Hydrogenolysis of Lignin and C–O Linkages Containing Lignin-Related Compounds over an Amorphous CoRuP/SiO2 Catalyst
Abstract
1. Introduction
2. Results and Discussion
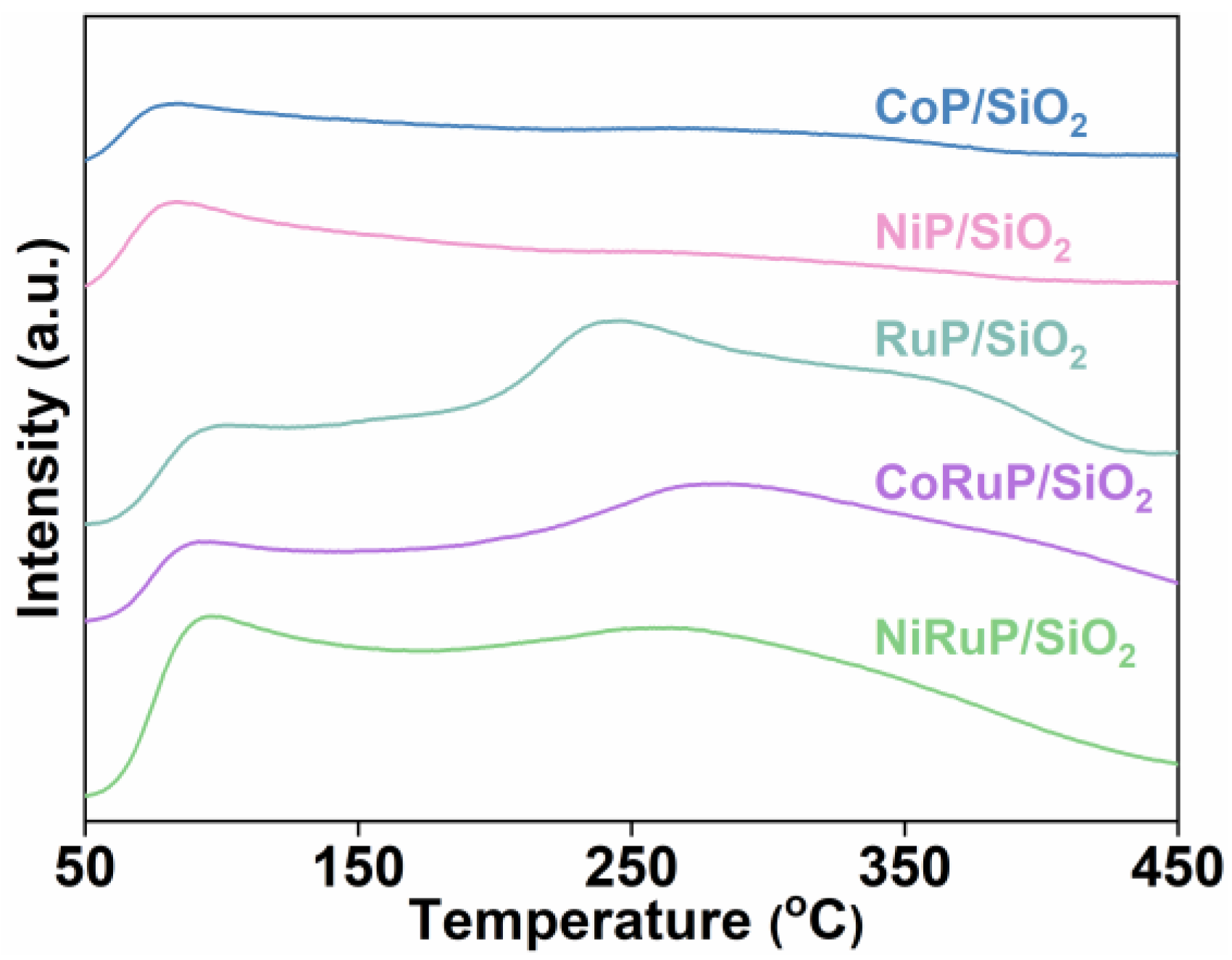
2.1. Characterization of Catalysts
2.2. Catalytic Performance
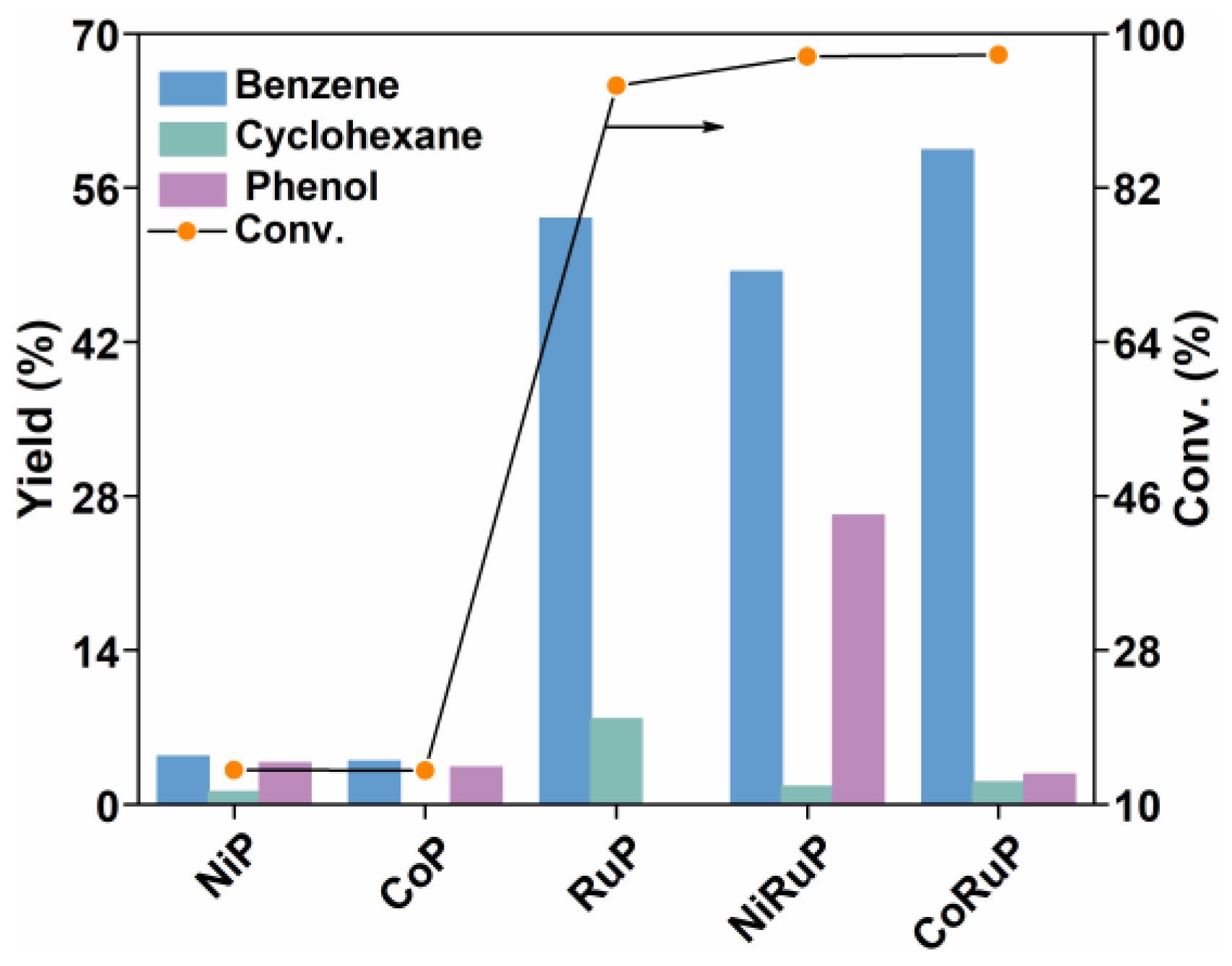
2.2.1. Screening of Catalysts
2.2.2. Influence of Initial H2 Partial Pressure on Hydrogenolysis Performance
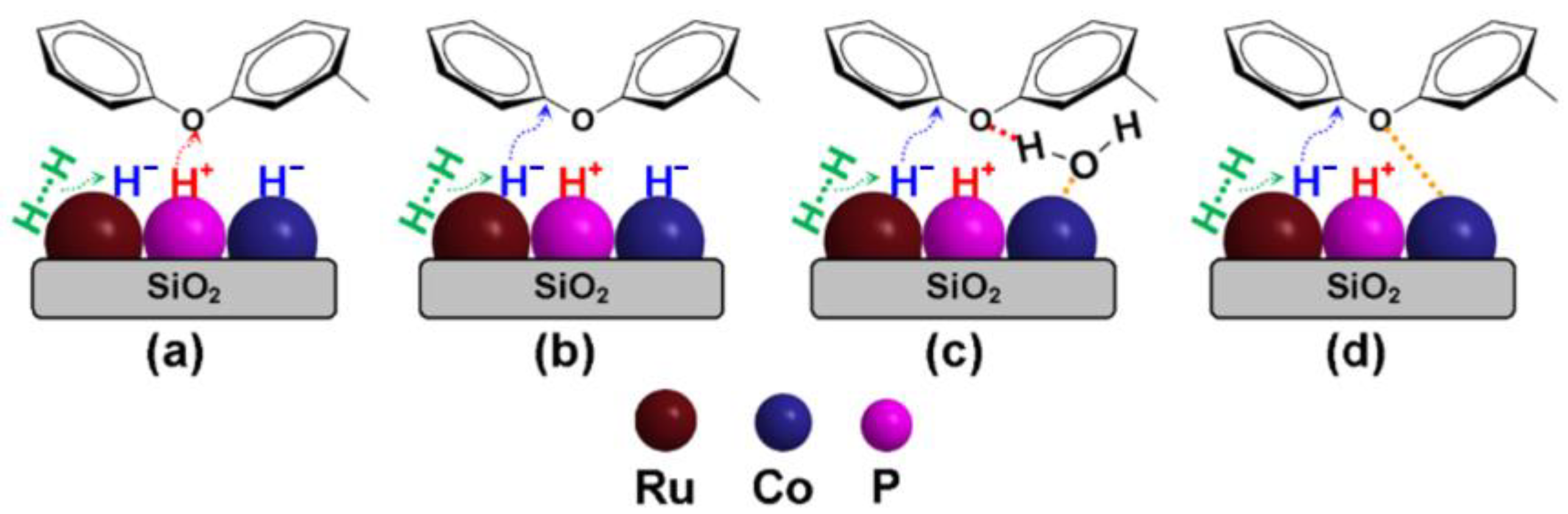
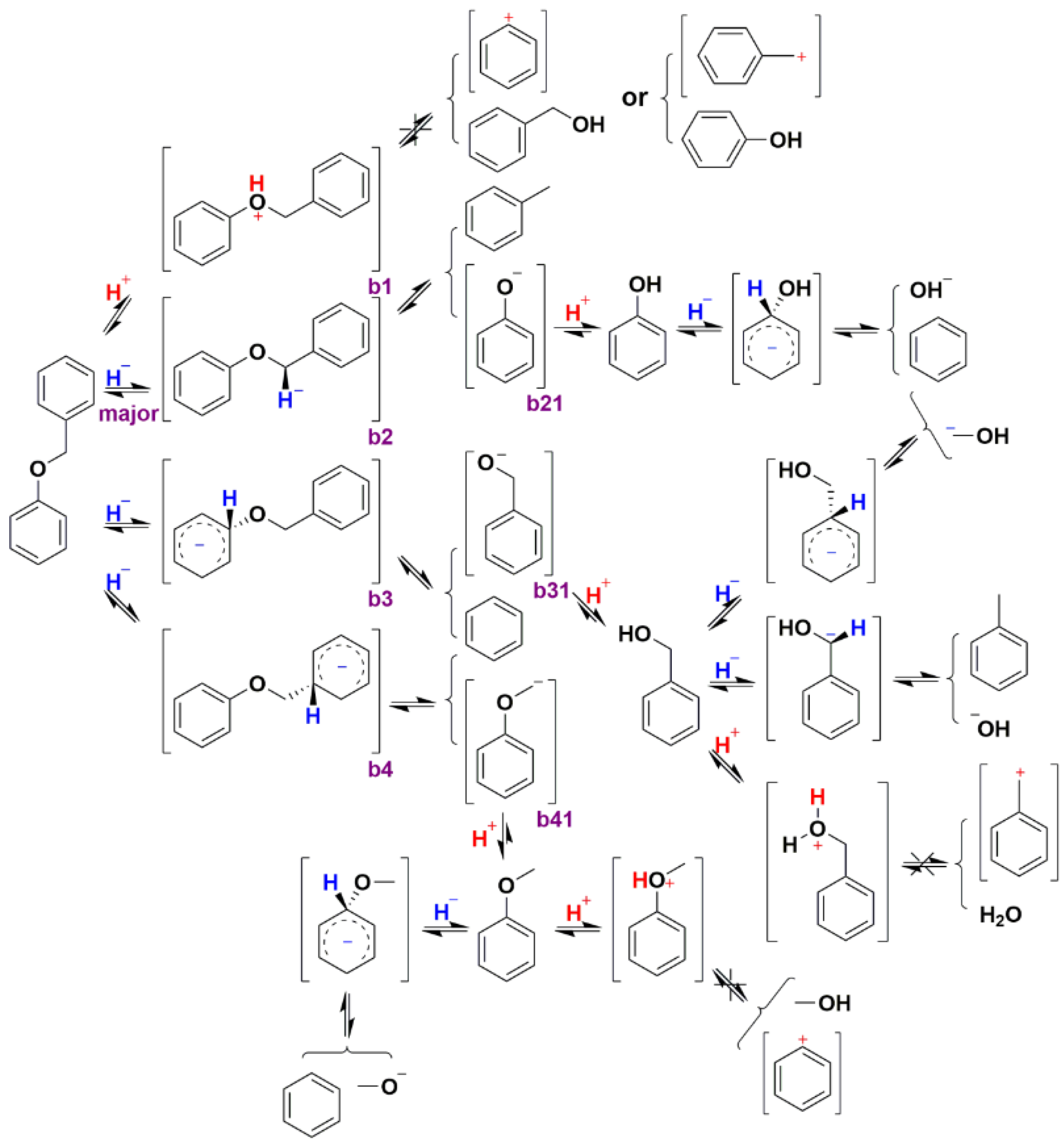
2.2.3. Hydrogenolysis Mechanism
2.2.4. Hydrogenolysis of Lignin over CoRuP/SiO2
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Hydrogenolysis of Model Compounds
3.5. Hydrogenolysis of Lignin
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, M.; Kumar, A. Production of renewable diesel through the hydroprocessing of lignocellulosic biomass-derived bio-oil: A review. Renew. Sustain. Energy Rev. 2016, 58, 1293–1307. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, T.R.; Hu, G.; Brown, R.C. Techno-economic analysis of two bio-oil upgrading pathways. Chem. Eng. J. 2013, 225, 895–904. [Google Scholar] [CrossRef]
- Teles, C.A.; de Souza, P.M.; Rabelo-Neto, R.C.; Griffin, M.B.; Mukarakate, C.; Orton, K.A.; Resasco, D.E.; Noronha, F.B. Catalytic upgrading of biomass pyrolysis vapors and model compounds using niobia supported Pd catalyst. Appl. Catal. B Environ. 2018, 238, 38–50. [Google Scholar] [CrossRef]
- Sirous-Rezaei, P.; Park, Y.K. Catalytic hydropyrolysis of lignin: Suppression of coke formation in mild hydrodeoxygenation of lignin-derived phenolics. Chem. Eng. J. 2020, 386, 121348. [Google Scholar] [CrossRef]
- Gao, F.; Webb, J.D.; Sorek, H.; Wemmer, D.E.; Hartwig, J.F. Fragmentation of lignin samples with commercial Pd/C under ambient pressure of hydrogen. ACS Catal. 2016, 6, 7385–7392. [Google Scholar] [CrossRef]
- Zhou, M.; Chen, C.; Liu, P.; Xia, H.; Li, J.; Sharma, B.K.; Jiang, J. Catalytic hydrotreatment of β–O–4 ether in lignin: Cleavage of the C-O bond and hydrodeoxygenation of lignin-derived phenols in one pot. ACS Sustain. Chem. Eng. 2020, 8, 14511–14523. [Google Scholar] [CrossRef]
- Li, S.; Liu, B.; Truong, J.; Luo, Z.; Ford, P.C.; Abu-Omar, M.M. One-pot hydrodeoxygenation (HDO) of lignin monomers to C9 hydrocarbons co-catalysed by Ru/C and Nb2O5. Green Chem. 2020, 22, 7406–7416. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, A.T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef]
- Zhao, M.X.; Wei, X.Y.; Qu, M.; Kong, J.; Li, Z.K.; Liu, J.; Zong, Z.M. Complete hydrocracking of dibenzyl ether over a solid acid under mild conditions. Fuel 2016, 183, 531–536. [Google Scholar] [CrossRef]
- Luo, H.; Wang, L.; Li, G.; Shang, S.; Lv, Y.; Niu, J.; Gao, S. Nitrogen-doped carbon-modified cobalt-nanoparticle-catalyzed oxidative cleavage of lignin β-O-4 model compounds under mild conditions. ACS Sustain. Chem. Eng. 2018, 6, 14188–14196. [Google Scholar] [CrossRef]
- Parthasarathi, R.; Romero, R.A.; Redondo, A.; Gnanakaran, S. Theoretical study of the remarkably diverse linkages in lignin. J. Phys. Chem. Lett. 2011, 2, 2660–2666. [Google Scholar] [CrossRef]
- Rizescu, C.; Sun, C.; Popescu, I.; Urdă, A.; Da Costa, P.; Marcu, I.C. Hydrodeoxygenation of benzyl alcohol on transition-metal-containing mixed oxides derived from layered double hydroxide precursors. Catal. Today 2021, 366, 235–244. [Google Scholar] [CrossRef]
- Li, X.; Zhang, B.; Pan, X.; Ji, J.; Ren, Y.; Wang, H.; Ji, N.; Liu, Q.; Li, C. One-pot conversion of lignin into naphthenes catalyzed by a heterogeneous rhenium oxide-modified iridium compound. ChemSusChem 2020, 13, 4409–4419. [Google Scholar] [CrossRef]
- Yang, X.; Feng, M.; Choi, J.S.; Meyer, H.M., III; Yang, B. Depolymerization of corn stover lignin with bulk molybdenum carbide catalysts. Fuel 2019, 244, 528–535. [Google Scholar] [CrossRef]
- Yan, B.; Lin, X.; Chen, Z.; Cai, Q.; Zhang, S. Selective production of phenolic monomers via high efficient lignin depolymerization with a carbon based nickel-iron-molybdenum carbide catalyst under mild conditions. Bioresour. Technol. 2020, 321, 124503. [Google Scholar] [CrossRef]
- Molinari, V.; Clavel, G.; Graglia, M.; Antonietti, M.; Esposito, D. Mild continuous hydrogenolysis of kraft lignin over titanium nitride-nickel catalyst. ACS Catal. 2016, 6, 1663–1670. [Google Scholar] [CrossRef]
- Yoosuk, B.; Sanggam, P.; Wiengket, S.; Prasassarakich, P. Hydrodeoxygenation of oleic acid and palmitic acid to hydrocarbon-like biofuel over unsupported Ni-Mo and Co-Mo sulfide catalysts. Renew. Energy 2019, 139, 1391–1399. [Google Scholar] [CrossRef]
- Song, W.; Lai, W.; Lian, Y.; Jiang, X.; Yang, W. Sulfated ZrO2 supported CoMo sulfide catalyst by surface exsolution for enhanced hydrodeoxygenation of lignin-derived ethers to aromatics. Fuel 2019, 263, 116705. [Google Scholar] [CrossRef]
- Rensel, D.J.; Kim, J.; Jain, V.; Bonita, Y.; Rai, N.; Hicks, J.C. Composition-directed FexMo2-xP bimetallic catalysts for hydrodeoxygenation reactions. Catal. Sci. Technol. 2017, 7, 1857–1867. [Google Scholar] [CrossRef]
- Jain, V.; Bonita, Y.; Brown, A.; Taconi, A.; Hicks, J.C.; Rai, N. Mechanistic insights into hydrodeoxygenation of phenol on bimetallic phosphide catalysts. Catal. Sci. Technol. 2018, 8, 4083–4096. [Google Scholar] [CrossRef]
- Bonita, Y.; Hicks, J.C. Periodic Trends from metal substitution in bimetallic Mo-based phosphides for hydrodeoxygenation and hydrogenation reactions. J. Phys. Chem. C 2018, 122, 13322–13332. [Google Scholar] [CrossRef]
- Li, K.; Wang, R.; Chen, J. Hydrodeoxygenation of anisole over silica-supported Ni2P, MoP, and NiMoP catalysts. Energy Fuel 2011, 25, 854–863. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Zhu, L.; Zhang, H.; Chen, B. Hydrodeoxygenation of phenol as a bio-oil model compound over intimate contact noble metal-Ni2P/SiO2 catalysts. RSC Adv. 2015, 5, 80388–80396. [Google Scholar] [CrossRef]
- Bonita, Y.; O’Connell, T.P.; Miller, H.E.; Hicks, J.C. Revealing the hydrogenation performance of RuMo phosphide for chemoselective reduction of functionalized aromatic hydrocarbons. Ind. Eng. Chem. Res. 2019, 8, 3650–3658. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, A.; Liu, S.; Yao, Y.; Sun, Z.; Li, X.; Liu, Y.; Wang, Y.; Camaioni, D.M.; Lercher, J.A. Hydrodeoxygenation of phenolic compounds to cycloalkanes over supported nickel phosphides. Catal. Today 2019, 319, 48–56. [Google Scholar] [CrossRef]
- Gutiérrez-Rubio, S.; Berenguer, A.; Přech, J.; Opanasenko, M.; Ochoa-Hernández, C.; Pizarro, P.; Čejka, J.; Serrano, D.P.; Coronado, J.M.; Moreno, I. Guaiacol hydrodeoxygenation over Ni2P supported on 2D-zeolites. Catal. Today 2020, 345, 48–58. [Google Scholar] [CrossRef]
- Nkabinde, S.S.; Ndala, Z.B.; Shumbula, N.P.; Kolokoto, T.; Nchoe, O.; Ngubeni, G.N.; Mubiayi, K.P.; Moloto, N. Delineating the role of crystallinity in the electrocatalytic activity of colloidally synthesized MoP nanocrystals. New J. Chem. 2020, 44, 14041–14049. [Google Scholar] [CrossRef]
- Chen, Q.; Cai, C.; Zhang, X.; Zhang, Q.; Chen, L.; Li, Y.; Wang, C.; Ma, L. Amorphous FeNi-ZrO2-catalyzed hydrodeoxygenation of lignin-derived phenolic compounds to naphthenic fuel. ACS Sustain. Chem. Eng. 2020, 8, 9335–9345. [Google Scholar] [CrossRef]
- Guo, C.; Rao, K.T.V.; Yuan, Z.; He, S.Q.; Rohani, S.; Xu, C.C. Hydrodeoxygenation of fast pyrolysis oil with novel activated carbon-supported NiP and CoP catalysts. Chem. Eng. Sci. 2017, 178, 248–259. [Google Scholar] [CrossRef]
- Rodríguez-Aguado, E.; Infantes-Molina, A.; Cecilia, J.A.; Ballesteros-Plata, D.; López-Olmo, R.; Rodríguez-Castellón, E. CoxPy Catalysts in HDO of phenol and dibenzofuran: Effect of P content. Top. Catal. 2017, 60, 1094–1107. [Google Scholar] [CrossRef]
- Chakroune, N.; Viau, G.; Ammar, S.; Poul, L.; Veautier, D.; Chehimi, M.M.; Mangeney, C.; Villain, F.; Fiévet, F. Acetate-and thiol-capped monodisperse ruthenium nanoparticles: XPS, XAS, and HRTEM studies. Langmuir 2005, 21, 6788–6796. [Google Scholar] [CrossRef] [PubMed]
- Topalian, P.J.; Carrillo, B.A.; Cochran, P.M.; Takemura, M.F.; Bussell, M.E. Synthesis and hydrodesulfurization properties of silica-supported nickel-ruthenium phosphide catalysts. J. Catal. 2021, 403, 173–180. [Google Scholar] [CrossRef]
- Galindo-Ortega, Y.I.; Infantes-Molina, A.; Huirache-Acuña, R.; Barroso-Martín, I.; Rodríguez-Castellón, E.; Fuentes, S.; Alonso-Nuñez, G.; Zepeda, T.A. Active ruthenium phosphide as selective sulfur removal catalyst of gasoline model compounds. Fuel Process. Technol. 2020, 208, 106507. [Google Scholar] [CrossRef]
- Bowker, R.H.; Smith, M.C.; Pease, M.L.; Slenkamp, K.M.; Kovarik, L.; Bussell, M.E. Synthesis and hydrodeoxygenation properties of ruthenium phosphide catalysts. ACS Catal. 2011, 1, 917–922. [Google Scholar] [CrossRef]
- Nelson, R.C.; Baek, B.; Ruiz, P.; Goundie, B.; Brooks, A.; Wheeler, M.C.; Frederick, B.G.; Grabow, L.C.; Austin, R.N. Experimental and theoretical insights into the hydrogen-efficient direct hydrodeoxygenation mechanism of phenol over Ru/TiO2. ACS Catal. 2015, 5, 6509–6523. [Google Scholar] [CrossRef]
- Cao, J.P.; Xie, T.; Zhao, X.Y.; Zhu, C.; Jiang, W.; Zhao, M.; Zhao, Y.P.; Wei, X.Y. Selective cleavage of ether C–O bond in lignin-derived compounds over Ru system under different H-sources. Fuel 2021, 284, 119027. [Google Scholar] [CrossRef]
- Zeng, G.; Guo, Y.; Li, S. New insights into the molecular mechanism of H2 activation. In Computational Organometallic Chemistry; Wiest, O., Wu, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–60. [Google Scholar]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Infantes-Molina, A.; Sanmartín-Donoso, J.; Rodríguez-Aguado, E.; Ballesteros-Plata, D.; Rodríguez-Castellón, E. Enhanced HDO activity of Ni2P promoted with noble metals. Catal. Sci. Technol. 2016, 6, 7323–7333. [Google Scholar] [CrossRef]
- Liu, Z.Q.; Wei, X.Y.; Wu, H.H.; Li, W.T.; Zhang, Y.Y.; Zong, Z.M.; Ma, F.Y.; Liu, J.M. Difunctional nickel/microfiber attapulgite modified with an acidic ionic liquid for catalytic hydroconversion of lignite-related model compounds. Fuel 2017, 204, 236–242. [Google Scholar] [CrossRef]
- Zhao, C.; Lercher, J.A. Upgrading pyrolysis oil over Ni/HZSM-5 by cascade reactions. Angew. Chem. Int. Ed. 2012, 51, 5935–5940. [Google Scholar] [CrossRef]
- Liu, X.X.; Zong, Z.M.; Li, W.T.; Li, X.; Li, Z.K.; Wang, S.K.; Wei, X.Y. A recyclable and highly active magnetic solid superbase for hydrocracking C-O bridged bonds in sawdust. Fuel Process. Technol. 2017, 159, 396–403. [Google Scholar] [CrossRef]
- Sergeev, A.G.; Hartwig, J.F. Selective, Nickel-catalyzed hydrogenolysis of aryl ethers. Science 2011, 332, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; Sun, S.N.; Xu, F.; Sun, R.C. Characterization of lignin structures and lignin–carbohydrate complex (LCC) linkages by quantitative 13C and 2D HSQC NMR spectroscopy. J. Agric. Food Chem. 2011, 59, 10604–10614. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, B.; Li, C.; Peng, C.; Dai, T.; Xie, H.; Wang, A.; Zhang, T. Tungsten Carbide: A remarkably efficient catalyst for the selective cleavage of lignin C-O bonds. ChemSusChem 2016, 9, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; McClelland, D.J.; Azarpira, A.; Ralph, J.; Luo, Z.Y.; Huber, G.W. Low temperature hydrogenation of pyrolytic lignin over Ru/TiO2: 2D HSQC and 13C NMR study of reactants and products. Green Chem. 2016, 18, 271–281. [Google Scholar] [CrossRef]
- Ren, X.; Wang, P.; Han, X.; Zhang, G.; Gu, J.; Ding, C.; Zheng, X.; Cao, F. Depolymerization of lignin to aromatics by selectively oxidizing cleavage of C–C and C–O bonds using CuCl2/polybenzoxazine catalysts at room temperature. ACS Sustain. Chem. Eng. 2017, 5, 6548–6556. [Google Scholar] [CrossRef]
- Diao, Z.J.; Huang, L.Q.; Chen, B.; Gao, T.; Cao, Z.Z.; Ren, X.D.; Zhao, S.J.; Li, S. Amorphous Ni-Ru bimetallic phosphide composites as efficient catalysts for the hydrogenolysis of diphenyl ether and lignin. Fuel 2022, 324, 124489. [Google Scholar] [CrossRef]
- Fukuhara, K.; Ishikawa, K.; Yasuda, S.; Kishishita, Y.; Kim, H.K.; Kakeda, T.; Yamamoto, M.; Norii, T.; Ishikawa, T. Intracerebroventricular 4-methylcatechol (4-MC) ameliorates chronic pain associated with depression-like behavior via induction of brain-derived neurotrophic factor (BDNF). Cell. Mol. Neurobiol. 2011, 32, 971–977. [Google Scholar] [CrossRef]
- Mann, J.; Wilde, P.D.; Finch, M.W. Synthesis and reactions of 2-aryl-8-oxabicycloc [3.2.1]oct-6-en-3-ones. Tetrahedron 1987, 43, 5431–5441. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, S.I.; Go, B.; Oh, U.H.; Kim, C.S.; Jung, Y.H.; Lee, J.; Kim, J.H. 2-methoxy-4-vinylphenol attenuates migration of human pancreatic cancer cells via blockade of fak and akt signaling. Anticancer Res. 2019, 39, 6685–6691. [Google Scholar] [CrossRef] [PubMed]
- Stefanska, J.; Sarniak, A.; Wlodarczyk, A.; Sokolowska, M.; Doniec, Z.; Bialasiewicz, P.; Nowak, D.; Pawliczak, R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm. Pharmacol. Ther. 2012, 25, 343–348. [Google Scholar] [CrossRef]
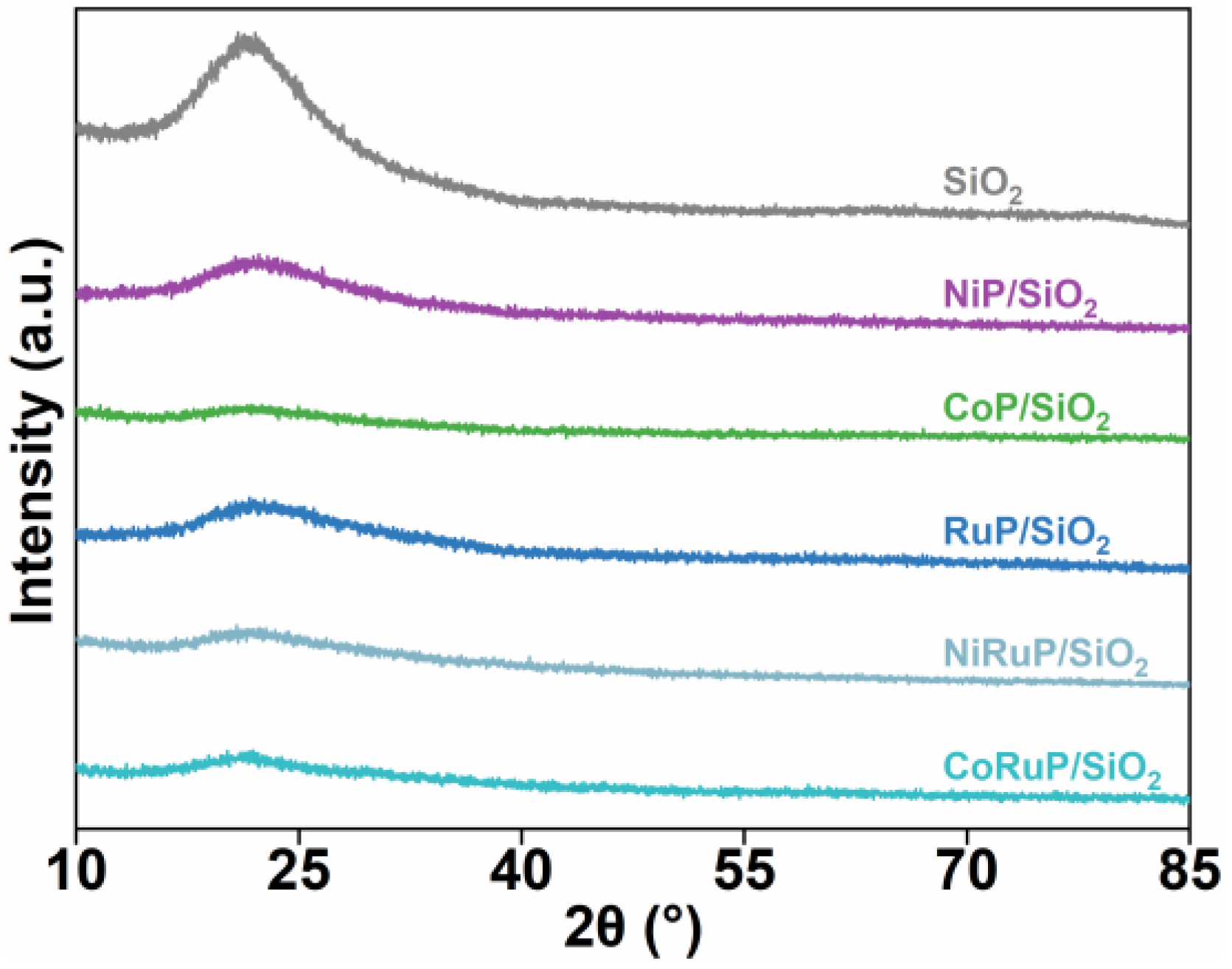
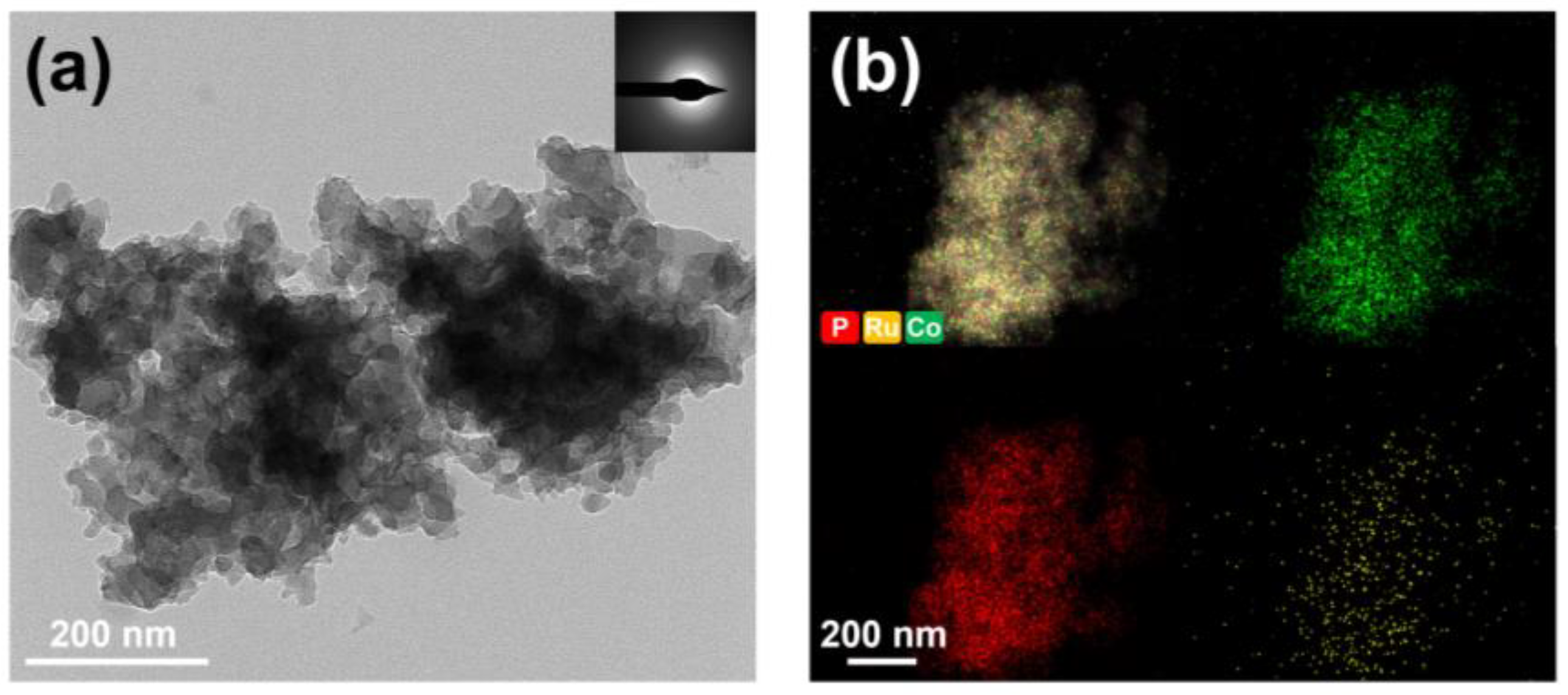
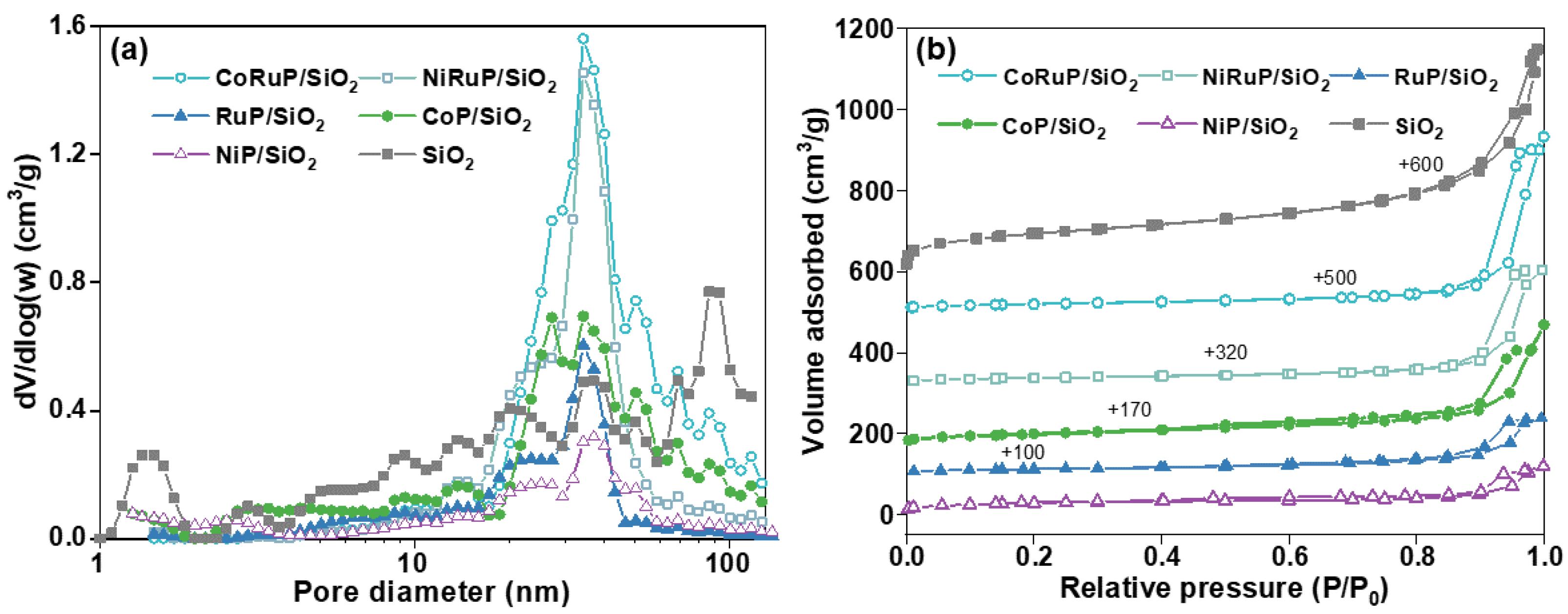
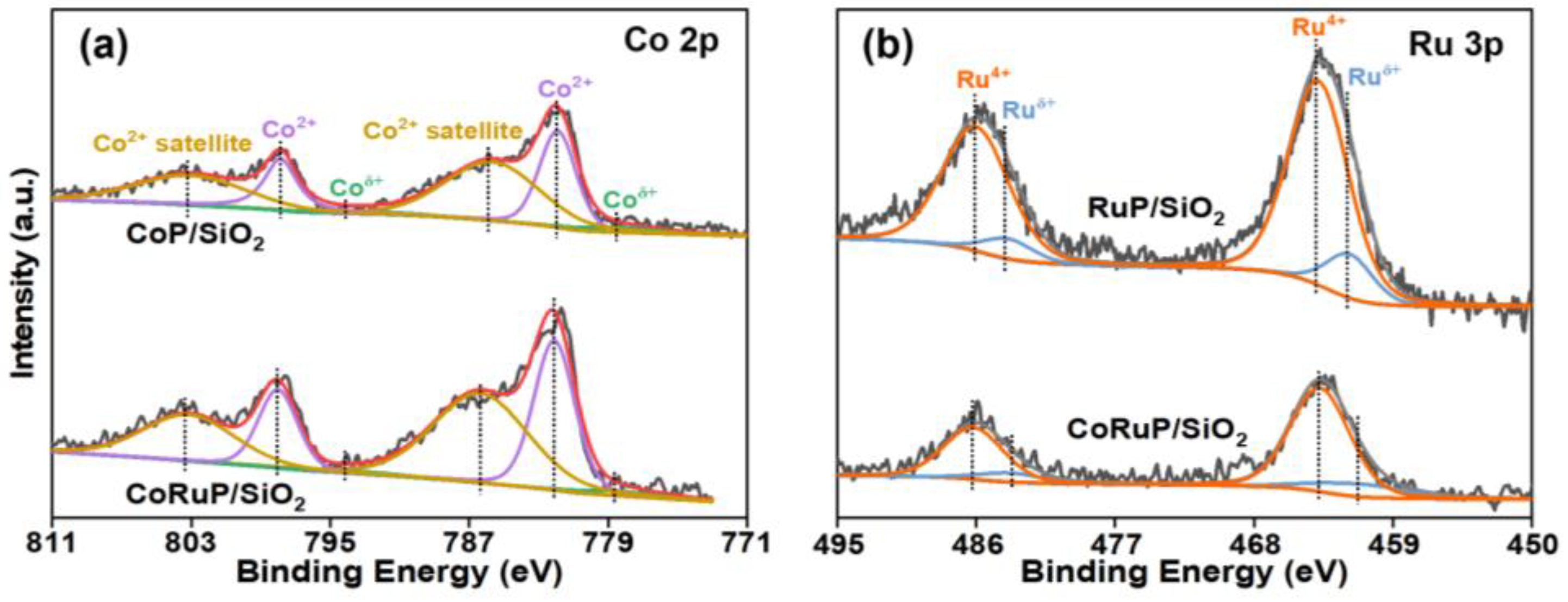






| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|
| SiO2 | 300.86 | 0.038 |
| NiP/SiO2 | 97.67 | 0.036 |
| CoP/SiO2 | 94.59 | 0.011 |
| RuP/SiO2 | 42.47 | 0.0004 |
| NiRuP/SiO2 | 60.07 | 0.0065 |
| CoRuP/SiO2 | 65.06 | 0.0054 |
| Sample | CoP/SiO2 | NiP/SiO2 | RuP/SiO2 | CoRuP/SiO2 | NiRuP/SiO2 |
|---|---|---|---|---|---|
| NH3 acidity/mmol·g−1 | 1.1 | 1.1 | 2.3 | 2.4 | 3.2 |
| Entry | Substrate | Conv./% | Yield/% | |||
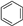 | 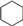 | 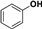 |  | |||
| 1 | 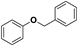 | 98.8 | 8.7 | 0.6 | 21.7 | 34.8 |
| 2 |  | 99.0 | 23.5 | 0.1 | - | 52.8 |
| Name | Yield (μg) | |
|---|---|---|
| Organic Phase | Aqueous phase | |
| 2,4-Dimethoxybenzaldehyde | 1.0 | - |
| 2,3-Dihydroxytoluene | 35.3 | 17.1 |
| 2,6-Dimethoxyphenol | 29.6 | 15.9 |
| 2-Methoxy-4-vinylphenol | 116.2 | 45.8 |
| 3-(3,4-Dimethoxyphenyl) propionic acid | 5.1 | 3.3 |
| 4-Hydroxy-3-methoxyphenylacetone | 116.7 | 57.1 |
| 4-Methylcatechol | 488.5 | 194.7 |
| Acetosyringone | 2.9 | 2.4 |
| Acetovanillone | 93.6 | 42.5 |
| Isoeugenol | 4.3 | - |
| Phenyl acetate | 22.6 | 5.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-Q.; Diao, Z.-J.; Chen, B.; Du, Q.-P.; Duan, K.-Y.; Zhao, S.-J. Hydrogenolysis of Lignin and C–O Linkages Containing Lignin-Related Compounds over an Amorphous CoRuP/SiO2 Catalyst. Catalysts 2022, 12, 1328. https://doi.org/10.3390/catal12111328
Huang L-Q, Diao Z-J, Chen B, Du Q-P, Duan K-Y, Zhao S-J. Hydrogenolysis of Lignin and C–O Linkages Containing Lignin-Related Compounds over an Amorphous CoRuP/SiO2 Catalyst. Catalysts. 2022; 12(11):1328. https://doi.org/10.3390/catal12111328
Chicago/Turabian StyleHuang, Liang-Qiu, Zhi-Jun Diao, Bo Chen, Qing-Pan Du, Kai-Yang Duan, and Si-Jia Zhao. 2022. "Hydrogenolysis of Lignin and C–O Linkages Containing Lignin-Related Compounds over an Amorphous CoRuP/SiO2 Catalyst" Catalysts 12, no. 11: 1328. https://doi.org/10.3390/catal12111328
APA StyleHuang, L.-Q., Diao, Z.-J., Chen, B., Du, Q.-P., Duan, K.-Y., & Zhao, S.-J. (2022). Hydrogenolysis of Lignin and C–O Linkages Containing Lignin-Related Compounds over an Amorphous CoRuP/SiO2 Catalyst. Catalysts, 12(11), 1328. https://doi.org/10.3390/catal12111328






