Acid-Modified Sepiolite-Supported Pt (Noble Metal) Catalysts for HCHO Oxidation at Ambient Temperature
Abstract
:1. Introduction
2. Results and Discussion
2.1. Textural Properties of Modified Sepiolite
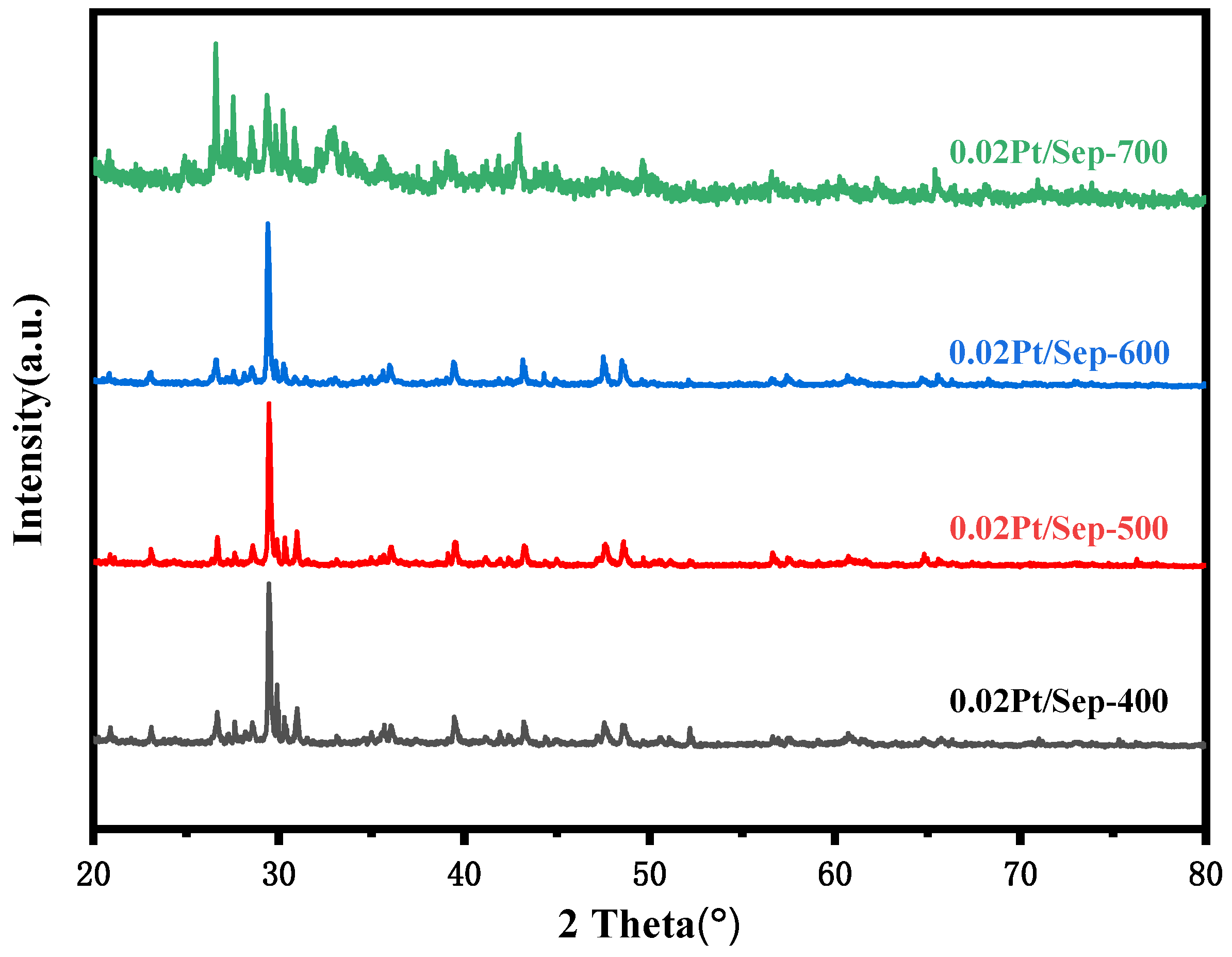
2.2. The Influence of Calcination Temperature on Material Structure
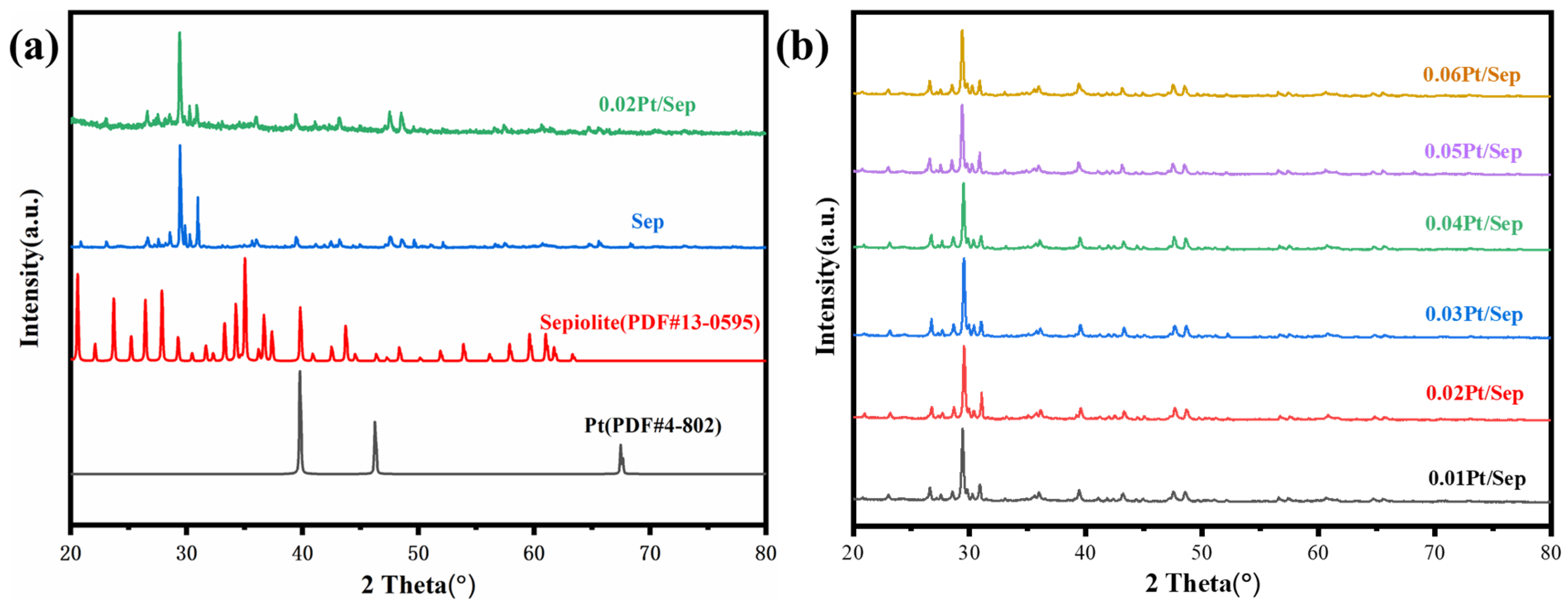
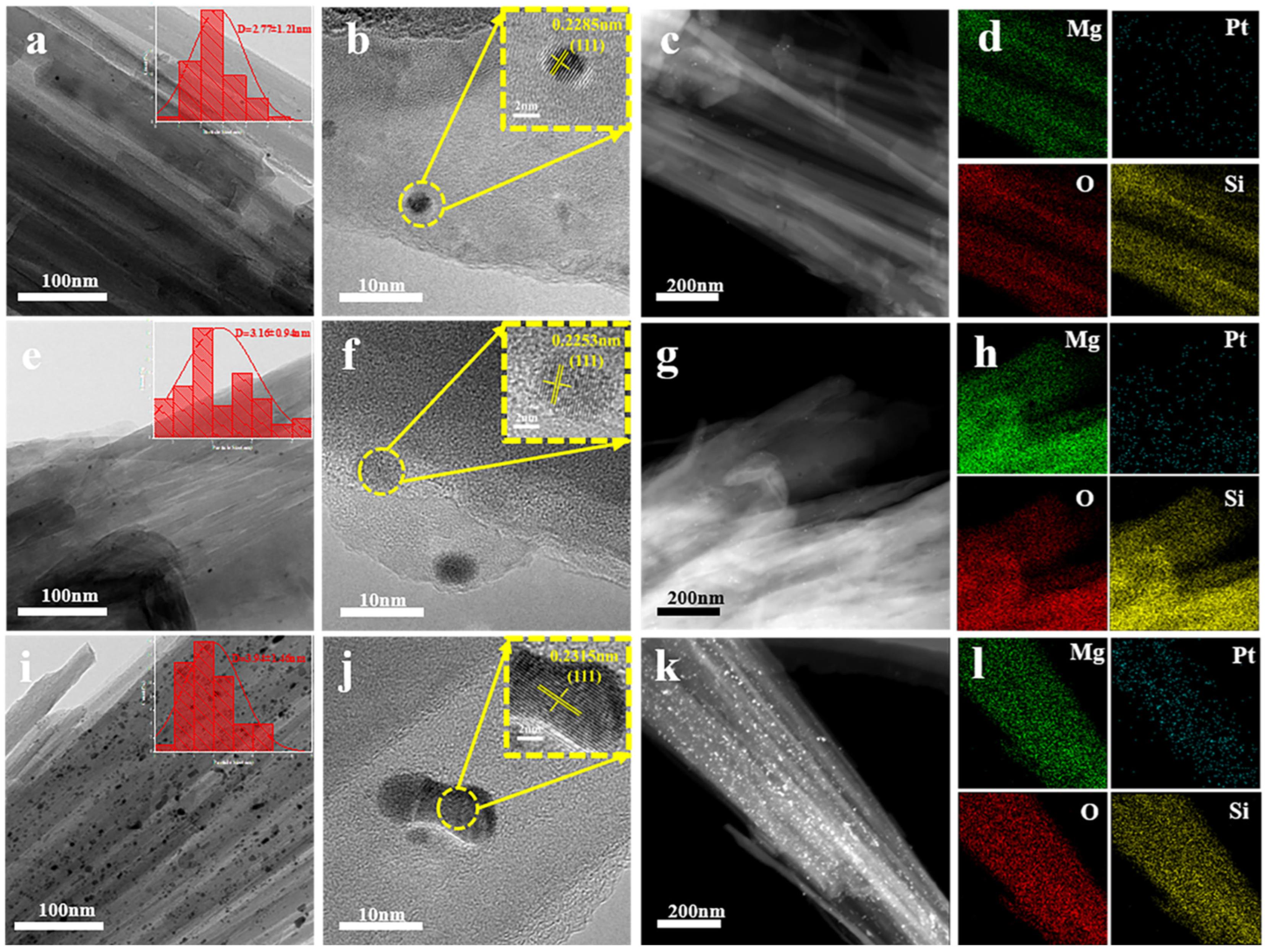
2.3. The Influence of Pt Loading on Structure and Morphology
2.4. HCHO Catalytic Oxidation
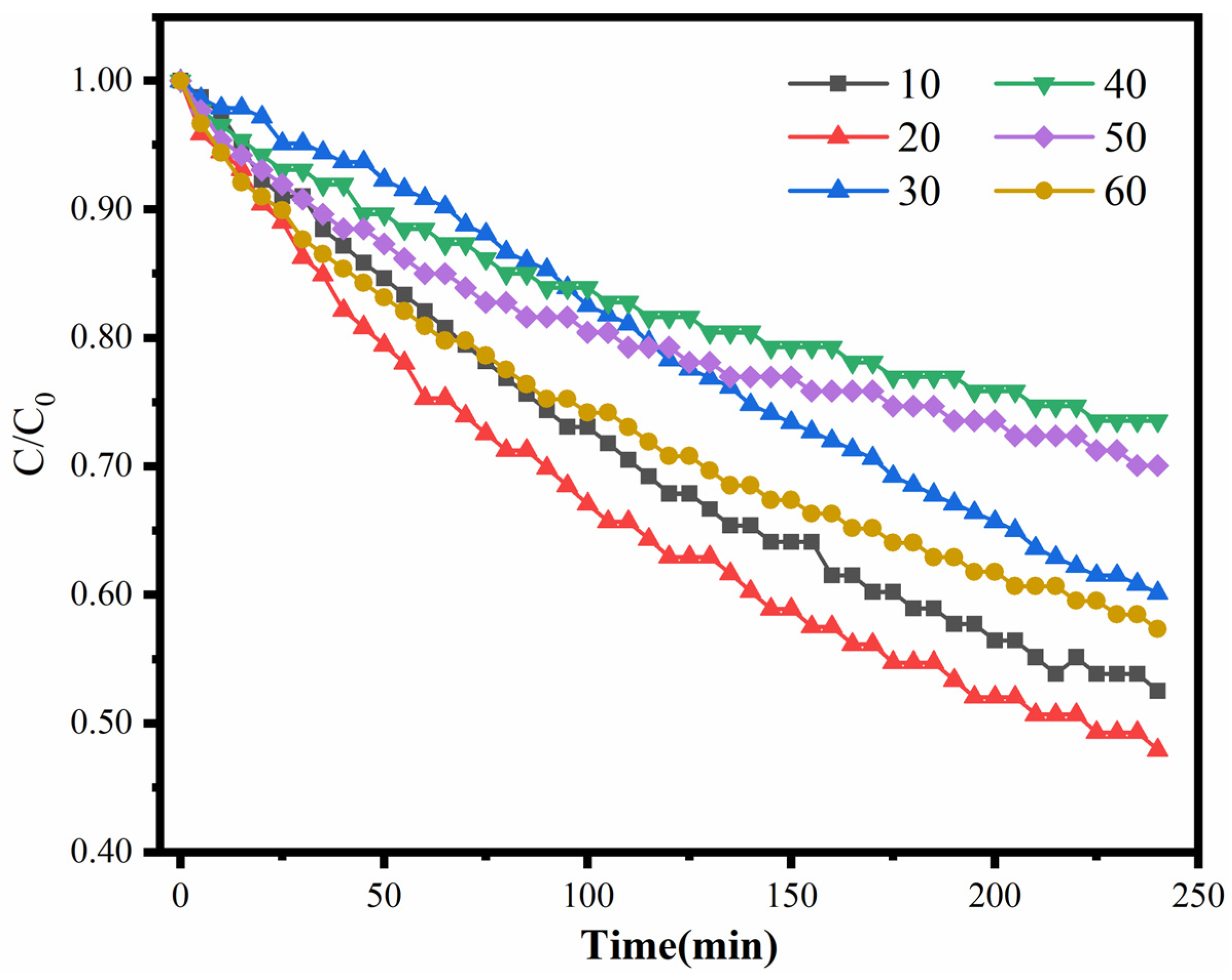
2.4.1. Effect of Pt Loading
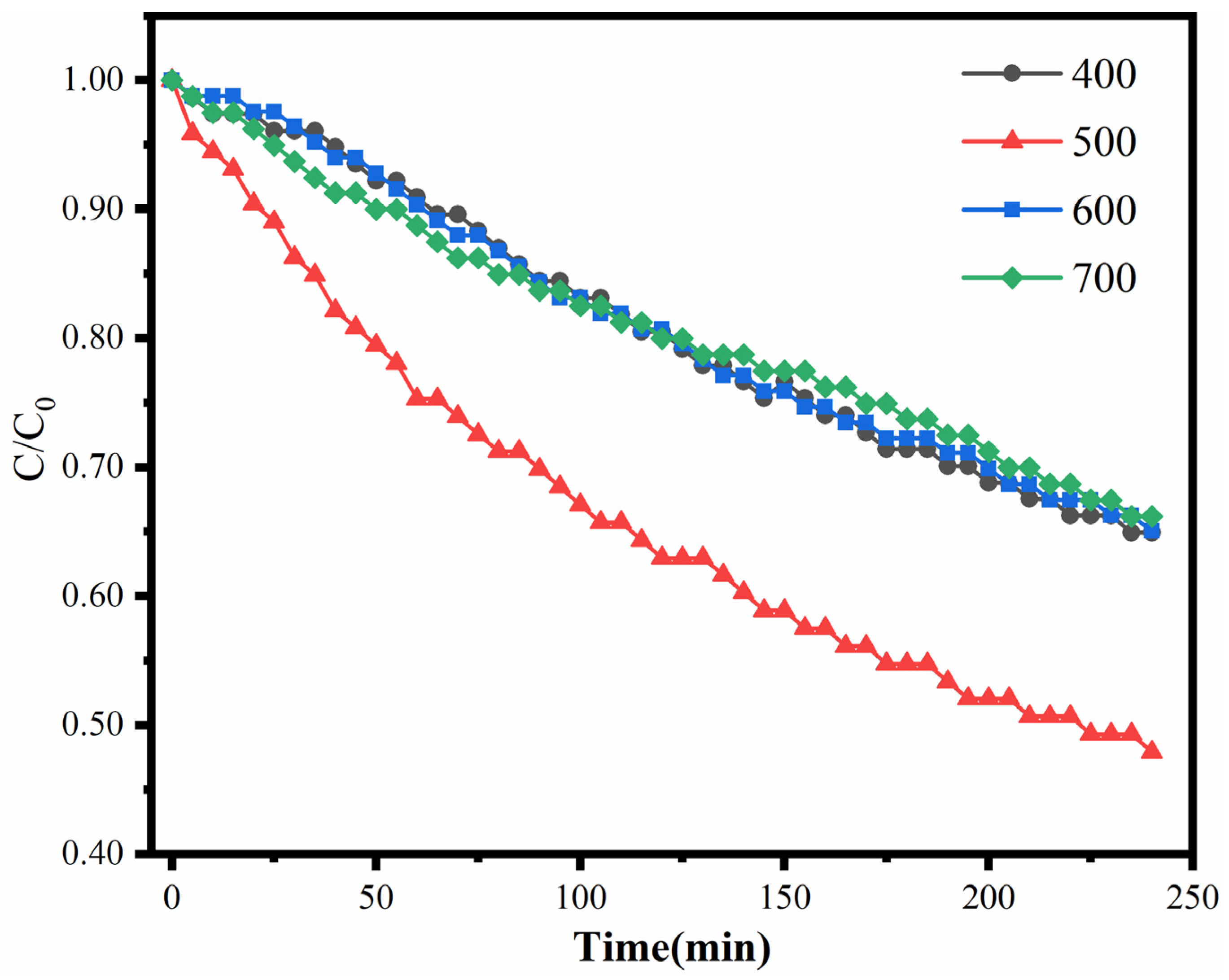
2.4.2. Effect of Calcination Temperature
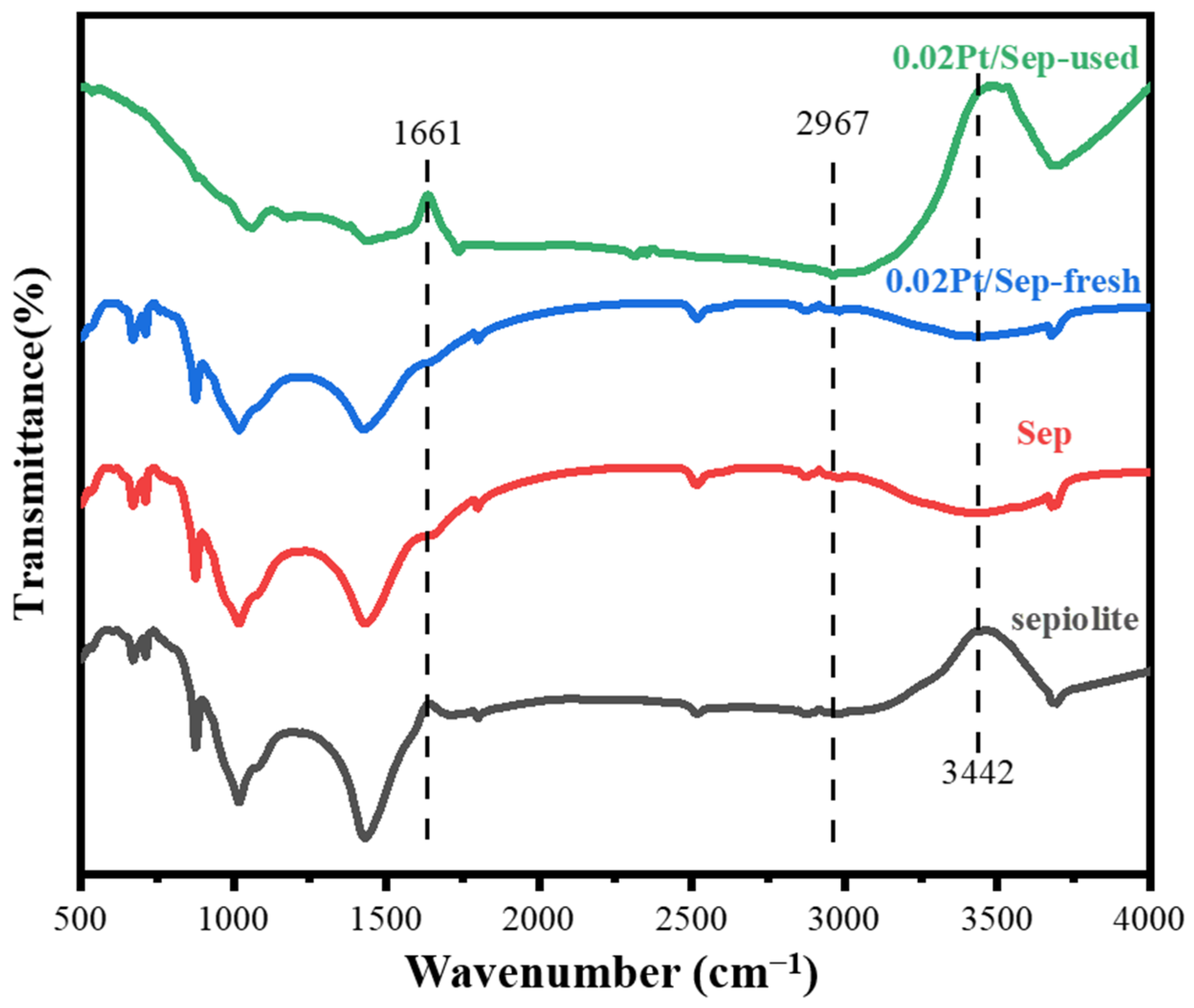
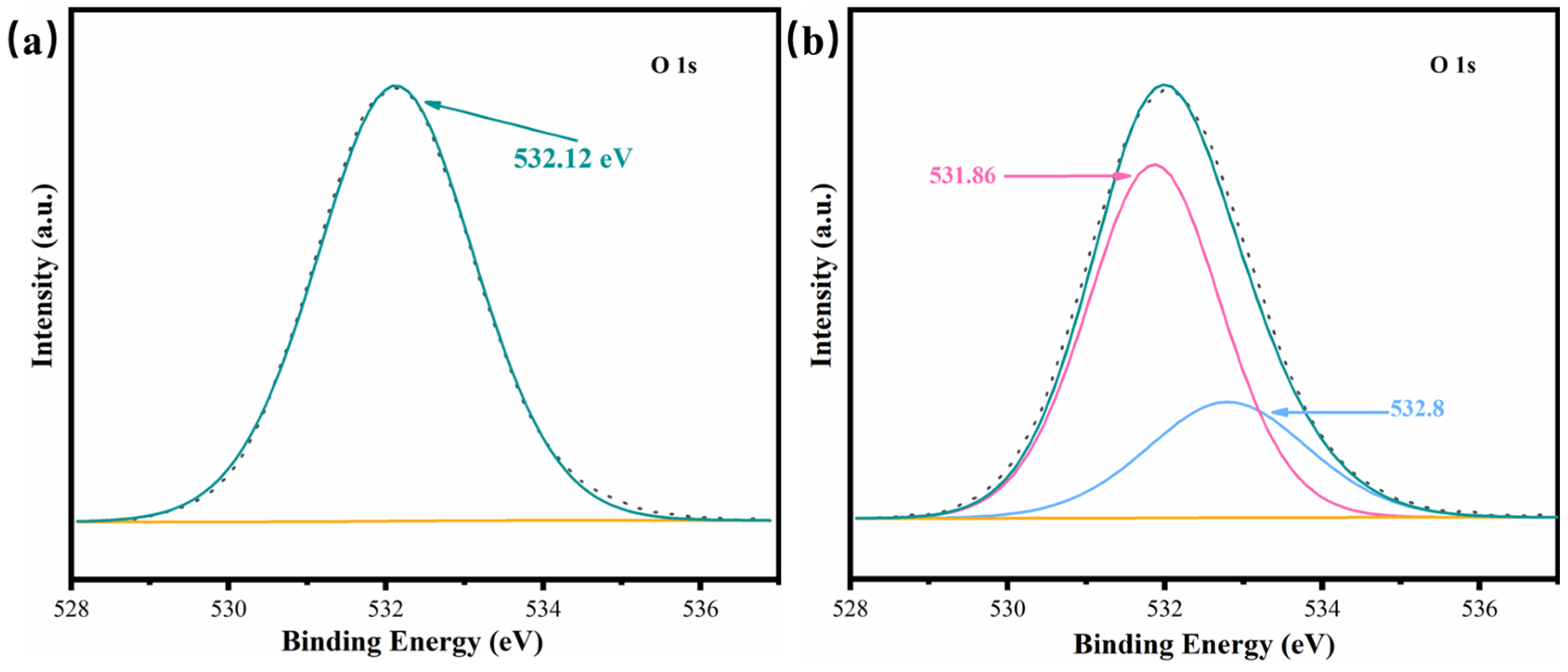
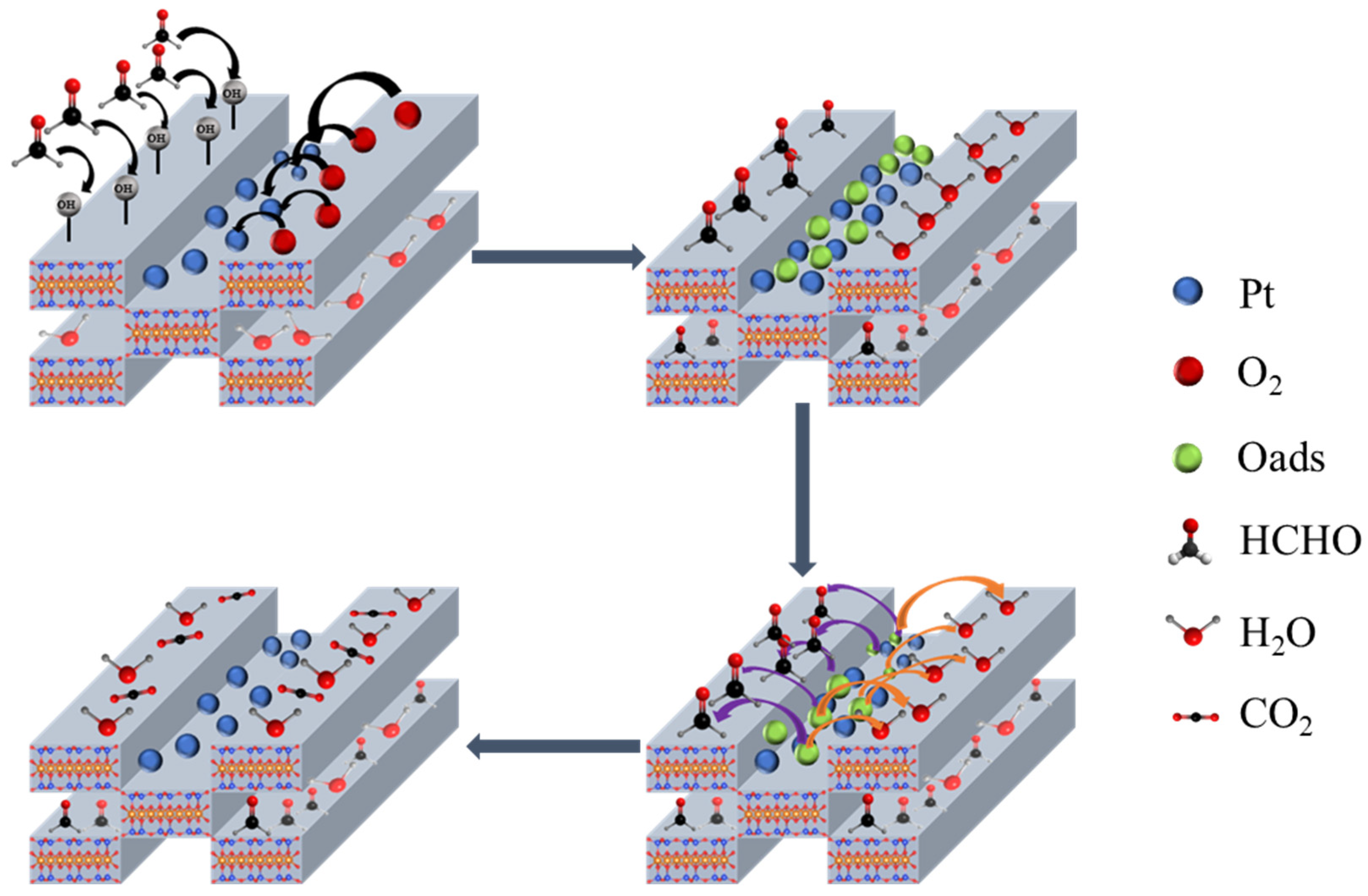
2.5. Catalyst Oxidation Mechanism
3. Materials and Methods
3.1. Catalyst Preparation
3.1.1. Sepiolite Acid Treatment
3.1.2. Catalyst Preparation
3.2. Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, B.; Lan, S. Experimental research of pulsed discharge plasma and TiO2/Zeolite coupling technology for formaldehyde removal. J. Phys. Conf. 2013, 418, 012121. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Han, K.; Yu, H. Efficient elimination of formaldehyde over Pt/Fe3O4 catalyst at room temperature. J. Environ. Chem. Eng. 2020, 8, 104041. [Google Scholar] [CrossRef]
- Ye, J.; Yu, Y.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Room-temperature formaldehyde catalytic decomposition. Environ. Sci. Nano 2020, 7, 3655–3709. [Google Scholar] [CrossRef]
- Huang, M.; Li, Y.; Li, M.; Zhao, J.; Zhu, Y.; Wang, C.; Sharma, V.K. Active Site Directed Tandem Catalysis on Single Platinum Nanoparticles for Efficient and Stable Oxidation of Formaldehyde at Room Temperature. Environ. Sci. Technol. 2019, 53, 3610–3619. [Google Scholar] [CrossRef]
- Tu, S.; Chen, Y.; Zhang, X.; Yao, J.; Xia, Q. Complete catalytic oxidation of formaldehyde at room temperature on MnxCo3−xO4 catalysts derived from metal-organic frameworks. Appl. Catal. A Gen. 2021, 611, 117975. [Google Scholar] [CrossRef]
- Yang, Z.; Miao, H.; Rui, Z.; Ji, H. Enhanced Formaldehyde Removal from Air Using Fully Biodegradable Chitosan Grafted β-Cyclodextrin Adsorbent with Weak Chemical Interaction. Polymers 2019, 11, 276. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liew, K.M.; Pan, C. Influence of hydroxyl groups on the adsorption of HCHO on TiO2-B(100) surface by first-principles study. Phys. Chem. Chem. Phys. Pccp 2013, 15, 3866–3880. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, C. First Principles Study of HCHO Adsorption on Hydroxylated TiO2-B(100) Surfaces. Chin. J. Comput. Phys. 2019, 36, 363. [Google Scholar]
- Zhang, Y.; Kan, W.; Miao, J.; Cheng, M.; Jing, Z. Hydrothermal Synthesis of Amino-PVC/DE Composite and Its Adsorption Performance for Formaldehyde. Ind. Eng. Chem. Res. 2021, 60, 12934–12943. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Q.; Ma, Y.; Zhu, R.; Fan, Y.; Huang, J.; Niu, H.; Dong, Y.; Li, D.; Zhang, Y.; et al. Highly efficient photocatalytic hydrogen evolution and simultaneous formaldehyde degradation over Z-scheme ZnIn2S4-NiO/BiVO4 hierarchical heterojunction under visible light irradiation. Chem. Eng. J. 2021, 423, 130164. [Google Scholar] [CrossRef]
- Wang, W.; Yu, H.; Li, K.; Lin, F.; Hou, L.A. Insoluble matrix proteins from shell waste for synthesis of visible-light response photocatalyst to mineralize indoor gaseous formaldehyde. J. Hazard. Mater. 2021, 415, 125649. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, P.; Zheng, J.; Li, J.; Lv, S.; Jin, T.; Xu, P.; Cheng, C.; Zhang, Y. Degradation of gaseous HCHO in a rotating photocatalytic fuel cell system with an absorption efficiency of up to 94%. Chem. Eng. J. 2020, 392, 123634. [Google Scholar] [CrossRef]
- Asilevi, P.J.; Boakye, P.; Oduro-Kwarteng, S.; Fei-Baffoe, B.; Sokama-Neuyam, Y.A. Indoor air quality improvement and purification by atmospheric pressure Non-Thermal Plasma (NTP). Sci. Rep. 2021, 11, 22830. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, D.; Ma, C. Study on Effects of Nano-Photocatalysis and Non-Thermal Plasma on the Removal of Indoor HCHO. In Proceedings of the Asme Second International Conference on Micro/nanoscale Heat & Mass Transfer, Shanghai, China, 18–21 December 2009. [Google Scholar]
- Lu, Y.; Wang, D.; Wu, Y.; Ma, C.; Zhang, X.; Yang, C. Synergistic Effect of Nanophotocatalysis and Nonthermal Plasma on the Removal of Indoor HCHO. Int. J. Photoenergy 2012, 2012, 354032. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Zhang, G.; Wang, M.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Pt/MnO2 Nanoflowers Anchored to Boron Nitride Aerogels for Highly Efficient Enrichment and Catalytic Oxidation of Formaldehyde at Room Temperature. Angew. Chem. 2020, 60, 6377–6381. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Jiang, Q.; Yang, X.; Liu, D.; Yu, H. CoMn2O4 supported on carbon nanotubes for effective low-temperature HCHO removal. J. Alloy. Compd. 2020, 859, 157808. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Yang, X.; Deng, L.; Zeng, T.; Huang, H. Preparation of Porous MnO2/CeO2 Composite for the Effective Removal of Formaldehyde from Air. Mater. Res. Bull. 2022, 150, 111751. [Google Scholar] [CrossRef]
- Cui, W.; Hui, J.; Tan, N. Research progress on catalytic oxidation of formaldehyde over supported platinum catalysts. Chem. Ind. Eng. Prog. 2017, 36, 3711–3719. [Google Scholar]
- Wang, C.; Li, Y.; Zheng, L.; Zhang, C.; He, H. A Nonoxide Catalyst System Study: Alkali Metal-Promoted Pt/AC Catalyst for Formaldehyde Oxidation at Ambient Temperature. ACS Catal. 2020, 11, 456–465. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Li, Y.; Peng, Y.; Chen, F.; Wang, L.; Zhang, C.; Meng, X.; He, H.; Xiao, F.-S. Complete oxidation of formaldehyde at room temperature over an Al-rich Beta zeolite supported platinum catalyst. Appl. Catal. B Environ. 2017, 219, 200–208. [Google Scholar] [CrossRef]
- Li, N.; Huang, B.; Dong, X.; Luo, J.; Wang, Y.; Wang, H.; Miao, D.; Pan, Y.; Jiao, F.; Xiao, J.; et al. Bifunctional zeolites-silver catalyst enabled tandem oxidation of formaldehyde at low temperatures. Nat. Commun. 2022, 13, 2209. [Google Scholar] [CrossRef]
- Guo, J.; Lin, C.; Jiang, C.; Zhang, P. Review on noble metal-based catalysts for formaldehyde oxidation at room temperature—ScienceDirect. Appl. Surf. Sci. 2019, 475, 237–255. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Zhao, X.; Wei, T.; Xiao, H. Excellent Low-Temperature Formaldehyde Decomposition Performance over Pt Nanoparticles Directly Loaded on Cellulose Triacetate. Ind. Eng. Chem. Res. 2020, 59, 21720–21728. [Google Scholar] [CrossRef]
- Selvitepe, N.; Balbay, A.; Saka, C. Optimisation of sepiolite clay with phosphoric acid treatment as support material for CoB catalyst and application to produce hydrogen from the NaBH4 hydrolysis. Int. J. Hydrogen Energy 2019, 44, 16387–16399. [Google Scholar] [CrossRef]
- Bai, B.; Qiao, Q.; Li, J.; Hao, J. Progress in research on catalysts for catalytic oxidation of formaldehyde. Chin. J. Catal. 2016, 37, 102–122. [Google Scholar] [CrossRef]
- Dong, N.; Ye, Q.; Chen, M.; Cheng, S.; Dai, H. Catalytic Oxidation of HCHO over the Sodium-Treated Sepiolite-Supported Rare Earth (La, Eu, Dy, and Tm) Oxide Catalysts. Catalysts 2020, 10, 328. [Google Scholar] [CrossRef] [Green Version]
- Aslan, S.; Aka, N.; Karaoglu, M.H. NaOH impregnated sepiolite based heterogeneous catalyst and its utilization for the production of biodiesel from canola oil. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 41, 290–297. [Google Scholar] [CrossRef]
- Meşe, E.; Figen, A.K.; Filiz, B.C.; Pişkin, S. Cobalt-boron loaded thermal activated Turkish sepiolite composites (Co-B@tSe) as a catalyst for hydrogen delivery. Appl. Clay Sci. 2018, 153, 95–106. [Google Scholar] [CrossRef]
- Xu, Z.; Jiang, H.; Yu, Y.; Xu, J.; Liang, J.; Zhou, L.; Hu, F. Activation and β-FeOOH modification of sepiolite in one-step hydrothermal reaction and its simulated solar light catalytic reduction of Cr(VI). Appl. Clay Sci. 2017, 135, 547–553. [Google Scholar] [CrossRef]
- Karaolu, M.H.; Zholobenko, V.; Aslan, S.; Karaolu, M.H.; Zholobenko, V.; Aslan, S. Preparation and application of KOH impregnated sepiolite as a solid base catalyst for biodiesel production using microwave irradiation. J. Chil. Chem. Soc. 2021, 66, 5320–5323. [Google Scholar]
- Du, Z.; Yang, B.B.; Duan, E.H.; Guo, B. Adsorption of Styrene on Hydrochloric Acid-Modified Sepiolite. Adv. Mater. Res. 2012, 518, 2058–2063. [Google Scholar] [CrossRef]
- Ardakani, M.B.; Mahabadi, H.A.; Jafari, A.J. Catalytic Removal of Toluene from Air Streams by Cobalt Oxide Supported on Sepiolite. J. Braz. Chem. Soc. 2019, 30, 1933–1940. [Google Scholar] [CrossRef]
- Moncea, M.; Deak, G.; Ana-Maria, P.; Baraitaru, A.; Dumitru, F.D. Handy and sustainable method of nano-Ca(OH)2 synthesis used in conservation and consolidation works. Int. J. Conserv. Sci. 2020, 11, 531–538. [Google Scholar]
- Nie, L.; Yu, J.; Li, X.; Cheng, B.; Liu, G.; Jaroniec, M. Enhanced performance of NaOH-modified Pt/TiO2 toward room temperature selective oxidation of formaldehyde. Environ. Sci. Technol. 2013, 47, 2777–2783. [Google Scholar] [CrossRef] [PubMed]
- Romanchenko, A.; Likhatski, M.; Mikhlin, Y. X-ray Photoelectron Spectroscopy (XPS) Study of the Products Formed on Sulfide Minerals Upon the Interaction with Aqueous Platinum (IV) Chloride Complexes. Minerals 2018, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, G.; Zhang, Z.; Chen, H. Influence of the interaction between surfactants and sepiolite on the rheological properties and thermal stability of organo-sepiolite in oil-based drilling fluids. Microporous Mesoporous Mater. 2018, 272, 143–154. [Google Scholar] [CrossRef]
- Yu, J.; Wei, D.; Zheng, Z.; Yu, W.; Shen, H.; Qu, Y.; Wen, S.; Kwon, Y.U.; Zhao, Y. Facile synthesis of Nafion-supported Pt nanoparticles with ultra-low loading as a high-performance electrocatalyst for hydrogen evolution reaction. J. Colloid Interface Sci. 2020, 566, 505–512. [Google Scholar] [CrossRef]
- Wu, B.; Du, F.; Wang, H.; Wu, C.; Chu, J.; Wang, X.; Xiong, S. Effects of annealing temperature of PtCu/MWCNT catalysts on their electrocatalytic performance of electrooxidation of methanol. Ionics 2021, 28, 369–382. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Chen, B.-B.; Shi, C.; Li, Y.; Wang, C.; Han, S.; Pan, S.; Wang, L.; Meng, X.; et al. Exceptional activity for formaldehyde combustion using siliceous Beta zeolite as a catalyst support. Catal. Today 2020, 339, 174–180. [Google Scholar] [CrossRef]
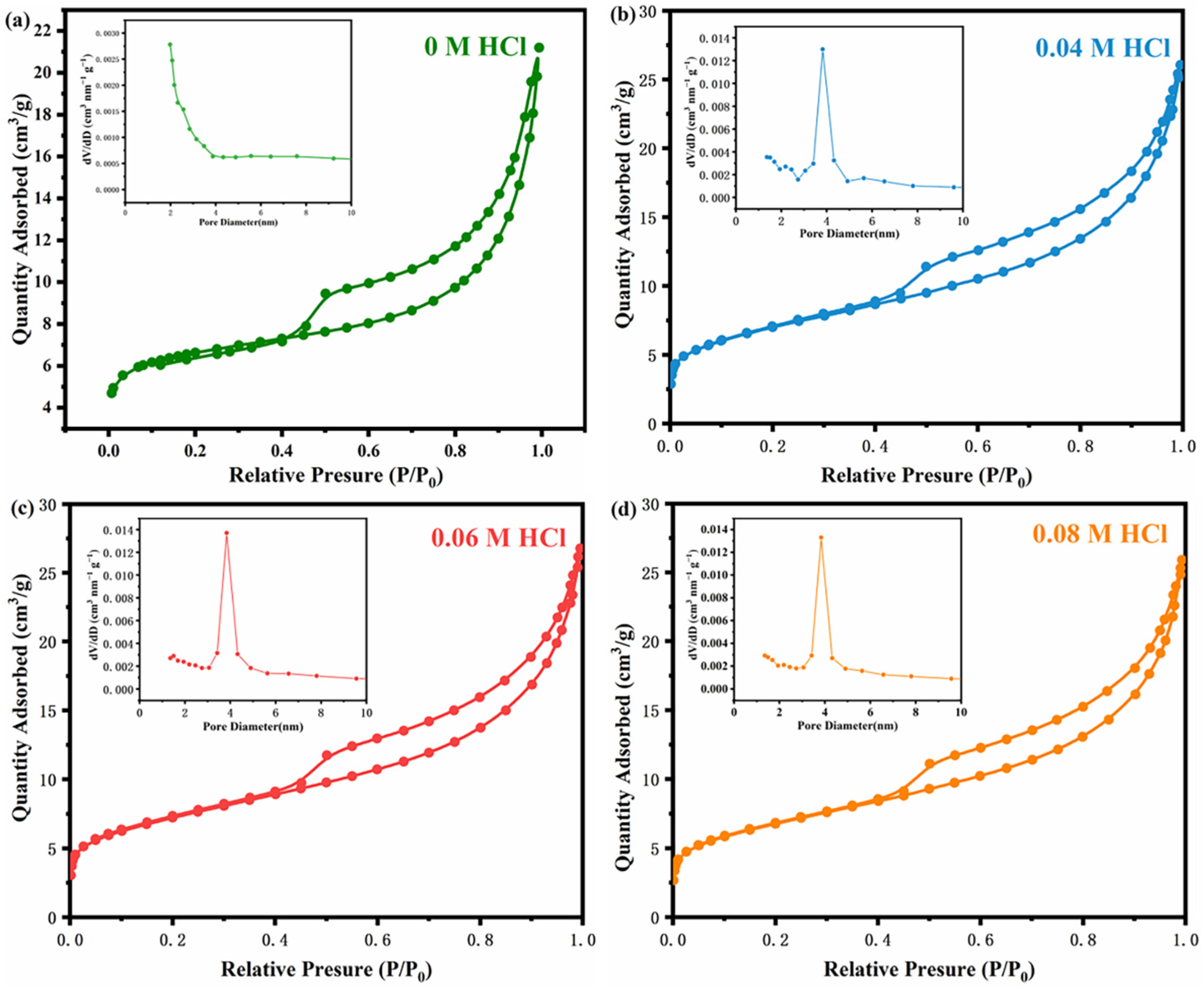

| C(HCl)/mol L−1 | 0 | 0.04 | 0.06 | 0.08 |
|---|---|---|---|---|
| Specific Surface Area (m2/g) | 23.995 | 24.853 | 25.581 | 23.374 |
| Pore Volume (cm3/g) | 0.032 | 0.040 | 0.040 | 0.039 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Min, X.; Wang, L.; Zhao, Y.; Yang, B.; Wu, X.; Zhang, D.; Hou, X.; Liu, Y.; Fang, M.; et al. Acid-Modified Sepiolite-Supported Pt (Noble Metal) Catalysts for HCHO Oxidation at Ambient Temperature. Catalysts 2022, 12, 1299. https://doi.org/10.3390/catal12111299
Zhou Y, Min X, Wang L, Zhao Y, Yang B, Wu X, Zhang D, Hou X, Liu Y, Fang M, et al. Acid-Modified Sepiolite-Supported Pt (Noble Metal) Catalysts for HCHO Oxidation at Ambient Temperature. Catalysts. 2022; 12(11):1299. https://doi.org/10.3390/catal12111299
Chicago/Turabian StyleZhou, Yidi, Xin Min, Lijuan Wang, Yajing Zhao, Bozhi Yang, Xiaoxian Wu, Dan Zhang, Xifeng Hou, Yan’gai Liu, Minghao Fang, and et al. 2022. "Acid-Modified Sepiolite-Supported Pt (Noble Metal) Catalysts for HCHO Oxidation at Ambient Temperature" Catalysts 12, no. 11: 1299. https://doi.org/10.3390/catal12111299





