The Role of Nanoparticle Catalysis in the Nylon Production
Abstract
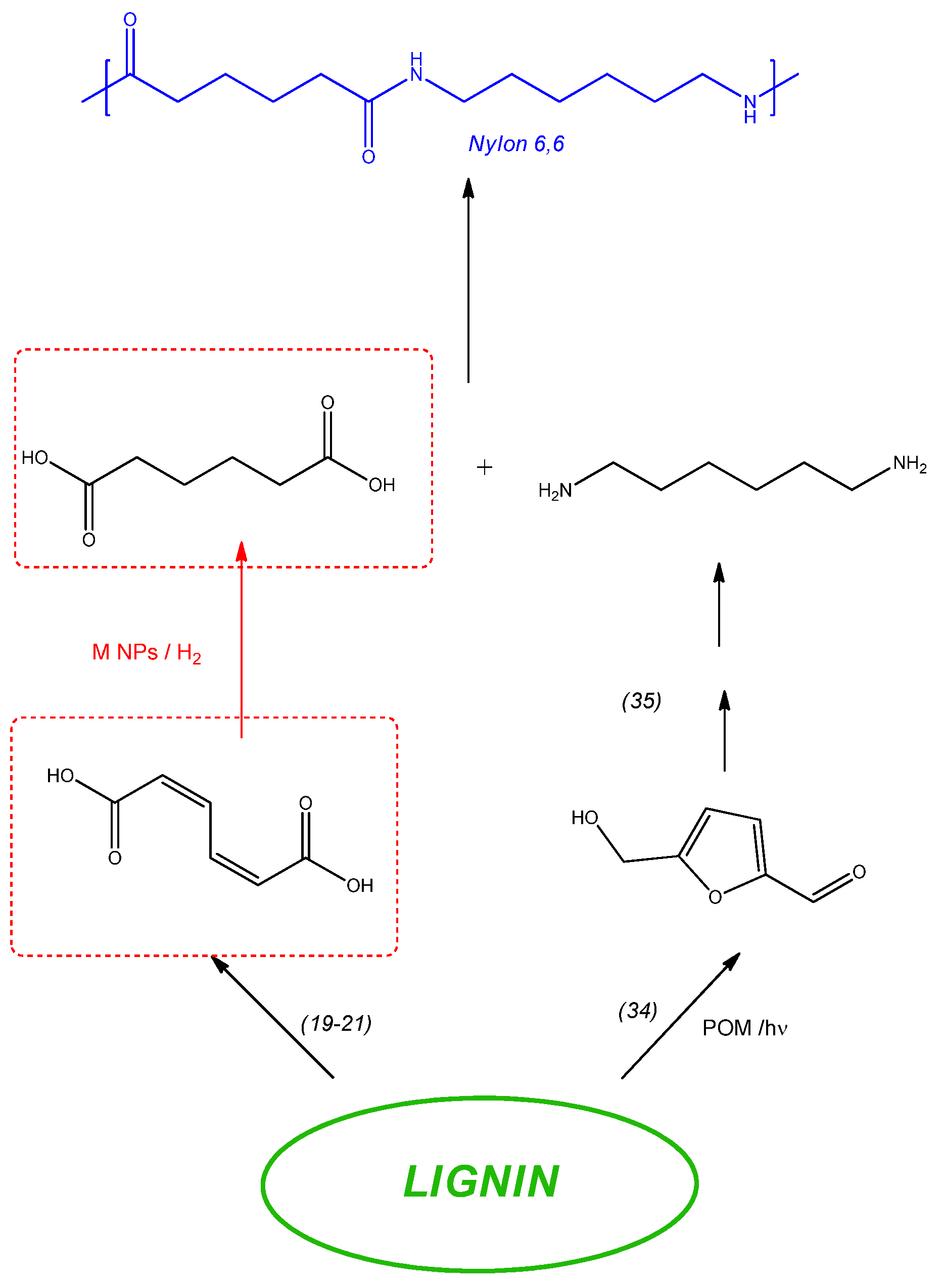
:1. Introduction
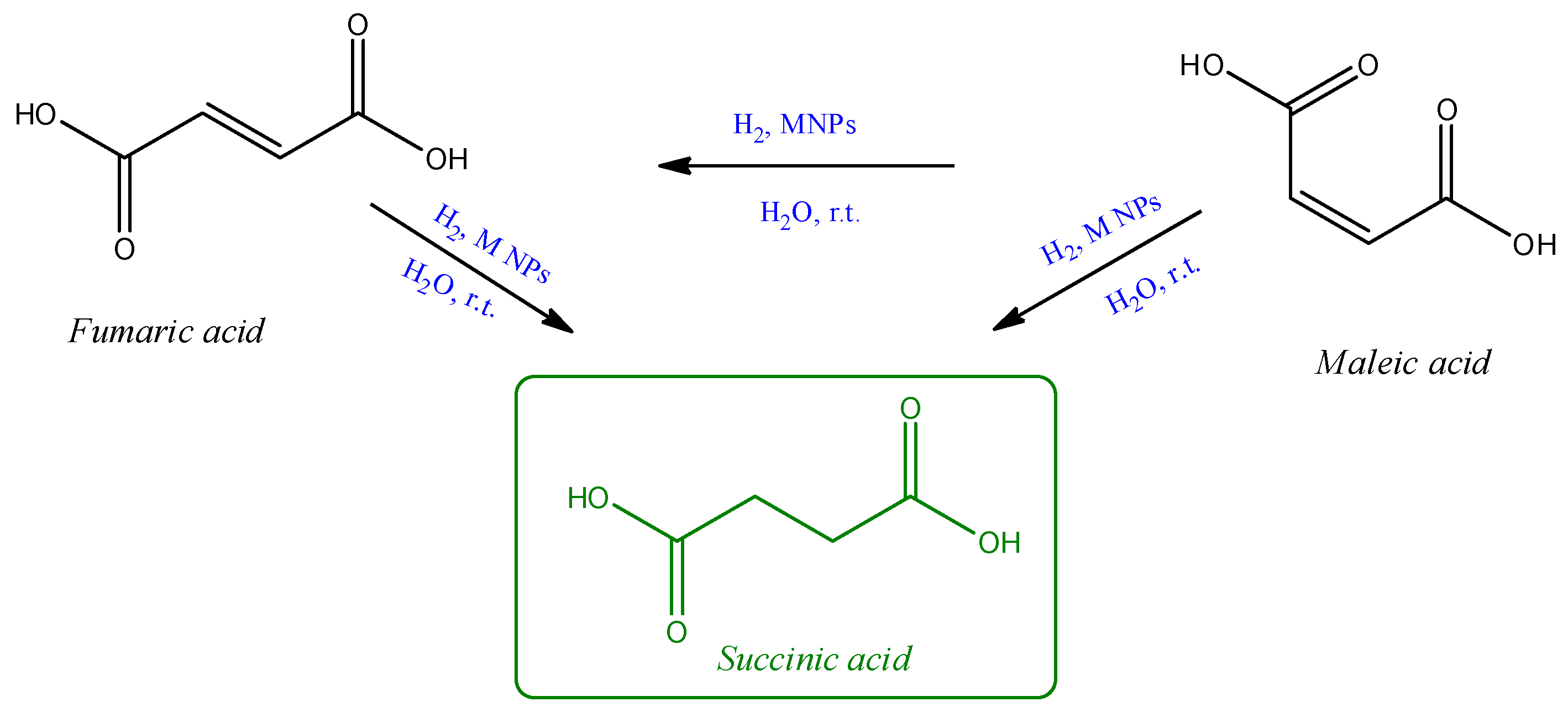
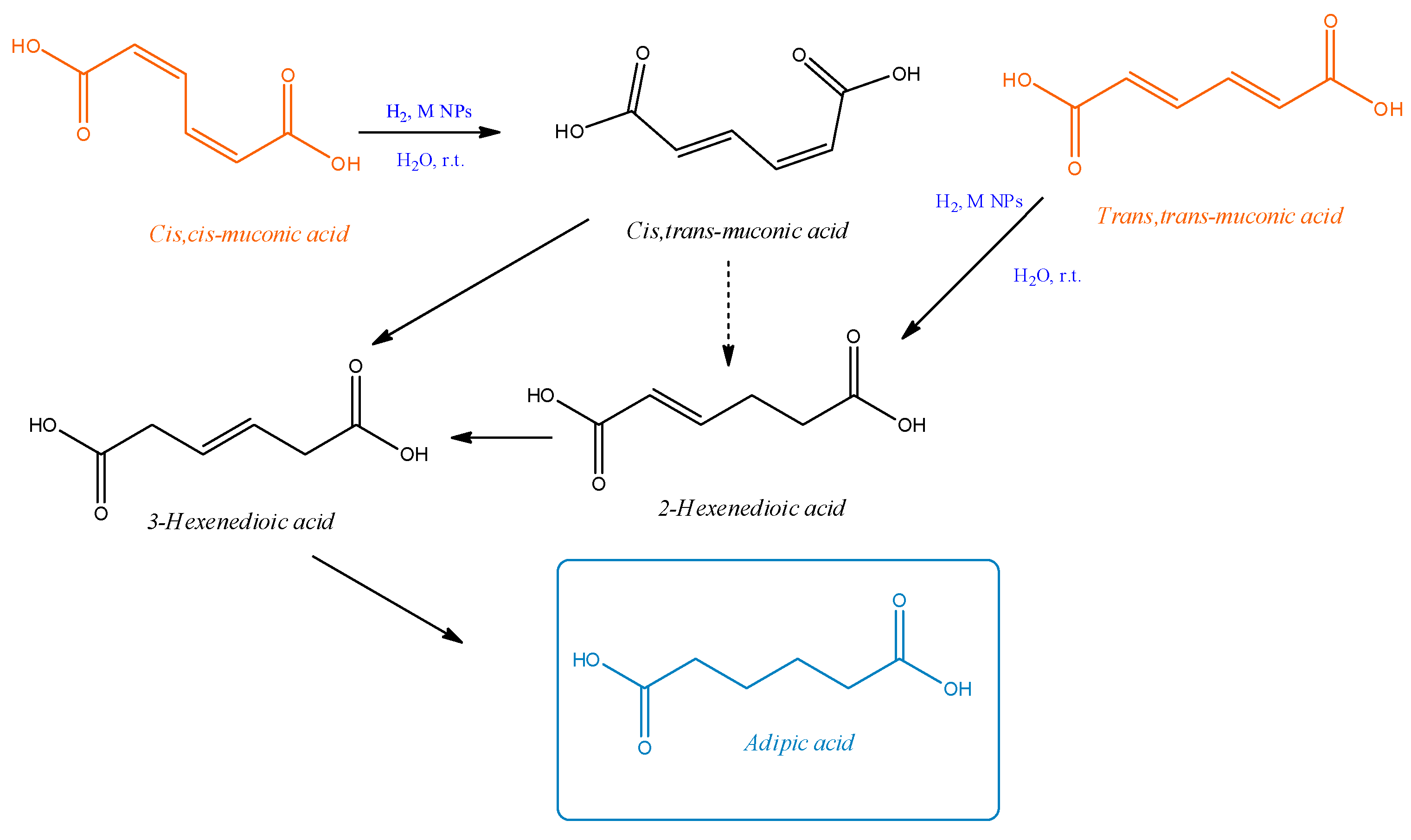
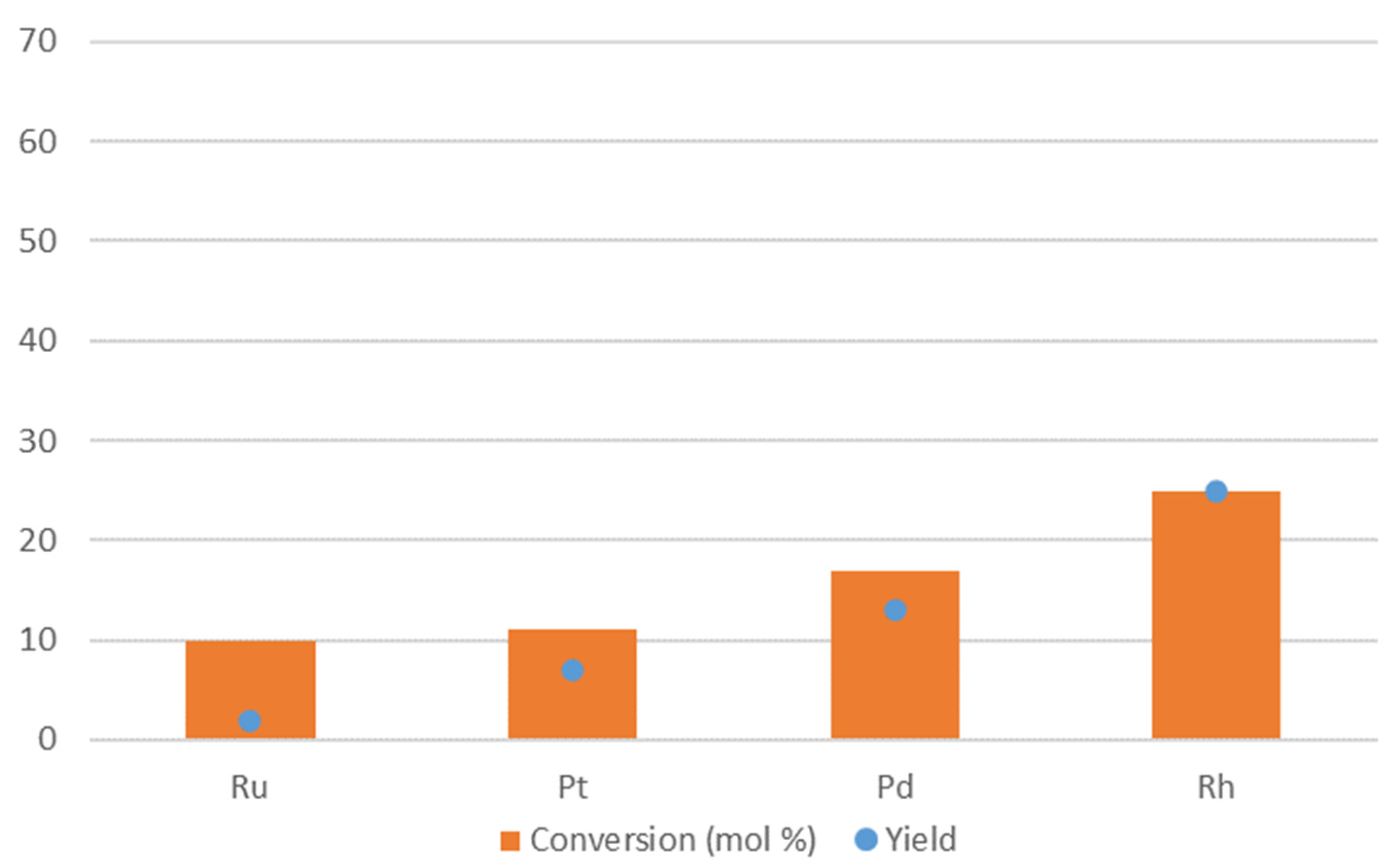
2. Results and Discussion
3. Materials and Methods
3.1. Materials
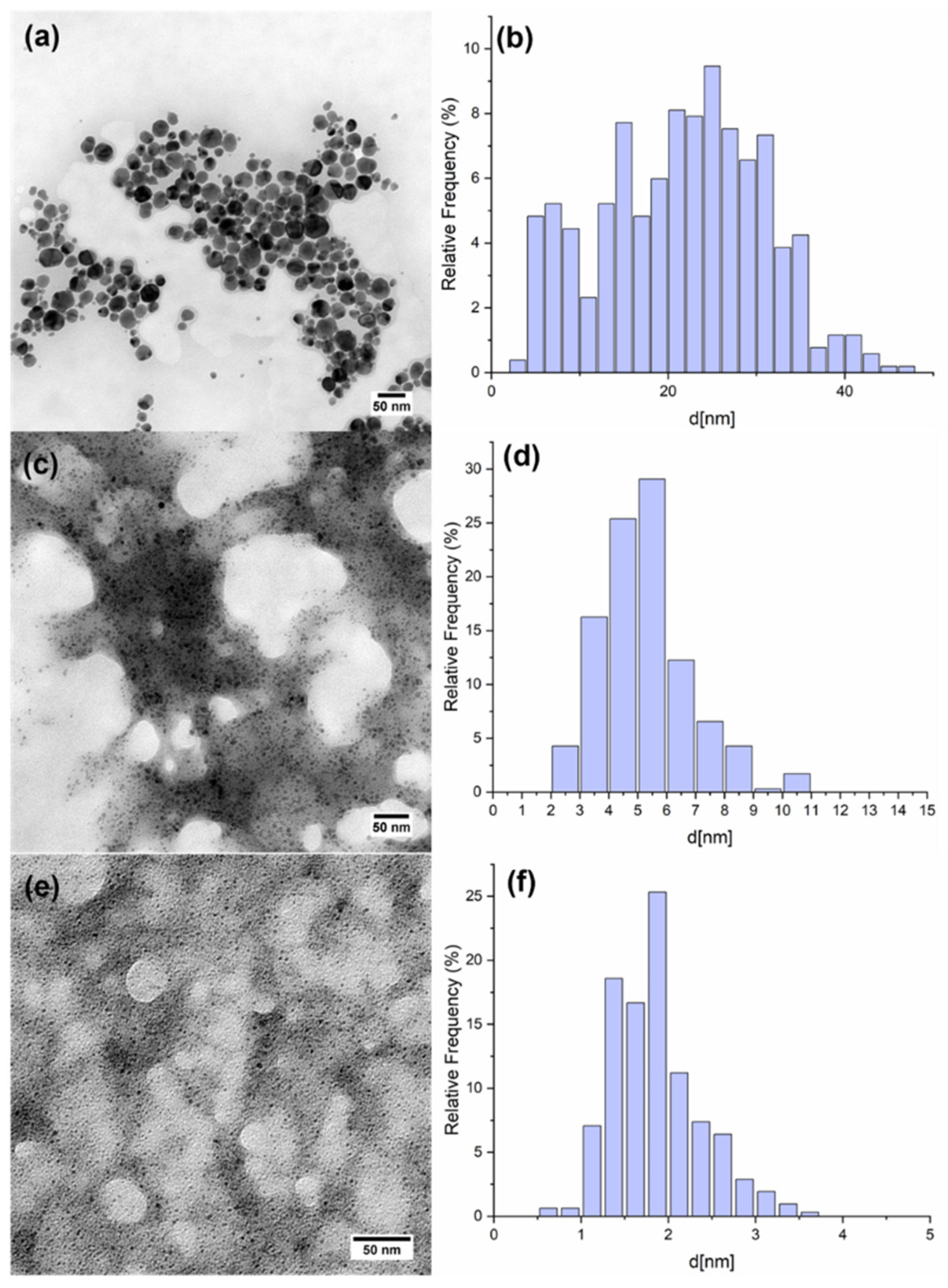
3.2. Synthesis and Characterization of M NPs
3.3. Hydrogenation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grand View Research. Nylon Market Size, Share & Trends Analysis Report By Product (Nylon 6, Nylon 66), By Application (Automobile, Electrical & Electronics, Engineering Plastics, Textiles), By Region, And Segment Forecasts, Grand View Research, San Francisco, US. Vol. GVR-1-6803. 2022–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/nylon-6-6-market (accessed on 12 September 2022).
- Tullo, A. Industry braces for nylon 6,6 shortage. CEN Glob. Enterp. 2018, 96, 22–23. [Google Scholar] [CrossRef]
- European Bioplastics. Market Update 2020: Bioplastics Continue to Become Mainstream as the Global Bioplastics Market Is Set to Grow by 36 Percent over the next 5 Years; European Bioplastics: Berlin, Germany, 2020. [Google Scholar]
- Van de Vyver, S.; Román-Leshkov, Y. Emerging catalytic processes for the production of adipic acid. Catal. Sci. Technol. 2013, 3, 1465–1479. [Google Scholar] [CrossRef] [Green Version]
- Reimer, R.A.; Slaten, C.S.; Seapan, M.; Koch, T.A.; Triner, V.G. Adipic Acid Industry—N2O Abatement. In Non-CO2 Greenhouse Gases: Scientific Understanding, Control and Implementation; Springer: Dordrecht, The Netherlands, 2000; pp. 347–358. [Google Scholar]
- Portmann, R.W.; Daniel, J.S.; Ravishankara, A.R. Stratospheric ozone depletion due to nitrous oxide: Influences of other gases. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1256–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donen, S.; Hash, K.; Smith, T.; Jensen, K. Nitric Acid Oxidation Processes. U.S. Patent 9,187,398, 12 March 2014. [Google Scholar]
- Zimmerman, J.B.; Anastas, P.T.; Erythropel, H.C.; Leitner, W. Designing for a green chemistry future. Science 2020, 367, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Capece, N.; Sadier, A.; Ferraz, C.P.; Thuriot-Roukos, J.; Pietrowski, M.; Zieliński, M.; Paul, S.; Cavani, F.; Wojcieszak, R. Aerobic oxidation of 1,6-hexanediol to adipic acid over Au-based catalysts: The role of basic supports. Catal. Sci. Technol. 2020, 10, 2644–2651. [Google Scholar] [CrossRef]
- D’Alessandro, N.; Liberatore, L.; Tonucci, L.; Morvillo, A.; Bressan, M. Direct synthesis of adipic acid by mono-persulfate oxidation of cyclohexane, cyclohexanone or cyclohexanol catalyzed by water-soluble transition-metal complexes. New J. Chem. 2001, 25, 1319–1324. [Google Scholar] [CrossRef]
- Mazzi, A.; Paul, S.; Cavani, F.; Wojcieszak, R. Cyclohexane Oxidation to Adipic Acid Under Green Conditions: A Scalable and Sustainable Process. ChemCatChem 2018, 10, 3680–3682. [Google Scholar] [CrossRef]
- Yan, W.; Zhang, G.; Wang, J.; Liu, M.; Sun, Y.; Zhou, Z.; Zhang, W.; Zhang, S.; Xu, X.; Shen, J.; et al. Recent Progress in Adipic Acid Synthesis Over Heterogeneous Catalysts. Front. Chem. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Lesage, G.; Peñate, I.Q.; Franceschi, S.; Perez, E.; Garrigues, J.-C.; Poux, M.; Cognet, P. Sustainable process for adipic acid production from cyclohexene in microemulsion. Catal. Today 2020, 346, 40–45. [Google Scholar] [CrossRef]
- Braga, M.; Ferreira, P.M.; Almeida, J.R.M. Screening method to prioritize relevant bio-based acids and their biochemical processes using recent patent information. Biofuels Bioprod. Biorefining 2021, 15, 231–249. [Google Scholar] [CrossRef]
- Polen, T.; Spelberg, M.; Bott, M. Toward biotechnological production of adipic acid and precursors from biorenewables. J. Biotechnol. 2013, 167, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Van Duuren, J.B.J.H.; Wijte, D.; Karge, B.; dos Santos, V.A.M.; Yang, Y.; Mars, A.E.; Eggink, G. pH-stat fed-batch process to enhance the production of cis, cis-muconate from benzoate by Pseudomonas putida KT2440-JD1. Biotechnol. Prog. 2012, 28, 85–92. [Google Scholar] [CrossRef]
- Draths, K.M.; Frost, J.W. Environmentally compatible synthesis of adipic acid from D-glucose. J. Am. Chem. Soc. 1994, 116, 399–400. [Google Scholar] [CrossRef]
- Becker, J.; Lange, A.; Fabarius, J.; Wittmann, C. Top value platform chemicals: Bio-based production of organic acids. Curr. Opin. Biotechnol. 2015, 36, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Sonoki, T.; Takahashi, K.; Sugita, H.; Hatamura, M.; Azuma, Y.; Sato, T.; Suzuki, S.; Kamimura, N.; Masai, E. Glucose-Free cis,cis-Muconic Acid Production via New Metabolic Designs Corresponding to the Heterogeneity of Lignin. ACS Sustain. Chem. Eng. 2018, 6, 1256–1264. [Google Scholar] [CrossRef]
- Vardon, D.R.; Franden, M.A.; Johnson, C.W.; Karp, E.M.; Guarnieri, M.T.; Linger, J.G.; Salm, M.J.; Strathmann, T.J.; Beckham, G.T. Adipic acid production from lignin. Energy Environ. Sci. 2015, 8, 617–628. [Google Scholar] [CrossRef]
- Johnson, C.W.; Abraham, P.E.; Linger, J.G.; Khanna, P.; Hettich, R.L.; Beckham, G.T. Eliminating a global regulator of carbon catabolite repression enhances the conversion of aromatic lignin monomers to muconate in Pseudomonas putida KT2440. Metab. Eng. Commun. 2017, 5, 19–25. [Google Scholar] [CrossRef]
- Rosengart, A.; Capelli, S.; Pirola, C.; Citterioa, A.; Bianchi, C.L.; Prati, L.; Villa, A. Renewable adipic acid from the hydrogenation of trans, trans-muconic acid: Selection of a three phases kinetic model. Chem. Eng. Trans. 2017, 57, 931–936. [Google Scholar] [CrossRef]
- Carraher, J.M.; Pfennig, T.; Rao, R.G.; Shanks, B.H.; Tessonnier, J.-P. cis,cis-Muconic acid isomerization and catalytic conversion to biobased cyclic-C6-1,4-diacid monomers. Green Chem. 2017, 19, 3042–3050. [Google Scholar] [CrossRef] [Green Version]
- Carraher, J.M.; Carter, P.; Rao, R.G.; Forrester, M.J.; Pfennig, T.; Shanks, B.H.; Cochran, E.W.; Tessonnier, J.-P. Solvent-driven isomerization of cis,cis-muconic acid for the production of specialty and performance-advantaged cyclic biobased monomers. Green Chem. 2020, 22, 6444–6454. [Google Scholar] [CrossRef]
- Matthiesen, J.E.; Carraher, J.M.; Vasiliu, M.; Dixon, D.A.; Tessonnier, J.-P. Electrochemical Conversion of Muconic Acid to Biobased Diacid Monomers. ACS Sustain. Chem. Eng. 2016, 4, 3575–3585. [Google Scholar] [CrossRef]
- She, X.; Brown, H.M.; Zhang, X.; Ahring, B.K.; Wang, Y. Selective Hydrogenation of Trans,Trans-Muconic Acid to Adipic Acid over a Titania-Supported Rhenium Catalyst. ChemSusChem 2011, 4, 1071–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Su, X.; Ang, E.L.; Zhang, Y.; Zhao, H. Production of Adipic Acid from Sugar Beet Residue by Combined Biological and Chemical Catalysis. ChemCatChem 2016, 8, 1500–1506. [Google Scholar] [CrossRef]
- Capelli, S.; Motta, D.; Evangelisti, C.; Dimitratos, N.; Prati, L.; Pirola, C.; Villa, A. Bio Adipic Acid Production from Sodium Muconate and Muconic Acid: A Comparison of two Systems. ChemCatChem 2019, 11, 3075–3084. [Google Scholar] [CrossRef]
- Capelli, S.; Motta, D.; Evangelisti, C.; Dimitratos, N.; Prati, L.; Pirola, C.; Villa, A. Effect of Carbon Support, Capping Agent Amount, and Pd NPs Size for Bio-Adipic Acid Production from Muconic Acid and Sodium Muconate. Nanomaterials 2020, 10, 505. [Google Scholar] [CrossRef] [Green Version]
- Vardon, D.R.; Rorrer, N.A.; Salvachúa, D.; Settle, A.E.; Johnson, C.W.; Menart, M.J.; Cleveland, N.S.; Ciesielski, P.N.; Steirer, K.X.; Dorgan, J.R.; et al. cis,cis-Muconic acid: Separation and catalysis to bio-adipic acid for nylon-6,6 polymerization. Green Chem. 2016, 18, 3397–3413. [Google Scholar] [CrossRef]
- Scelfo, S.; Pirone, R.; Russo, N. Highly efficient catalysts for the synthesis of adipic acid from cis,cis-muconic acid. Catal. Commun. 2016, 84, 98–102. [Google Scholar] [CrossRef]
- Hočevar, B.; Prašnikar, A.; Huš, M.; Grilc, M.; Likozar, B. H2 -Free Re-Based Catalytic Dehydroxylation of Aldaric Acid to Muconic and Adipic Acid Esters. Angew. Chem. Int. Ed. 2021, 60, 1244–1253. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Tonucci, L.; Coccia, F.; Bressan, M.; D’Alessandro, N. Mild Photocatalysed and Catalysed Green Oxidation of Lignin: A Useful Pathway to Low-Molecular-Weight Derivatives. Waste Biomass Valorization 2011, 3, 165–174. [Google Scholar] [CrossRef]
- Dros, A.B.; Larue, O.; Reimond, A.; De Campo, F.; Pera-Titus, M. Hexamethylenediamine (HMDA) from fossil- vs. bio-based routes: An economic and life cycle assessment comparative study. Green Chem. 2015, 17, 4760–4772. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Danby, A.M.; Ramanathan, A.; Chaudhari, R.V.; Motagamwala, A.H.; Dumesic, J.A.; Subramaniam, B. Enhanced Acid-Catalyzed Lignin Depolymerization in a Continuous Reactor with Stable Activity. ACS Sustain. Chem. Eng. 2020, 8, 4096–4106. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.-J.; Yu, H.; Sytu, M.R.; Wang, Y.; Beeri, D.; Zheng, W.; Sherman, B.D.; Yoo, C.G.; Leem, G. Solar-Driven Lignin Oxidation via Hydrogen Atom Transfer with a Dye-Sensitized TiO2 Photoanode. ACS Energy Lett. 2019, 5, 777–784. [Google Scholar] [CrossRef]
- Sun, Z.; Barta, K. Cleave and couple: Toward fully sustainable catalytic conversion of lignocellulose to value added building blocks and fuels. Chem. Commun. 2018, 54, 7725–7745. [Google Scholar] [CrossRef] [PubMed]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Ma, S.; Xu, C.; Liu, Y.; Dai, J.; Wang, Z.; Liu, X.; Chen, J.; Shen, X.; Wei, J.; et al. Vanillin-Derived High-Performance Flame Retardant Epoxy Resins: Facile Synthesis and Properties. Macromolecules 2017, 50, 1892–1901. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Cao, Z.; Dierks, M.; Clough, M.T.; de Castro, I.B.D.; Rinaldi, R. A Convergent Approach for a Deep Converting Lignin-First Biorefinery Rendering High-Energy-Density Drop-in Fuels. Joule 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Yang, B.; Zhang, Q.; Zhu, W. Catalytic routes for the conversion of lignocellulosic biomass to aviation fuel range hydrocarbons. Renew. Sustain. Energy Rev. 2020, 120, 109612. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; Bosco, D.; Bressan, M.; D’Alessandro, N. One-pot synthesis of lignin-stabilised platinum and palladium nanoparticles and their catalytic behaviour in oxidation and reduction reactions. Green Chem. 2012, 14, 1073–1078. [Google Scholar] [CrossRef]
- Coccia, F.; Tonucci, L.; D’Alessandro, N.; D’Ambrosio, P.; Bressan, M. Palladium nanoparticles, stabilized by lignin, as catalyst for cross-coupling reactions in water. Inorg. Chim. Acta 2013, 399, 12–18. [Google Scholar] [CrossRef]
- Di Pietrantonio, K.; Coccia, F.; Tonucci, L.; D’Alessandro, N.; Bressan, M. Hydrogenation of allyl alcohols catalyzed by aqueous palladium and platinum nanoparticles. RSC Adv. 2015, 5, 68493–68499. [Google Scholar] [CrossRef] [Green Version]
- Coccia, F.; Tonucci, L.; Del Boccio, P.; Caporali, S.; Hollmann, F.; D’Alessandro, N. Stereoselective Double Reduction of 3-Methyl-2-cyclohexenone, by Use of Palladium and Platinum Nanoparticles, in Tandem with Alcohol Dehydrogenase. Nanomaterials 2018, 8, 853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gherib, H.; Boudjahem, A.G.; Medjahdi, G. Effect of Preparation Method and Support on Catalytic Behavior of Rhodium Nanoparticles in Styrene Hydrogenation. Kinet. Catal. 2020, 61, 466–472. [Google Scholar] [CrossRef]
- Akbayrak, S. Rhodium(0) nanoparticles supported on ceria as catalysts in hydrogenation of neat benzene at room temperature. J. Colloid Interface Sci. 2018, 530, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ciganda, R.; Wang, C.; Escobar, A.; Martinez-Villacorta, A.M.; Ramírez, M.D.L.; Hernández, R.; Moya, S.E.; Ruiz, J.; Hamon, J.-R.; et al. High catalytic activity of Rh nanoparticles generated from cobaltocene and RhCl3 in aqueous solution. Inorg. Chem. Front. 2019, 6, 2704–2708. [Google Scholar] [CrossRef]
- Moos, G.; Emondts, M.; Bordet, A.; Leitner, W. Selective Hydrogenation and Hydrodeoxygenation of Aromatic Ketones to Cyclohexane Derivatives Using a Rh@SILP Catalyst. Angew. Chem. Int. Ed. 2020, 59, 11977–11983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, M.S.; Alhanash, A.M.; Benaissa, M.; Alsalme, A.; Alharthi, F.A.; Al-Zaqri, N. Rhodium Nanoparticles Incorporated Mesoporous Silica as an Active Catalyst for Cyclohexene Hydrogenation under Ambient Conditions. Catalysts 2020, 10, 925. [Google Scholar] [CrossRef]
- Morfin, F.; Blondeau, L.; Provost, K.; Malouche, A.; Piccolo, L.; Zlotea, C. Absorbed hydrogen enhances the catalytic hydrogenation activity of Rh-based nanocatalysts. Catal. Sci. Technol. 2018, 8, 2707–2715. [Google Scholar] [CrossRef]
- Crespo, I.; Dominguez, O.; Baricelli, P.; Borusiak, M.; Omaña, O.; Castro, W.; Rosales, M. Biphasic hydrogenation of eugenol with ruthenium and rhodium nanoparticles stabilized in ionic liquids. J. Chil. Chem. Soc. 2020, 65, 4982–4987. [Google Scholar] [CrossRef]
- Borodziński, A.; Bonarowska, M. Relation between Crystallite Size and Dispersion on Supported Metal Catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Papp, S.; Patakfalvi, R.; Dékánya, I. Formation and Stabilization of Noble Metal Nanoparticles. Croat. Chem. Acta 2007, 80, 493–502. [Google Scholar]
- Zhang, H.; Xia, X.; Li, W.; Zeng, J.; Dai, Y.; Yang, D.; Xia, Y. Facile Synthesis of Five-fold Twinned, Starfish-like Rhodium Nanocrystals by Eliminating Oxidative Etching with a Chloride-Free Precursor. Angew. Chem. Int. Ed. 2010, 49, 5296–5300. [Google Scholar] [CrossRef]
- Gopiraman, M.; Saravanamoorthy, S.; Ullah, S.; Ilangovan, A.; Kim, I.S.; Chung, I.M. Reducing-agent-free facile preparation of Rh-nanoparticles uniformly anchored on onion-like fullerene for catalytic applications. RSC Adv. 2020, 10, 2545–2559. [Google Scholar] [CrossRef] [Green Version]
- Gopiraman, M.; Karvembu, R.; Kim, I.S. Highly Active, Selective, and Reusable RuO2/SWCNT Catalyst for Heck Olefination of Aryl Halides. ACS Catal. 2014, 4, 2118–2129. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonucci, L.; Mascitti, A.; Ferretti, A.M.; Coccia, F.; d’Alessandro, N. The Role of Nanoparticle Catalysis in the Nylon Production. Catalysts 2022, 12, 1206. https://doi.org/10.3390/catal12101206
Tonucci L, Mascitti A, Ferretti AM, Coccia F, d’Alessandro N. The Role of Nanoparticle Catalysis in the Nylon Production. Catalysts. 2022; 12(10):1206. https://doi.org/10.3390/catal12101206
Chicago/Turabian StyleTonucci, Lucia, Andrea Mascitti, Anna M. Ferretti, Francesca Coccia, and Nicola d’Alessandro. 2022. "The Role of Nanoparticle Catalysis in the Nylon Production" Catalysts 12, no. 10: 1206. https://doi.org/10.3390/catal12101206
APA StyleTonucci, L., Mascitti, A., Ferretti, A. M., Coccia, F., & d’Alessandro, N. (2022). The Role of Nanoparticle Catalysis in the Nylon Production. Catalysts, 12(10), 1206. https://doi.org/10.3390/catal12101206








