Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
2.2. Water Oxidation Studies
3. Experimental
3.1. Materials
3.2. Synthesis of Na10[Co4(H2O)2(PW9O34)2] (Co4POM)
3.3. Electrode Fabrication
3.4. Electrochemical Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen reduction electrocatalysts toward practical fuel cells: Progress and perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Tian, X.; Su, Y.-Q.; Cai, W.; Yan, Y.; Qi, R.; Douka, A.I.; Chen, S.; You, B.; Liu, H.; et al. Direct integration of ultralow-platinum alloy into nanocarbon architectures for efficient oxygen reduction in fuel cells. Sci. Bull. 2021, 66, 2207–2216. [Google Scholar] [CrossRef]
- Iftikhar, M.; Ali, B.; Nisar, T.; Wagner, V.; Haider, A.; Rehman, A.; Hussain, S.; Bahadar, A.; Saleem, M.; Abbas, S.M. Improving lithium-ion half/full cell performance of WO3 protected SnO2 core-shell nanoarchitectures. ChemSusChem 2021, 14, 917–928. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 5, 886–906. [Google Scholar] [CrossRef]
- Zaman, S.; Su, Y.Q.; Dong, C.L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.C.; Li, F.M.; You, B.; Guo, W.; et al. Scalable molten salt synthesis of platinum alloys planted in metal-nitrogen-graphene for efficient oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, 202115835–202115843. [Google Scholar] [CrossRef]
- Li, J.; Triana, C.A.; Wan, W.; Saseendran, D.A.; Zhao, Y.; Balaghi, S.E.; Heidari, S.; Patzke, G.R. Molecular and heterogeneous water oxidation catalysts: Recent progress and joint perspectives. Chem. Soc. Rev. 2021, 50, 2444–2485. [Google Scholar] [CrossRef]
- Ishaq, T.; Yousaf, M.; Bhatti, I.A.; Batool, A.; Asghar, M.A.; Mohsin, M.; Ahmad, M. A perspective on possible amendments in semiconductors for enhanced photocatalytic hydrogen generation by water splitting. Int. J. Hydrogen Energy 2021, 46, 39036–39057. [Google Scholar] [CrossRef]
- Dau, H.; Limberg, C.; Reir, T.; Risch, M.; Roggan, S.; Strasser, P. The mechanism of water oxidation: From electrolysis via homogeneous to biological catalysis. ChemCatChem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Favaro, M.; Yang, J.; Nappini, S.; Magnano, E.; Toma, F.M.; Crumlin, E.J.; Yano, J.; Sharp, I.D. Understanding the oxygen evolution reaction mechanism on CoOx using operando ambient-pressure X-ray photoelectron spectroscopy. J. Am. Chem. Soc. 2017, 139, 8960–8970. [Google Scholar] [CrossRef] [Green Version]
- Lyons, M.E.G.; Floquet, S. Mechanism of oxygen reactions at porous oxide electrodes. Part 2—Oxygen evolution at RuO2, IrO2 and IrxRu1− xO2 electrodes in aqueous acid and alkaline solution. Phys. Chem. Chem. Phys. 2011, 13, 5314–5335. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Q.; Li, K.; Zhang, S.; Ma, X. First-row transition metal oxide oxygen evolution electrocatalysts: Regulation strategies and mechanistic understandings. Sustain. Energy Fuels 2020, 4, 5417–5432. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the rational design of non-precious transition metal oxides for oxygen electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef] [Green Version]
- Mansoor, M.A.; Mazhar, M.; McKee, V.; Arifin, Z. Mn2O3–4TiO2 semiconducting composite thin films for photoelectrochemical water splitting. Polyhedron 2014, 75, 135–140. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ali, A.; Haider, A.; Zaheer, M.; Nisar, T.; Wagner, V.; Akhter, Z. Electrochemically deposited amorphous cobalt–nickel-doped copper oxide as an efficient electrocatalyst toward water oxidation reaction. ACS Omega 2021, 6, 19419–19426. [Google Scholar] [CrossRef] [PubMed]
- Nasir, J.A.; ur Rehman, Z.; Shah, S.N.A.; Khan, A.; Butler, I.S.; Catlow, C.R.A. Recent developments and perspectives in CdS-based photocatalysts for water splitting. J. Mater. Chem. A 2020, 8, 20752–20780. [Google Scholar] [CrossRef]
- Peng, L.; Shah, S.S.A.; Wei, Z. Recent developments in metal phosphide and sulfide electrocatalysts for oxygen evolution reaction Chin. J. Catal. 2018, 39, 1575–1593. [Google Scholar]
- Peng, X.; Yan, Y.; Jin, X.; Huang, C.; Jin, W.; Gao, B.; Chu, P.K. Recent advance and prospectives of electrocatalysts based on transition metal selenides for efficient water splitting. Nano Energy 2020, 78, 105234. [Google Scholar] [CrossRef]
- Hu, Y.; Mao, L.; Guan, X.; Tucker, K.A.; Xie, H.; Wu, X.; Shi, J. Layered perovskite oxides and their derivative nanosheets adopting different modification strategies towards better photocatalytic performance of water splitting. Renew. Sustain. Energy Rev. 2020, 119, 109527. [Google Scholar] [CrossRef]
- Gershinsky, Y.; Zitoun, D. Direct chemical synthesis of lithium sub-stochiometric olivine Li0.7Co0.75Fe0.25PO4 coated with reduced graphene oxide as oxygen evolution reaction electrocatalyst. ACS Catalysis. 2018, 8, 8715–8725. [Google Scholar] [CrossRef]
- Zhao, Z.; Lamoureux, P.S.; Kulkarni, A.; Bajdich, M. Trends in oxygen electrocatalysis of 3d-layered (oxy) (hydro) oxides. ChemCatChem 2019, 11, 3423–3431. [Google Scholar] [CrossRef]
- Iqbal, W.; Batool, M.; Hameed, A.; Abbas, S.; Nadeem, M.A. Boosting the activity of FeOOH via integration of ZIF-12 and graphene to efficiently catalyze the oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 25050–25059. [Google Scholar] [CrossRef]
- Gao, M.R.; Xu, Y.F.; Jiang, J.; Zheng, Y.R.; Yu, S.H. Water oxidation electrocatalyzed by an efficient Mn3O4/CoSe2 nanocomposite. J. Am. Chem. Soc. 2012, 134, 2930–2933. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.T. Heteropoly and Isopoly Oxometalates; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic−inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef]
- Sartorel, A.; Carraro, M.; Scorrano, G.; De Zorzi, R.; Geremia, S.; McDaniel, N.D.; Bernhard, S.; Bonchio, M. Polyoxometalate embedding of a tetraruthenium(IV)-oxo-core by template-directed metalation of [γ-SiW10O36]8−: A totally inorganic oxygen-evolving catalyst. J. Am. Chem. Soc. 2008, 130, 5006–5007. [Google Scholar] [CrossRef] [Green Version]
- Geletii, Y.V.; Botar, B.; Kögerler, P.; Hillesheim, D.A.; Musaev, D.G.; Hill, C.L. An all-inorganic, stable, and highly active tetraruthenium homogeneous catalyst for water oxidation. Angew. Chem. Int. Ed. 2008, 47, 3896–3899. [Google Scholar] [CrossRef]
- Lv, H.; Geletii, Y.V.; Zhao, C.; Vickers, J.W.; Zhu, G.; Luo, Z.; Song, J.; Lian, T.; Musaev, D.G.; Hill, C.L. Polyoxometalate water oxidation catalysts and the production of green fuel. Chem. Soc. Rev. 2012, 41, 7572–7589. [Google Scholar] [CrossRef]
- Irfan, U.; Munir, A.; Haider, A.; Ullah, N.; Hussain, I. Supported polyoxometalates as emerging nanohybrid materials for photochemical and photoelectrochemical water splitting. Nanophotonics 2021, 10, 1595–1620. [Google Scholar]
- Li, N.; Liu, J.; Dong, B.X.; Lan, Y.Q. Polyoxometalate-based compounds for photo- and electrocatalytic applications. Angew. Chem. Int. Ed. 2020, 59, 20779–20793. [Google Scholar] [CrossRef]
- Weakley, T.J.R.; Evans, H.T.; Showell, J.S.; Tourné, G.F.; Tourné, C.M. 18-Tungstotetracobalto(II)diphosphate and related anions: A novel structural class of heteropolyanions. J. Chem. Soc. Chem. Commun. 1973, 4, 139–140. [Google Scholar] [CrossRef]
- Yin, Q.; Tan, J.M.; Besson, C.; Geletii, Y.V.; Musaev, D.G.; Kuznetsov, A.E.; Luo, Z.; Hardcastle, K.I.; Hill, C.L. A Fast Soluble Carbon-Free Molecular Water Oxidation Catalyst Based on Abundant Metals. Science 2010, 328, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Luo, Z.; Geletii, Y.V.; Vickers, J.W.; Yin, Q.; Wu, D.; Hou, Y.; Ding, Y.; Song, J.; Musaev, D.G.; et al. Efficient light-driven carbon-free cobalt-based molecular catalyst for water oxidation. J. Am. Chem. Soc. 2011, 133, 2068–2071. [Google Scholar] [CrossRef] [PubMed]
- Stracke, J.J.; Finke, R.G. Electrocatalytic water oxidation beginning with the cobalt polyoxometalate [Co4(H2O)2(PW9O34)2]10-: Identification of heterogeneous CoOx as the dominant catalyst. J. Am. Chem. Soc. 2011, 133, 14872–14875. [Google Scholar] [CrossRef] [PubMed]
- Natali, M.; Berardi, S.; Sartorel, A.; Bonchio, M.; Campagna, S.; Scandola, F. Is [Co4(H2O)2(α-PW9O34)2]10− a Genuine molecular catalyst in photochemical water oxidation? Answers from time-resolved hole scavenging experiments Chem. Commun. 2012, 48, 8808–8810. [Google Scholar]
- Zhu, G.; Geletii, Y.V.; Kögerler, P.; Schilder, H.; Song, J.; Lense, S.; Zhao, C.; Hardcastle, K.I.; Musaev, D.G.; Hill, C.L. Water oxidation catalyzed by a new tetracobalt-substituted polyoxometalate complex: [{Co4(μ-OH)(H2O)3}(Si2W19O70)]11−. Dalton Trans. 2012, 41, 2084–2090. [Google Scholar] [CrossRef] [Green Version]
- Vickers, J.W.; Lv, H.; Sumliner, J.M.; Zhu, G.; Luo, Z.; Musaev, D.G.; Geletii, Y.V.; Hill, C.L. Differentiating homogeneous and heterogeneous water oxidation catalysis: Confirmation that [Co4(H2O)2(α-PW9O34)2]10– is a molecular water oxidation catalyst. J. Am. Chem. Soc. 2013, 135, 14110–14118. [Google Scholar] [CrossRef]
- Stracke, J.J.; Finke, R.G. Water Oxidation Catalysis Beginning with Co4(H2O)2(PW9O34)210– When driven by the chemical oxidant ruthenium(III)tris(2,2′-bipyridine): Stoichiometry, kinetic, and mechanistic studies en route to identifying the true catalyst. ACS Catal. 2014, 4, 79–89. [Google Scholar] [CrossRef]
- Stracke, J.J.; Finke, R.G. Water oxidation catalysis beginning with 2.5 μM [Co4(H2O)2(PW9O34)2]10–: Investigation of the true electrochemically driven catalyst at ≥600 mV overpotential at a glassy carbon electrode. ACS Catal. 2013, 3, 1209–1219. [Google Scholar] [CrossRef]
- Schiwon, R.; Klingan, K.; Dau, H.; Limberg, C. Shining light on integrity of a tetracobalt-polyoxometalate water oxidation catalyst by X-ray spectroscopy before and after catalysis. Chem. Commun. 2014, 50, 100–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, H.; Song, J.; Geletii, Y.V.; Vickers, J.W.; Sumliner, J.M.; Musaev, D.G.; Kögerler, P.; Zhuk, P.F.; Bacsa, J.; Zhu, G.; et al. An exceptionally fast homogeneous carbon-free cobalt-based water oxidation catalyst. J. Am. Chem. Soc. 2014, 136, 9268–9271. [Google Scholar] [CrossRef]
- Goberna-Ferrón, S.; Soriano-López, J.; Galán-Mascarós, J.R.; Nyman, M. Solution speciation and stability of Cobalt-polyoxometalate water oxidation catalysts by X-ray scattering. Eur. J. Inorg. Chem. 2015, 2015, 2833–2840. [Google Scholar] [CrossRef]
- Soriano-López, J.; Musaev, D.G.; Hill, C.L.; Galán-Mascarós, J.R.; Carbó, J.J.; Poblet, J.M. Tetracobalt-polyoxometalate catalysts for water oxidation: Key mechanistic details. J. Catal. 2017, 350, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Folkman, S.J.; Soriano-Lopez, J.; Galán-Mascarós, J.R.; Finke, R.G. Electrochemically driven water-oxidation catalysis Beginning with six exemplary cobalt polyoxometalates: Is it molecular, homogeneous catalysis or electrode-bound, heterogeneous CoOx catalysis. J. Am. Chem. Soc. 2018, 140, 12040–12055. [Google Scholar] [CrossRef] [Green Version]
- Arens, J.T.; Blasco-Ahicart, M.; Azmani, K.; Soriano-López, J.; García-Eguizábal, A.; Poblet, J.M.; Galán-Mascarós, J.R. Water oxidation electrocatalysis in acidic media with Co-containing polyoxometalates. J. Catal. 2020, 389, 345–351. [Google Scholar] [CrossRef]
- Gong, R.; Gao, D.; Liu, R.; Sorsche, D.; Biskupek, J.; Kaiser, U.; Rau, S.; Streb, C. Self-activation of a polyoxometalate-derived composite electrocatalyst for the oxygen evolution reaction. ACS Appl. Energy Mater. 2021, 4, 12671–12676. [Google Scholar]
- Azmani, K.; Besora, M.; Soriano-López, J.; Landolsi, M.; Teillout, A.L.; de Oliveira, P.; Mbomekall’e, I.M.; Poblet, J.M.; Galán-Mascarós, J.R. Understanding polyoxometalates as water oxidation catalysts through iron vs. cobalt reactivity. Chem. Sci. 2021, 12, 8755–8766. [Google Scholar] [CrossRef]
- Ahmed, T.; Asghar, M.A.; Ali, A.; Akhter, Z.; Ali, S.; Ullah, I.; Nisar, T.; Wagner, V.; Touseef, S.; Hussain, A.; et al. High-nuclearity cobalt(II)-containing polyoxometalate anchored on nickel foam as electrocatalyst for electrochemical water oxidation studies. J. Alloys Compd. 2022, 909, 164709. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, W.Z.; Wang, D.Z.; Ren, Z.F.; Chou, T.W. Carbon nanotube/carbon fiber hybrid multiscale composites. J. Appl. Phys. 2002, 91, 6034–6037. [Google Scholar] [CrossRef]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Ali, A.; Shah, S.M.; Bozar, S.; Kazici, M.; Keskin, B.; Kaleli, M.; Akyürekli, S.; Günes, S. Metal-free polymer/MWCNT composite fiber as an efficient counter electrode in fiber shape dye-sensitized solar cells. Nanotechnology 2016, 27, 384003. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Akyuz, D.; Asghar, M.A.; Koca, A.; Keskin, B. Free-standing carbon nanotubes as non-metal electrocatalyst for oxygen evolution reaction in water splitting. Int. J. Hydrogen Energy 2018, 43, 1123–1128. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical properties of carbon nanotube based fibers and their future use in electrical wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Chhetri, K.; Muthurasu, A.; Dahal, B.; Kim, T.; Mukhiya, T.; Chae, S.-H.; Ko, T.H.; Choi, Y.C.; Kim, H.Y. Engineering the abundant heterointerfaces of integrated bimetallic sulfide-coupled 2D MOF-derived mesoporous CoS2 nanoarray hybrids for electrocatalytic water splitting. Mater. Today Nano 2022, 17, 100146. [Google Scholar] [CrossRef]
- Kandel, M.R.; Pan, U.N.; Paudel, D.R.; Dhakal, P.P.; Kim, N.H.; Lee, J.H. Hybridized bimetallic phosphides of Ni-Mo, Co-Mo, and Co-Ni in a single ultrathin-3D-nanosheets for efficient HER and OER in alkaline media. Compos. B Eng. 2022, 239, 109992. [Google Scholar] [CrossRef]
- Suen, N.T.; Hung, S.F.; Quan, Q.; Zhang, N.; Xu, Y.J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Design of electrocatalysts for oxygen-and hydrogen-involving energy conversion reactions. Chem. Soc. Rev. 2015, 44, 2060–2086. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Wang, E.; Mao, B.; Su, Z.; Gao, L.; Lian, S.; Xu, L. Controllable fabrication of carbon nanotube and nanobelt with a polyoxometalate-assisted mild hydrothermal process. J. Am. Chem. Soc. 2005, 127, 6534–6535. [Google Scholar] [CrossRef] [PubMed]
- Grewe, T.; Deng, X.; Tüysüz, X. Influence of Fe doping on structure and water oxidation activity of nanocast Co3O4. Chem. Mater. 2014, 26, 3162–3168. [Google Scholar] [CrossRef]
- Sivanantham, A.; Ganesan, P.; Shanmugam, S. Hierarchical NiCo2S4 nanowire arrays supported on Ni foam: An efficient and durable bifunctional electrocatalyst for oxygen and hydrogen evolution reactions. Adv. Funct. Mater. 2016, 26, 4661–4672. [Google Scholar] [CrossRef]
- Luo, W.; Hu, J.; Diao, H.; Schwarz, B.; Streb, C.; Song, Y.F. Robust polyoxometalate/nickel foam composite electrodes for sustained electrochemical oxygen evolution at high pH. Angew. Chem. Int. Ed. 2017, 56, 4941–4944. [Google Scholar] [CrossRef] [PubMed]
- Tantraviwat, D.; Anuchai, S.; Ounnunkad, K.; Saipanya, S.; Aroonyadet, N.; Rujijanagul, G.; Inceesungvorn, B. Structural properties of tungsten-doped cobalt molybdate and its application in electrochemical oxygen evolution reaction. J. Mater. Sci. Mater. Electron. 2018, 29, 13103–13111. [Google Scholar] [CrossRef]
- Zhang, L.; Ding, X.; Cong, M.; Wang, Y.; Zhang, X. Self-adaptive amorphous Co2P@Co2P/Co-polyoxometalate/nickel foam as an effective electrode for electrocatalytic water splitting in alkaline electrolyte. Int. J. Hydrog. Energy 2019, 44, 9203–9209. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.S.; Pang, H. Core–shell-type ZIF-8@ ZIF-67@ POM hybrids as efficient electrocatalysts for the oxygen evolution reaction. Inorg. Chem. Front. 2019, 6, 2514–2520. [Google Scholar] [CrossRef]
- Li, Q.Y.; Zhang, L.; Xu, Y.X.; Li, Q.; Xue, H.; Pang, H. Smart yolk/shell ZIF-67@ POM hybrids as efficient electrocatalysts for the oxygen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 5027–5033. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhang, L.; Liu, C.S.; Pang, H. PBA@ POM hybrids as efficient electrocatalysts for the oxygen evolution reaction. Chem.: Asian J. 2019, 14, 2790–2795. [Google Scholar]
- Park, K.R.; Jeon, J.E.; Ali, G.; Ko, Y.H.; Lee, J.; Han, H.; Mhin, S. Oxygen evolution reaction of Co-Mn-O electrocatalyst prepared by solution combustion synthesis. Catalysts 2019, 9, 564. [Google Scholar] [CrossRef]



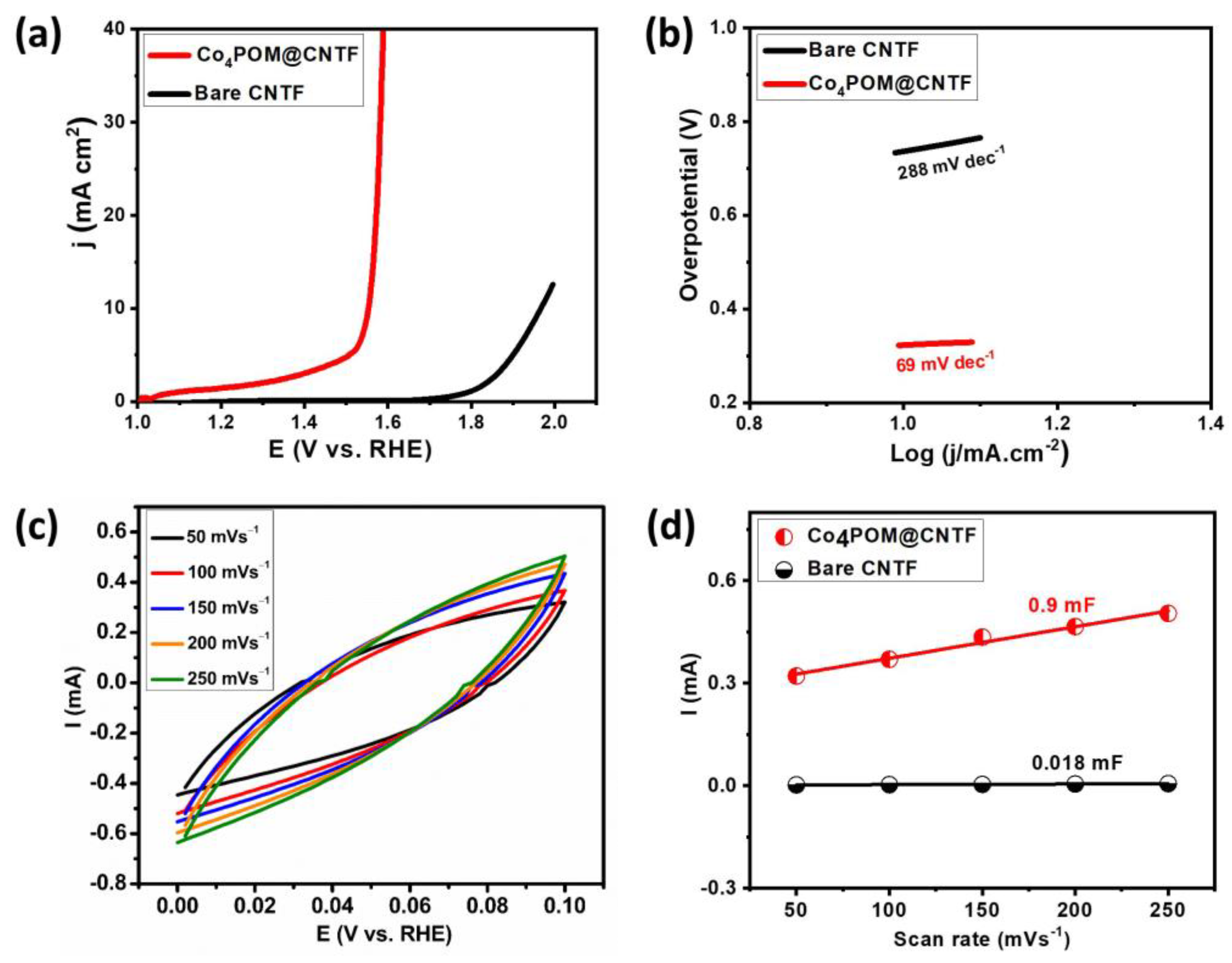


| Electrocatalysts | η at 10 mA/cm2 (mV) | Tafel Slope (mV dec−1) | Cdl (mF) | ECSA (cm2) | Rf |
|---|---|---|---|---|---|
| Bare CNTF | 737 | 288 | 0.018 | 0.45 | 8 |
| Co4POM@CNTF | 323 | 69 | 0.9 | 22.5 | 402 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tariq, I.; Asghar, M.A.; Ali, A.; Badshah, A.; Abbas, S.M.; Iqbal, W.; Zubair, M.; Haider, A.; Zaman, S. Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction. Catalysts 2022, 12, 1242. https://doi.org/10.3390/catal12101242
Tariq I, Asghar MA, Ali A, Badshah A, Abbas SM, Iqbal W, Zubair M, Haider A, Zaman S. Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction. Catalysts. 2022; 12(10):1242. https://doi.org/10.3390/catal12101242
Chicago/Turabian StyleTariq, Irsa, Muhammad Adeel Asghar, Abid Ali, Amin Badshah, Syed Mustansar Abbas, Waheed Iqbal, Muhammad Zubair, Ali Haider, and Shahid Zaman. 2022. "Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction" Catalysts 12, no. 10: 1242. https://doi.org/10.3390/catal12101242
APA StyleTariq, I., Asghar, M. A., Ali, A., Badshah, A., Abbas, S. M., Iqbal, W., Zubair, M., Haider, A., & Zaman, S. (2022). Surface Reconstruction of Cobalt-Based Polyoxometalate and CNT Fiber Composite for Efficient Oxygen Evolution Reaction. Catalysts, 12(10), 1242. https://doi.org/10.3390/catal12101242