Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin
Abstract
1. Introduction
2. Results
2.1. Spectroscopic Assessment
2.2. Morphology Assessment
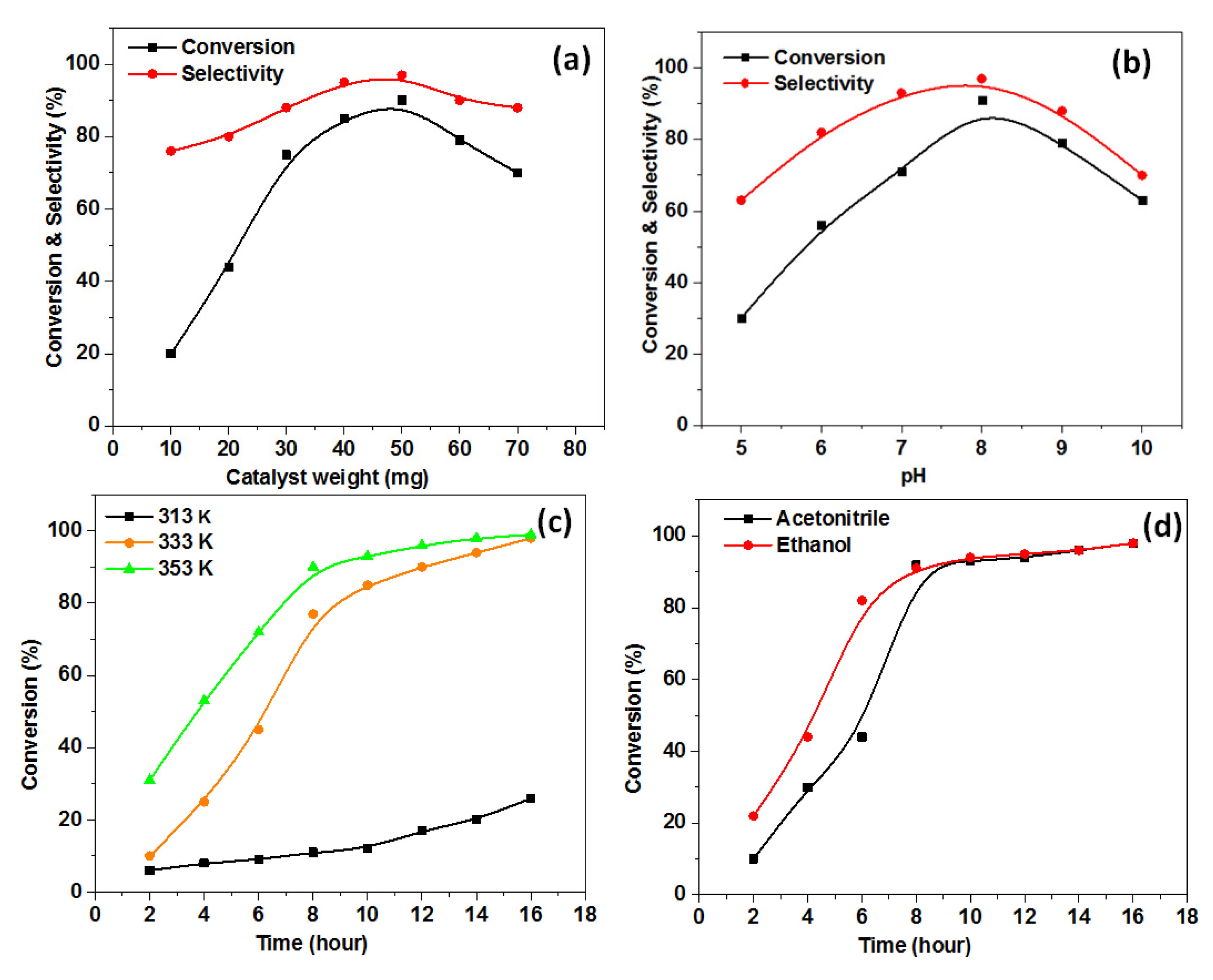
2.3. Catalytic Performance
2.3.1. Impact of Catalyst Dose
2.3.2. Impact of pH
2.3.3. Impact of the Epoxidation Temperature
2.3.4. Impact of the Solvents
2.3.5. The Scope for Epoxidation Reaction
2.3.6. Recycling Process
2.3.7. Comparison Assessment
3. Materials and Methods
3.1. Chemicals
3.2. Fabrication of Carbon Spheres (CSs)
3.3. Synthesis of Ni@CS Composite
3.4. Characterization
3.5. Epoxidation Performance
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Madubuonu, N.; Aisida, S.O.; Ali, A.; Ahmad, I.; Zhao, T.-K.; Botha, S.; Maaza, M.; Ezema, F.I. Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol. B Biol. 2019, 199, 111601. [Google Scholar] [CrossRef] [PubMed]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.-K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Berseth, P.A.; Harter, A.G.; Zidan, R.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R.; Jena, P. Carbon Nanomaterials as Catalysts for Hydrogen Uptake and Release in NaAlH4. Nano Lett. 2009, 9, 1501–1505. [Google Scholar] [CrossRef] [PubMed]
- Baharudin, L.; Yip, A.; Golovko, V.; Watson, M.J. Potential of metal monoliths with grown carbon nanomaterials as catalyst support in intensified steam reformer: A perspective. Rev. Chem. Eng. 2018, 36, 459–491. [Google Scholar] [CrossRef]
- Testa, C.; Zammataro, A.; Pappalardo, A.; Sfrazzetto, G.T. Catalysis with carbon nanoparticles. RSC Adv. 2019, 9, 27659–27664. [Google Scholar] [CrossRef]
- González-González, R.B.; Rodríguez-Hernández, J.A.; Araújo, R.G.; Sharma, P.; Parra-Saldívar, R.; Ramirez-Mendoza, R.A.; Bilal, M.; Iqbal, H.M. Prospecting carbon-based nanomaterials for the treatment and degradation of endocrine-disrupting pollutants. Chemosphere 2022, 297, 134172. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.; Li, H.; Huang, P.; Li, Q.; Wang, Y. Carbon-based and carbon-supported nanomaterials for the catalytic conversion of biomass: A review. Environ. Chem. Lett. 2022, 20, 1719–1744. [Google Scholar] [CrossRef]
- Chattopadhyay, J.; Pathak, T.S.; Pak, D. Heteroatom-Doped Metal-Free Carbon Nanomaterials as Potential Electrocatalysts. Molecules 2022, 27, 670. [Google Scholar] [CrossRef]
- Behera, P.; Subudhi, S.; Tripathy, S.P.; Parida, K. MOF derived nano-materials: A recent progress in strategic fabrication, characterization and mechanistic insight towards divergent photocatalytic applications. Co-ord. Chem. Rev. 2022, 456, 214392. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Lajgut, G.G.; Kocsis, T.; Kótai, L.; Kauthale, S.; Tekale, S.; Pawar, R. Copper nanoparticles grafted on carbon microspheres as novel heterogeneous catalysts and their application for the reduction of nitrophenol and one-pot multicomponent synthesis of hexahydroquinolines. New J. Chem. 2018, 42, 1092–1098. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Modarres, M. Clicked graphene oxide as new support for the immobilization of peroxophosphotungstate: Efficient catalysts for the epoxidation of olefins. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 886–892. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Mirshekar, S. Covalent functionalization of graphene oxide with molybdenum-carboxylate complexes: New reusable catalysts for the epoxidation of olefins. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 387–392. [Google Scholar] [CrossRef]
- Weerakkody, C.; Biswas, S.; Song, W.; He, J.; Wasalathanthri, N.; Dissanayake, S.; Kriz, D.A.; Dutta, B.; Suib, S.L. Controllable synthesis of mesoporous cobalt oxide for peroxide free catalytic epoxidation of alkenes under aerobic conditions. Appl. Catal. B Environ. 2018, 221, 681–690. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Wang, F.; Wei, W.; Sun, Y. Preparation of immobilized chromium Schiff base complexes and their catalytic performance for cyclohexene epoxidation. Chin. J. Catal. 2011, 32, 1812–1821. [Google Scholar] [CrossRef]
- Nasseri, M.A.; Allahresani, A.; Raissi, H. Mild oxidation of alkenes catalyzed by Fe3O4/SiO2 nanoparticles. React. Kinet. Mech. Catal. 2014, 112, 397–408. [Google Scholar] [CrossRef]
- Ghiami, S.; Nasseri, M.A.; Allahresani, A.; Kazemnejadi, M. FeNi3@SiO2 nanoparticles: An efficient and selective heterogeneous catalyst for the epoxidation of olefins and the doxidation of sulfides in the presence of meta-chloroperoxybenzoic acid at room temperature. React. Kinet. Mech. Catal. 2019, 126, 383–398. [Google Scholar] [CrossRef]
- Samanta, S.; Laha, S.; Mal, N.; Bhaumik, A. Co(III)-containing mesoporous silica as an efficient catalyst in selective dihydroxylation of cyclohexene. J. Mol. Catal. A Chem. 2004, 222, 235–241. [Google Scholar] [CrossRef]
- Samanta, S.; Mal, N.; Bhaumik, A. Mesoporous Cr-MCM-41: An efficient catalyst for selective oxidation of cycloalkanes. J. Mol. Catal. A Chem. 2005, 236, 7–11. [Google Scholar] [CrossRef]
- Held, A.; Kowalska-Kuś, J.; Nowińska, K.; Góra-Marek, K. MCF material as an attractive support for vanadium oxide applied as a catalyst for propene epoxidation with N2O. Catal. Lett. 2018, 148, 2058–2068. [Google Scholar] [CrossRef]
- De Vos, D.E.; de Wildeman, S.; Sels, B.F.; Grobet, P.J.; Jacobs, P.A. Selective Alkene Oxidation with H2O2 and a Heterogenized Mn Catalyst: Epoxidation and a New Entry to Vicinal cis-Diols. Angew. Chem. Int. Ed. 1999, 38, 980–983. [Google Scholar] [CrossRef]
- Hosoya, N.; Hatayama, A.; Irie, R.; Sasaki, H.; Katsuki, T. Rational design of Mn-Salen epoxidation catalysts: Preliminary results. Tetrahedron 1994, 50, 4311–4322. [Google Scholar] [CrossRef]
- Mohamed, S.K.; Abuelhamd, M.; Allam, N.K.; Shahat, A.; Ramadan, M.; Hassan, H.M. Eco-friendly facile synthesis of glucose–derived microporous carbon spheres electrodes with enhanced performance for water capacitive deionization. Desalination 2020, 477, 114278. [Google Scholar] [CrossRef]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.-W.; Yin, L.-C.; Li, N.; Li, F.; Cheng, H.-M. Oxygen Bridges between NiO Nanosheets and Graphene for Improvement of Lithium Storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef]
- Peng, C.; Lu, X.-H.; Ma, X.-T.; Shen, Y.; Wei, C.-C.; He, J.; Zhou, D.; Xia, Q.-H. Highly efficient epoxidation of cyclohexene with aqueous H2O2 over powdered anion-resin supported solid catalysts. J. Mol. Catal. A Chem. 2016, 423, 393–399. [Google Scholar] [CrossRef]
- Zhan, W.; Guo, Y.; Wang, Y.; Liu, X.; Guo, Y.; Wang, Y.; Zhang, Z.; Lu, G. Synthesis of lanthanum-doped MCM-48 molecular sieves and its catalytic performance for the oxidation of styrene. J. Phys. Chem. B 2007, 111, 12103–12110. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Jacobs, P.A. A high-throughput experimentation study of the epoxidation of alkenes with transition-metal-free heterogeneous catalysts. Catal. Today 2008, 137, 52–60. [Google Scholar] [CrossRef]
- Valand, J.; Parekh, H.; Friedrich, H.B. Mixed Cu–Ni–Co nano-metal oxides: A new class of catalysts for styrene oxidation. Catal. Comm. 2013, 40, 149–153. [Google Scholar] [CrossRef]
- Sinclair, P.E.; Catlow, C.R.A. Quantum Chemical Study of the Mechanism of Partial Oxidation Reactivity in Titanosilicate Catalysts: Active Site Formation, Oxygen Transfer, and Catalyst Deactivation. J. Phys. Chem. B 1999, 103, 1084–1095. [Google Scholar] [CrossRef]
- Hassan, H.; Betiha, M.A.; El-Sharkawy, E.; Elshaarawy, R.F.; El-Assy, N.; Essawy, A.A.; Tolba, A.; Rabie, A.M. Highly selective epoxidation of olefins using vanadium (IV) schiff base- amine-tagged graphene oxide composite. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 591, 124520. [Google Scholar] [CrossRef]
- Moghe, K.; Sutar, A.K.; Kang, I.K.; Gupta, K.C. Poly(vinylbenzyl chloride-co-divinyl benzene) polyHIPE monolith-supported o-hydroxynaphthaldehyde propylenediamine Schiff base ligand complex of copper(ii) ions as a catalyst for the epoxidation of cyclohexene. RSC Adv. 2019, 9, 30823–30834. [Google Scholar] [CrossRef] [PubMed]
- Sreethawong, T.; Yamada, Y.; Kobayashi, T.; Yoshikawa, S. Catalysis of nanocrystalline mesoporous TiO2 on cyclohexene epoxidation with H2O2: Effects of mesoporosity and metal oxide additives. J. Mol. Catal. A Chem. 2005, 241, 23–32. [Google Scholar] [CrossRef]
- Jhung, S.H.; Lee, J.-H.; Cheetham, A.K.; Férey, G.; Chang, J.-S. A shape-selective catalyst for epoxidation of cyclic olefins: The nanoporous nickel phosphate VSB-5. J. Catal. 2006, 239, 97–104. [Google Scholar] [CrossRef]
- Ebadi, A.; Mozaffari, M.; Shojaei, S. Aerobic oxidation of cyclohexene catalyzed by NiO/MCM-41 nanocomposites in the gas phase. J. Chem. Sci. 2014, 126, 989–996. [Google Scholar] [CrossRef]
- Hassan, H.M.; Saad, E.M.; Soltan, M.S.; Betiha, M.A.; Butler, I.S.; Mostafa, S.I. A palladium(II) 4-hydroxysalicylidene Schiff-base complex anchored on functionalized MCM-41: An efficient heterogeneous catalyst for the epoxidation of olefins. Appl. Catal. A Gen. 2014, 488, 148–159. [Google Scholar] [CrossRef]
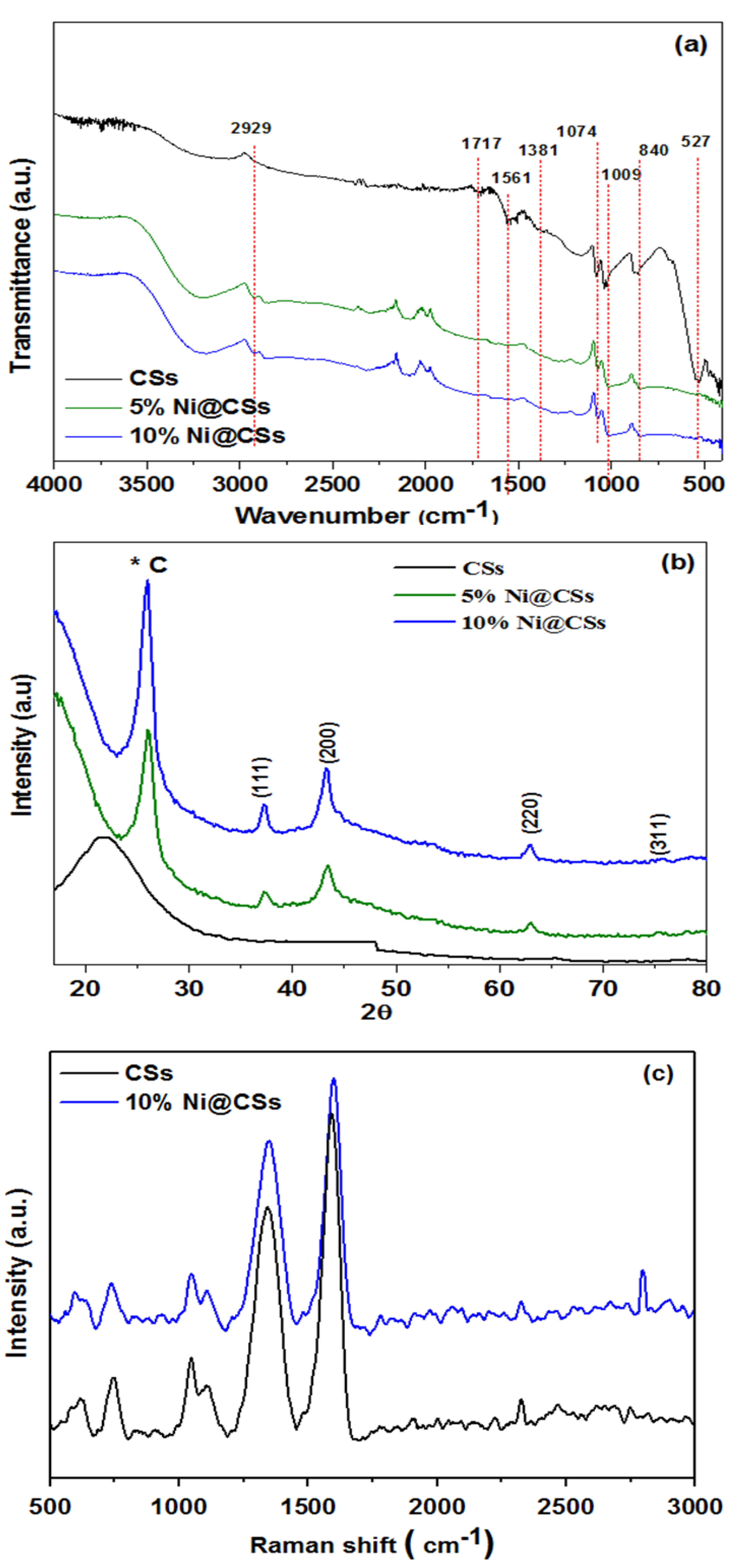




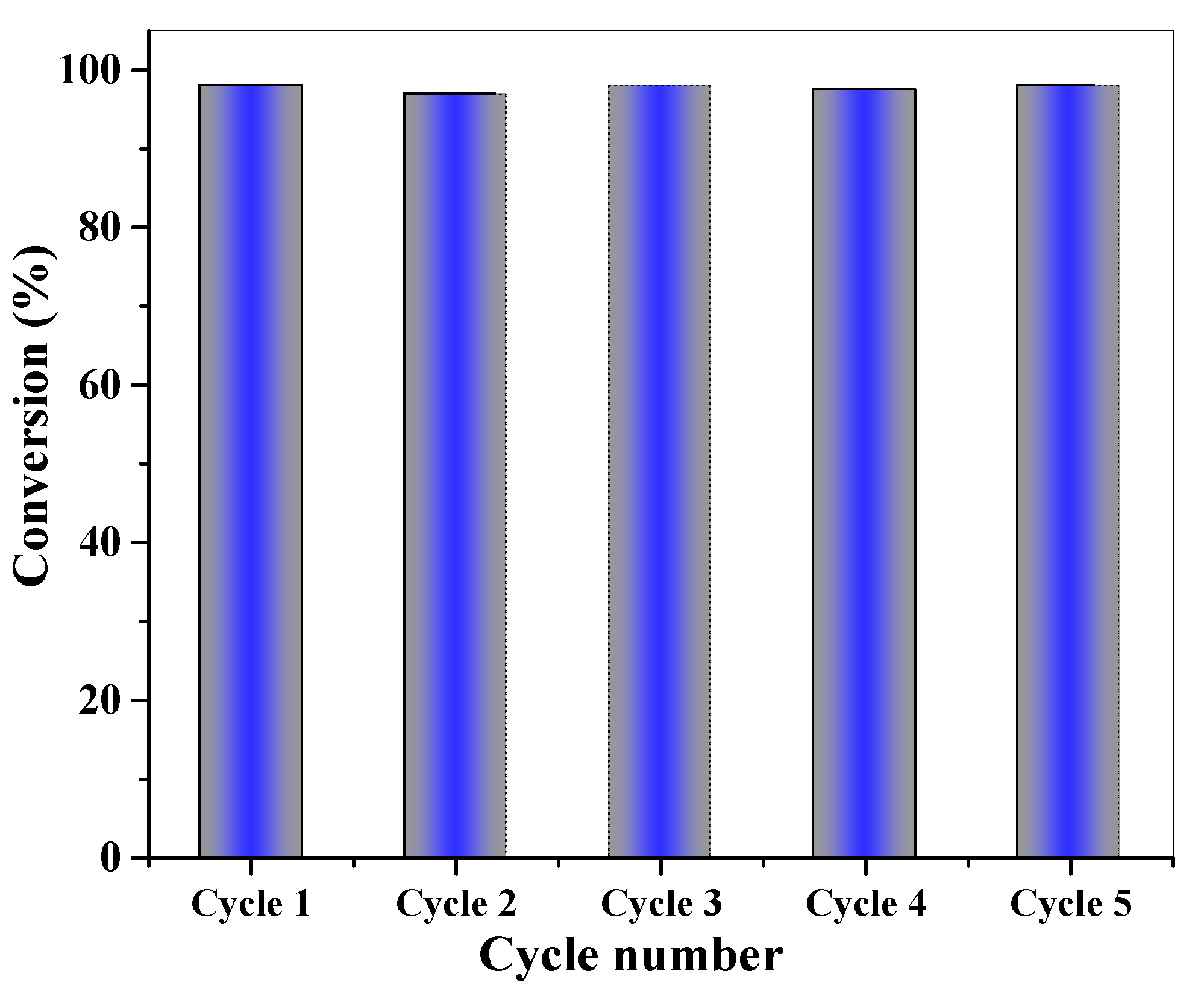
| Sample | C1s a | O1s a | Ni2p a | Actual at% Ni | Found at% Ni b |
|---|---|---|---|---|---|
| CSs | 283.3 (86.1) | 530.8 (13.64) | - | - | - |
| 5% Ni@CSs | 284.1 (55.1) | 531.2 (6.50) | 856 (0.9) | 5 | 4.93 |
| 10% Ni@CSs | 284.2 (84.1) | 531.9 (14.40) | 856 (1.5) | 10 | 9.20 |
| Olefin | Time (h) | Product | Conversion (%) | Selectivity (%) |
|---|---|---|---|---|
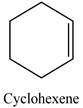 | 14 |  | 98 | 55 |
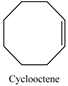 | 14 |  | 99 | 93 |
 | 14 | 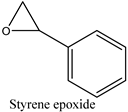 | 89 | 80 |
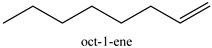 | 14 |  | 93 | 80 |
 | 14 |  | 60 | 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhumaimess, M.S. Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin. Catalysts 2022, 12, 1246. https://doi.org/10.3390/catal12101246
Alhumaimess MS. Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin. Catalysts. 2022; 12(10):1246. https://doi.org/10.3390/catal12101246
Chicago/Turabian StyleAlhumaimess, Mosaed S. 2022. "Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin" Catalysts 12, no. 10: 1246. https://doi.org/10.3390/catal12101246
APA StyleAlhumaimess, M. S. (2022). Nickel Nanoparticles Decorated on Glucose-Derived Carbon Spheres as a Novel, Non-Palladium Catalyst for Epoxidation of Olefin. Catalysts, 12(10), 1246. https://doi.org/10.3390/catal12101246








