Protection Effect of Ammonia on CeNbTi NH3-SCR Catalyst from SO2 Poisoning
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Activity Measurement
2.3. Catalyst Characterization
3. Results
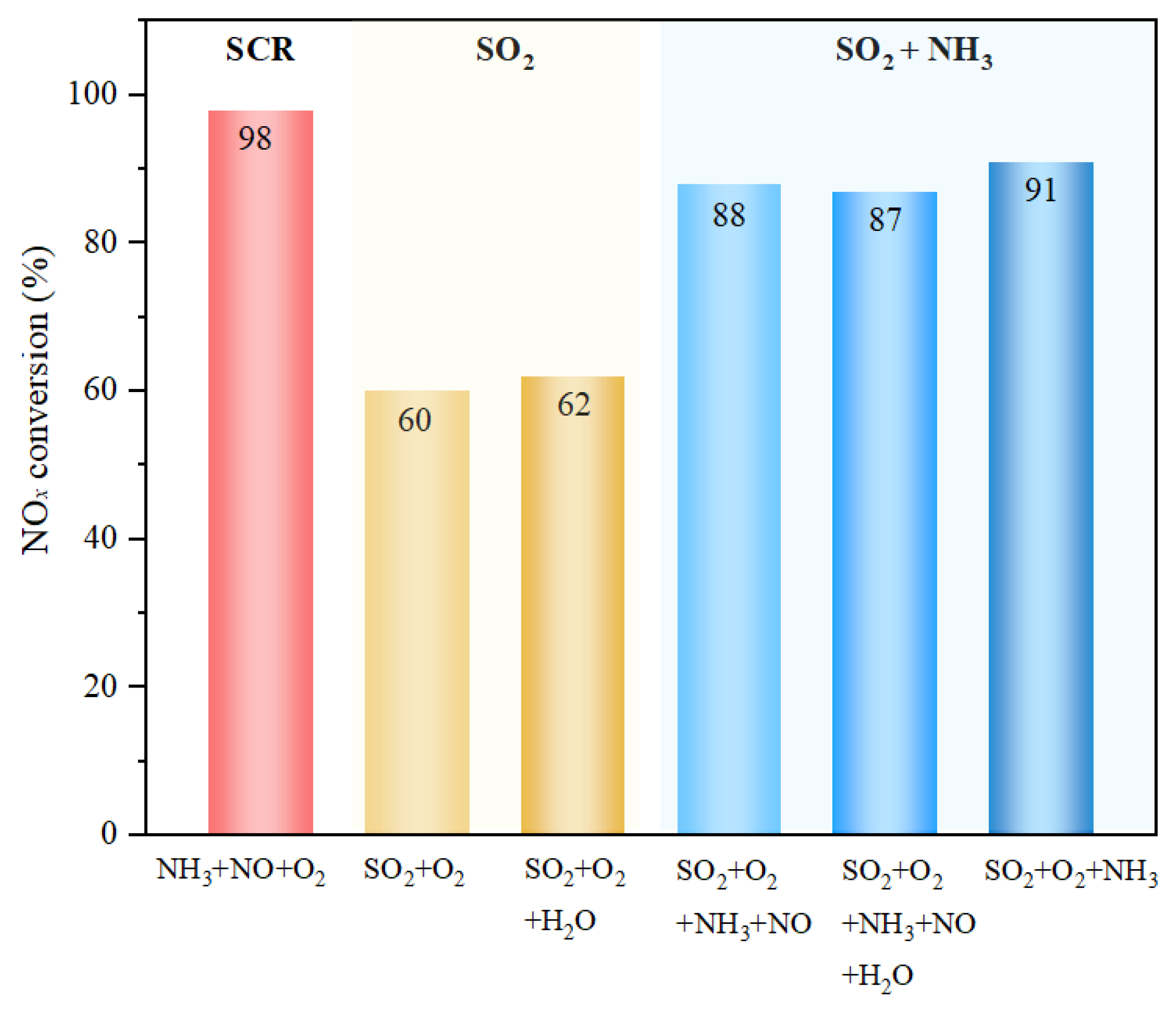
3.1. NH3-SCR Activity
3.2. TPD Analyses
3.2.1. Identification of Surface Deposits
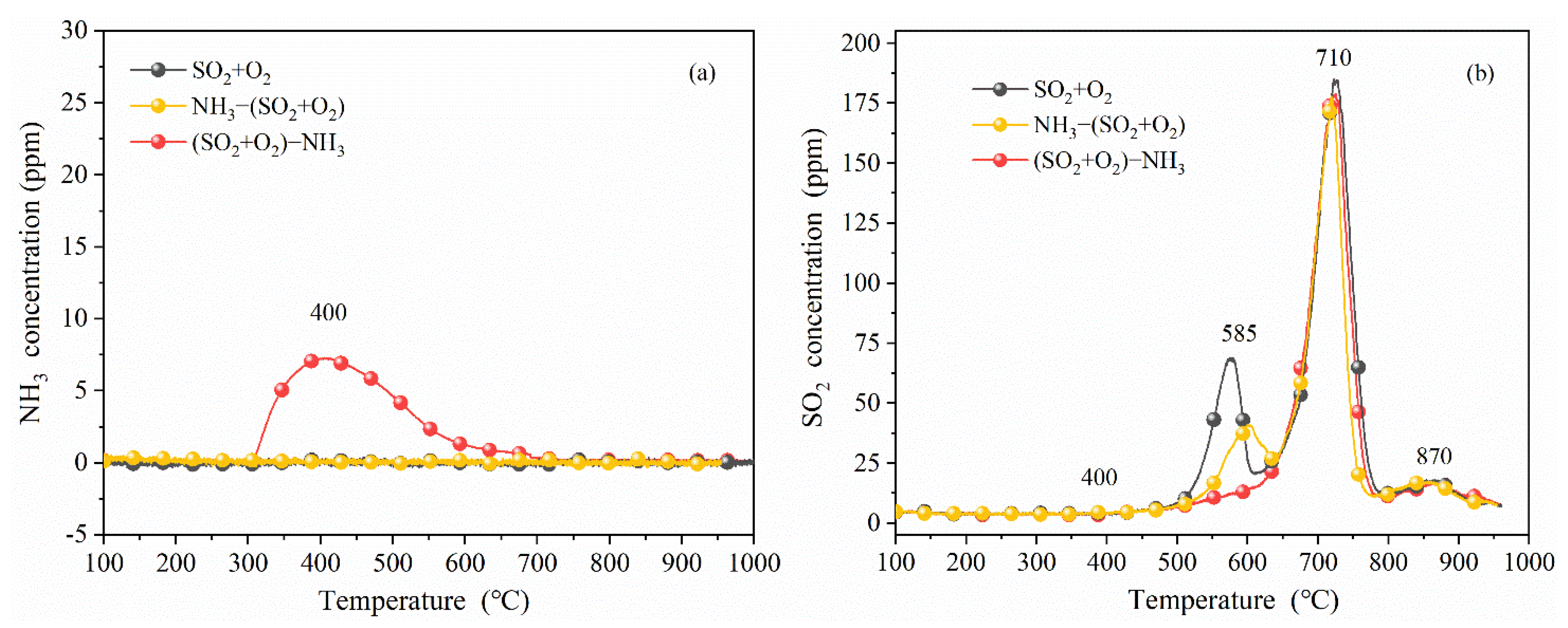
3.2.2. Effect of Ammonia on Surface Deposits
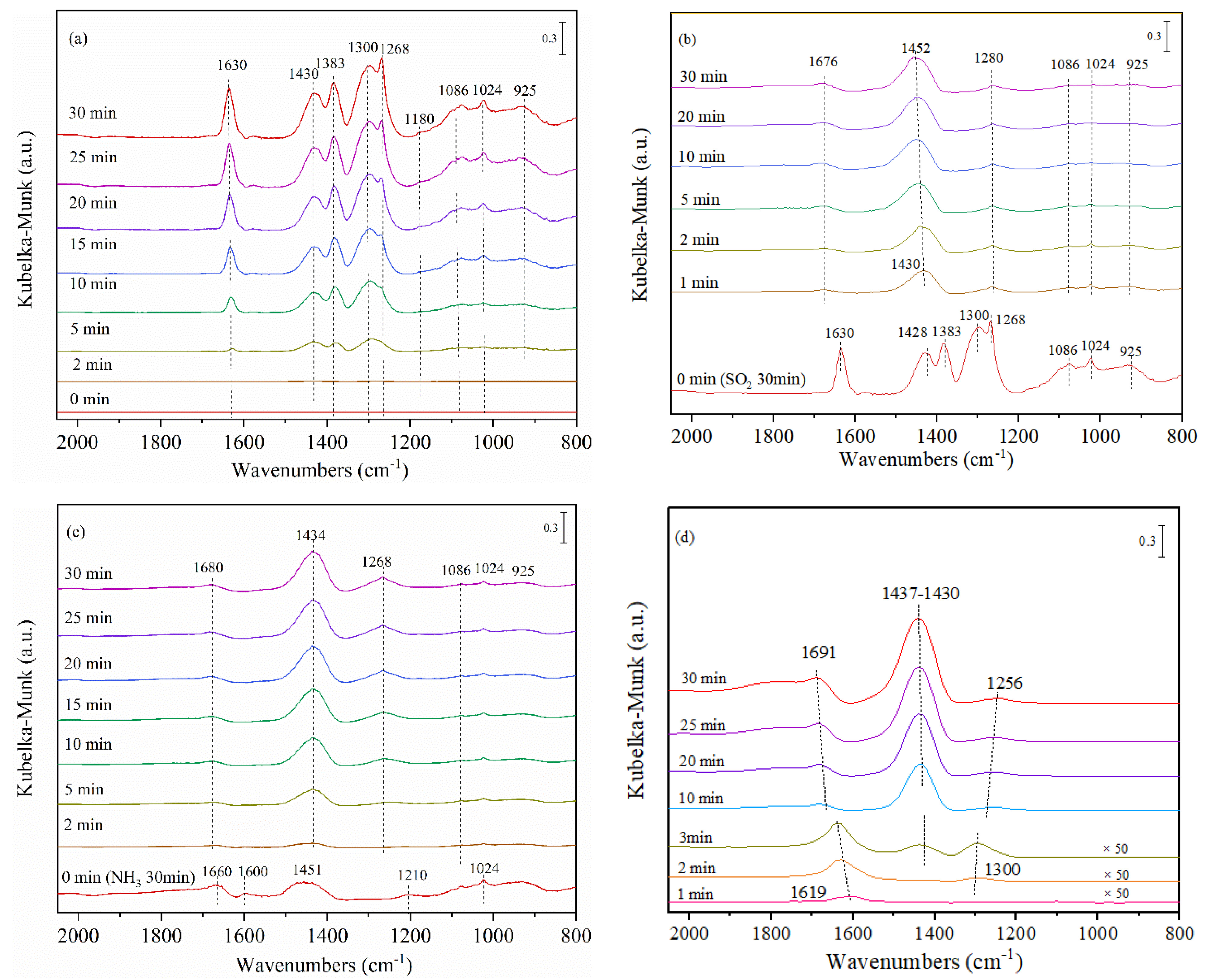
3.3. Infrared Studies
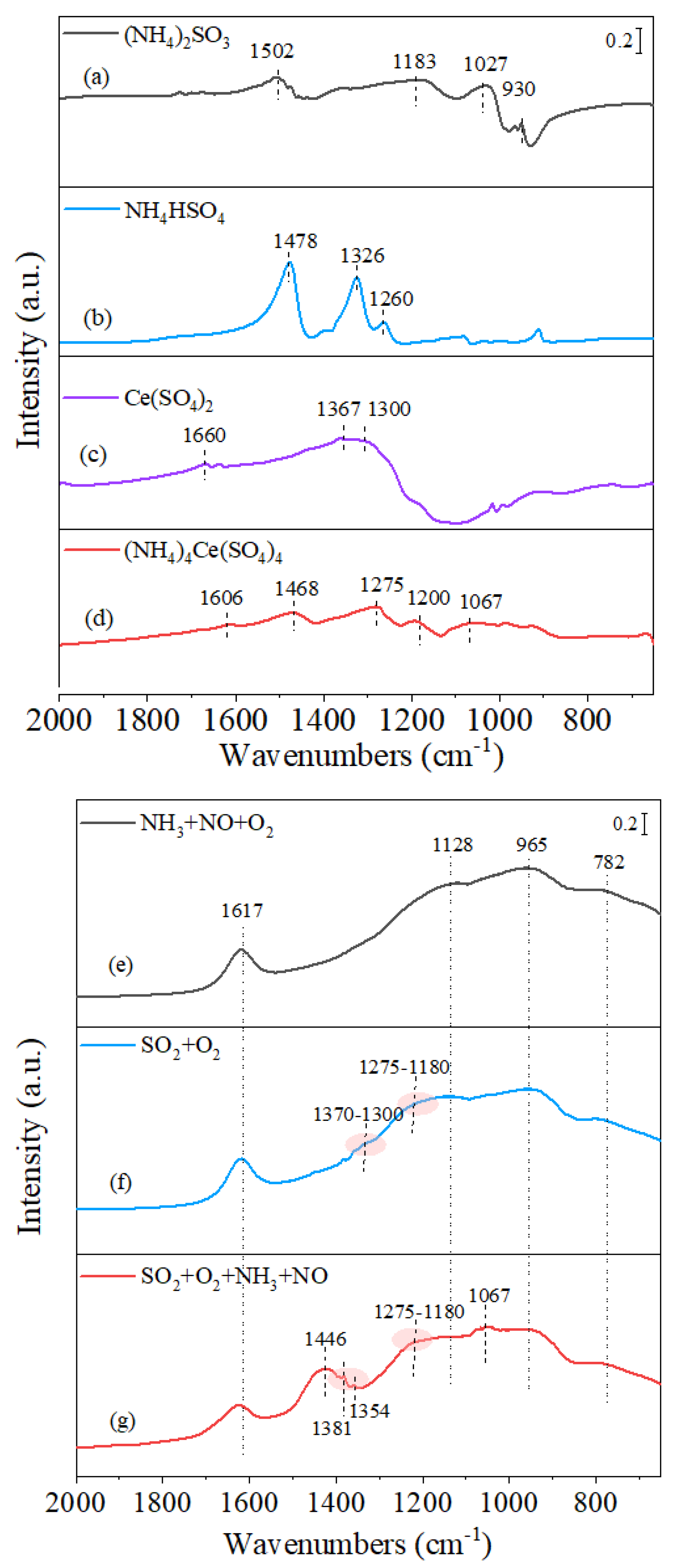
3.3.1. Surface Groups Identification
3.3.2. In Situ SO2 + O2 Adsorption
3.3.3. In Situ Surface Reaction between Ad-Species and NH3/SO2
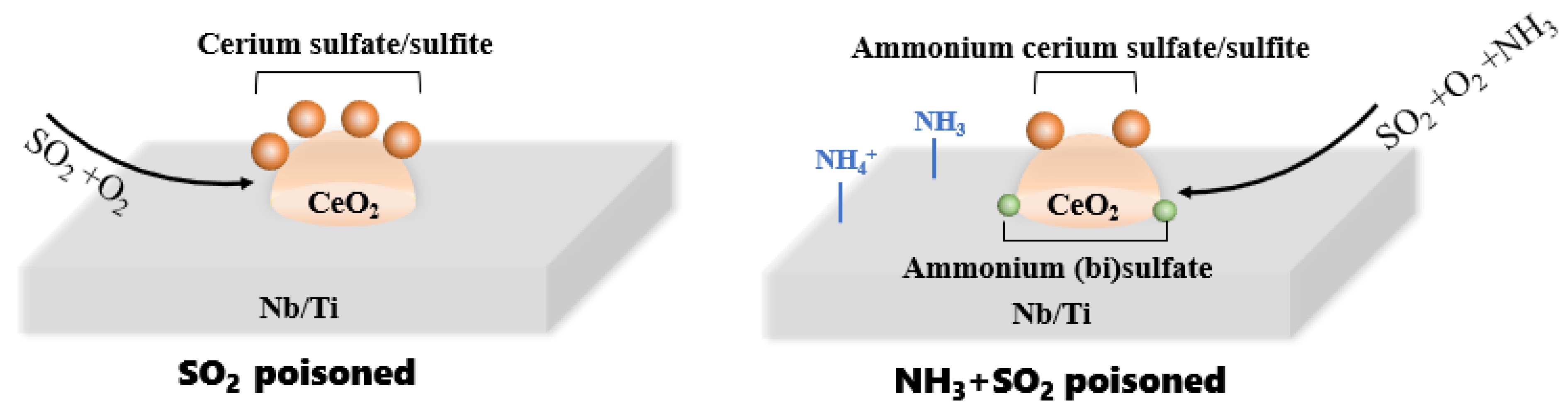
4. Discussion
5. Conclusions
- (1)
- After exposure to SO2 + O2 for 6 h, the NOx conversion at 300 °C of the catalyst decreases from almost 100% to ca. 60%, while the presence of NH3 in the poisoning atmospheres can reserve the catalyst with about 90% NH3-SCR activity. Additionally, humid conditions do not result in any obvious changes in the deactivation degree of sulfur poisoning with or without NH3.
- (2)
- The types and amounts of sulfates/sulfites depend importantly on the sulfur poisoning atmospheres. When the poisoning is performed in the presence of NH3, the total amount of sulfites and sulfates that are deposited on the catalyst is reduced by 44% compared with that in absence of ammonia. Competitive adsorption between NH3 and SO2 is suggested to be one of the dominating factors for the decreased surface deposits. The pre-occupying NH3 is confirmed to protect ceria active sites from reacting with SO2.
- (3)
- With the introduction of ammonia, not only the amounts of sulfates/sulfites decrease significantly, but also the types of sulfates change a lot from metal sulfates to ammonium sulfates. The ready decomposition behaviors of NH4HSO4 (and (NH4)2SO4), as well as the transformation of cerium sulfates with more sulfate radicals that are bonded per cerium atom, can also transform to cerium ammonium sulfates which facilitate the decrease in sulfate deposits. Compared with inert metal sulfates, cerium ammonium sulfates even contribute to NH3-SCR reaction with the redox cycle between Ce3+ and Ce4+ in the ammonium sulfates. Additionally, metal sulfites can be partially converted into ammonium sulfite in a NH3-containing atmosphere which is easy to decompose even at low temperatures, resulting in fewer sulfite deposits.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kwon, D.W.; Park, K.H.; Ha, H.P.; Hong, S.C. The role of molybdenum on the enhanced performance and SO2 resistance of V/Mo-Ti catalysts for NH3-SCR. Appl. Surf. Sci. 2019, 481, 1167–1177. [Google Scholar] [CrossRef]
- Han, L.P.; Cai, S.X.; Gao, M.; Hasegawa, J.Y.; Wang, P.L.; Zhang, J.P.; Shi, L.Y.; Zhang, D.S. Selective catalytic reduction of NOx with NH3 by using novel catalysts: State of the art and future prospects. Chem. Rev. 2019, 119, 10916–10976. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.C.; Wu, X.D.; Weng, D.; Ran, R.; Yu, J. Rare earth containing catalysts for selective catalytic reduction of NOx with ammonia: A Review. J. Rare Earth 2014, 32, 907–917. [Google Scholar] [CrossRef]
- Mosrati, J.; Atia, H.; Eckelt, R.; Lund, H.; Agostini, G.; Bentrup, U.; Rockstroh, N.; Keller, S.; Armbruster, U.; Mhamdi, M. Nb-modified Ce/Ti oxide catalyst for the selective catalytic reduction of NO with NH3 at low temperature. Catalysts 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Bao, C.; Liu, S.; Liang, G.; Lu, M.; Lai, C.; Shi, W.; Ma, S. Enhanced activity of Nb-modified CeO2/TiO2 catalyst for the selective catalytic reduction of NO with NH3. Aerosol Air Qual. Res. 2018, 18, 2121–2130. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; He, H.; Yu, Y.; Yan, Z.; Yang, W.; Shan, W. Quantitative study of the NH3-SCR pathway and the active site distribution over CeWOx at low temperatures. J. Catal. 2019, 369, 372–381. [Google Scholar] [CrossRef]
- Liu, J.J.; He, G.Z.; Shan, W.P.; Yu, Y.B.; Huo, Y.L.; Zhang, Y.; Wang, M.; Yu, R.; Liu, S.S.; He, H. Introducing tin to develop ternary metal oxides with excellent hydrothermal stability for NH3 selective catalytic reduction of NOx. Appl. Catal. B Environ. 2021, 291, 120125. [Google Scholar] [CrossRef]
- Shan, W.P.; Yu, Y.B.; Zhang, Y.; He, G.Z.; Peng, Y.; Li, J.H.; He, H. Theory and practice of metal oxide catalyst design for the selective catalytic reduction of NOx with NH3. Catal. Today 2021, 375, 292–301. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Jiang, J.; Tan, Y.; Wang, Q.; Gong, C.; Shen, D.; Wu, C. Temperature sensitivity of the selective catalytic reduction (SCR) performance of Ce–TiO2 in the presence of SO2. Chemosphere 2020, 243, 125419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zou, W.; Ma, K.; Cao, Y.; Xiong, Y.; Wu, S.; Tang, C.; Gao, F.; Dong, L. Sulfated temperature effects on the catalytic activity of CeO2 in NH3-selective catalytic reduction conditions. J. Phys. Chem. C 2015, 119, 1155–1163. [Google Scholar] [CrossRef]
- Wijayanti, K.; Xie, K.; Kumar, A.; Kamasamudram, K.; Olsson, L. Effect of gas compositions on SO2 poisoning over Cu/SSZ-13 used for NH3-SCR. Appl. Catal. B Environ. 2017, 219, 142–154. [Google Scholar] [CrossRef]
- Wijayanti, K.; Leistner, K.; Chand, S.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Deactivation of Cu-SSZ-13 by SO2 exposure under SCR conditions. Catal. Sci. Technol. 2016, 6, 2565–2579. [Google Scholar] [CrossRef]
- Wang, C.; Wang, J.; Wang, J.; Yu, T.; Shen, M.; Wang, W.; Li, W. The effect of sulfate species on the activity of NH3-SCR over Cu/SAPO-34. Appl. Catal. B Environ. 2017, 204, 239–249. [Google Scholar] [CrossRef]
- Jangjou, Y.; Wang, D.; Kumar, A.; Li, J.; Epling, W.S. SO2 poisoning of the NH3-SCR reaction over Cu-SAPO-34: Effect of ammonium sulfate versus other S-containing species. ACS Catal. 2016, 6, 6612–6622. [Google Scholar] [CrossRef]
- Wang, Y.; Yi, W.; Yu, J.; Zeng, J.; Chang, H. Novel methods for assessing the so2 poisoning effect and thermal regeneration possibility of MOx–WO3/TiO2 (M = Fe, Mn, Cu, and V) Catalysts for NH3-SCR. Environ. Sci. Technol. 2020, 54, 12612–12620. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.; Liu, F.; Shan, W.; He, H. Improvement of Nb doping on SO2 resistance of VOx/CeO2 catalyst for the selective catalytic reduction of NOx with NH3. J. Phys. Chem. C 2017, 121, 7803–7809. [Google Scholar] [CrossRef]
- An, D.; Yang, S.; Zou, W.; Sun, J.; Tan, W.; Ji, J.; Tong, Q.; Sun, C.; Li, D.; Dong, L. Unraveling the so2 poisoning effect over the lifetime of MeOx (Me = Ce, Fe, Mn) catalysts in low-temperature NH3-SCR: Interaction of reaction atmosphere with surface species. J. Phys. Chem. C 2022, 126, 12168–12177. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, S.; Wu, X.; Shi, Y.; Cao, L.; Liu, L.; Ran, R.; Si, Z.; Liu, J.; Weng, D. Low-temperature solid-state ion-exchange method for preparing Cu-SSZ-13 selective catalytic reduction catalyst. ACS Catal. 2019, 8, 6962–6973. [Google Scholar] [CrossRef]
- Ma, Z.; Weng, D.; Wu, X.; Si, Z.; Wang, B. A novel Nb–Ce/WOx–TiO2 catalyst with high NH3-SCR activity and stability. Catal. Commun. 2012, 27, 97–100. [Google Scholar] [CrossRef]
- Song, L.; Chao, J.; Fang, Y.; He, H.; Li, J.; Qiu, W.; Zhang, G. Promotion of ceria for decomposition of ammonia bisulfate over V2O5-MoO3/TiO2 catalyst for selective catalytic reduction. Chem. Eng. J. 2016, 303, 275–281. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Zheng, C.; Cen, K.; Gao, X. Mechanistic investigation of enhanced reactivity of NH4HSO4 and NO on Nb-and Sb-doped VW/Ti SCR catalysts. Appl. Catal. A Gen. 2018, 549, 310–319. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Zheng, C.; Gao, X.; Luo, Z.; Ma, N.; Cen, K. Investigation of the promotion effect of WO₃ on the decomposition and reactivity of NH₄HSO₄ with NO on V₂O₅–WO₃/TiO₂ SCR catalysts. RSC Adv. 2016, 6, 55584–55592. [Google Scholar] [CrossRef]
- Leistner, K.; Mihai, O.; Wijayanti, K.; Kumar, A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.L.; Olsson, L. Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions. Catal. Today 2015, 258, 49–55. [Google Scholar] [CrossRef]
- Udupa, M.R. Thermal decomposition of cerium (IV), cerium (III), chromium (III) and titanium (IV) sulphates. Thermochim. Acta 1982, 57, 377–381. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Lee, H.; Cho, S.J.; Kim, D.H. Effect of pore structure of TiO2 on the SO2 poisoning over V2O5/TiO2 catalysts for selective catalytic reduction of NOx with NH3. Catal. Today 2018, 303, 19–24. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Feng, Y.; Si, Z.; Weng, D.; Shi, L. Low-temperature SCR activity and SO2 deactivation mechanism of Ce-modified V2O5–WO3/TiO2 catalyst. Prog. Nat. Sci. Mater. 2015, 25, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiong, J.; Lin, Y.; Guo, J.; Zhu, T. Distribution of SO2 oxidation products in the SCR of NO over V2O5/TiO2 catalysts at different temperatures. Ind. Eng. Chem. Res. 2020, 59, 5177–5185. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.; Lim, L.G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.H.; Grabow, L.C.; Epling, W.S. Nature of Cu active centers in Cu-SSZ-13 and their responses to SO2 exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Shen, B.X.; Liu, T. Deactivation of MnOx-CeOx/ACF catalysts for low-temperature NH3-SCR in the presence of SO2. Acta Phys. Chim. Sin. 2010, 26, 3009–3016. [Google Scholar]
- Kwon, D.W.; Nam, K.B.; Hong, S.C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2015, 166, 37–44. [Google Scholar] [CrossRef]
- Qing, M.; Lei, S.; Kong, F.; Liu, L.; Zhang, W.; Wang, L.; Guo, T.; Su, S.; Hu, S.; Wang, Y.; et al. Analysis of ammonium bisulfate/sulfate generation and deposition characteristics as the by-product of SCR in coal-fired flue gas. Fuel 2022, 313, 122790. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Shen, Y.; Zheng, L.; Han, L.; Deng, J.; Zhang, J.P.; Wang, A.Y.; Ren, W.; Gao, F.; et al. Boosting SO2-Resistant NOx Reduction by modulating electronic interaction of short-range Fe–O coordination over Fe2O3/TiO2 catalysts. Environ. Sci. Technol. 2022, 56, 11646–11656. [Google Scholar] [CrossRef]
- Xu, Z.; Impeng, S.; Jia, X.; Wang, F.; Shen, Y.; Wang, P.; Zhang, D. SO2-Tolerant catalytic reduction of NOx by confining active species in TiO2 nanotubes. Environ. Sci. Nano 2022, 9, 2121. [Google Scholar] [CrossRef]
- Jin, R.; Liu, Y.; Wang, Y.; Cen, W.; Wu, Z.; Wang, H.; Weng, X. The role of cerium in the improved SO2 tolerance for NO reduction with NH3 over Mn-Ce/TiO2 catalyst at low temperature. Appl. Catal. B Environ. 2014, 148, 582–588. [Google Scholar] [CrossRef]
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.; Hao, J. Improvement of activity and SO2 tolerance of Sn-modified MnOx–CeO2 catalysts for NH3-SCR at low temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef]
- Ye, D.; Qu, R.; Song, H.; Gao, X.; Luo, Z.; Ni, M.; Cen, K. New insights into the various decomposition and reactivity behaviors of NH4HSO4 with NO on V2O5/TiO2 catalyst surfaces. Chem. Eng. J. 2016, 283, 846–854. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Li, J.; Zhao, S.; Wang, J.; Chu, C.; Li, C. Promotional mechanisms of activity and SO2 tolerance of Co-or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem. Eng. J. 2017, 317, 20–31. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, X.; Si, Z.; Weng, D.; Ma, J.; Xu, T. Impacts of niobia loading on active sites and surface acidity in NbOx/CeO2–ZrO2 NH3–SCR catalysts. Appl. Catal. B Environ. 2015, 179, 380–394. [Google Scholar] [CrossRef] [Green Version]
- Burcham, L.J.; Datka, J.; Wachs, I.E. In situ vibrational spectroscopy studies of supported niobium oxide catalysts. J. Phys. Chem. B. 1999, 103, 6015–6024. [Google Scholar] [CrossRef]
- Cao, L.; Chen, L.; Wu, X.; Ran, R.; Xu, T.; Chen, Z.; Weng, D. TRA and DRIFTS studies of the fast SCR reaction over CeO2/TiO2 catalyst at low temperatures. Appl. Catal. A Gen. 2018, 557, 46–54. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Jiang, J.; Tan, Y.; Wang, Q.; Gong, C.; Shen, D.; Wu, C. Sulfation effect of Ce/TiO2 catalyst for the selective catalytic reduction of NOx with NH3: Mechanism and kinetic studies. RSC Adv. 2019, 9, 32110. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Liu, F.; He, H.; Shi, X.; Zhang, C. A superior Ce-W-Ti mixed oxide catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2012, 115, 100–106. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D. DRIFT study of CuO–CeO2–TiO2 mixed oxides for NOx reduction with NH3 at low temperatures. ACS Appl. Mater. Interfaces 2014, 6, 8134–8145. [Google Scholar] [CrossRef] [PubMed]
- Lonyi, F.; Valyon, J. A TPD and IR study of the surface species formed from ammonia on zeolite H-ZSM-5, H-mordenite and H-beta. Thermochim. Acta 2001, 373, 53–57. [Google Scholar] [CrossRef]
- Tong, T.; Chen, J.; Xiong, S.; Yang, W.; Yang, Q.; Yang, L.; Li, J. Vanadium-density-dependent thermal decomposition of NH4HSO4 on V2O5/TiO2 SCR catalysts. Catal. Sci. Technol. 2019, 9, 3779–3787. [Google Scholar] [CrossRef]
- Casari, B.M.; Langer, V. Two Ce(SO4)2·4H2O polymorphs: Crystal structure and thermal behavior. J. Solid State Chem. 2007, 180, 1616–1622. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, R. Study on the thermal decomposition of tetrahydrated cerie sulphate. Thermochim. Acta 1992, 202, 301–306. [Google Scholar] [CrossRef]
- Singh Mudher, K.D.; Keskar, M.; Venugopal, V. Solid state reactions of CeO2, ThO2 and PuO2 with ammonium sulphate. J. Nucl. Mater. 1999, 265, 146–153. [Google Scholar] [CrossRef]
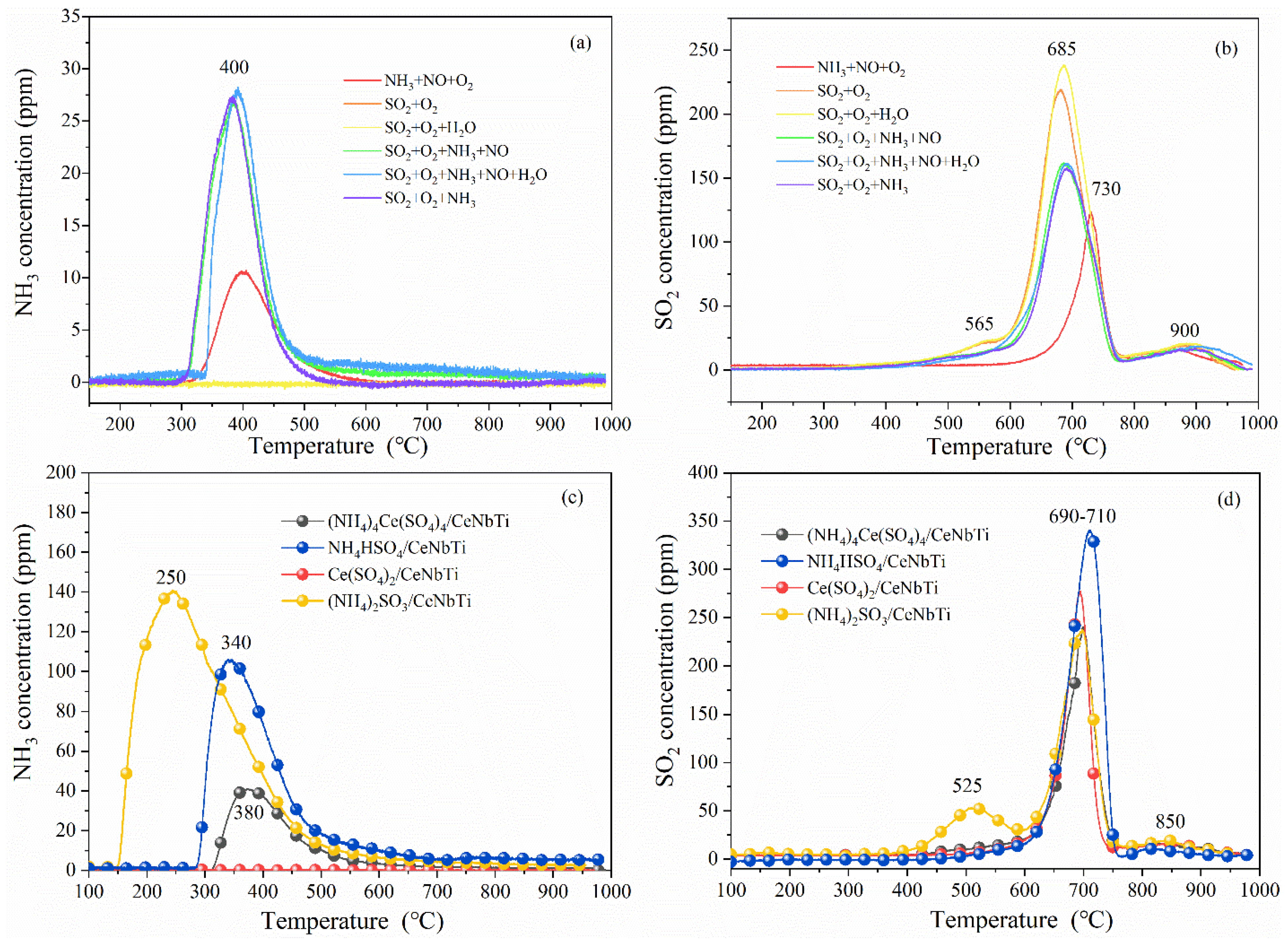

| Sample | SBET (m2/g) | NH3 (μmol/g) | SO2 (μmol/g) | ||||
|---|---|---|---|---|---|---|---|
| Peak Temperature (°C) | Deposit * | ||||||
| 565 | 685 | 730 | 900 | ||||
| NH3 + NO + O2 | 113 | 16 | 0 | 0 | 98 | 34 | - |
| SO2 + O2 | 85 | 0 | 35 | 185 | 98 | 34 | 220 |
| SO2 + O2 + H2O | 87 | 0 | 35 | 212 | 98 | 38 | 247 |
| SO2 + O2 + NH3 + NO | 93 | 35 | 17 | 131 | 98 | 37 | 148 |
| SO2 + O2 +NH3 + NO + H2O | 86 | 33 | 15 | 129 | 98 | 32 | 144 |
| SO2 + O2 + NH3 | 89 | 34 | 17 | 125 | 98 | 32 | 142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Cao, L.; Wu, X.; Zhang, X.; Ma, Z.; Ran, R.; Si, Z.; Weng, D.; Wang, B. Protection Effect of Ammonia on CeNbTi NH3-SCR Catalyst from SO2 Poisoning. Catalysts 2022, 12, 1430. https://doi.org/10.3390/catal12111430
Gao Y, Cao L, Wu X, Zhang X, Ma Z, Ran R, Si Z, Weng D, Wang B. Protection Effect of Ammonia on CeNbTi NH3-SCR Catalyst from SO2 Poisoning. Catalysts. 2022; 12(11):1430. https://doi.org/10.3390/catal12111430
Chicago/Turabian StyleGao, Yang, Li Cao, Xiaodong Wu, Xu Zhang, Ziran Ma, Rui Ran, Zhichun Si, Duan Weng, and Baodong Wang. 2022. "Protection Effect of Ammonia on CeNbTi NH3-SCR Catalyst from SO2 Poisoning" Catalysts 12, no. 11: 1430. https://doi.org/10.3390/catal12111430
APA StyleGao, Y., Cao, L., Wu, X., Zhang, X., Ma, Z., Ran, R., Si, Z., Weng, D., & Wang, B. (2022). Protection Effect of Ammonia on CeNbTi NH3-SCR Catalyst from SO2 Poisoning. Catalysts, 12(11), 1430. https://doi.org/10.3390/catal12111430








