A Novel Shift in the Absorbance Maxima of Methyl Orange with Calcination Temperature of Green Tin Dioxide Nanoparticle-Induced Photocatalytic Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. Surface Area and Pore Size Distribution
2.3. SEM Analysis
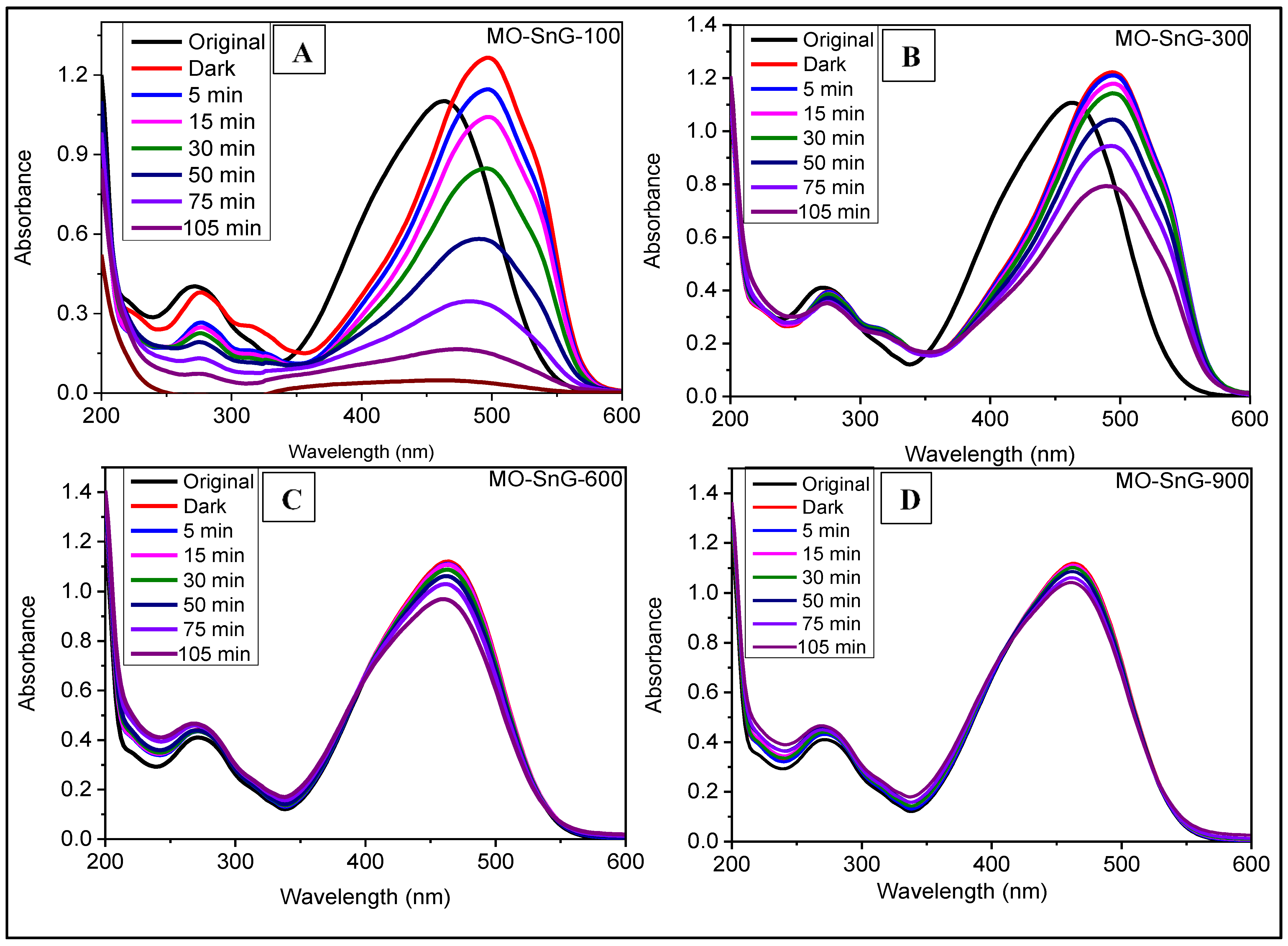
2.4. UV–Vis DRS Analysis
2.5. FTIR Analysis
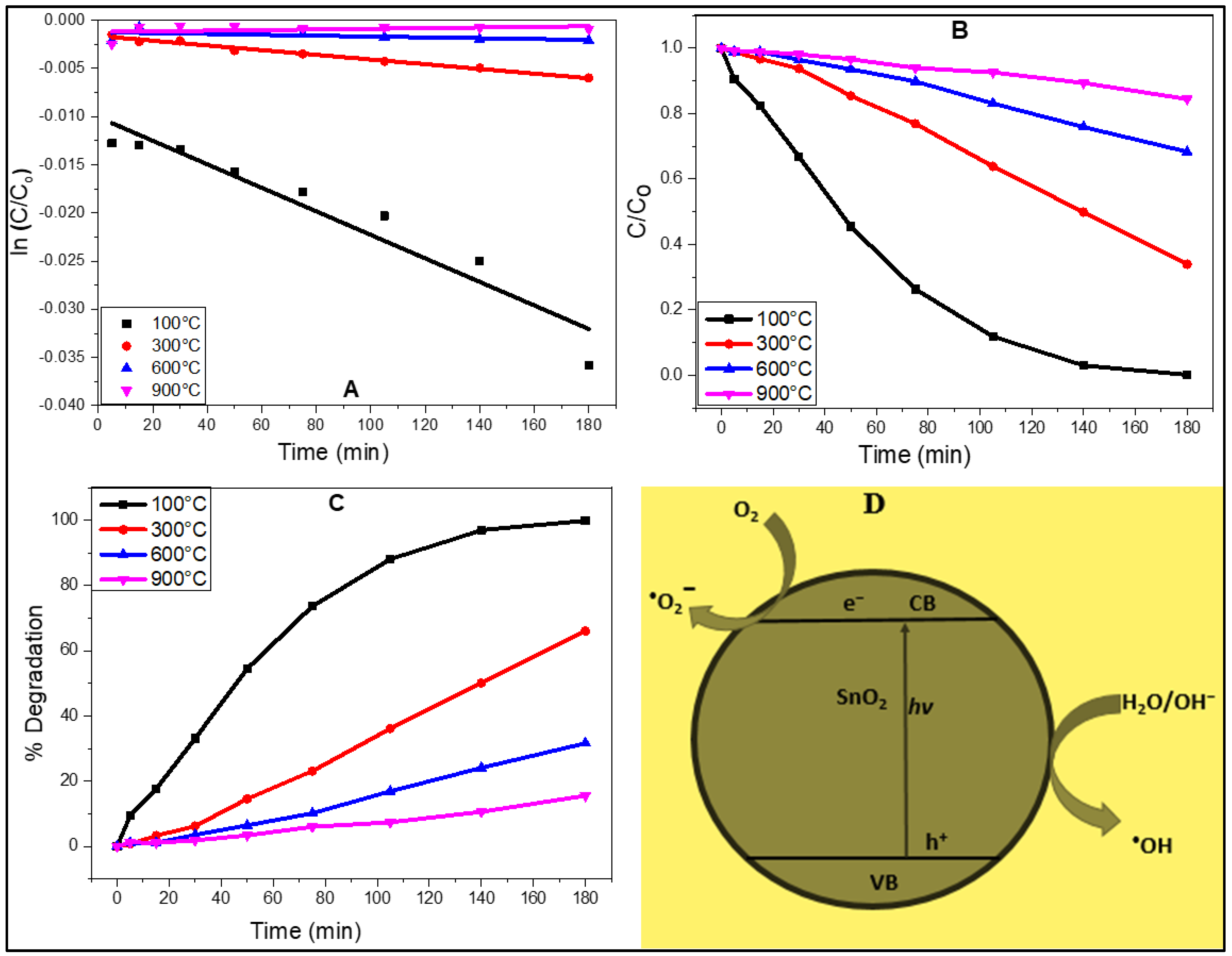
2.6. Photocatalytic Activity and Underlying Mechanism
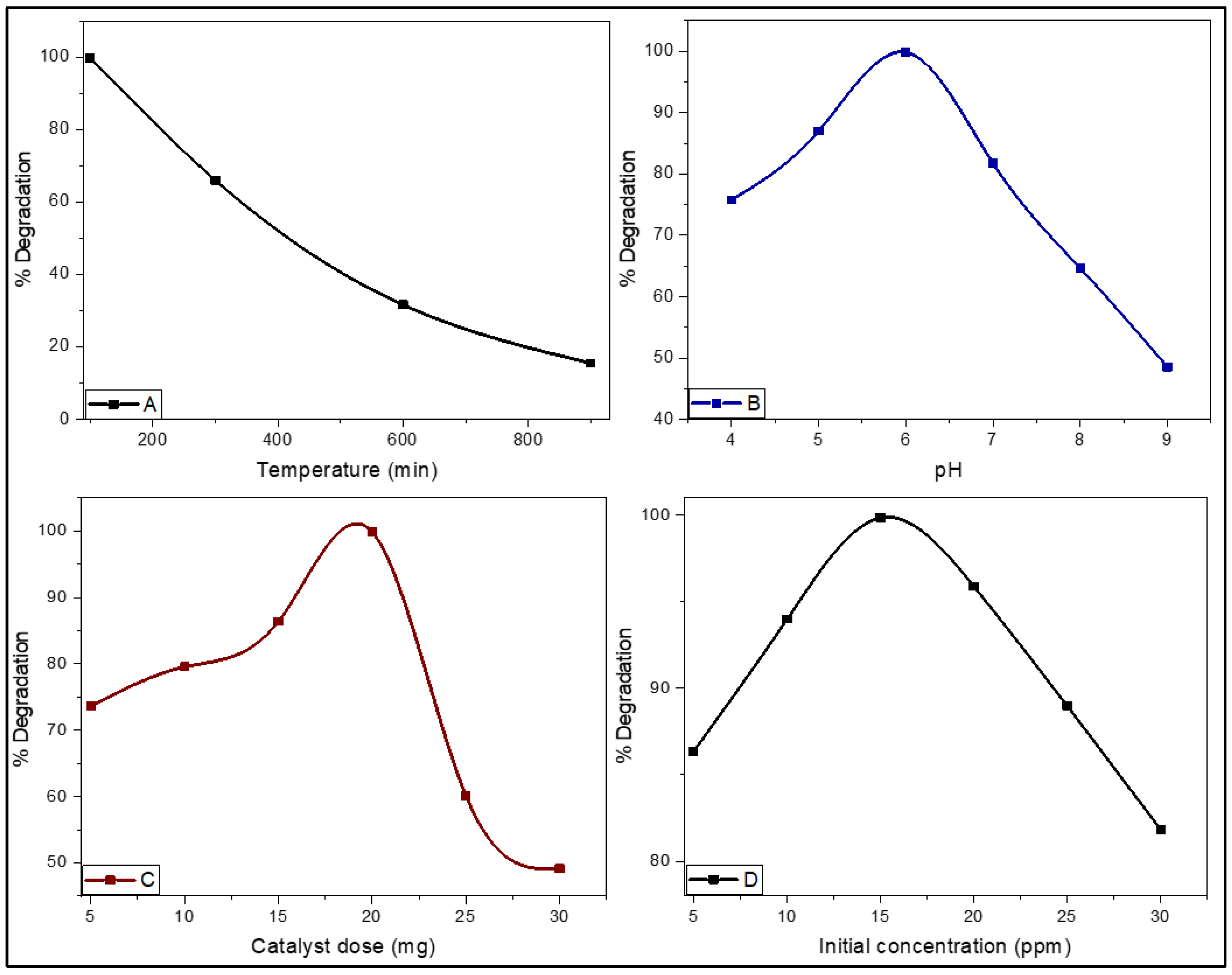
2.6.1. Factors Affecting the Photocatalytic Activity
Effect of pH
Effect of Photocatalysts Dosage
Effect of Initial Concentration of MO
3. Materials and Methods
3.1. Reagents
3.2. Plant
3.3. Preparation of the Plant Extract
3.4. Green Synthesis of SnO2 NPs
3.5. Physicochemical Characterizations
3.5.1. BET/BJH
3.5.2. XRD, SEM, UV–Vis, and FTIR
3.5.3. Photocatalytic Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haq, S.; Shoukat, S.; Rehman, W.; Waseem, M.; Shah, A. Green fabrication and physicochemical investigations of zinc-cobalt oxide nanocomposite for wastewater treatment. J. Mol. Liq. 2020, 318, 114260. [Google Scholar] [CrossRef]
- Tammina, S.K.; Mandal, B.K.; Kadiyala, N.K. Photocatalytic degradation of methylene blue dye by nonconventional synthesized SnO2 nanoparticles. Environ. Nanotechnol. Monit. Manag. 2018, 10, 339–350. [Google Scholar] [CrossRef]
- Khan, B.; Nawaz, M.; Waseem, M.; Hussain, R.; Arif, S.; Price, G.J. Adsorption of methylene blue onto size controlled magnetite nanoparticles Adsorption of methylene blue onto size controlled magnetite nanoparticles. Mater. Res. Express 2019, 6, 095511. [Google Scholar] [CrossRef]
- Zou, W.; Li, K.; Bai, H.; Shi, X.; Han, R. Enhanced Cationic Dyes Removal from Aqueous Solution by Oxalic Acid Modified Rice Husk. J. Chem. Eng. Data 2011, 56, 1882–1891. [Google Scholar] [CrossRef]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of pH Value on the Bandgap Energy and Particles Size for Biosynthesis of ZnO Nanoparticles: Efficiency for Photocatalytic Adsorption of Methyl Orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Harikumar, B.; Okla, M.K.; Alaraidh, I.A.; Mohebaldin, A.; Soufan, W.; Abdel-Maksoud, M.A.; Aufy, M.; Thomas, A.M.; Raju, L.L.; Khan, S.S. Robust visible light active CoNiO2–BiFeO3–NiS ternary nanocomposite for photo-fenton degradation of rhodamine B and methyl orange: Kinetics, degradation pathway and toxicity assessment. J. Environ. Manag. 2022, 317, 115321. [Google Scholar] [CrossRef]
- Jana, M.; Taheruddin, S.; Mitra, M.; Kulsi, C. Applied Surface Science Visible-light in fl uenced photocatalytic activity of polyaniline -bismuth selenide composites for the degradation of methyl orange, rhodamine B and malachite green dyes. Appl. Surf. Sci. 2019, 470, 472–483. [Google Scholar]
- Kazazi, M.; Moradi, B.; Delshad Chermahini, M. Enhanced photocatalytic degradation of methyl orange using Ag/Sn-doped CeO2 nanocomposite. J. Mater. Sci. Mater. Electron. 2019, 30, 6116–6126. [Google Scholar] [CrossRef]
- Sivakarthik, P.; Thangaraj, V.; Perumalraj, K.; Balaji, J. Synthesis of co-doped tin oxide nanoparticles for photo catalytic degradation of synthetic organic dyes. Dig. J. Nanomater. Biostruct. 2016, 11, 935–943. [Google Scholar]
- Lin, C.; Liu, H.; Guo, M.; Zhao, Y.; Su, X.; Zhang, P.; Zhang, Y. Plasmon-induced broad spectrum photocatalytic overall water splitting: Through non-noble bimetal nanoparticles hybrid with reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2022, 646, 128962. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, P.; Zhang, Y.; Li, X. Highly efficient photocatalytic overall water splitting on plasmonic Cu6Sn5/polyaniline nanocomposites. J. Colloid Interface Sci. 2022, 609, 785–793. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, D.; Qin, Y.; Xie, Y.; Ling, Y.; Ye, H.; Zhang, Y. Facile construction of BiOBr/CoAl-LDH heterojunctions with suppressed Z-axis growth for efficient photoreduction of CO2. Sep. Purif. Technol. 2022, 302, 122090. [Google Scholar] [CrossRef]
- Zafar, N.; Uzair, B.; Menaa, F.; Khan, B.A.; Niazi, M.B.K.; Alaryani, F.S.; Majrashi, K.A.; Sajjad, S. Moringa concanensis-Mediated Synthesis and Characterizations of Ciprofloxacin Encapsulated into Ag/TiO2/Fe2O3/CS Nanocomposite: A Therapeutic Solution against Multidrug Resistant E. coli Strains of Livestock Infectious Diseases. Pharmaceutics 2022, 14, 1719. [Google Scholar] [CrossRef]
- Wijesinghe, U.; Thiripuranathar, G.; Menaa, F.; Iqbal, H.; Razzaq, A.; Almukhlifi, H. Green synthesis, structural characterization and photocatalytic applications of ZnO nanoconjugates using Heliotropium indicum. Catalysts 2021, 11, 831. [Google Scholar] [CrossRef]
- Wijesinghe, U.; Thiripuranathar, G.; Iqbal, H.; Menaa, F. Biomimetic synthesis, characterization, and evaluation of fluorescence resonance energy transfer, photoluminescence, and photocatalytic activity of zinc oxide nanoparticles. Sustainability 2021, 13, 2004. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Khan, A.R.; Hussain, S.; Zain-ul-Abdin; Din, S.U.; Hafeez, M.; et al. Effect of Annealing Temperature on Structural Phase Transformations and Band Gap Reduction for Photocatalytic Activity of Mesopores TiO2 Nanocatalysts. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1312–1322. [Google Scholar] [CrossRef]
- Haq, S.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Rehman, W.; Waseem, M.; Din, S.U. Antibacterial, antioxidant and physicochemical investigations of tin dioxide nanoparticles synthesized via microemulsion method. Mater. Res. Express 2021, 8, 035013. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Shah, A.; Khan, A.R. Green synthesis and characterization of tin dioxide nanoparticles for photocatalytic and antimicrobial studies. Mater. Res. Express 2020, 7, 025012. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Bibi, N.; Haq, S.; Rehman, W.; Waseem, M.; Rehman, M.U.; Shah, A.; Khan, B.; Rasheed, P. Low temperature fabrication of SnO2, ZnO and Zn2SnO4 nanostructures for the degradation of Rhodamine 6G: Characterization. Biointerface Res. Appl. Chem. 2020, 10, 5895–5900. [Google Scholar]
- Yao, W.; Wu, S.; Zhan, L.; Wang, Y. Two-dimensional porous carbon-coated sandwich-like mesoporous SnO2/graphene/mesoporous SnO2 nanosheets towards high-rate and long cycle life lithium-ion batteries. Chem. Eng. J. 2019, 361, 329–341. [Google Scholar] [CrossRef]
- Elango, G.; Roopan, S.M. Efficacy of SnO2 nanoparticles towards photocatalytic degradation of methylene blue dye. J. Photochem. Photobiol. B Biol. 2015, 155, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef]
- Haq, S.; Yasin, K.A.; Rehman, W.; Waseem, M.; Ahmed, M.N.; Shahzad, M.I.; Shahzad, N.; Shah, A.; Rehman, M.U.; Khan, B. Green Synthesis of Silver Oxide Nanostructures and Investigation of Their Synergistic Effect with Moxifloxacin Against Selected Microorganisms. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1134–1142. [Google Scholar] [CrossRef]
- Haq, S.; Afsar, H.; Ali, M.B.; Almalki, M.; Albogami, B.; Hedfi, A. Green Synthesis and Characterization of a ZnO–ZrO2 Heterojunction for Environmental and Biological Applications. Crystals 2021, 11, 1502. [Google Scholar] [CrossRef]
- Nandeesh, R.; Ashok Kumar, B.S.; Lakshman, K.; Ranganayakulu, D.; Manoj, B.; Ganapathy, S. Histological and Physico-Chemical Evaluation of Buxus wallichiana Baill. In World Journal of Fungal and Plant Biology; Idress Hamad Attitalla, Sandra Ann Ingela Wright, Eds.; IDOSI Publications: Faisalabad, Pakistan, 2010; Volume 1, pp. 46–50. ISSN 2219-4312. [Google Scholar]
- Cheng, B.; Russell, J.M.; Shi, W.; Zhang, L.; Samulski, E.T. Large-Scale, Solution-Phase Growth of Single-Crystalline SnO2 Nanorods. J. Am. Chem. Soc. 2004, 126, 5972–5973. [Google Scholar] [CrossRef]
- Aziz, M.; Saber Abbas, S.; Wan Baharom, W.R. Size-controlled synthesis of SnO2 nanoparticles by sol-gel method. Mater. Lett. 2013, 91, 31–34. [Google Scholar] [CrossRef]
- Yu, J.-G.; Yu, H.-G.; Cheng, B.; Zhao, X.-J.; Yu, J.C.; Ho, W.K. The Effect of Calcination Temperature on the Surface Microstructure and Photocatalytic Activity of TiO2 Thin Films Prepared by Liquid Phase Deposition. J. Phys. Chem. B 2003, 107, 13871–13879. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Javed, R.; Mahfooz-ur-Rehman; Shahid, M. Effect of heating on the structural and optical properties of TiO2 nanoparticles: Antibacterial activity. Appl. Nanosci. 2018, 8, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Haq, S.; Rehman, W.; Waseem, M.; Rehman, M.; Shah, K.H. Modeling, Thermodynamic Study and Sorption Mechanism of Cadmium Ions onto Isopropyl Alcohol Mediated Tin Dioxide Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2019, 30, 1198. [Google Scholar] [CrossRef]
- Manjula, P.; Boppella, R.; Manorama, S.V. A facile and green approach for the controlled synthesis of porous SnO2 nanospheres: Application as an efficient photocatalyst and an excellent gas sensing material. ACS Appl. Mater. Interfaces 2012, 4, 6252–6260. [Google Scholar] [CrossRef]
- Khan, M.M.; Pradhan, D.; Sohn, Y. Springer Series on Polymer and Composite Materials Nanocomposites for Visible Light-induced Photocatalysis. In Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 19–40. [Google Scholar] [CrossRef]
- Fu, L.; Zheng, Y.; Ren, Q.; Wang, A.; Deng, B. Green biosynthesis of SnO2 nanoparticles by plectranthus amboinicus leaf extract their photocatalytic activity toward rhodamine B degradation. J. Ovonic Res. 2015, 11, 21–26. [Google Scholar]
- Kulkarni, R.M.; Malladi, R.S.; Hanagadakar, M.S. Cu-ZnO nanoparticles for degradation of methyl orange photocatalytic. Adv. Mater. Proc. 2018, 3, 521–525. [Google Scholar] [CrossRef]
- Yuan, H.; Xu, J. Preparation, characterization and photocatalytic activity of nanometer SnO2. Int. J. Chem. Eng. Appl. 2010, 1, 241–246. [Google Scholar] [CrossRef]
- Kansal, S.K.; Singh, M.; Sud, D. Studies on photodegradation of two commercial dyes in aqueous phase using different photocatalysts. J. Hazard. Mater. 2007, 141, 581–590. [Google Scholar] [CrossRef]
- He, Y.; Chen, Q.; Tian, Y.; Yan, C.; He, Y.; Li, K. Estimation of shale pore-size-distribution from N2 adsorption characteristics employing modified BJH algorithm. Pet. Sci. Technol. 2021, 39, 843–859. [Google Scholar] [CrossRef]
- Rasheed, P.; Haq, S.; Waseem, M.; Rehman, S.U.; Rehman, W. Green synthesis of vanadium oxide-zirconium oxide nanocomposite for the degradation of methyl orange and picloram. Mater. Res. Express 2020, 7, 025011. [Google Scholar] [CrossRef]
- Shoukat, S.; Haq, S.; Rehman, W.; Waseem, M.; Hafeez, M.; Din, S.U.; Zain-ul-Abdin; Ahmad, P.; Rehman, M.U.; Shah, A.; et al. Remediation of Chromium (VI) and Rhodamine 6G via Mixed Phase Nickel-Zinc Nanocomposite: Synthesis and Characterization. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1565–1575. [Google Scholar] [CrossRef]
- Haq, S.; Afsar, H.; Din, I.U.; Ahmad, P.; Khandaker, M.U.; Osman, H.; Alamri, S.; Shahzad, M.I.; Shahzad, N.; Rehman, W.; et al. Enhanced photocatalytic activity of ficus elastica mediated zinc oxide-zirconium dioxide nanocatalyst at elevated calcination temperature: Physicochemical study. Catalysts 2021, 11, 1481. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Saeed, S.; Iqbal, N. Fabrication of pure and moxifloxacin functionalized silver oxide nanoparticles for photocatalytic and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2018, 186, 116–124. [Google Scholar] [CrossRef]

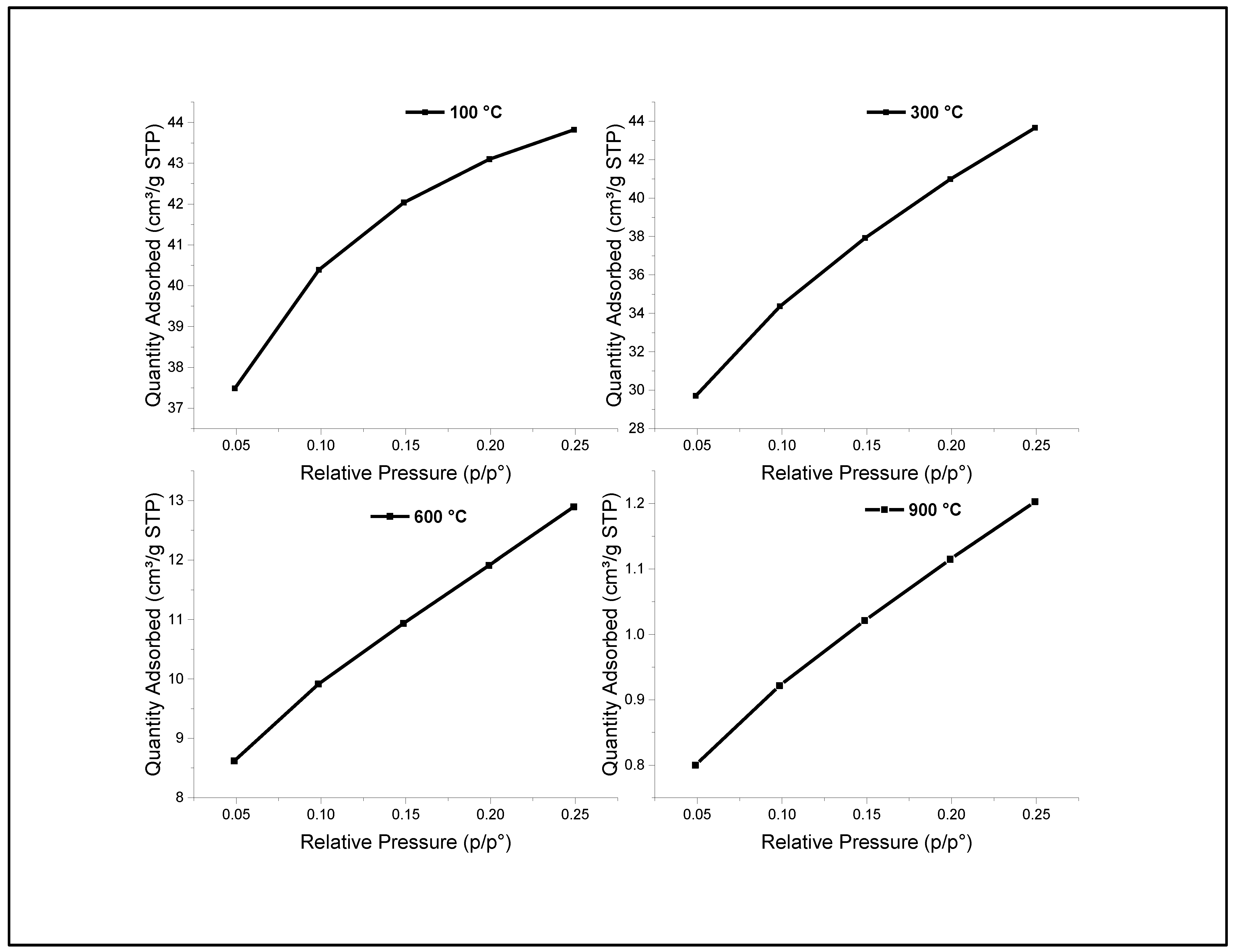



| Cal. Temp (°C) | SBET (m2/g) | Pore Size (Ǻ) | Pore Volume (cm3/g) |
|---|---|---|---|
| 100 | 143.20 | 19.89 | 0.0180 |
| 300 | 142.67 | 21.22 | 0.0938 |
| 600 | 42.51 | 21.28 | 0.0272 |
| 900 | 4.08 | 21.06 | 0.0025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haq, S.; Ehsan, R.; Menaa, F.; Shahzad, N.; Din, S.U.; Shahzad, M.I.; Rehman, W.; Waseem, M.; Alrhabi, W.; Almukhlifi, H.A.; et al. A Novel Shift in the Absorbance Maxima of Methyl Orange with Calcination Temperature of Green Tin Dioxide Nanoparticle-Induced Photocatalytic Activity. Catalysts 2022, 12, 1397. https://doi.org/10.3390/catal12111397
Haq S, Ehsan R, Menaa F, Shahzad N, Din SU, Shahzad MI, Rehman W, Waseem M, Alrhabi W, Almukhlifi HA, et al. A Novel Shift in the Absorbance Maxima of Methyl Orange with Calcination Temperature of Green Tin Dioxide Nanoparticle-Induced Photocatalytic Activity. Catalysts. 2022; 12(11):1397. https://doi.org/10.3390/catal12111397
Chicago/Turabian StyleHaq, Sirajul, Rimsha Ehsan, Farid Menaa, Nadia Shahzad, Salah Ud Din, Muhammad Imran Shahzad, Wajid Rehman, Muhammad Waseem, Walaa Alrhabi, Hanadi A. Almukhlifi, and et al. 2022. "A Novel Shift in the Absorbance Maxima of Methyl Orange with Calcination Temperature of Green Tin Dioxide Nanoparticle-Induced Photocatalytic Activity" Catalysts 12, no. 11: 1397. https://doi.org/10.3390/catal12111397
APA StyleHaq, S., Ehsan, R., Menaa, F., Shahzad, N., Din, S. U., Shahzad, M. I., Rehman, W., Waseem, M., Alrhabi, W., Almukhlifi, H. A., & Alsharef, S. A. (2022). A Novel Shift in the Absorbance Maxima of Methyl Orange with Calcination Temperature of Green Tin Dioxide Nanoparticle-Induced Photocatalytic Activity. Catalysts, 12(11), 1397. https://doi.org/10.3390/catal12111397









