Effects of Preparation Methods of Pd Supported on (001) Crystal Facets Exposed TiO2 Nanosheets for Toluene Catalytic Combustion
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Performance of Toluene Oxidation
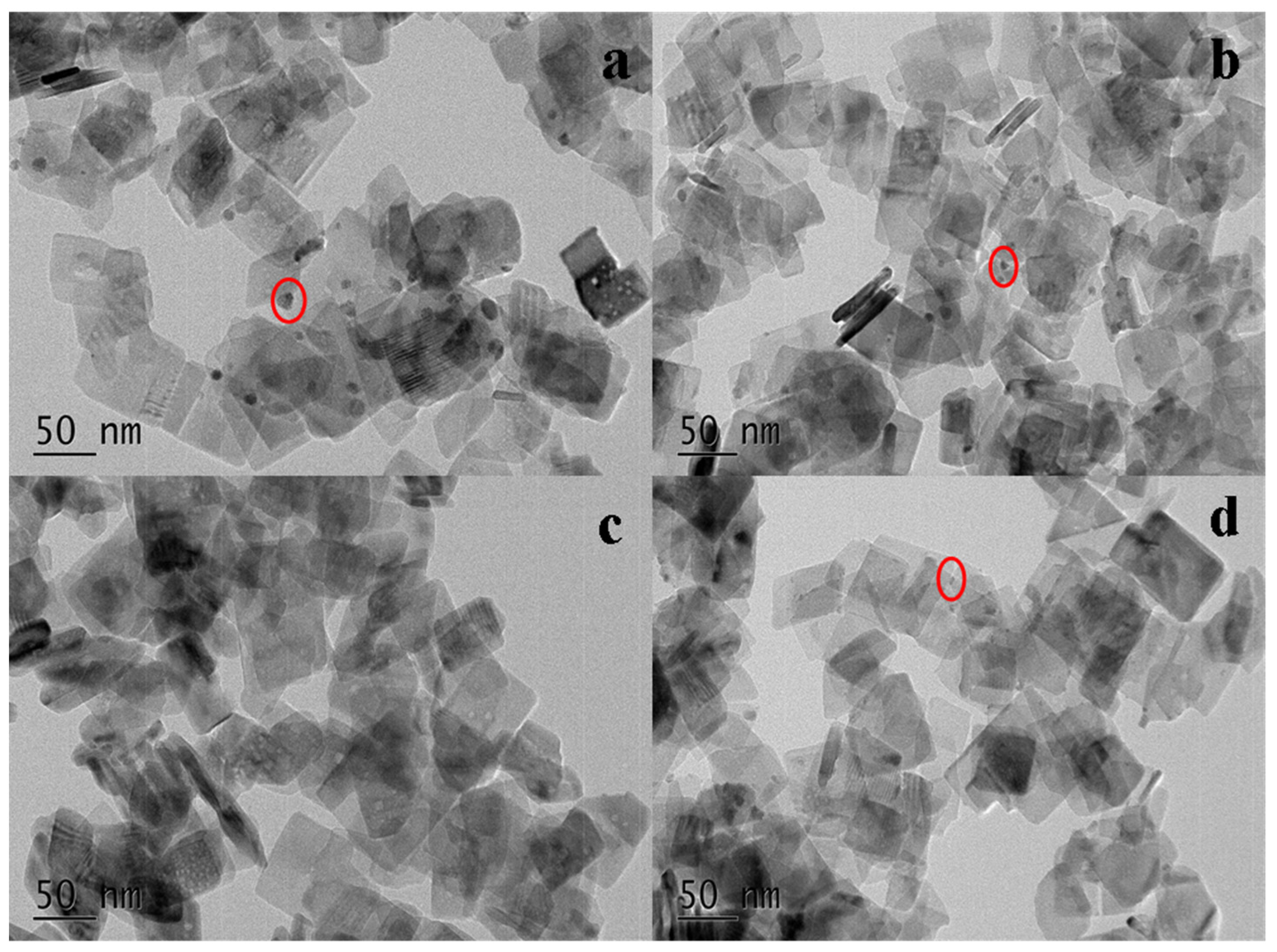
2.2. BET and TEM Analysis
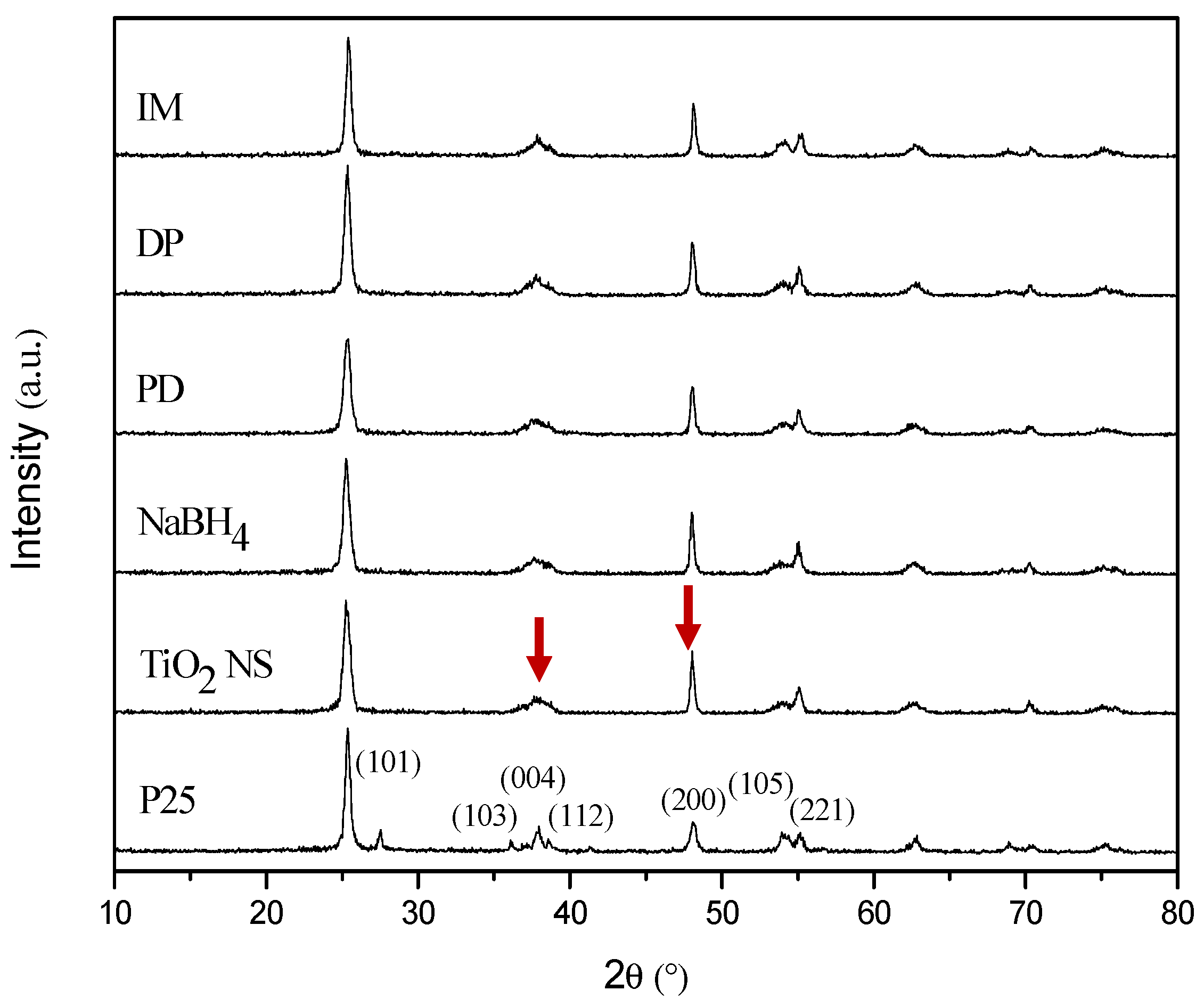
2.3. XRD Analysis
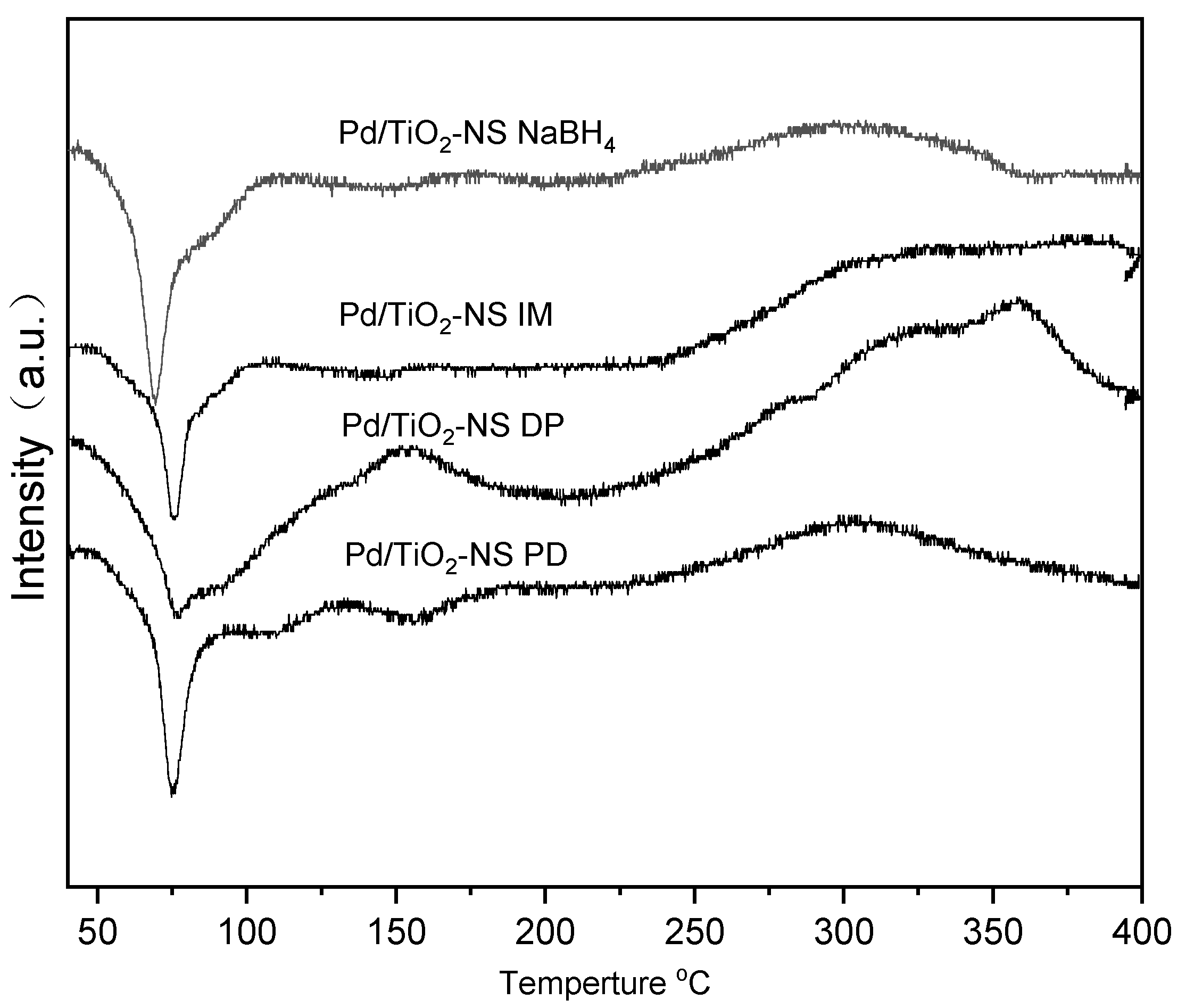
2.4. H2-TPR Analysis
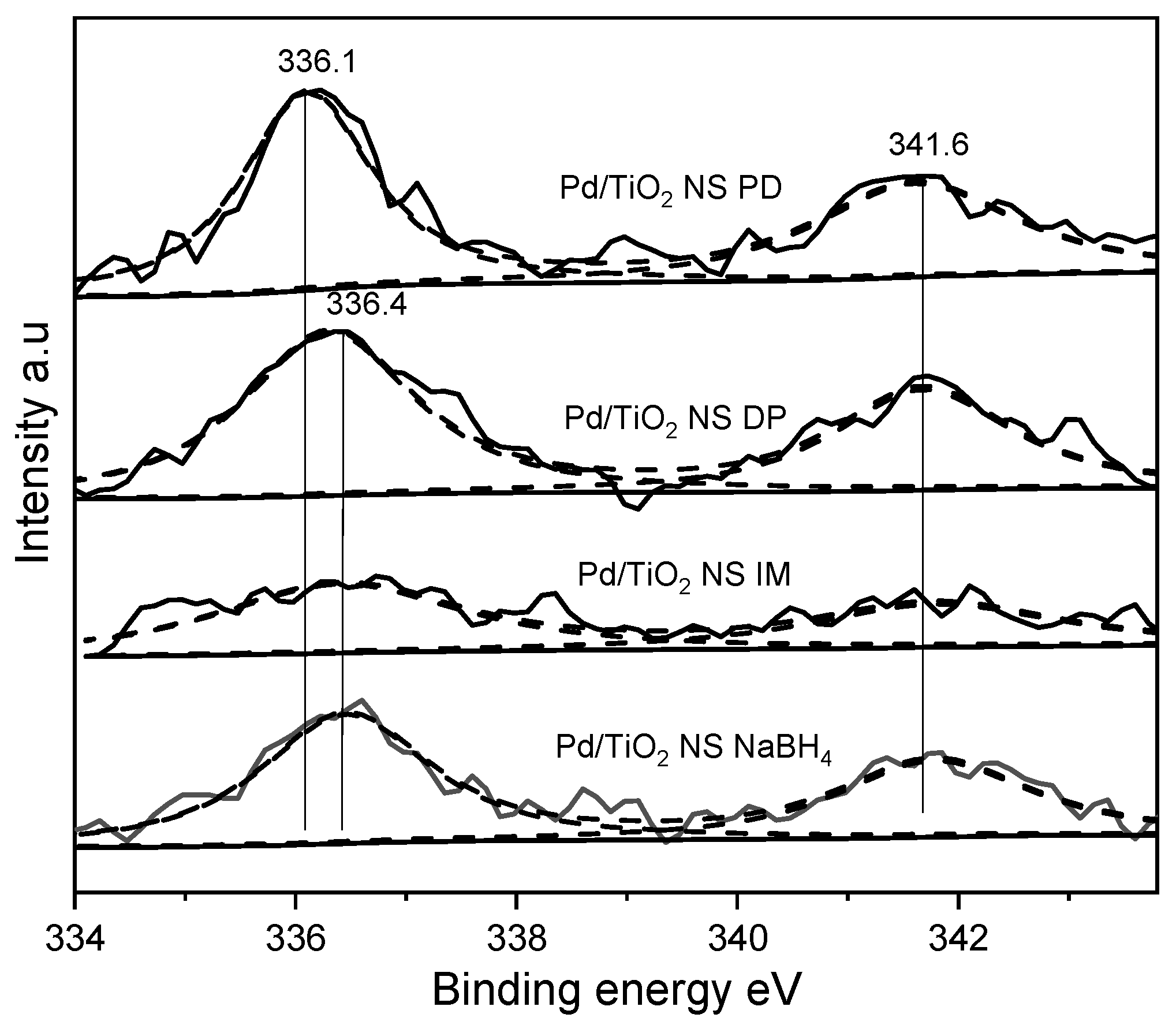
2.5. XPS Analysis
3. Materials and Methods
3.1. Catalyst Preparation
- Preparation of TiO2 Nanosheets (TiO2 NS)
- Preparation of the Pd/TiO2 NS and Pd/P25 Catalysts
3.2. Catalyst Characterization
3.3. Catalytic Performance Test
4. Conclusions
- (1)
- Pd/TiO2 catalysts were synthesized by different methods to study the effects of different preparation methods on the catalytic combustion activity of toluene. Firstly, we compared the catalytic activity of toluene with that of ordinary commercial P25 and TiO2 nanometer tablets after impregnating the Pd. The results showed that Pd/TiO2 NS with {001} crystal surface as the exposed crystal surface was significantly better than Pd/P25, and the temperature of 100% complete transformation of toluene was 40 °C lower. This is mainly because the {001} crystal surface of nano-sheet TiO2 is easier to form oxygen vacancy;
- (2)
- According to the experimental results, the catalyst prepared by the impregnation method had smaller Pd particles and more active sites. The smaller the Pd particles, the better the catalytic performance. The 100% conversion of toluene was achieved at 210 °C on Pd/ TiO2-IM. The catalytic activity of Pd / TiO2 catalyst prepared by the illumination (Pd/TiO2-PD) and deposition–precipitation (Pd/ TiO2-DP) methods was lower than that of Pd / TiO2 catalyst prepared by immersion method. Compared with the other synthesis methods, Pd in the impregnation method may be dispersed better in the synthesis process and the particles are smaller, so that more Pd active sites can be spread on the TiO2 nano-sheet carrier.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujimoto, T.M.; Ponczek, M.; Rochetto, U.L.; Tomaz, E. Photocatalytic oxidation of selected gas-phase VOCs using UV light, TiO2, and TiO2/Pd. Environ. Sci. Pollut. Res. 2017, 24, 6390–6396. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Q.; Chen, Y.W. Complete oxidation of toluene on Pd/modified-CeO2 catalysts. J. Taiwan Inst. Chem. Eng. 2016, 67, 69–73. [Google Scholar] [CrossRef]
- Shao, Y.; Xia, Q.; Liu, X.; Lu, G.; Wang, Y. Pd/Nb2O5/SiO2 catalyst for the direct hydrodeoxygenation of biomass-related compounds to liquid alkanes under mild conditions. ChemSusChem 2015, 8, 1761–1767. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, H.F.; Zhang, H.H.; Chen, Y.F. Catalytic properties of LaBO3 perovskite catalysts in VOCs combustion. China Environ. Sci. 2012, 32, 1772–1777. [Google Scholar]
- Zhang, C.; Hong, H.; Tanaka, K.I. Catalytic performance and mechanism of a Pt/TiO2 catalyst for the oxidation of formaldehyde at room temperature. Appl. Catal. B Environ. 2006, 65, 37–43. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Hou, W.; Zou, Z. Two-dimensional titanium/niobium metal oxide-based materials for photocatalytic application. Sol. RRL 2020, 4, 2000070. [Google Scholar] [CrossRef]
- Liu, C.; Sun, T.; Wu, L.; Liang, J.; Huang, Q.; Chen, J.; Hou, W. N-doped Na2Ti6O13@TiO2 core-shell nanobelts with exposed {1 0 1} anatase facets and enhanced visible light photocatalytic performance. Appl. Catal. B Environ. 2015, 170, 17–24. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Shangguan, W. Low-temperature SCR of NO with propylene in excess oxygen over the Pt/TiO2 catalyst. Catal. Commun. 2009, 10, 1330–1333. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Chen, M.X.; Jiang, Z.; Shangguan, W.F. Performance and mechanism study for low-temperature SCR of NO with propylene in excess oxygen over Pt/TiO2 catalyst. J. Environ. Sci. 2010, 22, 1441–1446. [Google Scholar] [CrossRef]
- Li, Y.; Liu, F.; Fan, Y.; Cheng, G.; Song, W.; Zhou, J. Silver palladium bimetallic core-shell structure catalyst supported on TiO2 for toluene oxidation. Appl. Surf. Sci. 2018, 462, 207–212. [Google Scholar] [CrossRef]
- Hosseini, M.; Siffert, S.; Tidahy, H.L.; Cousin, R.; Lamonier, J.F.; Aboukais, A.; Vantomme, A.; Roussel, M.; Su, B.L. Promotional effect of gold added to palladium supported on a new mesoporous TiO2 for total oxidation of volatile organic compounds. Catal. Today 2007, 122, 391–396. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent advances in designing ZnIn2S4-based heterostructured photocatalysts for hydrogen evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Wang, Z.F.; Chen, H.Y.; Chen, Y.; Murakami, N.; Ohno, T. Synthesis of anatase TiO2 with exposed {001} and {101} facets and photocatalytic activity. Rare Met. 2019, 4, 287–291. [Google Scholar] [CrossRef]
- Liu, L.C.; Gu, X.R.; Ji, Z.Y. Crystal-Plane Effects on the Catalytic Properties of Au/TiO2. ACS Catal. 2013, 3, 2768–2775. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, B.; Su, D.; Huang, W. Titania Morphology-Dependent Gold–Titania Interaction, Structure, and Catalytic Performance of Gold/Titania Catalysts. Chemcatchem 2015, 7, 3290–3298. [Google Scholar] [CrossRef]
- Zeng, Y.; Jiang, L.; Zhang, X.; Xie, S.; Pei, Y.; Zhou, G.; Hua, W.; Qiao, M.; Li, Z.H.; Zong, B. Effect of Titania Polymorphs on the Structure and Catalytic Performance of the Pt-WOx/TiO2 Catalyst in Glycerol Hydrogenolysis to 1,3-Propanediol. ACS Sustain. Chem. Eng. 2022, 10, 9532–9545. [Google Scholar] [CrossRef]
- Su, Y.; Ji, K.; Xun, J.; Zhang, K.; Liu, P.; Zhao, L. Catalytic oxidation of low concentration formaldehyde over Pt/TiO2 catalyst. Chin. J. Chem. Eng. 2021, 29, 190–195. [Google Scholar] [CrossRef]
- Feng, L.; Zheng PFu, Y.L. Pt/TiO2 Nanosheets Array Dominated by {001} Facets with Enhanced Photocatalytic Activity. Chin. J. Chem. Phys. 2014, 27, 530–534. [Google Scholar]
- Gao, Z.; Cui, Z.; Zhu, S.; Liang, Y.; Li, Z.; Yang, X. Fabrication, characterization, and photocatalytic properties of anatase TiO2 nanoplates with exposed {001} facets. J. Nanopart Res. 2014, 16, 2191. [Google Scholar] [CrossRef]
- Tang, K.; Wang, Z.; Zou, W.; Guo, H.; Wu, Y.; Pu, Y.; Tong, Q.; Wan, H.; Gu, X.; Dong, L.; et al. Advantageous Role of Ir0 Supported on TiO2 Nanosheets in Photocatalytic CO2 Reduction to CH4: Fast Electron Transfer and Rich Surface Hydroxyl Groups. ACS Appl. Mater. Interfaces 2021, 13, 6219–6228. [Google Scholar] [CrossRef]
- Deng, S.C.; Fan, Y.Y.; Meng, T.T.; Gao, B.L.; Ding, F. Advanced MnOx/TiO2 Catalyst with Preferentially Exposed Anatase {001} Facet for Low-temperature SCR of NO. ACS Catal. 2016, 6, 5807–5815. [Google Scholar] [CrossRef]
- Guila, G.; Gracia, F.; Araya, P. CuO and CeO2 catalysts supported on Al2O3, ZrO2, and SiO2 in the oxidation of CO at low temperature. Appl. Catal. A Gen. 2008, 1, 16–24. [Google Scholar]
- Sun, J.; Ge, C.; Yao, X.; Zou, W.; Hong, X.; Tang, C.; Dong, L. Influence of different impregnation modes on the properties of CuO CeO2/Al2O3 catalysts for NO reduction by CO. Appl. Surf. Sci. 2017, 426, 279–286. [Google Scholar] [CrossRef]
- Huang, W.; Zuo, Z.; Han, P.; Li, Z.; Zhao, T. XPS and XRD investigation of Co/Pd/TiO2 catalysts by different preparation methods. J. Electron Spectrosc. Relat. Phenom. 2009, 173, 88–95. [Google Scholar] [CrossRef]
- Ataloglou, T.; Vakros, J.; Bourikas, K.; Fountzoula, C.; Kordulis, C.; Lycourghiotis, A. Influence of the preparation method on the structure–activity of cobalt oxide catalysts supported on alumina for complete benzene oxidation. Appl. Catal. B Environ. 2005, 57, 299–312. [Google Scholar] [CrossRef]
- Yang, X.; Su, Y.X.; Qian, W.Y.; Yuan, M.H.; Zhou, H.; Deng, W.Y.; Zhao, B.T. Experimental study on selective catalytic reduction of NO by C3H6 over Fe-Ag/Al2O3 catalysts. J. Fuel Chem. Technol. 2017, 45, 1365–1375. [Google Scholar] [CrossRef]
- Ge, C.; Yu, Y.; An, D.; Tong, Q.; Tang, C.; Gao, F.; Sun, J.; Dong, L. Surface configuration modulation for FeOx-CeO2/γ-Al2O3 catalysts and its influence in CO oxidation. J. Catal. 2020, 386, 139–150. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, H.; Jia, B.; Wang, Z.H.; Liu, Z.M. Selective catalytic reduction of NOx by H2 over Pd/TiO2 catalyst. Chin. J. Catal. 2019, 40, 849–855. [Google Scholar] [CrossRef]
- Li, L.; Jiang, F.; Wan, H.; Zheng, S. TiO2 Nanosheet: Synthesis and Photocatalytic Performance for Phenol Degradation. Chin. J. Inorg. Cheemistry 2011, 27, 1041–1046. [Google Scholar]
- Denkwitz, Y.; Makosch, M.; Geserick, J.; Hörmann, U.; Selve, S.; Kaiser, U.; Hüsing, N.; Behm, R.J. Influence of the crystalline phase and surface area of the TiO2 support on the CO oxidation activity of mesoporous Au/TiO2 catalysts. Appl. Catal. B Environ. 2009, 91, 470–480. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Zhen, L.; Sun, C.; Hua, G.Y.; Li, C. Hydrothermal Stability of {001} Faceted Anatase TiO2. Chem. Mater. 2011, 23, 3486–3494. [Google Scholar]
- Shi, T.; Duan, Y.; Lv, K.; Hu, Z.; Li, Q.; Li, M.; Li, X. Photocatalytic Oxidation of Acetone Over High Thermally Stable TiO2 Nanosheets With Exposed (001) Facets. Front. Chem. 2018, 6, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, L.; Li, Z.; Lee, B.; Park, D.W. An alternative atmospheric-pressure cold plasma method for synthesizing Pd/P25 catalysts with the assistance of ethanol. Int. J. Hydrog. Energy 2017, 42, 11372–11378. [Google Scholar] [CrossRef]
- Komhom, S.; Mekasuwandumrong, O.; Praserthdam, P.; Panpranot, J. Improvement of Pd/Al2O3 catalyst performance in selective acetylene hydrogenation using mixed phases Al2O3 support. Catal. Commun. 2008, 10, 86–91. [Google Scholar] [CrossRef]
- Zhao, B. Characterization of Pd Catalysts by Temperature Programmed Reduction and Temperature Programmed Desorption. Precious Met. 2011, 32, 1–3. [Google Scholar]
- Chen, H.; Shao, Y.; Xu, Z.; Wan, H.; Wan, Y.; Zheng, S.; Zhu, D. Effective catalytic reduction of Cr(VI) over TiO2 nanotube supported Pd catalysts. Appl. Catal. B Environ. 2011, 105, 255–262. [Google Scholar] [CrossRef]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic Reduction of CO2 in Aqueous Solution on Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets. Energy Fuels 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- Lpez, E.; Ordfiez, S.; Diez, F.V.; Sastre, H. Hydrodechlorination of chlorobenzene tetrachloroethylene mixtures over a Pd/Al2O3 csatalyst. Stud. Surf. Sci. Catal. 2001, 133, 521–526. [Google Scholar]
- Fagherazzi, G.; Benedetti, A.; Polizzi, S.; Mario, A.; Pinna, F.; Signoretto, M.; Pernicone, N. Structural investigation on the stoichiometry of β-PdHxin Pd/SiO2 catalysts as a function of metal dispersion. React. Kinet. Catal. Lett. 1995, 32, 293–303. [Google Scholar] [CrossRef]
- Pinna, F.; Signoretto, M.; Strukul, G.; Polizzi, S.; Pernicone, N. Pd-SiO2 catalysts. stability of β-PdHxas a function of Pd dispersion. React. Kinet. CataI. Lett. 1997, 60, 9–13. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Wu, J.; Dai, H.; Ma, C.; Zhang, Q.; Zou, Z. Ag-Pd alloy decorated ZnIn2S4 microspheres with optimal Schottky barrier height for boosting visible-light-driven hydrogen evolution. J. Mater. Sci. Technol. 2022, 114, 81–89. [Google Scholar] [CrossRef]
- Liu, C.; Han, Z.; Feng, Y.; Dai, H.; Zhao, Y.; Han, N.; Zhang, Q.; Zou, Z. Ultrathin direct Z-scheme 2D/2D N-doped HTiNbO5 nanosheets/g-C3N4 porous composites for efficient photocatalytic degradation and H2 generation under visible light. J. Colloid Interface Sci. 2021, 583, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Feng, Y.; Han, Z.; Sun, Y.; Wang, X.; Zhang, Q.; Zou, Z. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2021, 42, 164–174. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Z.; He, F.; Xu, G. XPS study on the deactivated Pdd Pde2O3 catalyst for CO coupling reaction with ammonia. J. Tianjin Inst. Technol. 2003, 19, 24–27. [Google Scholar]
- Gao, Z.; Li, H.; Xu, J.; Zeng, Y. XPS analysis and gas sensing properties of Pd doped SnO2 films. Electron. Compon. Mater. 2011, 30, 27–30. [Google Scholar]
- Karhu, H.; Kalantar, A.; Väyrynen, I.J.; Salmi, T.; Murzin, D.Y. XPS analysis of chlorine residues in supported Pt and Pd catalysts with low metal loading. Appl. Catal. B Environ. 2003, 247, 283–294. [Google Scholar] [CrossRef]
- Weng, X.; Shi, B.; Liu, A.; Sun, J.; Xiong, Y.; Wan, H.; Zheng, S.; Dong, L.; Chen, Y. Highly dispersed Pd/modified-Al2O3 catalyst on complete oxidation of toluene: Role of basic sites and mechanism insight. Appl. Surf. Sci. 2019, 497, 143747. [Google Scholar] [CrossRef]


| Catalyst | Surface Area m2/g | Pore Volume cm3/g | Pore Size nm |
|---|---|---|---|
| P25 | 50 | 0.4 | 21 |
| TiO2 NS | 92 | 0.43 | 18.1 |
| Pd/TiO2 NS-DP | 80 | 0.38 | 20.1 |
| Pd/TiO2 NS-PD | 77 | 0.38 | 17.9 |
| Pd/TiO2 NS-NaBH4 | 74 | 0.37 | 20.1 |
| Pd/TiO2 NS-IM | 52 | 0.22 | 25.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Ge, C.; Wan, H. Effects of Preparation Methods of Pd Supported on (001) Crystal Facets Exposed TiO2 Nanosheets for Toluene Catalytic Combustion. Catalysts 2022, 12, 1406. https://doi.org/10.3390/catal12111406
Yu G, Ge C, Wan H. Effects of Preparation Methods of Pd Supported on (001) Crystal Facets Exposed TiO2 Nanosheets for Toluene Catalytic Combustion. Catalysts. 2022; 12(11):1406. https://doi.org/10.3390/catal12111406
Chicago/Turabian StyleYu, Guiyun, Chengyan Ge, and Haiqin Wan. 2022. "Effects of Preparation Methods of Pd Supported on (001) Crystal Facets Exposed TiO2 Nanosheets for Toluene Catalytic Combustion" Catalysts 12, no. 11: 1406. https://doi.org/10.3390/catal12111406





