev. 2012, 16, 3286–3301. [Google Scholar] [CrossRef]
- Lynd, L.R.; van Zyl, W.H.; McBride, J.E.; Laser, M. Consolidated Bioprocessing of Cellulosic Biomass: An Update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef]
- Christakopoulos, P.; Macris, B.J.; Kekos, D. Direct Fermentation of Cellulose to Ethanol by Fusarium Oxysporum. Enzym. Microb. Technol. 1989, 11, 236–239. [Google Scholar] [CrossRef]
- de Almeida, M.N.; Guimarães, V.M.; Falkoski, D.L.; Visser, E.M.; Siqueira, G.A.; Milagres, A.M.F.; de Rezende, S.T. Direct Ethanol Production from Glucose, Xylose and Sugarcane Bagasse by the Corn Endophytic Fungi Fusarium Verticillioides and Acremonium Zeae. J. Biotechnol. 2013, 168, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Hu, L.; Xia, J.; Wu, Z.; Xu, N.; Dai, B.; Wu, B. A Novel Ionic Liquid-Tolerant Fusarium Oxysporum BN Secreting Ionic Liquid-Stable Cellulase: Consolidated Bioprocessing of Pretreated Lignocellulose Containing Residual Ionic Liquid. Bioresour. Technol. 2015, 181, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, V.; Keskar, S.; Mishra, C.; Rao, M. Direct Conversion of Cellulose to Ethanol by Neurospora Crassa. Enzym. Microb Technol 1986, 8, 149–152. [Google Scholar] [CrossRef]
- Dogaris, I.; Gkounta, O.; Mamma, D.; Kekos, D. Bioconversion of Dilute-Acid Pretreated Sorghum Bagasse to Ethanol by Neurospora Crassa. Appl. Microbiol. Biotechnol. 2012, 95, 541–550. [Google Scholar] [CrossRef]
- Rao, M.; Mishra, C.; Keskar, S.; Srinivasan, M.C. Production of Ethanol from Wood and Agricultural Residues by Neurospora Crassa. Enzyme Microb. Technol. 1985, 7, 625–628. [Google Scholar] [CrossRef]
- Gong, C.; Maun, C.; Tsao, G. Direct Fermentation of Cellulose to Ethanol by a Cellulolytic Filamentous Fungi, Monilia sp. Biotechnol. Lett. 1981, 3, 77–82. [Google Scholar] [CrossRef]
- Kamei, I.; Hirota, Y.; Meguro, S. Integrated Delignification and Simultaneous Saccharification and Fermentation of Hard Wood by a White-Rot Fungus, Phlebia sp. MG-60. Bioresour. Technol. 2012, 126, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kamei, I.; Hirota, Y.; Mori, T.; Hirai, H.; Meguro, S.; Kondo, R. Direct Ethanol Production from Cellulosic Materials by the Hypersaline-Tolerant White-Rot Fungus Phlebia sp. MG-60. Bioresour. Technol. 2012, 112, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Khuong, L.D.; Kondo, R.; De Leon, R.; Kim Anh, T.; Shimizu, K.; Kamei, I. Bioethanol Production from Alkaline-Pretreated Sugarcane Bagasse by Consolidated Bioprocessing Using Phlebia sp. MG-60. Int. Biodeterior. Biodegrad. 2014, 88, 62–68. [Google Scholar] [CrossRef]
- Okamoto, K.; Nitta, Y.; Maekawa, N.; Yanase, H. Direct Ethanol Production from Starch, Wheat Bran and Rice Straw by the White Rot Fungus Trametes Hirsuta. Enzym. Microb. Technol. 2011, 48, 273–277. [Google Scholar] [CrossRef]
- Okamoto, K.; Uchii, A.; Kanawaku, R.; Yanase, H. Bioconversion of Xylose, Hexoses and Biomass to Ethanol by a New Isolate of the White Rot Basidiomycete Trametes Versicolor. Springerplus 2014, 3, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isroi; Millati, R.; Niklasson, C.; Cahyanto, M.N.; Lundquist, K.; Taherzadeh, M. Biological Pretreatment of Lignocelluloses with White-Rot Fungi and Its Applications: A Review. BioResources 2011, 6, 5224–5259. [Google Scholar] [CrossRef]
- Singhania, R.R.; Sukumaran, R.K.; Pillai, A.; Prema, P.; Szakacs, G.; Pandey, A. Solid-State Fermentation of Lignocellulosic Substrates for Cellulase Production by Trichoderma Reesei NRRL 11460. Indian J. Biotechnol. 2006, 5, 332–336. [Google Scholar]
- Chahal, D.S. Solid-State Fermentation with Trichoderma Reesei for Cellulase Production. Appl. Environ. Microbiol. 1985, 49, 205–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skory, C.D.; Freer, S.N.; Bothast, R.J. Screening for Ethanol-Producing Filamentous Fungi. Biotechnol. Lett. 1997, 19, 203–206. [Google Scholar] [CrossRef]
- Mizuno, R.; Ichinose, H.; Maehara, T.; Takabatake, K.; Kaneko, S. Properties of Ethanol Fermentation by Flammulina Velutipes. Biosci. Biotechnol. Biochem. 2009, 73, 2240–2245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maehara, T.; Ichinose, H.; Furukawa, T.; Ogasawara, W.; Takabatake, K.; Kaneko, S. Ethanol Production from High Cellulose Concentration by the Basidiomycete Fungus Flammulina Velutipes. Fungal Biol. 2013, 117, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Suihko, M.L.; Enari, T.M. The Production of Ethanol from D-Glucose and D-Xylose by Different Fusarium Strains. Biotechnol. Lett. 1981, 3, 723–728. [Google Scholar] [CrossRef]
- Nait M’Barek, H.; Arif, S.; Taidi, B.; Hajjaj, H. Consolidated Bioethanol Production from Olive Mill Waste: Wood-Decay Fungi from Central Morocco as Promising Decomposition and Fermentation Biocatalysts. Biotechnol. Reports 2020, 28, e0054. [Google Scholar] [CrossRef] [PubMed]
- Suihko, M.L. The Fermentation of Different Carbon Sources by Fusarium Oxysporum. Biotechnol. Lett. 1983, 5, 721–724. [Google Scholar] [CrossRef]
- Millati, R.; Edebo, L.; Taherzadeh, M.J. Performance of Rhizopus, Rhizomucor, and Mucor in Ethanol Production from Glucose, Xylose, and Wood Hydrolyzates. Enzym. Microb. Technol. 2004, 36, 294–300. [Google Scholar] [CrossRef]
- Okamoto, K.; Kanawaku, R.; Masumoto, M.; Yanase, H. Efficient Xylose Fermentation by the Brown Rot Fungus Neolentinus Lepideus. Enzym. Microb. Technol. 2012, 50, 96–100. [Google Scholar] [CrossRef]
- Wu, J.F.; Lastick, S.M.; Updegraff, D.M. Ethanol Production from Sugars Derived from Plant Biomass by a Novel Fungus. Nature 1986, 324, 227–231. [Google Scholar] [CrossRef]
- Zerva, A.; Savvides, A.L.; Katsifas, E.A.; Karagouni, A.D.; Hatzinikolaou, D.G. Evaluation of Paecilomyces Variotii Potential in Bioethanol Production from Lignocellulose through Consolidated Bioprocessing. Bioresour. Technol. 2014, 162, 294–299. [Google Scholar] [CrossRef]
- Okamoto, K.; Imashiro, K.; Akizawa, Y.; Onimura, A.; Yoneda, M.; Nitta, Y.; Maekawa, N.; Yanase, H. Production of Ethanol by the White-Rot Basidiomycetes Peniophora Cinerea and Trametes Suaveolens. Biotechnol. Lett. 2010, 32, 909–913. [Google Scholar] [CrossRef]
- Okamoto, K.; Goda, T.; Yamada, T.; Nagoshi, M. Direct Ethanol Production from Xylan and Acorn Using the Starch-Fermenting Basidiomycete Fungus Phlebia Acerina. Fermentation 2021, 7, 116. [Google Scholar] [CrossRef]
- Wikandari, R.; Millati, R.; Lennartsson, P.R.; Harmayani, E.; Taherzadeh, M.J. Isolation and Characterization of Zygomycetes Fungi from Tempe for Ethanol Production and Biomass Applications. Appl. Biochem. Biotechnol. 2012, 167, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, S.; Ando, H.; Ariga, O.; Sakuma, Y. Direct Ethanol Production from Cellulosic Materials by Consolidated Biological Processing Using the Wood Rot Fungus Schizophyllum Commune. Bioresour. Technol. 2015, 197, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Romero, I.; Moya, M.; Sanchez, S.; Bravo, V.; Castro, E. Sugar Fermentation by Fusarium Oxysporum to Produce Ethanol. World J. Microbiol. Biotechnol. 2007, 23, 259–267. [Google Scholar] [CrossRef]
- Somerville, C.; Youngs, H.; Taylor, C.; Davis, S.C.; Long, S.P. Feedstocks for Lignocellulosic Biofuels. Science 2010, 329, 790–792. [Google Scholar] [CrossRef] [Green Version]
- Laurent, A.; Pelzer, E.; Loyce, C.; Makowski, D. Ranking Yields of Energy Crops: A Meta-Analysis Using Direct and Indirect Comparisons. Renew. Sustain. Energy Rev. 2015, 46, 41–50. [Google Scholar] [CrossRef]
- Del Río, J.C.; Pepijin, P.; Rencoret, J.; Nieto, L.; Jimenez-Barbero, J.; Ralph, J.; Martinez, A.; Gutierrez, A. Structural Characterization of the Lignin in the Cortex and Pith of Elephant Grass ( Pennisetum Purpureum ) Stems. J. Agric. Food Chem. 2012, 60, 3619–3634. [Google Scholar] [CrossRef] [Green Version]
- Sutherland, J.B.; Pometto III, A.L.; Crawford, D.L. Lignocellulose Degradation by Fusarium Species. Botany 1983, 61, 1194–1198. [Google Scholar] [CrossRef]
- Chang, A.J.; Fan, J.; Wen, X. Screening of Fungi Capable of Highly Selective Degradation of Lignin in Rice Straw. Int. Biodeterior. Biodegrad. 2012, 72, 26–30. [Google Scholar] [CrossRef]
- Zacchi, G.; Axelsson, A. Economic Evaluation of Preconcentration in Production of Ethanol from Dilute Sugar Solutions. Biotechnol. Bioeng. 1989, 34, 223–233. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; National Renewable Energy Laboratory: Golden, CO, USA, 2011.






| Culture Condition | Fungi | Cellulose Consumed | Ethanol Concentration | Ethanol Yield | Theoretical Yield | Conversion Efficiency |
|---|---|---|---|---|---|---|
| (g) | (g/L) | (g/g Substrate) | (%) | (%) | ||
| Solid state | F. moniliforme BIOTECH 3170 | 0.55 ± 0.01 | 25.2 ± 0.7 | 0.072 ± 0.001 | 6.34 ± 0.07 | 46.3 ± 0.3 |
| F. oxysporum BIOTECH 3429 | 0.33 ± 0.00 | 0.0 ± 0.0 | 0.000 ± 0.000 | 0.00 ± 0.00 | 0.0 ± 0.1 | |
| F. culmorum | 0.27 ± 0.00 | 0.6 ± 0.0 | 0.002 ± 0.000 | 0.16 ± 0.00 | 2.4 ± 0.0 | |
| Submerged | F. moniliforme BIOTECH 3170 | 0.34 ± 0.03 | 5.9 ± 0.3 | 0.058 ± 0.002 | 5.12 ± 0.19 | 60.2 ± 3.6 |
| F. oxysporum BIOTECH 3429 | 0.22 ± 0.02 | 0.5 ± 0.3 | 0.005 ± 0.003 | 0.44 ± 0.26 | 7.7 ± 3.9 | |
| F. culmorum | 0.09 ± 0.05 | 0.5 ± 0.3 | 0.005 ± 0.002 | 0.41 ± 0.22 | 19.1 ± 1.2 |
| Fungi | Strain | Glucose | Xylose | Cellobiose | Cellulose | Lignocellulose | Reference | |
|---|---|---|---|---|---|---|---|---|
| %TY | %TY | %TY | %TY (EC) | Feedstock a | BY (EC) | |||
| A. oryzae | NRRL 694 | 95.5 | 18.4 | 2.1 (0.6) | [19] | |||
| F. velutipes | Fv-1 | 87 | 1.0 | 83 | 0 (0) | [20,21] | ||
| F. clamydosporium | VTT-D-77055 | 82.2 | 43.1 | [22] | ||||
| F. culmorum | VTT-D-72012 | 54.8 | 47.0 | [22] | ||||
| F. culmorum | 80.5 | 25.9 | 64.7 | 0.2 (0.6) | Napier grass * | 0.001 (1.5) | Present work | |
| F. graminearum | VTT-D-76013 | 82.2 | 23.5 | [22] | ||||
| F. moniliforme | BIOTECH 3170 | 86.4 | 68.6 | 45.4 | 6.3 (25.2) | Napier grass * | 0.032 (10.5) | Present work |
| F. oxysporum | ATCC 10960 | 90.0 | 54.8 | [22] | ||||
| F. oxysporum | BIOTECH 3429 | 83.8 | 41.1 | 11.3 | 0.1 (0.3) | Napier grass * | 0.001 (1.5) | Present work |
| F. oxysporum | BN | 97.8 | 58.7 | ILP rice straw | 0.155 (9.3) | [6] | ||
| F. oxysporum | F3 | 80.2 | 48 | 82.7 | 89.2 (6.9) | AP wheat straw | 0.160 (11.2) | [4] |
| 53.9 (14.5) | BM wheat straw | 0.280 (8.4) | ||||||
| Corn cob * | 0.048 (1.9) | |||||||
| Brewer’s spent grain | 0.048 (3.6) | |||||||
| Brewer’s spent grain | 0.069 (5.2) | |||||||
| AP Brewer spent grain | 0.107 (8) | |||||||
| AP Brewer spent grain | 0.109 (8.2) | |||||||
| AP wheat straw | 0.210 (8.4) | |||||||
| F. oxysporum | MK956809 | (1.52) | Milled olive waste | (2.47) | [23] | |||
| F. oxysporum | VTT-D-80134 | 97.8 | 86.1 | 64.5 | 0 (0) | [24] | ||
| F. oxysporum | VTT-D-80135 | 86.1 | 50.9 | [22] | ||||
| F. solani | VTT-D-77057 | 90.0 | 43.1 | [22] | ||||
| F. sporotrichioides | VTT-D-77058 | 86.1 | 15.7 | [22] | ||||
| F. sporotrichioides | VTT-D-80138 | 86.1 | 15.7 | [22] | ||||
| F. verticillioides | 92.4 | 55.6 | AP sugarcane bagasse | 0.115 (4.6) | [5] | |||
| Monilia sp. | 90.0 | 43.1 | 70 (17) | [10] | ||||
| 60 (14) | ||||||||
| M. corticolous | CCUG 0481 | 84.15 | 29.35 | [25] | ||||
| N. lepideus | 74.4 | 66.5 | 66.9 | [26] | ||||
| N. crassa | NCIM 870 | 86.1 | 91 (9.9) | CEAP bagasse | 0.260 (13) | [7] | ||
| 91 (9.9) | CEAP straw | 0.240 (12) | ||||||
| 77 (16.9) | CAP Mesta Wood | 0.220 (11) | ||||||
| 54 (11.9) | CAP Su Babul | 0.200 (10) | ||||||
| 36 (9.9) | CAP Mesta Wood | 0.350 (7) | ||||||
| 36 (9.9) | CAP Su Babul | 0.300 (6) | ||||||
| Paecilomyces sp. | NF1 | 80.0 | 77.9 | 74.7 | [27] | |||
| P. variotii | ATHUM 8891 | 80.9 | 90.7 | Corb cob * | <0.030 (0.6) | [28] | ||
| Brewer’s spent grain | <0.030 (1.2) | |||||||
| P. cinerea | 80 | 17.6 | 52.9 (3) | [29] | ||||
| Phlebia sp. | MG-60 | 86.9 | 65 | 70.6 | 28.3 (2.8) | AU hardwood Kraft Pulp | 0.42 (8.4) | [11,12,13] |
| Oakwood * | 0.159 (7) | |||||||
| Sugarcane bagasse | 0.225 | |||||||
| AP sugarcane bagasse | 0.34 (6.16) | |||||||
| Newspaper | 0.2 (4.2) | |||||||
| Phlebia acerina | SF 23754 | 93.9 | 78.3 | 92.3 | [30] | |||
| Rhizomucor sp. | CCUG 61146 | 92.3 | 24.9 | [31] | ||||
| Rhizomucor sp. | CCUG 61147 | 89.4 | 22.0 | [31] | ||||
| R. javanicus | NRRL 13161 | 92.0 | 9.0 | 15.2 | 3.2 (0.9) | [19] | ||
| R. javanicus | NRRL 13162 | 85.3 | 6.7 | 14.1 | 1.8 (0.5) | [19] | ||
| R. oryzae | CCUG 22420 | 84.1 | 54.8 | [25] | ||||
| R. oryzae | CCUG 28958 | 80.2 | 31.3 | [25] | ||||
| R. oryzae | NRRL 13480 | 90.8 | 11.7 | 73.6 | 4.2 (1.2) | [19] | ||
| R. oryzae | NRRL 1501 | 98.2 | 7.4 | 68.4 | 4.6 (1.3) | [19] | ||
| R. oryzae | NRRL 2625 | 95.9 | 8.6 | 14.5 | 3.2 (0.9) | [19] | ||
| R. oryzae | NRRL 6201 | 99.4 | 22.3 | 26.0 | 3.2 (0.9) | [19] | ||
| S. commune | 80.5 | 52.4 | 81.2 | 45.6 (0.09) | [32] | |||
| T. hirsuta | 95.9 | 38.2 | 92.2 | BM rice straw | 0.17 (3.4) | [14] | ||
| Rice straw * | 0.15 (3) | |||||||
| T. versicolor | KT9427 | 90.0 | 86.1 | 96.1 | 41.4 (4.7) | Rice straw * | 0.218 (4.8) | [15] |
| Day | Oxygen Condition | Biomass (Solid) | Supernatant (Liquid) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Residual Mass (g/2 g Napier Grass) | Percent Degradation (%) | Concentration (g/L) * | ||||||||||
| Total Solid Biomass | Glucan | Xylan | Lignin | Total Solid Biomass | Glucan | Xylan | Lignin | Ethanol | Glucose | Xylose | ||
| 0 | Aerobic | 1.87 | 0.75 | 0.40 | 0.34 | 0.0 | 0.0 | 0.0 | 0.0 | 0.00 | n.d. | n.d. |
| 7 | Anaerobic | 1.57 | 0.54 | 0.31 | 0.30 | 16.1 | 27.4 | 23.1 | 14.0 | 0.00 | n.d. | n.d. |
| 14 | Anaerobic | 1.42 | 0.47 | 0.27 | 0.28 | 24.1 | 36.9 | 32.6 | 18.6 | 6.33 | n.d. | n.d. |
| 21 | Anaerobic | 1.34 | 0.46 | 0.25 | 0.27 | 28.3 | 39.2 | 37.8 | 20.3 | 6.69 | n.d. | n.d. |
| 28 | Anaerobic | 1.31 | 0.45 | 0.23 | 0.28 | 30.1 | 40.3 | 42.1 | 19.9 | 10.54 | n.d. | n.d. |
| 35 | Anaerobic | 1.26 | 0.44 | 0.21 | 0.27 | 32.5 | 41.7 | 47.4 | 21.4 | 9.02 | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, M.; Alfafara, C.; de Leon, R. Screening of Fusarium moniliforme as Potential Fungus for Integrated Biodelignification and Consolidated Bioprocessing of Napier Grass for Bioethanol Production. Catalysts 2022, 12, 1204. https://doi.org/10.3390/catal12101204
Lao M, Alfafara C, de Leon R. Screening of Fusarium moniliforme as Potential Fungus for Integrated Biodelignification and Consolidated Bioprocessing of Napier Grass for Bioethanol Production. Catalysts. 2022; 12(10):1204. https://doi.org/10.3390/catal12101204
Chicago/Turabian StyleLao, Marco, Catalino Alfafara, and Rizalinda de Leon. 2022. "Screening of Fusarium moniliforme as Potential Fungus for Integrated Biodelignification and Consolidated Bioprocessing of Napier Grass for Bioethanol Production" Catalysts 12, no. 10: 1204. https://doi.org/10.3390/catal12101204
APA StyleLao, M., Alfafara, C., & de Leon, R. (2022). Screening of Fusarium moniliforme as Potential Fungus for Integrated Biodelignification and Consolidated Bioprocessing of Napier Grass for Bioethanol Production. Catalysts, 12(10), 1204. https://doi.org/10.3390/catal12101204






