Abstract
Using the bioresources of the Regional Specialised Collection of Alkanotrophic Microorganisms (acronym IEGM, Perm, Russia; WFCC # 285), R. rhodochrous IEGM 757 was selected, which catalyzed the C5, C22, and C23 functionalization of pentacyclic triterpenoid oleanolic acid (OA, 3β-hydroxyolean-12-en-28-oic acid, 1.0 g/L) to form a new 5α,22α-dihydroxy derivative of gypsogenic acid (3β,5α,22α-trihydroxyolean-12-ene-23,28-dioic acid) for 5 days. In silico analysis showed that, compared to the native triterpenoid, the OA metabolite may be more soluble in water and less ecotoxic, act as an apoptosis agonist and insulin promoter, and have chemopreventive and analgesic effects. Phase-contrast, fluorescent, scanning, and transmission electron microscopy and X-ray spectroscopy demonstrated the high resistance of R. rhodochrous IEGM 757 to OA. This creates opportunities for further research and development of a method for the production of the OA metabolite. New-generation sequencing of the R. rhodochrous IEGM 757 whole genome, annotation and bioinformatics analysis of the obtained sequences, and real-time PCR were applied. As a result, 24 genes encoding CYP450 enzymes were found, which are highly likely to be involved in the process of OA oxidation.
1. Introduction
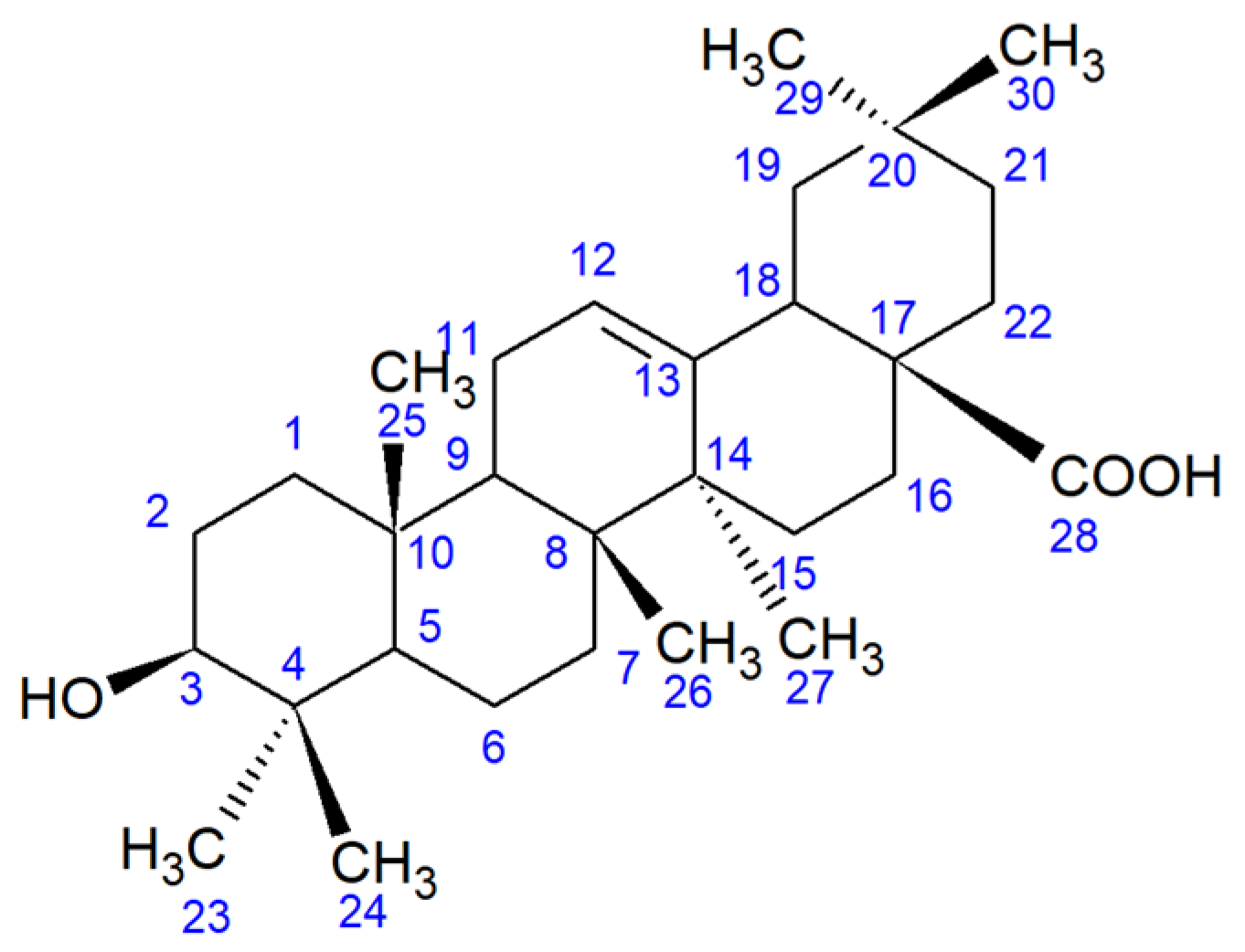
Now, amidst the deficit of effective pharmacological agents for socially significant diseases (cancer, infectious and neurodegenerative diseases), an essential task is to synthesize new chemical compounds with potential biological activities, including those derived from plant terpenoids [1]. Plant pentacyclic triterpenoids, in particular oleanolic acid (OA, 3β-hydroxyolean-12-en-28-oic acid, Figure 1), are one of the intensively developed groups of compounds in this field [2]. OA is used to obtain derivatives with pronounced antiviral, antimicrobial, anti-inflammatory, antitumor, and hepatoprotective activities [3,4,5]. At present, the conversion of triterpenoids is mainly carried out by chemical methods that require extreme acidity and temperature, and expensive catalysts to protect the reactive centers of the molecule [6].

Figure 1.
Structural formula of OA.
Along with chemical modification, there have been attempts to biologically transform OA. Due to the current state of enzymatic chemistry and organic synthesis, the processes of biotransformation of polycyclic compounds are no longer considered exotic. Enzyme systems, the entire metabolic system of a microbial cell, and semi-synthetic bioreagents are often used as stable catalysts to transform these substances. This is due to their exceptional chemo- and stereoselectivity, a variety of metabolizable substrates, minimal side reactions, and unnecessary multiple protection and deprotection steps. The vast majority of the processes of biological transformation of OA are carried out using filamentous fungi and results in the formation of various hydroxy-, oxo-, and glycoside derivatives [7,8,9]. However, fungus-catalyzed processes are characterized by relatively low (2.6 to 43.2%) substrate conversion, are technologically impractical, and are unsafe due to the mycelial growth of fungi and the mycotoxins with pronounced mutagenic and carcinogenic effects they can produce. Examples of bacterial transformations of OA are rare and include processes catalyzed by representatives of the genera Bacillus, Nocardia, and Streptomyces, with many strains having pathogenic properties. In addition, bacteria exhibit catalytic activity against OA at a triterpenoid concentration no higher than 0.3 g/L [10,11,12]. In this regard, it seems timely to search for new non-pathogenic bacterial catalysts for the directed transformation of OA.
One of the groups of microorganisms that has been actively developed in biotechnology is non-pathogenic actinomycetes of ecologically significant species capable of transforming a variety of complex hydrophobic substrates [13]. These microorganisms are characterized by typical bacterial growth, a labile metabolic system, polyfunctionality, the synthesis of biosurfactants, growth on minimal media, and high catabolic activity under extreme environmental conditions. Taken together, these properties offer encouraging prospects for the use of non-pathogenic actinomycetes for OA biotransformation. Moreover, we have previously shown the ability of some members of Rhodococcus spp. for the directed conversion of pentacyclic triterpenoids, on the example of betulin, resulting in betulone with a pronounced biological activity [14].
2. Results and Discussion
2.1. Biotransformation of OA and Effects of OA on Rhodococcal Cells
In this study, 148 actinomycete strains from the Regional Specialised Collection of Alkanotrophic Microorganisms (acronym IEGM, Perm, Russia; WFCC # 285) were employed. An initial screening demonstrated that only a few strains belonging to the species R. jostii (1 strain), R. opacus (4 strains), R. rhodochrous (5 strains), and R. ruber (2 strains) were able to utilize 1.0 g/L OA as a sole source of carbon and energy. R. rhodochrous IEGM 757, isolated from oil-contaminated soil (Perm Krai, Russia), proved to be the most effective OA-transforming strain and was selected for further studies.
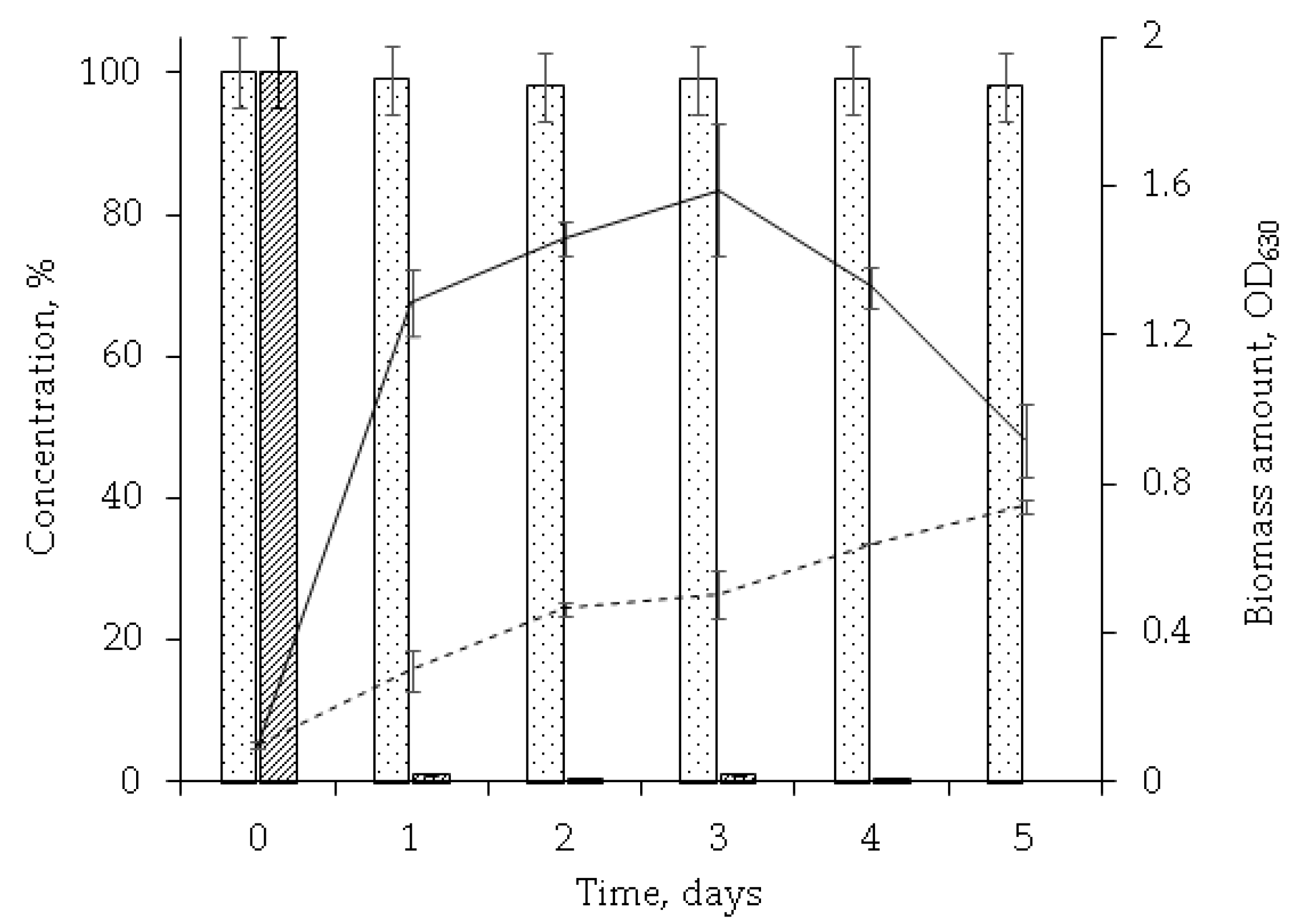
Studies of the dynamics of the OA concentration decrease and cell biomass increase showed that the maximum catalytic activity of rhodococci was on the first day accompanied by nearly complete (more than 98%) OA degradation and significantly increased biomass (OD630 0.1 to OD630 1.3) (Figure 2). The fifth day featured a decreased number of viable cells (to OD630 0.9) while no residual OA was detected in the extracts. It was considered as the completion of the biotransformation process.
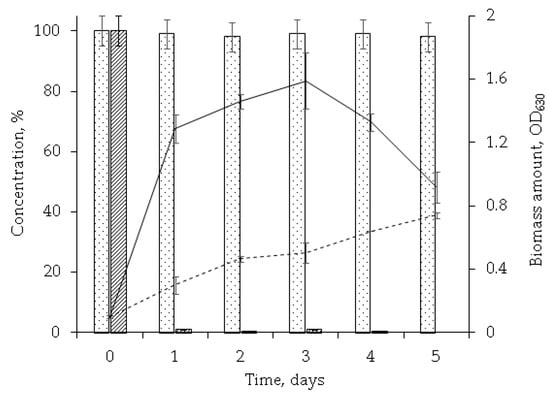
Figure 2.
The dynamics of OA transformation by R. rhodochrous IEGM 757 ( ), abiotic control (
), abiotic control ( ). OD630 in the presence of OA (
). OD630 in the presence of OA ( ) and without it (
) and without it ( ). HPLC data of the post-cultural extracts are presented.
). HPLC data of the post-cultural extracts are presented.
 ), abiotic control (
), abiotic control ( ). OD630 in the presence of OA (
). OD630 in the presence of OA ( ) and without it (
) and without it ( ). HPLC data of the post-cultural extracts are presented.
). HPLC data of the post-cultural extracts are presented.
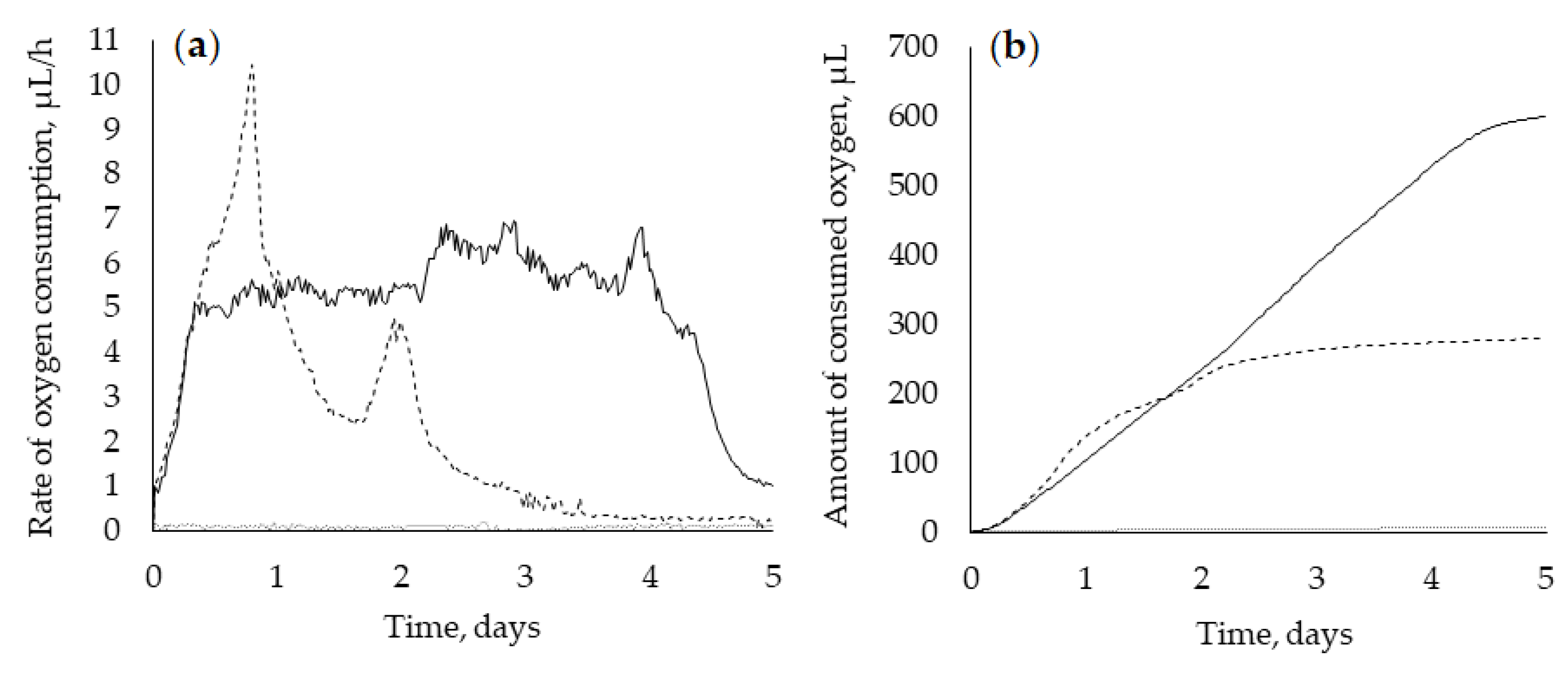
The respiratory activity of bacterial cells during the OA biotransformation was characterized by a sharp increase (0.9 to 5 µL/h) in the rate of oxygen consumption during the first day, which correlates with the maximum catalytic activity of the cells. The same trend of maximum respiration and maximum catalytic activity correlation was previously revealed during the rhodococcal transformation of another triterpenoid betulin [14]. The values of the rate of oxygen consumption remained at the same level during the next four days (Figure 3a) while the decreased amount of biomass on the fifth day (see Figure 2) was accompanied by a drastic drop in the rate of oxygen consumption (Figure 3a). The total amount of oxygen consumed on fourth and fifth days in the presence of OA was twice as much as in the biotic control (Figure 3b). This indicates stable metabolic activity and high viability of R. rhodochrous IEGM 757 cells in the presence of a structurally complicated hydrophobic triterpene substrate.

Figure 3.
The rate of oxygen consumption (a) and amount of oxygen consumed (b) by R. rhodochrous IEGM 757 during OA biotransformation ( ). Biotic (
). Biotic ( ) and abiotic (
) and abiotic ( ) controls.
) controls.
 ). Biotic (
). Biotic (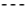 ) and abiotic (
) and abiotic ( ) controls.
) controls.
It should be noted that in the abiotic control, respiration and the rate of OA transformation were almost zero (Figure 2 and Figure 3), which suggests biocatalytic oxidation of the triterpenoid.
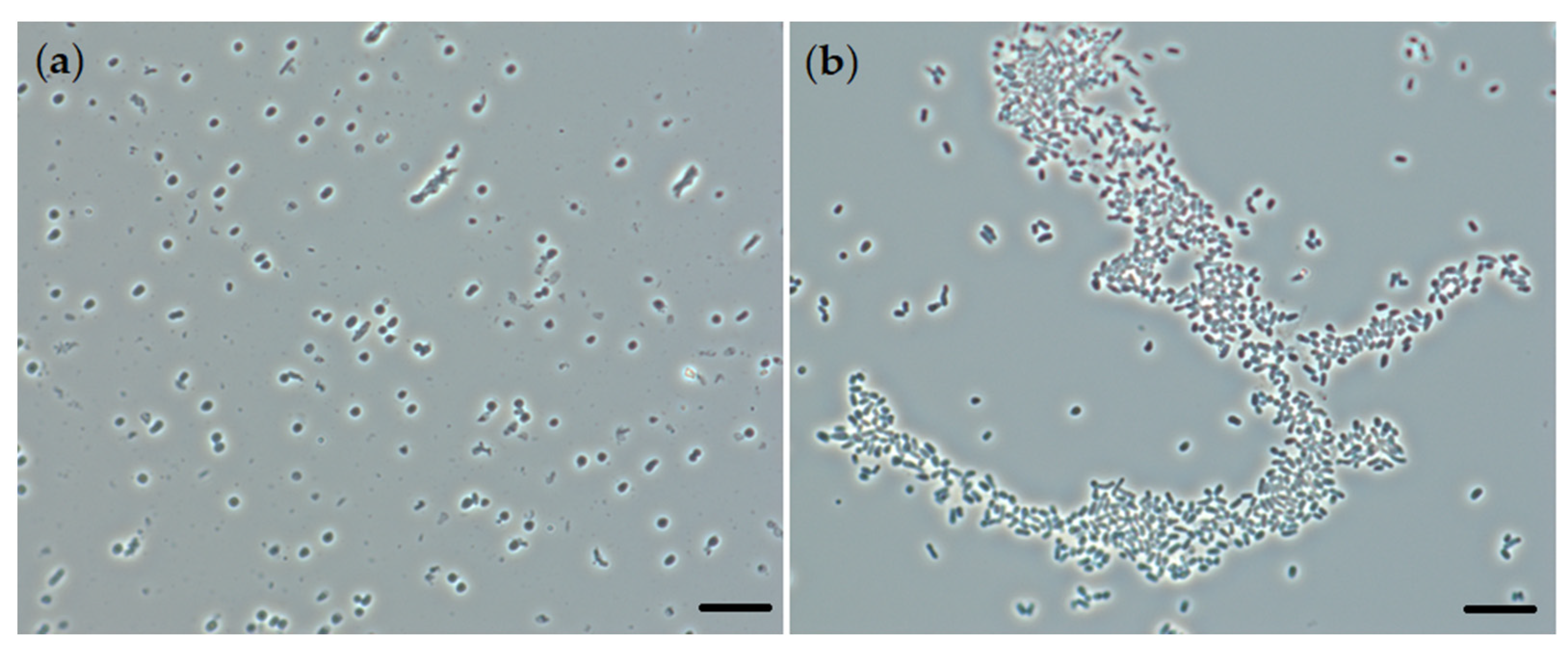
Using phase-contrast microscopy, it was shown that rhodococci form isolated cell aggregates on the surface of crystalline particles of the triterpenoid during OA biotransformation (Figure 4). Aggregation is one of the typical response mechanisms of bacterial cells to non-optimal environmental conditions [15,16]. Apparently, the formation of aggregates determines the stable metabolic activity of rhodococci in relation to structurally complicated hydrophobic substrates, providing high catalytic activity under conditions when single cells are not able to divide and convert the substrate. Analysis of the morphometric parameters of rhodococci did not reveal significant changes in the cell size in the presence of OA (Table 1).

Figure 4.
Phase-contrast microscopy (×1000) of R. rhodochrous IEGM 757 cells ((a), biotic control) in the presence of OA (b). Bars: 4 µm.

Table 1.
Morphometric parameters of R. rhodochrous IEGM 757 cells.
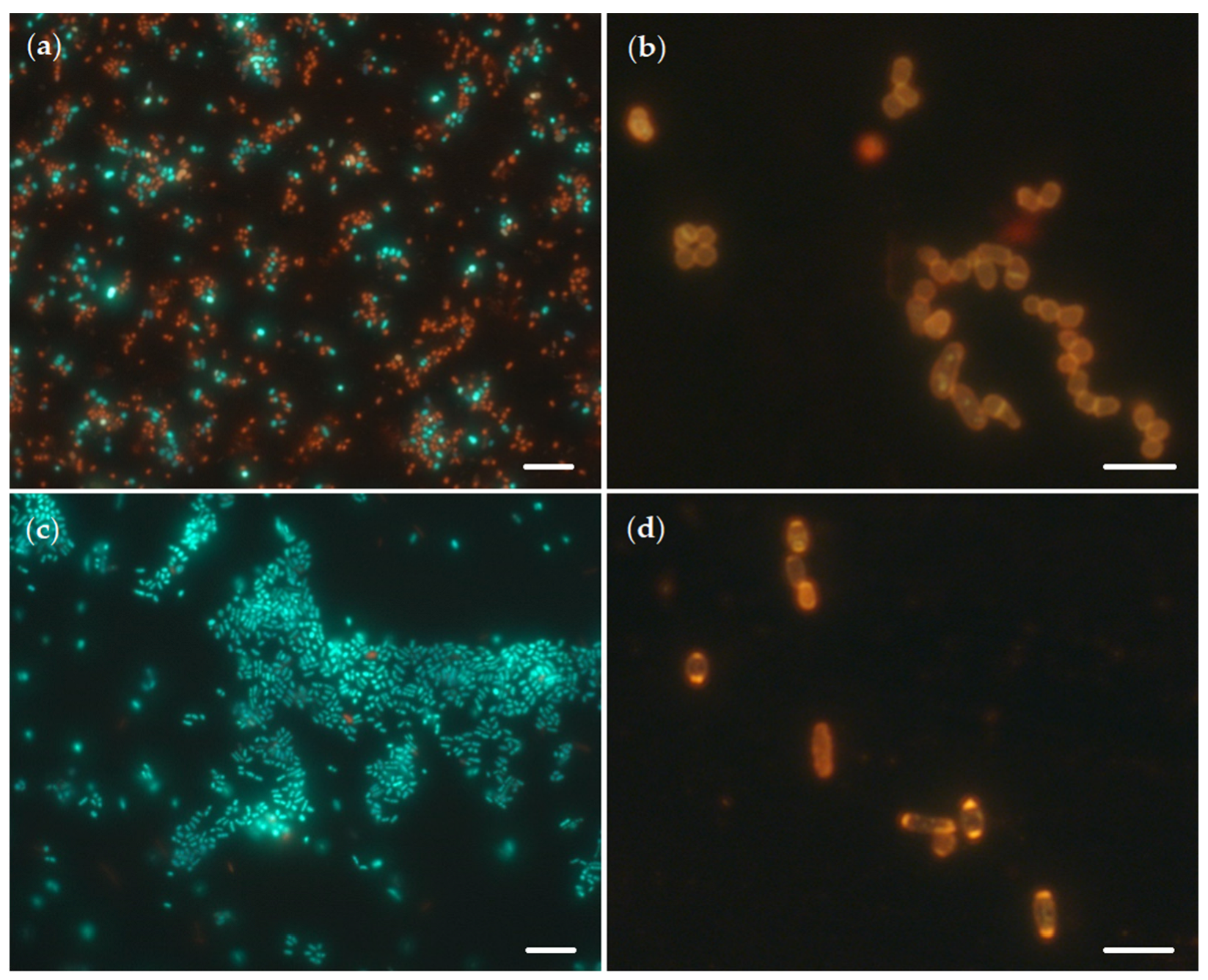
Staining of R. rhodochrous IEGM 757 cells with Live/Dead and Nile Red dyes and subsequent fluorescent microscopy confirmed our assumption of high viability and resistance of rhodococci to OA (Figure 5). Thus, the formed aggregates mainly consisted of living (green) cells (Figure 5c); the number of intracellular lipid inclusions, as an indicator of the state of bacterial stress [17], did not differ from the control (Figure 5d).

Figure 5.
Fluorescent microscopy (×1000) of R. rhodochrous IEGM 757 cells stained with Live/Dead (a,c) and Nile Red (b,d) dyes in the presence of OA (c,d) and without it (a,b). Bars: 8 µm (a,c); 3 µm (b,d).
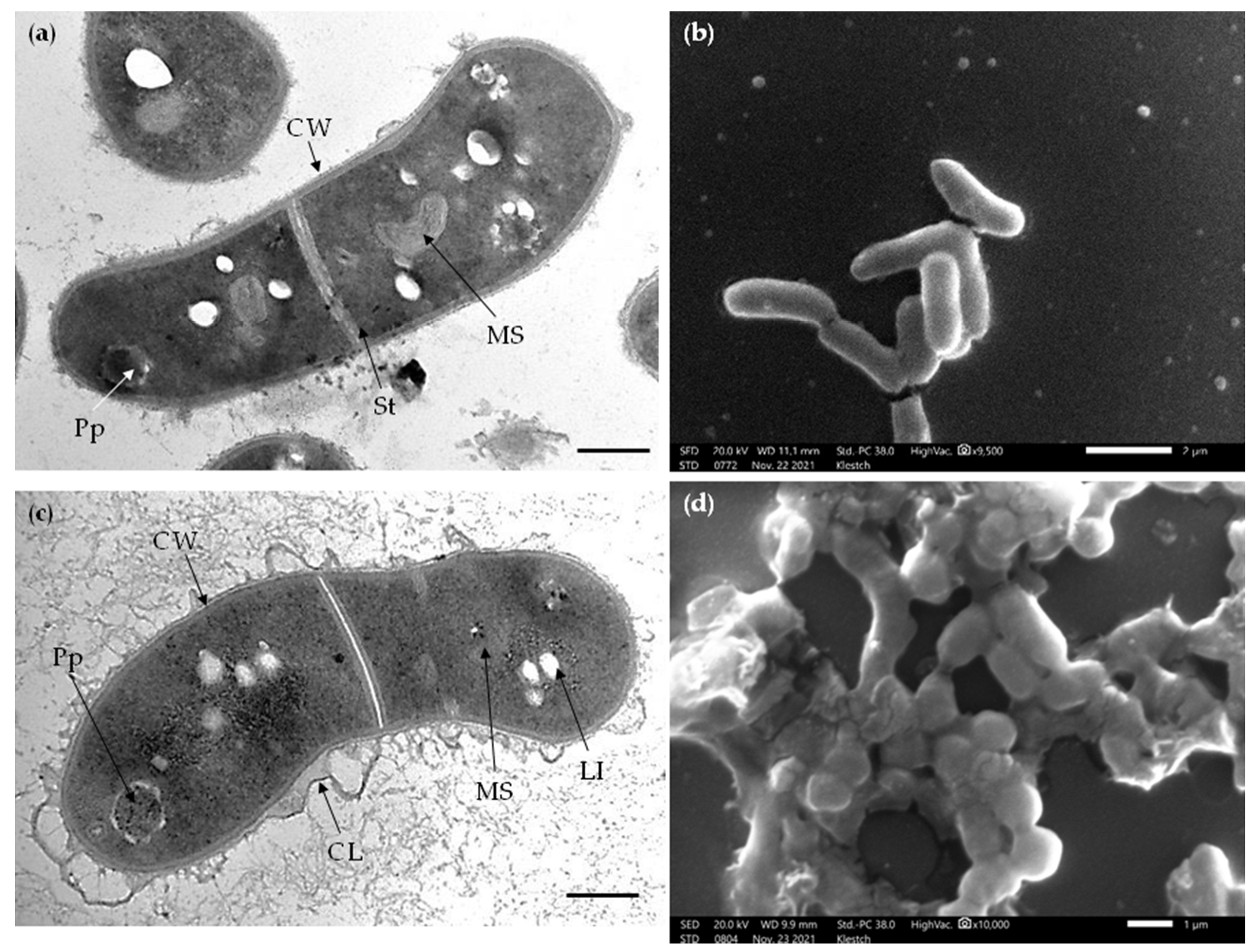
Transmission electron microscopy (TEM) of ultrathin sections showed similar morphological features of intact R. rhodochrous IEGM 757 cells grown in the control medium and in the presence of OA (Figure 6). Dividing cells had the outer capsular layer (visible as outgrowths) and the stratified cell wall with layers of varying electron density, common for rhodococci. A finely grained and dense cytoplasm contained small membrane-like structures and few electron-dense particles (probably, polyphosphates) and electron-transparent lipid inclusions. Cell division septa were located near the mid-cell and are visible as two electron-dense and transparent layers (Figure 6). Notably, OA-grown cells had a more curved cell wall profile and numerous capsular outgrowths and were surrounded by vast material with a net-like structure than the cells in the control variant (Figure 6). In addition, scanning electron microscopy (SEM) imaging showed a rougher cellular surface and a more typical agglomeration of cells with adhesion signs in OA-amended cultures than in the control (Figure 6).
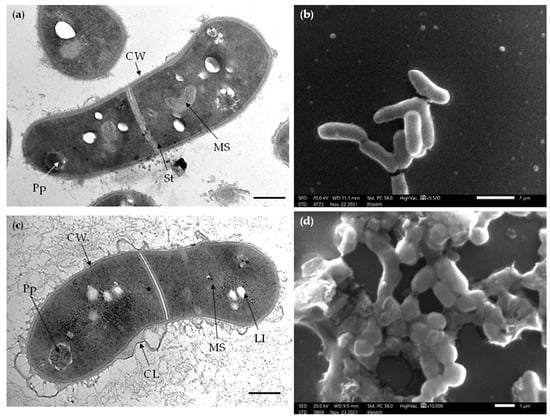
Figure 6.
TEM images of ultrathin sections (a,c) and SEM images of whole cells (b,d) of R. rhodochrous IEGM 757 grown in the control medium (a,b) and in the presence of OA (c,d). Designations: CL, capsular layer; CW, cell wall; Pp, polyphosphate granules; LI, lipid inclusions; MS, membrane-like structures; St, cell division septa. Bars: (a,c) 200 nm; (b) 2 µm; (d) 1 µm.
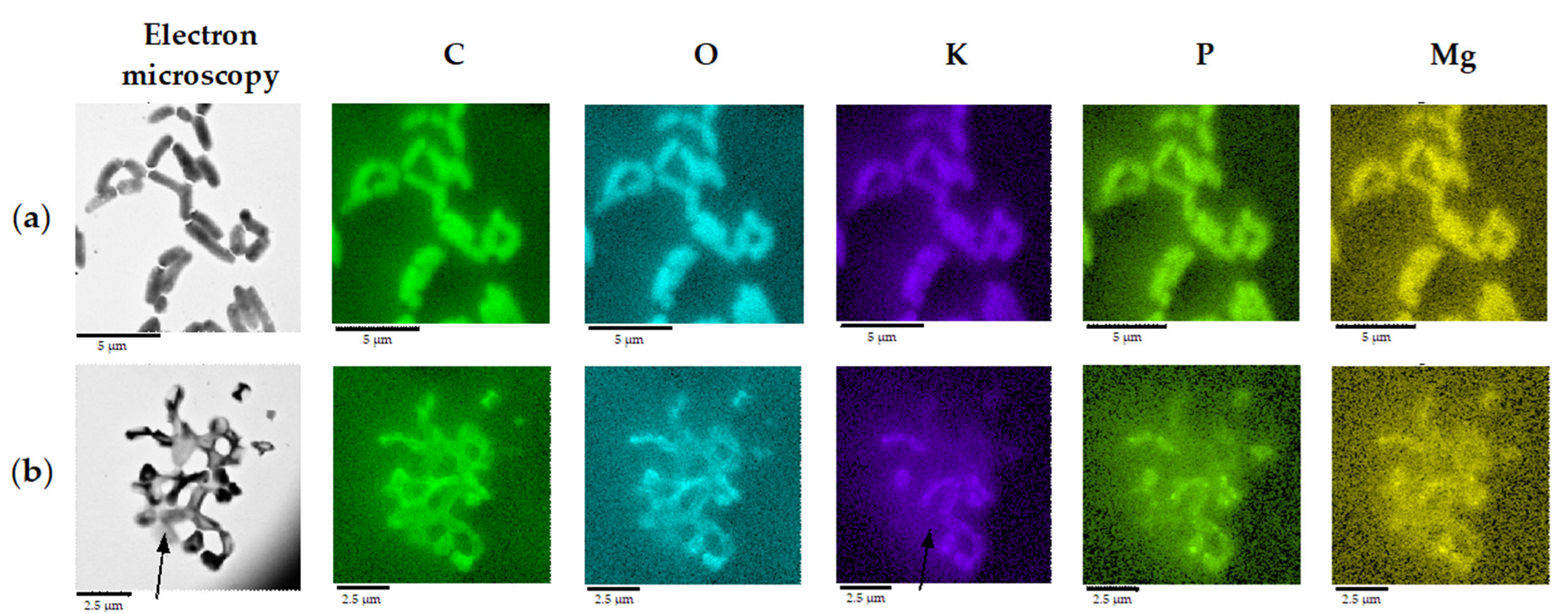
TEM with energy-dispersive X-ray spectroscopy (TEM-EDX) and elemental mapping demonstrated that a minor part of cells in OA cultures underwent a cytotoxic effect as judged by a depleted K+ pool (Figure 7), likely due to a loss of cytoplasmic membrane integrity. Numerous intact cells had zones of increased intracellular P and Mg accumulation near the cell pole (Figure 7), where electron-dense granules composed of polyphosphates were resolved by TEM (Figure 6).
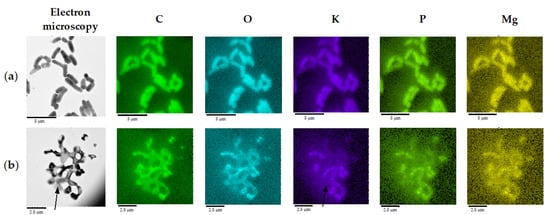
Figure 7.
Elemental composition of R. rhodochrous IEGM 757, as determined by mapping using X-ray microanalysis. The distribution of individual chemical elements is marked with colors. Cells were grown in: (a) the control medium; and (b) in the presence of OA. Arrows indicate a cell with an extinct K+ pool. Loci of increased intracellular P and Mg accumulation are seen as bright dots. Bars: (a) 5 µm; (b) 2.5 µm.
Hence, the results of our microscopy examinations highlight some ultrastructural features important for R. rhodochrous physiology and adaptation. The accumulation of intracellular lipid granules and the production of polyhydroxyalkanoates (PHAs) have already been reported for rhodococci grown on hydrocarbon substrates, and PHAs can be used as a reserve, enabling cells to function normally under stressful conditions or a nutritional imbalance or deficiency [17]. An expanded total cell surface, as with cultures grown with OA, can be important for binding and further transformation of a hydrophobic substrate. The formation of polyphosphate granules within cells is a common case when bacterial cultures grow on rich nutrient or selective media with hydrocarbons. Polyphosphates can be a P reserve and provide a competitive advantage for rhodococci in natural and under suboptimal conditions [18].
2.2. Identification and In Silico Analysis of OA Metabolite
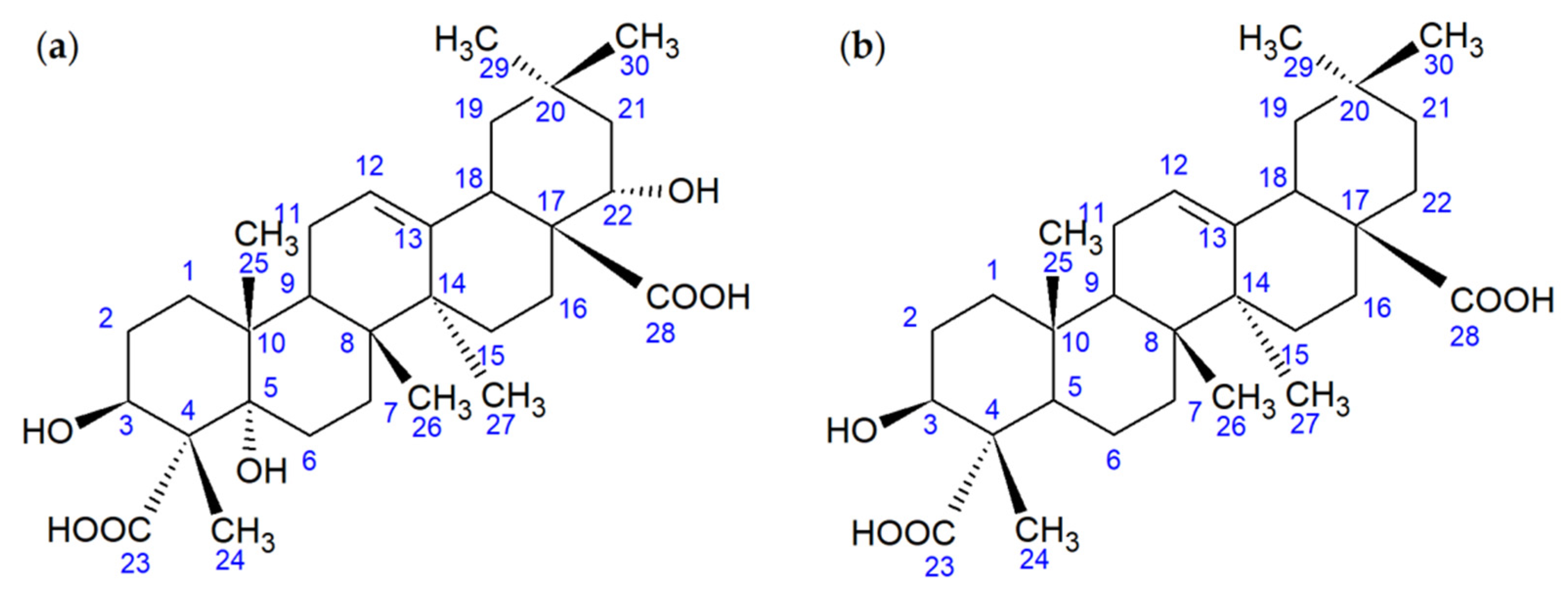
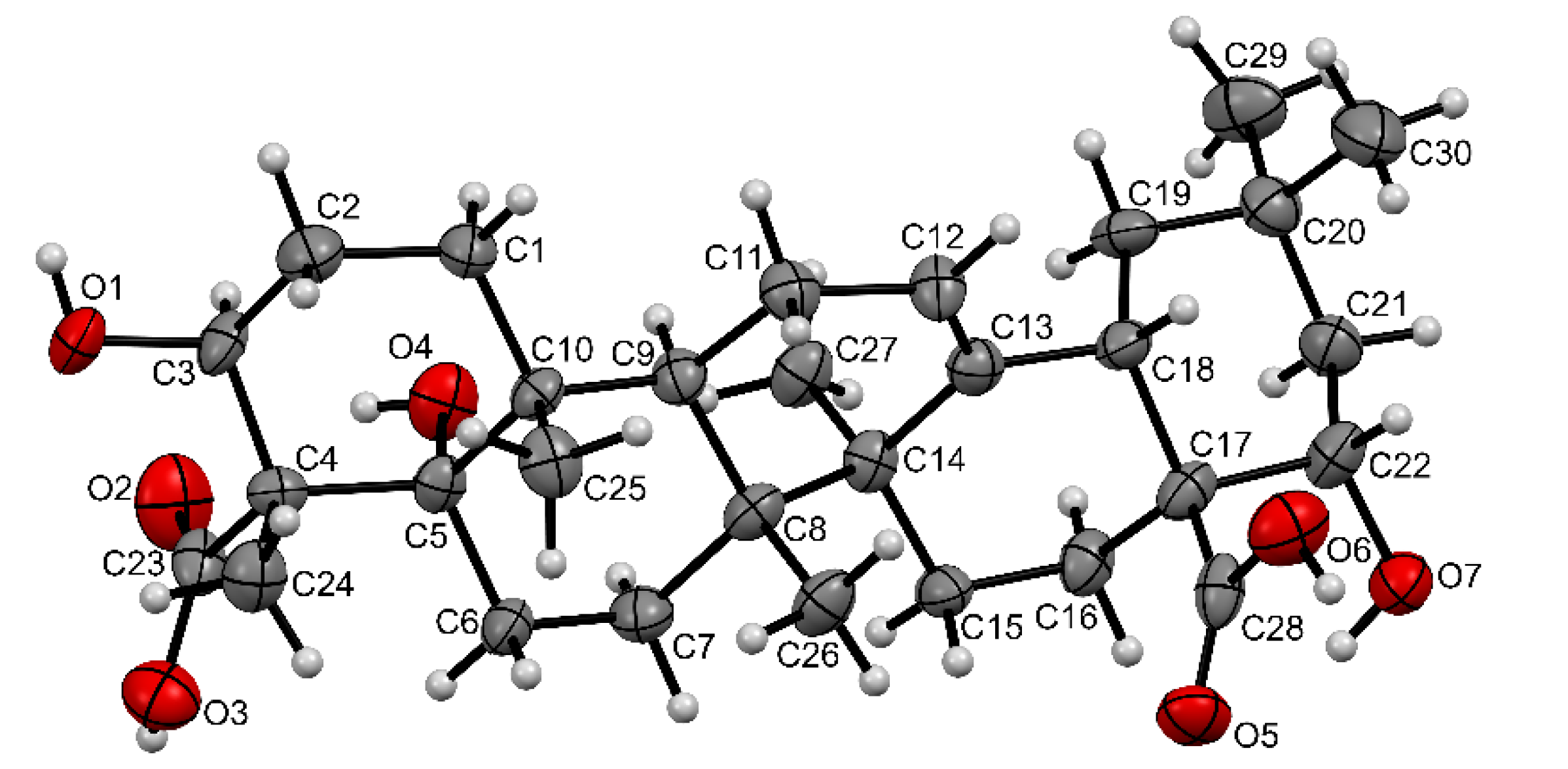
When studying the extracts obtained at the end of the biotransformation process (the fifth day), the formation of a compound with an Rf value of 0.01 was detected by TLC. The compound was isolated individually by column chromatography. Using NMR and X-ray diffraction methods, this OA metabolite was identified as a 5α,22α-dihydroxy derivative of gypsogenic acid (3β,5α,22α-trihydroxyolean-12-ene-23,28-dioic acid, Figure 8).
Figure 8.
Structural formulas of the OA metabolite (a) and gypsogenic acid (b).
It was found that the 13C NMR spectrum was the most informative for determining the structural changes of OA during biotransformation. This spectrum revealed the characteristic signals of three carbon atoms (76.50, 70.21, and 69.70 ppm) associated with tertiary or secondary hydroxyl groups, the signals of the carbon atoms of two carboxyl groups (179.92 and 177.16 ppm), and the signals of the carbon atoms of the double bond (143.53 and 121.46 ppm) in the structure of the OA metabolite. In turn, the 1H NMR spectrum contained the signals of only six methyl groups (1.20, 1.15, 1.02, 0.96, 0.92, and 0.75 ppm), a characteristic signal of the olefinic proton at 5.17 ppm, and two proton signals at carbon atoms close to secondary hydroxyl groups: a doublet of doublets with a center at 3.79 ppm and a multiplet with a center at 4.35 ppm. The final assignment of the OA metabolite structure as a new 5α,22α-dihydroxy derivative of gypsogenic acid (3β,5α,22α-trihydroxyolean-12-ene-23,28-dioic acid) was performed using the X-ray diffraction method (Figure 9).
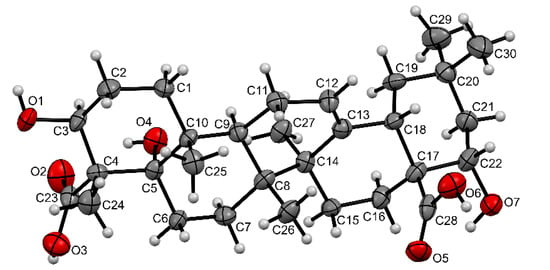
Figure 9.
Molecular structure of the OA metabolite as represented by non-hydrogen atoms (30% probability ellipsoids). Solvent and water molecules are not shown.
It should be noted that OA biohydroxylation at C5 by microorganisms was found for the first time. Although, we registered a similar reaction during the bioconversion of the tricyclic diterpenoid dehydroabietic acid by R. erythropolis IEGM 267 cells [19]. Microbial carboxylation of OA at C23 was also observed for the first time, allowing the production of gypsogenic acid derivatives. Gypsogenic acid is a rare triterpenoid produced in small amounts by Miconia [20] and Gypsophila [21] species, and by a transgenic yeast strain expressing plant cytochromes [22]. At the same time, the formation of gypsogenic acid and its derivatives from the more accessible OA is of practical interest. According to literature data, gypsogenic acid exhibited moderate cytotoxic, antibacterial, and tryponocidal properties [20,21,23], which can be enhanced by additional functionalization, in particular, the introduction of hydroxyl groups.
In silico analysis of the obtained metabolite showed that 5α,22α-dihydroxy-derivative of gypsogenic acid presumably has significantly (more than 700 times) increased solubility in water compared to the native OA and is also characterized by the reduced acute and chronic ecotoxicity in relation to water bodies (Table 2).

Table 2.
Estimated ecotoxicity and solubility of OA and its metabolite.
The assessment of potential bioactivity (Table 3) showed that the OA metabolite can act as an apoptosis agonist (0.908) and an insulin promoter (0.906) and can exhibit chemoprophylactic (0.892) and analgesic effects (0.778) with a high degree of probability.

Table 3.
Estimated biological activity of OA and its metabolite.
2.3. Determination of Enzyme Systems Involved in OA Bioconversion
Currently, the main enzyme systems that catalyze the transformation of OA in plant tissues have been widely studied [24], whereas data on microbial enzymes responsible for the conversion of triterpenoids and their localization are few and fragmentary [25]. Using individual cell fractions of R. rhodochrous IEGM 757, it was shown that membrane-bound enzymes are involved in the process of OA conversion. Enzymes extracted with a detergent catalyzed the degradation of the triterpenoid not leading to metabolites while enzyme complexes firmly bound to the membrane catalyzed the conversion of OA with the formation of derivatives (Table 4).

Table 4.
Composition of extracts obtained after OA biotransformation by individual cell fractions.
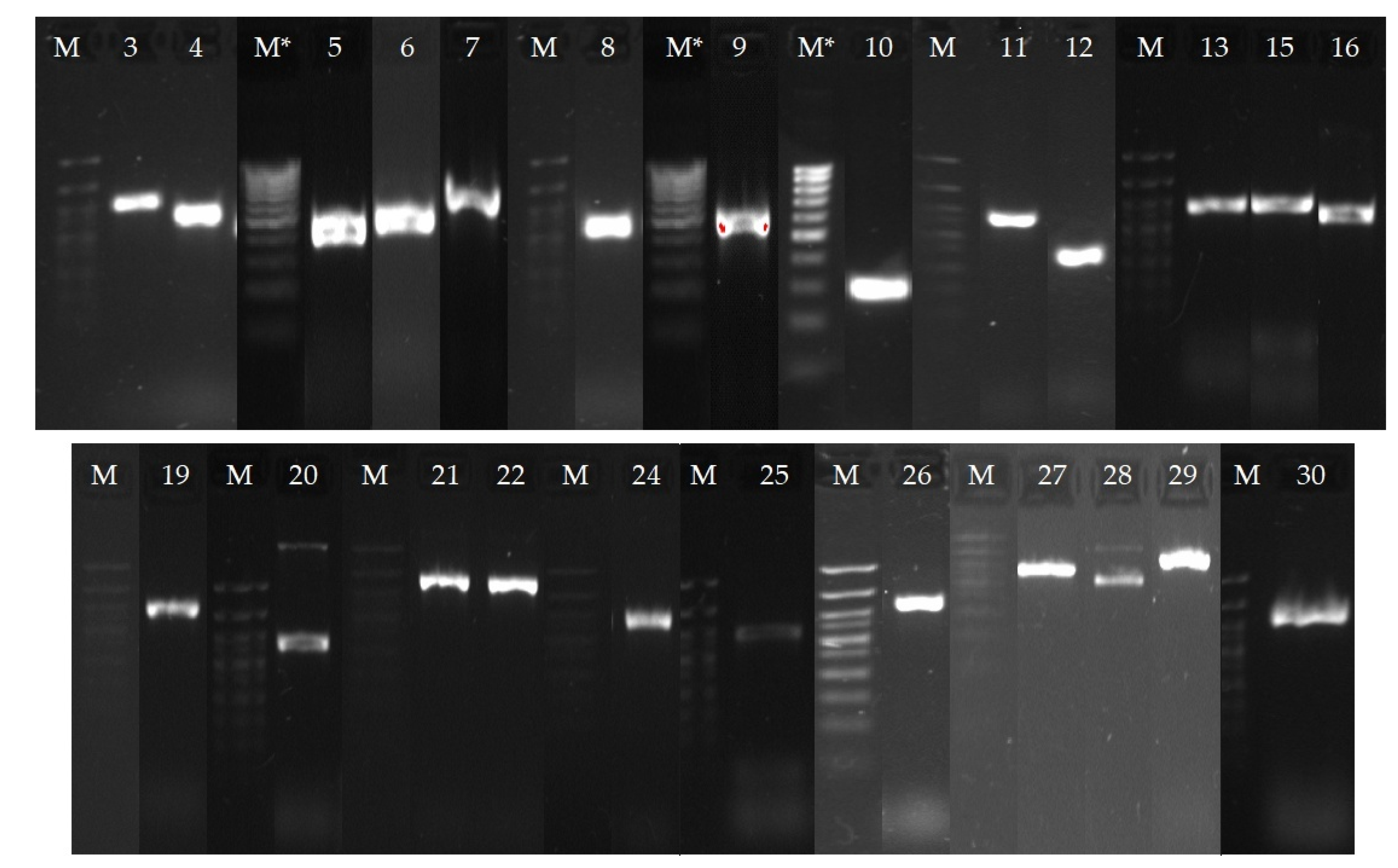
NGS and annotation of the resulting sequences in the NCBI database made it possible to carry out a bioinformatics search for genes presumably involved in the process of OA bioconversion. It is known that the processes of actinomycelial transformation of terpenes involve enzymes of the CYP450 family, which catalyze various oxidation reactions [26]. We detected 30 genes encoding CYP450 in the genome of R. rhodochrous IEGM 757 (Table 5). Using specific primers (Table S1), real-time PCR and subsequent gel electrophoresis allowed the detection of 24 of the putative genes in the genome (Figure 10). The data obtained provided the prerequisites for further study of rhodococci transcriptomes in order to determine the functional genes of OA conversion.

Table 5.
R. rhodochrous IEGM 757 genes encoding the CYP450 enzymes.
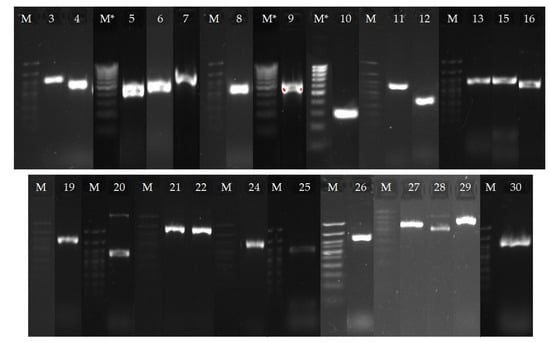
Figure 10.
Gel electrophoresis of real-time PCR products with specific primers for genes encoding CYP450: M is a marker of DNA lengths from 700 to 50 bp (bands from top to bottom: 700, 500, 400, 350, 300, 250, 200, 150, 100, 50 bp), M * is a marker of DNA lengths from 1000 to 100 bp (bands from top to bottom: 1000, 900, 800, 700, 600, 500, 400, 300, 200, 100 bp). The gene bands should be compared with the corresponding marker bands on the left. The gene numbers are the same as the gene numbers in Table 5.
3. Materials and Methods
3.1. Microorganisms
Initial screenings included 148 strains, catalytically active towards complex hydrophobic organic compounds, from the Regional Specialised Collection of Alkanotrophic Microorganisms (acronym IEGM, Perm, Russia; World Federation for Culture Collections # 285, http://www.iegmcol.ru/strains, accessed on 20 September 2022) [27]. Previously, the strains were taxonomically affiliated based on their phenotypic and chemotaxonomic properties and were represented by Brevibacterium sp. (3 strains), Corynebacterium ammoniagenes (1 strain), C. glutamicum (1 strain), Glutamicibacter nicotinas (1 strain), Gordonia terrae (4 strains), Micrococcus luteus (2 strains), M. lylae (1 strain), Micrococcus sp. (2 strains), Paeniglutamicibacter sulfureus (1 strain), Rhodococcus aetherivorans (1 strain), R. cercidiphylli (1 strain), R. erythropolis (32 strains), R. fascians (19 strains), R. jostii (3 strains), R. koreensis (1 strain), R. opacus (15 strains), R. qingshengii (2 strains), R. rhodochrous (27 strains), and R. ruber (31 strains) (Table S2).
3.2. Reagents
High-purity (≥97%) OA (CAS 508-02-1) manufactured by Acros Organics (Branchburg, NJ, USA) was used in the experiments. Chemical reagents, including acetonitrile, dimethyl sulfoxide (DMSO), diethyl ether, methanol, chloroform, ethyl acetate, and n-hexane, were of chemically pure or analytically pure grades (Cryochrome, St. Petersburg, Russia; Merck, Darmstadt, Germany; Sigma-Aldrich, St. Louis, MO, USA). A Millipore Simplicity Personal Ultrapure Water System (Millipore, Burlington, MA, USA) was used to obtain ultrapure water.
3.3. Cultivation Conditions
Biotransformation was carried out under batch cultivation conditions on a Certomat IS orbital shaker (Sartorius, Göttingen, Germany) at 28 °C and 160 rpm in mineral medium K containing (g/L): K2HPO4—1.0, KH2PO4—1.0, KNO3—1.0, NaCl—1.0, MgSO4—0.2, CaCl2—0.02, and Postgate trace element solution (0.1 vol.%) [28]. OA was dissolved in DMSO (1 mg:10 µL) and used at a concentration of 1.0 g/L.
3.4. Controls
An inoculated medium with yeast extract (0.1 g/L, FBIS SRCAMB, Obolensk, Russia) was used as a biotic control. Non-inoculated medium with OA was used as an abiotic control.
3.5. Preparation of Individual Cell Fractions
Rhodococcal cells pre-grown for 2 days in the meat-peptone broth were washed three times and resuspended in the phosphate-alkaline buffer (pH 7.0). The cell suspension was homogenized using a Soniprep 150 ultrasonic disintegrator (MSE, Heathfield, UK, 10 µm amplitude for 45 min). The cell homogenate was centrifuged at 6000× g rpm and 4 °C for 15 min to obtain cytoplasmic enzymes (supernatant) (I). Membrane-bound enzymes were solubilized by resuspending the precipitate in 100 mL of a 1% Triton X-100 solution (Sigma-Aldrich, St. Louis, MO, USA) in the phosphate-alkaline buffer (pH 7.0) and stirring on the orbital shaker for 30 min. The supernatant with extracted membrane-bound enzymes (II) was obtained by centrifugation. After sonication, the cell precipitate with enzymes strongly bound to the membrane and non-extractable with a detergent (III) was resuspended in 100 mL of the phosphate-alkaline buffer (pH 7.0). The prepared cell fractions included (I) a supernatant with cytoplasmic enzymes; (II) a supernatant with extracted membrane-bound enzymes; and (III) a resuspended cell precipitate with non-extractable enzymes.
3.6. Microscopy
3.6.1. Phase-Contrast and Fluorescent Microscopy
Bacterial cells were visualized, and cell sizes were measured using an Axio Imager M2 optical microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) in a phase-contrast or fluorescent mode (light filters FS 106) with ×1000 magnification. The volume (V) and area (S) of cells were calculated using Equations (1) and (2) [29]:
where r is ½ of the cell width, µm; π is 3.14; and h is the cell length, µm.
V = r2πh;
S = 2r2π + πrh,
3.6.2. Scanning and Transmission Electron Microscopy
Cells were harvested from the cultures grown for 72 h on agar media, washed, and suspended in sterile water.
For SEM, 10- to 1000-fold diluted cell suspensions were fixed with 1.5% (w/v) glutaraldehyde and washed, dropped onto coverslips, and air-dried. The coverslips were mounted on stubs and coated with Au in a JFC-1100 ion sputter (Jeol, Tokyo, Japan). Specimens were examined in a JSM-IT200 electron microscope (Jeol, Tokyo, Japan).
For TEM, cells were pelleted by centrifugation (3400× g) and fixed in 2.5% glutaraldehyde (w/v) in 0.1 M sodium cacodylate buffer (pH 7.2) for 2.5 h, and then post-fixed in 1% (w/v) osmium tetroxide in the same buffer. The fixed material was dehydrated through a series of ethanol solutions, including absolute ethanol saturated with uranyl acetate, and embedded in araldite. Thin sections were prepared on an 8800 Ultrotome III (LKB-Produkter, Stockholm, Sweden) and stained with lead citrate. Ultrathin sections were examined in a JEM-1400 electron microscope (Jeol, Tokyo, Japan).
3.7. Cell Viability Tests
To assess the abundance of metabolically active cells, the cell suspension (100 µL per well) and a 0.2% aqueous solution of iodonitrotetrazolium chloride (50 µL per well) were added to microplates and incubated at 28 °C for 2 h. The optical density (λ = 630 nm) of formazane was recorded on a Multiskan Ascent microplate spectrophotometer (Thermo Electron Corporation, Waltham, MA, USA).
To differentiate living and dead cells, the bacterial suspensions were stained with a fluorescent dye Live/Dead® BacLightTM Bacterial Viability Kit (Invitrogen, Carlsbad, CA, USA), air dried in the dark for 10–15 min, and washed with deionized water to remove residual dye. The stained cells were visualized by fluorescent microscopy.
3.8. Nile Red Staining of Bacterial Cells
To detect intracellular lipid inclusions, bacterial cells were stained with 0.08% Nile Red solution in DMSO (Nanjing Dulai Biotechnology Co., Nanjing, China) as previously described [30]. For this, the cell suspension (1 mL) was centrifuged at 12,000× g rpm for 5 min. The precipitated cells were resuspended in 1 mL of distilled water and supplemented with 40 μL of the working solution of Nile Red (0.3 μg/mL, final concentration). The resulting suspension was incubated at 28 °C for 40 min with shaking at 160 rpm. Cells were separated from the reaction medium by centrifugation and resuspended in 1 mL of distilled water. The cell suspension (0.02 mL) was spread on a clean glass slide, and fluorescence was read at two spectral settings on an Axio Imager M2 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany): yellow-gold fluorescence using a 450–500 nm band pass exciter filter and red fluorescence using a 515–560 nm band pass exciter filter.
3.9. Energy-Dispersive X-Ray Spectroscopy with Elemental Mapping
Unfixed suspensions of cells in sterile water were applied onto Formvar-coated and carbon-reinforced copper grids and air-dried. TEM-EDX with elemental mapping was performed using a JEM-1400 microscope (Jeol, Tokyo, Japan) equipped with an energy-dispersive X-ray analysis system (EDXA, Inca Energy-350, Oxford Instruments, Abingdon, UK), operating at accelerating voltage of 80 keV (tilt angle, 15°). EDX spectra and elemental maps were obtained using AZtec software (Oxford Instruments, Abingdon, UK).
3.10. Respiration Activity
The respiration activity of cells (100 mL per 300-mL vial) was recorded using a 10-channel Columbus Micro-Oxymax respirometer (Columbus Instruments, Columbus, OH, USA) with stirring (300 rpm, 28 ± 2 °C) on an Ikamag® RO10 power magnetic stirrer (IKA-Werke, Staufen, Germany). The amount (µL) and rate (µL/h) of oxygen consumed were automatically recorded every 30 min.
3.11. New-Generation Sequencing
Whole-genome sequencing was performed by the biotechnology company CeGaT GmbH (Tübingen, Germany) using the Illumina NovaSeq (Illumina, San Diego, CA, USA) and the SPAdes v. 3.14.1 genome assembly method. The complete genome of R. rhodochrous IEGM 757 was included in the NCBI database under the accession number JAJNCO000000000.1.
3.12. Bioinformatics Analysis
The search for functional genes encoding enzymes of the CYP450 group, presumably involved in the process of triterpenoid biotransformation, was carried out in the NCBI database based on an automatically annotated complete genome of the strain capable of biotransformation. Pairwise comparison of sequences and selection of primer pairs were performed using the BLASTN and Primer-BLAST services, respectively, available from the NCBI website.
3.13. Real-Time PCR and Gel Electrophoresis
DNA isolation was performed using the biomass obtained by preliminary cultivation in meat-peptone broth for 2 days according to the standard protocol of the ExtractDNA Blood genomic DNA isolation kit (Eurogen, Moscow, Russia). The resulting purified DNA was used for real-time PCR with primers selected by bioinformatics analysis on a Real-time CFX Connect amplifier (Bio-Rad, Hercules, CA, USA). Species-specific primers for the 16S rRNA of Rhodococcus rhodochrous served as a positive control. The PCR protocol included the following steps and conditions:
- Step 1
- 95.0 °C; 3 min.
- Step 2
- 95.0 °C; 30 s.
- Step 3
- Gradient 55.0–65.0 °C; 30 s.
- Step 4
- 72.0 °C; 1:30 min.
Steps 2, 3, and 4 included 34 cycles.
- Step 5
- Melt Curve from 65.0 to 95.0 °C, increment of 0.5 °C; 5 s.
- Step 6
- 72.0 °C; 10 min.
The size of the nucleic acids and the presence of amplicons in the reaction mixture after PCR were determined by horizontal agarose gel electrophoresis (1.5% agarose in TBE buffer) using the Bio-Rad Gel Doc XR+ gel documentation system (Bio-Rad, Hercules, CA, USA). Electrophoretic separation was carried out at a voltage of 70 V for 40 min. Nucleic acids were stained with GelRed (Biotium, Fremont, CA, USA).
3.14. Quantitative and Qualitative Analysis of OA and Its Derivative
To extract the residual OA and its derivative, an equivalent volume of ethyl acetate was added to the cell suspension; after separation of the resulting emulsion, the ethyl acetate fraction was taken. Ethyl acetate was added to the remaining aqueous fraction for re-extraction; the procedure for separation and selection of fractions was repeated twice. Qualitative analysis was carried out by thin layer chromatography (TLC) on Alugram® Xtra SIL G/UV254 plates (Macherey-Nagel, Duren, Germany) in the n-hexane/ethyl acetate (1:1) system. For the detection of compounds, the plates were sprayed by 15% H2SO4 and heated at 100–120 °C for 2–3 min.
The OA degradation was assessed using high-performance liquid chromatography (HPLC) on a Kromasil 100-5-C18 reversed phase column (C18, 5 µm particle size, 100 Å pore size, 250 mm × 4.6 mm, Eka Chemicals AB, Sundsvall, Sweden). The eluent was a mixture of acetonitrile:deionized water in the ratio of 80:20. The flow rate was 1 mL/min, the temperature of the column thermostat was 40 °C, and the injected sample volume was 20 µL. The samples were preliminarily dissolved in isopropanol (Cryochrome, St. Petersburg, Russia). Calculation of the OA concentration during biotransformation was carried out according to Equation (3) derived from the calibration curve of the dependence of the OA concentration on the peak area:
where y is peak area, mAU; x is the OA concentration, %.
y = 7 × 10−6 × x − 1.2389,
3.15. Isolation and Identification of OA Metabolite
To extract the OA derivative, an equivalent volume of ethyl acetate was added to the cell suspension. After separation of the resulting emulsion, the ethyl acetate fraction was taken. Ethyl acetate was added to the remaining aqueous fraction for re-extraction; the procedure for the separation and selection of fractions was repeated twice. The ethyl acetate fractions were combined and evaporated on a Laborota 4000 rotary evaporator (Heidolph, Schwabach, Germany). The resulting residue (65.0 mg) was separated by column chromatography on Macherey-Nagel silica gel (60–200 μm, Duren, Germany). Residual OA (0.5 mg) and the OA metabolite (58.0 mg) were successively isolated by the mixture of n-hexane and ethyl acetate (from 9:1 to 4:1, v/v) as an eluent.
Optical rotation was measured on a Perkin Elmer 341 polarimeter (Perkin Elmer, Waltham, MA, USA) at 589 nm for a solution of the OA metabolite in CHCl3. The melting point was recorded using an OptiMelt MPA100 automated melting point system (Stanford Research Systems, Sunnyvale, CA, USA) with a heating rate of 1 °C/min. 1H, 13C, and DEPT NMR spectra were recorded on a Bruker AVANCE II 400 using DMSO-d6 as a solvent and hexamethyldisiloxane as an internal standard at 400 and 100 MHz, respectively.
3β,5α,22α-Trihydroxyolean-12-ene-23,28-dioic acid. Yellow needle crystal, mp 201 °C (ethyl acetate/chloroform, 1:0.1), Rf 0,01 (n-hexane/ethyl acetate, 1:1), = +20.8 (c 0.5, CHCl3). 1H NMR (400 MHz, DMSO-d6, δ, ppm, J/Hz): 5.17 (1H, m, H-12), 4.35 (1H, m, H-22), 3.79 (1H, dd, J = 11.2, 5.5 Hz, H-3), 1.20, 1.15, 1.02, 0.96, 0.92, 0.75 (each 3H, 6s, 6CH3). 13C NMR (100 MHz, DMSO-d6, δ, ppm): 179.92 (s, C-28), 177.16 (s, C-23), 143.53 (s, C-13), 121.46 (d, C-12), 95.39, 76.50 (s, C-5), 70.21 (d, C-3), 69.70 (d, C-22), 56.43, 51.45, 44.88, 42.55, 42.40, 42.11, 38.12, 38.01, 32.91, 31.43, 30.87, 26.46 (3C), 26.00, 25.74, 24.65, 23.11, 17.56, 16.89, 15.30, 12.98.
X-ray crystallographic data of the OA metabolite were obtained on an Xcalibur Ruby diffractometer (Agilent Technologies, Cheadle, UK) with a CCD detector by a standard method (MoKα-radiation, 295(2) K, ω-scan with step 1°). Absorption is taken into account empirically using the algorithm SCALE3 ABSPACK [31]. Crystal system (3β,5α,22α-trihydroxyolean-12-en-23,28-dioic acid dihydrate, C30H46O7∙2(H2O), M 554.70) is monoclinic, space group I2, a 14.588(5) Å, b 7.0874(19) Å, c 31.788(16) Å, β 102.25(4)°, V 3212(2) Å3, Z 4, dcalc 1.147 g·cm−3, μ 0.083 mm−1. The structures were solved by the SHELXS program [32] and refined by the full-matrix least-squares technique against F2 in the anisotropic-isotropic approximation using SHELXL [33] with a graphical interface OLEX2 [34]. The positions of H atoms were calculated with the riding model. Hydrogen atoms of water molecules are not localized but are taken into account in the empirical formula. A disordered molecule of an unidentified solvent was removed using the SQUEEZE procedure in the PLATON program [35] and was not taken into account in the empirical formula. Final parameters: R1 0.0890 (for 2140 reflections with I > 2σ(I)), wR2 0.2809 (for all 6600 independent reflections, Rint 0.0694), S 0.950. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 7 October 2022) with the CCDC number 2211937.
Crystal data of 3β,5α,22α-trihydroxyolean-12-ene-23,28-dioic acid: C30H46O7, M = 51,868, monoclinic, space group P21, at 296 K: a = 14.588(5), b = 7.0874(19), c = 31.788(16) Å, β = 102.25(4)°, V = 519.03(4) Å3, Z = 4, dcalc = 1.179 g·cm−3, µ = 0.083 mm−1, a total of 15,008 (θmax = 27,61°), 2411 unique (Rint = 0.0409), 2248 [I > 2σ(I)], 127 parameters. GooF = 1.015, R1 = 0.0332, wR2 = 0.0870 [I > 2σ(I)], R1 = 0.0365, wR2 = 0.0906 (all data), max/min diff. peak 0.18/−0.12 e·Å−3.
3.16. In Silico Analysis of OA and Its Metabolite
The ecotoxicity and solubility of OA and its derivative were calculated using the Ecological Structure Activity Relationship program (Ecological Structure Activity Relationship, EPA, Washington, DC, USA). The potential acute toxicity and chronic toxicity to aquatic organisms were predicted based on the available data on the toxic effects of organic compounds of various chemical classes using a computational analysis of structure-function relationship in molecules.
The estimated biological activities of OA and its metabolite were predicted based on their structural formulas using the PASS software (Prediction of Activity Spectra for Substances, http://www.pharmaexpert.ru/passonline/index.php accessed on 20 September 2022 [36]). Exploring the biological potential of substances generated a list of anticipated types of biological activity, including the evaluation of detection (Pa)/non-detection (Pi) probabilities of the latter. The highest probability of biological activity was taken as 1.
4. Conclusions
As a result of the experiments performed, the ability of Rhodococcus rhodochrous IEGM 757 to convert high (1.0 g/L) OA concentrations was shown. Cells of IEGM 757 catalyzed in one step the regio- and stereoselective introduction of two hydroxyl and one carboxyl groups into the non-activated positions of the molecule while retaining the native hydroxyl group and the double bond of OA with the formation of the not previously described compound 3β,5α,22α-trihydroxyolean-12-ene-23,28-dioic acid. The metabolite is highly likely to possess antitumor, antidiabetic, chemoprotective, and analgesic activities. The study of the morphophysiological features of the biocatalyst in the presence of OA showed that the concentration of OA used did not have negative effects on cells, giving grounds for the development of a method for the production of the OA metabolite. The use of next-generation sequencing in combination with bioinformatics analysis and real-time PCR allowed the identification of the genes encoding CYP450 enzymes in the R. rhodochrous IEGM 757 genome. The obtained data are useful for studying Rhodococcus transcriptomes in order to determine the functional genes of OA transformation and elucidate the biosynthetic pathways of the OA metabolite production.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12111352/s1, Table S1: Specific primers for R. rhodochrous IEGM 757 genes encoding CYP450 enzymes, Table S2: Collection strains used in the research.
Author Contributions
Conceptualization and methodology, I.B.I. and V.V.G.; writing—original draft preparation, N.A.L.; writing—review and editing, V.V.G. and I.B.I.; visualization, N.A.L., N.A.K., V.V.S. and A.L.M.; final conclusions, I.B.I., V.V.G. and N.A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 18-14-00140, and the Russian Foundation for Basic Research, grant number 20-34-90104, and partially supported by the Ministry of Science and Higher Education of the Russian Federation, State Assignment AAAA-A19-119112290008-4 and Agreement 075-15-2021-1051.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. The crystallographic data of the OA metabolite (CCDC 2211937) can be obtained free of charge from The Cambridge Crystallographic Data Centre.
Acknowledgments
The work was carried out using the equipment of the Core Facilities Centers “Regional Specialised Collection of Alkanotrophic Microorganisms” and “Research of Materials and Matter” at Perm Federal Research Center Ural Branch Russian Academy of Sciences. TEM and SEM microscopy and EDX analysis were performed in the Core Facility Center “UNIQEM Collection” at Research Center of Biotechnology, Russian Academy of Sciences. The authors gratefully acknowledge M.V. Dmitriev, Ph.D., Senior Researcher at Perm State National Research University, for X-ray diffraction analysis of OA metabolite.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91, e20190105. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Dubey, K.K. Hybrid approach for transformation for betulin (an anti-HIV molecule). In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–203. ISBN 9780444635044. [Google Scholar]
- Huang, L.R.; Luo, H.; Yang, X.S.; Chen, L.; Zhang, J.X.; Wang, D.P.; Hao, X.J. Enhancement of anti-bacterial and anti-tumor activities of pentacyclic triterpenes by introducing exocyclic α,β-unsaturated ketone moiety in ring A. Med. Chem. Res. 2014, 23, 4631–4641. [Google Scholar] [CrossRef]
- Wiemann, J.; Heller, L.; Csuk, R. Targeting cancer cells with oleanolic and ursolic acid derived hydroxamates. Bioorg. Med. Chem. Lett. 2016, 26, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.W.; Dou, T.Y.; Wang, P.; Lei, W.; Weng, Z.M.; Hou, J.; Wang, D.D.; Fan, Y.M.; Zhang, W.D.; Ge, G.B.; et al. Structure-activity relationships of pentacyclic triterpenoids as potent and selective inhibitors against human carboxylesterase 1. Front. Pharmacol. 2017, 8, 435. [Google Scholar] [CrossRef]
- Alho, D.P.S.; Salvador, J.A.R.; Cascante, M.; Marin, S. Synthesis and antiproliferative activity of novel heterocyclic glycyrrhetinic acid derivatives. Molecules 2019, 24, 766. [Google Scholar] [CrossRef]
- Capel, C.S.; de Souza, A.C.D.; de Carvalho, T.C.; de Sousa, J.P.B.; Ambrósio, S.R.; Martins, C.H.G.; Cunha, W.R.; Galán, R.H.; Furtado, N.A.J.C. Biotransformation using Mucor rouxii for the production of oleanolic acid derivatives and their antimicrobial activity against oral pathogens. J. Ind. Microbiol. Biotechnol. 2011, 38, 1493–1498. [Google Scholar] [CrossRef]
- Martinez, A.; Rivas, F.; Perojil, A.; Parra, A.; Garcia-Granados, A.; Fernandez-Vivas, A. Biotransformation of oleanolic and maslinic acids by Rhizomucor miehei. Phytochemistry 2013, 94, 229–237. [Google Scholar] [CrossRef]
- Gong, T.; Zheng, L.; Zhen, X.; He, H.X.; Zhu, H.X.; Zhu, P. Microbial transformation of oleanolic acid by Trichothecium roseum. J. Asian Nat. Prod. Res. 2014, 16, 383–386. [Google Scholar] [CrossRef]
- Ludwig, B.; Geib, D.; Haas, C.; Steingroewer, J.; Bley, T.; Muffler, K.; Ulber, R. Whole-cell biotransformation of oleanolic acid by free and immobilized cells of Nocardia iowensis: Characterization of new metabolites. Eng. Life Sci. 2015, 15, 108–115. [Google Scholar] [CrossRef]
- Xu, S.H.; Wang, W.W.; Zhang, C.; Liu, X.F.; Yu, B.Y.; Zhang, J. Site-selective oxidation of unactivated C–H sp3 bonds of oleanane triterpenes by Streptomyces griseus ATCC 13273. Tetrahedron 2017, 73, 3086–3092. [Google Scholar] [CrossRef]
- Xu, S.H.; Chen, H.L.; Fan, Y.; Xu, W.; Zhang, J. Application of tandem biotransformation for biosynthesis of new pentacyclic triterpenoid derivatives with neuroprotective effect. Bioorg. Med. Chem. Lett. 2020, 30, 126947. [Google Scholar] [CrossRef] [PubMed]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V. Hydrocarbon-oxidizing bacteria and their potential in eco-biotechnology and bioremediation. In Microbial Resources; Kurtboke, I., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–148. ISBN 978-0-12-804765-1. [Google Scholar]
- Grishko, V.V.; Tarasova, E.V.; Ivshina, I.B. Biotransformation of betulin to betulone by growing and resting cells of the actinobacterium Rhodococcus rhodochrous IEGM 66. Process. Biochem. 2013, 48, 1640–1644. [Google Scholar] [CrossRef]
- Corno, G.; Villiger, J.; Pernthaler, J. Coaggregation in a microbial predator–prey system affects competition and trophic transfer efficiency. Ecology 2013, 94, 870–881. [Google Scholar] [CrossRef]
- Belfiore, C.; Curia, M.V.; Farías, M.E. Characterization of Rhodococcus sp. A5wh isolated from a high altitude Andean lake to unravel the survival strategy under lithium stress. Rev. Argent. Microbiol. 2018, 50, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.M.; Mayer, F.; Frabritius, D.; Steinbüchel, A. Formation of intracytoplasmic lipid inclusions. Arch. Microbiol. 1996, 165, 377–386. [Google Scholar] [CrossRef]
- Hernández, M.A.; Mohn, W.W.; Martínez, E.; Rost, E.; Alvarez, A.F.; Alvarez, H.M. Biosynthesis of storage compounds by Rhodococcus jostii RHA1 and global identification of genes involved in their metabolism. BMC Genom. 2008, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Cheremnykh, K.M.; Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Bioconversion of ecotoxic dehydroabietic acid using Rhodococcus actinobacteria. J. Hazard. Mater. 2018, 346, 103–112. [Google Scholar] [CrossRef]
- Scalon Cunha, L.C.; Andrade e Silva, M.L.; Cardoso Furtado, N.A.J.; Vinhólis, A.H.C.; Gomes Martins, C.H.; da Silva Filho, A.A.; Cunha, W.R. Antibacterial activity of triterpene acids and semi-synthetic derivatives against oral pathogens. Z. Für Nat. C 2007, 62, 668–672. [Google Scholar] [CrossRef]
- Krasteva, I.; Yotova, M.; Yosifov, D.; Benbassat, N.; Jenett-Siems, K.; Konstantinov, S. Cytotoxicity of gypsogenic acid isolated from Gypsophila trichotoma. Pharmacogn. Mag. 2014, 10, 430. [Google Scholar] [CrossRef]
- Fukushima, E.O.; Seki, H.; Sawai, S.; Suzuki, M.; Ohyama, K.; Saito, K.; Muranaka, T. Combinatorial biosynthesis of legume natural and rare triterpenoids in engineered yeast. Plant Cell Physiol. 2013, 54, 740–749. [Google Scholar] [CrossRef]
- Cunha, W.R.; Martins, C.; da Silva Ferreira, D.; Miller Crotti, A.E.; Lopes, N.P.; Albuquerque, S. In vitro trypanocidal activity of triterpenes from Miconia species. Planta Med. 2003, 69, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, E.O.; Seki, H.; Ohyama, K.; Ono, E.; Umemoto, N.; Mizutani, M.; Saito, K.; Muranaka, T. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011, 52, 2050–2061. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, D.; Zapp, J.; Bernhardt, R. Hydroxylation of the triterpenoid dipterocarpol with CYP106A2 from Bacillus megaterium. FEBS J. 2012, 279, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Çelik, A.; Flitsch, S.L.; Turner, N.J. Efficient terpene hydroxylation catalysts based upon P450 enzymes derived from actinomycetes. Org. Biomol. Chem. 2005, 3, 2930–2934. [Google Scholar] [CrossRef] [PubMed]
- Catalogue of the Strains of Regional Specialised Collection of Alkanotrophic Microorganisms. Available online: http://www.iegmcol.ru/strains/index.html (accessed on 20 September 2022).
- Postgate, J.R. Differential media for sulphur bacteria. J. Sci. Food Agric. 1959, 10, 669–674. [Google Scholar] [CrossRef]
- Neumann, G.; Veeranagouda, Y.; Karegoudar, T.B.; Sahin, Ö; Mäusezahl, I.; Kabelitz,, N.; Kappelmeyer, U.; Heipieper, H.J. Cells of Pseudomonas putida and Enterobacter sp. adapt to toxic organic compounds by increasing their size. Extremophiles 2005, 9, 163–168. [Google Scholar] [CrossRef]
- Mrunalini, B.R.; Girisha, S.T. Screening and characterization of lipid inclusions in bacteria by fluorescence microscopy and mass spectrometry as a source for biofuel production. Indian J. Sci. Technol. 2017, 10, 104166501. [Google Scholar] [CrossRef]
- CrysAlisPro, Version 1.171.37.33 (release 27-03-2014 CrysAlis171.NET); Agilent Technologies: Yarnton, UK, 2014.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Way2Drug Predictive Services. PASS Online. Available online: http://www.pharmaexpert.ru/passonline/index.php (accessed on 20 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).