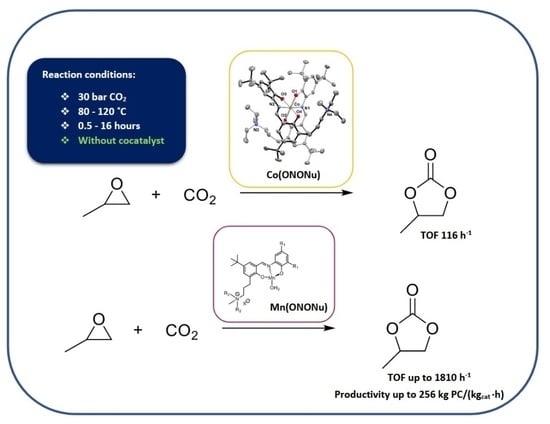
New Co and Mn Catalysts Bearing ONO Ligands Containing Nucleophile for the Coupling of CO2 and Propylene Oxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of (ONONu) Ligands
2.2. Preparation and Characterization of Cobalt and Manganese Complexes
2.2.1. Preparation and Characterization of Co(III) (ONONu) Complexes
2.2.2. Preparation and Characterization of Mn(II)(ONONu) Complexes
2.3. Catalytic Activity of the M(ONONu)X Complexes in the Carbon Dioxide/Epoxide Coupling Reactions
3. Materials and Methods
3.1. General Procedures
3.2. General Analysis and Methods
3.3. Preparation and Characterization of Ligands
3.3.1. General Procedure for Ligands L1–L4
3.3.2. General Procedure for Quaternization of the Ligands (Example for ligand L1-I)
3.4. Preparation and Characterization of Cobalt and Manganese Complexes
3.4.1. General procedure and Characterization of the Compounds [Co(ONONu)2(S)](Example for Ligand L1-I)
3.4.2. Synthesis and Characterization of the Compounds [Mn(ONONu)] (7–8)
3.5. X-ray Structure Determination
Crystal Data and Details of Refinement
3.6. Autoclave Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hasan, M.M.F.; Rossi, L.M.; Debecker, D.P.; Leonard, K.C.; Li, Z.; Makhubela, B.C.E.; Zhao, C.; Kleij, A. Can CO2 and Renewable Carbon Be Primary Resources for Sustainable Fuels and Chemicals? ACS Sustain. Chem. Eng. 2021, 9, 12427–12430. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; Qian, L.; Luo, D.; Dou, H.; Wen, G.; Yu, A.; Chen, Z. Emerging Trends in Sustainable CO2-Management Materials. Adv. Mater. 2022, 34, 2201547. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.L.; Sherwood, J.; Hunt, A.J.; Clark, J.H. Cyclic Carbonates as Green Alternative Solvents for the Heck Reaction. ACS Sustain. Chem. Eng. 2014, 2, 1739–1742. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Pescarmona, P.P. Cyclic carbonates synthesised from CO2: Applications, challenges and recent research trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Decortes, A.; Castilla, A.M.; Kleij, A.W. Salen-Complex-Mediated Formation of cyclic carbonates by cycloaddition of CO2 to epoxides. Angew. Chem. Int. Ed. 2010, 49, 9822. [Google Scholar] [CrossRef]
- Qin, Z.; Thomas, C.M.; Lee, S.; Coates, G.W. Cobalt-Based Complexes for the Copolymerization of Propylene Oxide and CO2: Active and Selective Catalysts for Polycarbonate Synthesis. Angew. Chem. Int. Ed. 2003, 42, 5484. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Escudero-Adán, E.C.; Martin, E.; Kleij, A.W. A powerful aluminum catalyst for the synthesis of highly functional organic carbonates. J. Am. Chem. Soc. 2013, 135, 1228. [Google Scholar] [CrossRef]
- Tian, D.; Liu, B.; Gan, Q.; Li, H.; Darensbourg, D.J. Formation of Cyclic Carbonates from Carbon Dioxide and Epoxides Coupling Reactions Efficiently Catalyzed by Robust, Recyclable One-Component Aluminum-Salen Complexes. ACS Catal. 2012, 2, 2029–2035. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R. Mechanism of cyclic carbonate synthesis from epoxides and CO2. Angew. Chem. Int. Ed. 2009, 48, 2946–2948. [Google Scholar] [CrossRef]
- Mercadé, E.; Zangrando, E.; Claver, C.; Godard, C. Robust Zinc complexes that contain pyrrolidine-based ligands as recyclable catalysts for the synthesis of cyclic carbonates from carbon dioxide and epoxides. ChemCatChem 2016, 8, 234. [Google Scholar] [CrossRef]
- Allen, S.D.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Highly-activity, single-site catalysts for the alternating copolymerization of CO2 and propylene oxide. J. Am. Chem. Soc. 2002, 124, 14284. [Google Scholar] [CrossRef]
- Kilic, A.; Durgun, M.; Ulusoy, M.; Tas, E. Conversion of CO2 into cyclic carbonates in presence of metal complexes as catalysts. J. Chem. Res. 2010, 2, 622–626. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Martin, E.; Belmonte, M.M.; Benet-Buchholz, J.; Kleij, A.W. An efficient iron catalysts for the synthesis of five- and six- membered organic carbonates under mild conditions. Adv. Synth. Catal. 2012, 354, 469. [Google Scholar] [CrossRef]
- Whiteoak, C.J.; Kielland, N.; Laserna, V.; Castro-Gómez, F.; Martin, E.; Escudero-Adán, E.C.; Bo, C.; Kleij, A.W. Highly Active Aluminium Catalysts for the Formation of Organic Carbonates from CO2 and Oxiranes. Chem.—A Eur. J. 2014, 20, 2264–2275. [Google Scholar] [CrossRef]
- Paddock, R.L.; Nguyen, S.T. Chemical CO2 Fixation: Cr(III) Salen Complexes as Highly Efficient Catalysts for the Coupling of CO2 and Epoxides. J. Am. Chem. Soc. 2001, 123, 11498–11499. [Google Scholar] [CrossRef]
- Buonerba, A.; de Nisi, A.; Grassi, A.; Milione, S.; Capacchione, C.; Vagin, S.; Rieger, B. Novel iron(III) catalyst for the efficient and selective coupling of carbon dioxide and epoxides to form cyclic carbonates. Catal. Sci. Technol. 2015, 5, 118–123. [Google Scholar] [CrossRef]
- Andrea, K.A.; Butler, E.D.; Brown, T.R.; Anderson, T.S.; Jagota, D.; Rose, C.; Lee, E.M.; Goulding, S.D.; Murphy, J.N.; Kerton, F.M.; et al. Iron Complexes for Cyclic Carbonate and Polycarbonate Formation: Selectivity Control from Ligand Design and Metal-Center Geometry. Inorg. Chem. 2019, 58, 11231–11240. [Google Scholar] [CrossRef]
- Srivastava, R.; Bennur, T.H.; Srinivas, D. Factors affecting activation and utilization of carbon dioxide in cyclic carbonates synthesis over Cu and Mn peraza macrocyclic complexes. J. Mol. Catal. A Chem. 2005, 226, 199–205. [Google Scholar] [CrossRef]
- Man, L.M.; Lam, K.C.; Sit, W.N.; Ng, S.M.; Zhou, Z.; Lin, Z.; Lau, C.P. Synthesis of heterobimetallic Ru-Mn complexes and the coupling reactions of epoxides with carbon dioxide catalyzed by these complexes. Chem.—A Eur. J. 2006, 12, 1004–1015. [Google Scholar] [CrossRef]
- Muñoz, B.K.; Viciano, M.; Godard, C.; Castillón, S.; García-Ruiz, M.; Blanco González, M.D.; Claver, C. Metal complexes bearing ONO ligands as highly active catalysts in carbon dioxide and epoxide coupling reactions. Inorganica Chim. Acta. 2021, 517, 120194. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y.-B.; Lu, X.-B.; Li, B.; Wang, H.; Sun, L.-C. Intramolecularly two-centered cooperation catalysis for the synthesis of cyclic carbonates from CO2 and epoxides. Tetrahedron Lett. 2008, 49, 6589. [Google Scholar] [CrossRef]
- Miao, C.-X.; Wang, J.-Q.; Wu, Y.; Du, Y.; He, L.-N. Bifunctional Metal-Salen Complexes as Efficient Catalysts for the Fixation of CO2 with Epoxides under Solvent-Free Conditions. ChemSusChem 2008, 1, 236. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Jin, L.; Jing, H. Bifunctional chiral catalysts for the synthesis of chiral cyclic carbonates from carbon dioxide and epoxides. ChemCatChem 2009, 1, 379–383. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Making plastics from carbon dioxide: Salen metal complexes as catalysts to produce polycarbonates from epoxides and CO2. Chem. Rev. 2007, 107, 2388–2410. [Google Scholar] [CrossRef]
- Chatterjee, C.; Chisholm, M.H. The influence of the metal (Al, Cr, and Co) and the substituents of the porphyrin in controlling the reactions involved in the copolymerization of propylene oxide and carbon dioxide by porphyrin metal (III) complexes. 1. Aluminum chemistry. Inorg. Chem. 2011, 50, 4481–4492. [Google Scholar] [CrossRef]
- Lu, X.-B.; Darensbourg, D.J. Cobalt catalysts for the coupling of CO2 and epoxides to provide polycarbonates and cyclic carbonates. Chem. Soc. Rev. 2012, 41, 1462–1484. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Salen metal complexes as catalysts for the synthesis of polycarbonates from cyclic ethers and carbon dioxide. In Synthetic Biodegradable Polymers; Rieger, B., Kunkel, A., Coates, G.W., Reichardt, R., Dinjus, E., Zevaco, T.A., Eds.; Advances in Polymer Science; Springer: Berlin, Germany, 2012; Volume 245, pp. 1–27. [Google Scholar]
- Chatterjee, C.; Chisholm, M.H. Influence of the metal (Al, Cr, and Co) and the substituents of the porphyrin in controlling the reactions involved in the copolymerization of propylene oxide and carbon dioxide by porphyrin metal(III) complexes. 2. Chromium chemistry. Inorg. Chem. 2012, 51, 12041–12052. [Google Scholar] [CrossRef]
- Nakano, K.; Kamada, T.; Nozaki, K. Selective formation of polycarbonate over cyclic carbonate: Copolymerization of epoxides with carbon dioxide catalyzed by a cobalt(III) complex with a piperidinium end-capping arm. Angew. Chem. Int. Ed. 2006, 45, 7274–7277. [Google Scholar] [CrossRef]
- Sujith, S.; Min, J.K.; Seong, J.E.; Na, S.J.; Lee, B.Y. A highly active and recyclable catalytic system for CO2 propylene oxide copolymerization. Angew. Chem. Int. Ed. 2008, 47, 7306–7309. [Google Scholar] [CrossRef]
- Ren, W.-M.; Liu, Z.-W.; Wen, Y.-Q.; Zhang, R.; Lu, X.-B. Mechanistics aspects of the copolymerization of CO2 with epoxides using a thermally stable single-site cobalt (III) catalysts. J. Am. Chem. Soc. 2009, 131, 11509. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, K.I.; Zagoraiou, E.; Zafiropoulos, T.F.; Raptopoulou, C.P.; Psycharis, V.; Terzis, A.; Perlepes, S.P. Mononuclear anionic octahedral cobalt (III) complexes based on N-salicylidene-o-aminophenol and its derivatives: Synthetic, structural and spectroscopic studies. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 122. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Chowdhury, A.R.; Saha, S.K.; Ghosh, M.; Pal, M.; Murmu, N.C.; Banerjee, P. Synthesis and characterization of redox non-innocent cobalt(III) complexes of a O,N,O donor ligand: Radical generation, semi-conductivity, antibacterial and anticancer activities. Inorganica Chim. Acta 2015, 429, 99. [Google Scholar] [CrossRef]
- Abdel Aziz, A.A.; Salem, A.N.M.; Sayed, M.A.; Aboaly, M.M. Synthesis, structural characterization, thermal studies, catalytic efficiency and antimicrobial activity of some M(II) complexes with ONO tridentate Schiff base N-salicylidene-o-aminophenol (saphH2). J. Mol. Struct. 2012, 1010, 130. [Google Scholar] [CrossRef]
- Belaid, S.; Landreau, A.; Djebbar, S.; Benali-Baitich, O.; Bouet, G.; Bouchara, J.P. Synthesis, characterization and antifungal activity of a series of manganese (II) and copper (II) complexes with ligands derived from reduced N,N′-O-phenylenebis(salicylideneimine). J. Inorg. Biochem. 2008, 102, 63–69. [Google Scholar] [CrossRef] [Green Version]
- İspir, E.; Kurtoğlu, M.; Purtaş, F.; Serin, S. Synthesis and Antimicrobial Activity of New Schiff Bases Having the -SiOR Group (R = CH3 or CH2CH3), and Their Transition Metal Complexes. Transit. Met. Chem. 2005, 30, 1042. [Google Scholar] [CrossRef]
- Ueno, K.; Martell, A.E. Infrared studies on Synthetic Oxigen Carriers. J. Phys. Chem. 1956, 60, 1270–1275. [Google Scholar] [CrossRef]
- Faniran, J.A.; Patel, K.S.; Bailar, J.C. Infrared spectra of N, N’-bis(salicylidene)-1,1-(dimethyl)ethylenediamine and its metal complexes. J. Inorg. Nucl. Chem. 1974, 36, 1547. [Google Scholar] [CrossRef]
- Aranha, P.E.; dos Santos, M.P.; Romera, S.; Dockal, E.R. Synthesis, characterization, and spectroscopic studies of tetradentate schiff base chromium (III) complexes. Polyhedron 2007, 26, 1373–1382. [Google Scholar] [CrossRef]
- Swamy, S.J.; Pola, S. Spectroscopic Studies on Co(II), Ni(II), Cu(II) and Zn(II) complexes with a N4-macrocyclic ligands. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 929–933. [Google Scholar] [CrossRef]
- Sebastian, M.; Arun, V.; Robinson, P.P.; Varghese, A.A.; Abraham, R.; Suresh, E.; Yusuff, K.K.M. Synthesis, structural characterization and catalytic activity study of Mn(II), Fe(III), Ni(II), Cu(II) and Zn(II) complexes of quinoxaline-2-carboxalidine-2-amino-5-methylphenol: Crystal structure of the nickel (II) complex. Polyhedron 2010, 29, 3014–3020. [Google Scholar] [CrossRef]
- Bailar, J.C.; Trotman-Dickenson, A.F. Comprehensive Inorganic Chemistry; Bailar, J.C., Emeleus, H.J., Nyholm, R., Trotman-Dickenson, A.F., Eds.; Pergamon Press: Oxford, UK, 1975; Volume 3, ISBN 9780080169880y0080169880. [Google Scholar]
- Cotton, F.A.; Wilkinson, G.; Murillo, C.A.; Bochmann, M. Advances in Inorganic Chemistry, 6th ed.; Wiley: New York, NY, USA, 1999; ISBN 978-0-471-19957-1. [Google Scholar]
- Cuesta-Aluja, L.; Castilla, J.; Masdeu-Bulto, A.M.; Henriques, C.A.; Calvete, M.J.F.; Pereira, M.M. Halogenated meso-phenyl Mn(III) porphyrins as highly efficient catalysts for the synthesis of polycarbonates and cyclic carbonates using carbon dioxide and epoxides. J. Molec. Catal. A Chem. 2016, 423, 489–494. [Google Scholar] [CrossRef]
- Sônego Milani, J.L.; Moreira Meireles, A.; Noschang Cabral, B.; de Almeida Bezerra, W.; Terra Martins, F.; da Silva Martins, D.C.; das Chagas, R.P. Highly active Mn(III) meso-tetrakis(2,3-dichlorophenyl)porphyrin catalysts for the cycloaddition of CO2 with epoxides. J. CO2 Util. 2019, 30, 100–106. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal Structure Determination and Refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112. [Google Scholar] [CrossRef] [Green Version]
- Farrugia, L. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849. [Google Scholar] [CrossRef]








| Co-N(1) | 1.893(3) | Co-O(2) | 1.890(2) |
| Co-N(2) | 1.887(3) | Co-O(3) | 1.906(2) |
| Co-O(1) | 1.903(2) | Co-O(4) | 1.904(2) |
| N(1)-Co-N(2) | 174.35(12) | N(2)-Co-O(4) | 92.83(12) |
| N(1)-Co-O(1) | 85.71(11) | O(1)-Co-O(2) | 179.01(10) |
| N(1)-Co-O(2) | 93.38(11) | O(1)-Co-O(3) | 87.82(11) |
| N(1)-Co-O(3) | 90.61(11) | O(1)-Co-O(4) | 92.30(11) |
| N(1)-Co-O(4) | 91.35(11) | O(2)-Co-O(3) | 91.78(11) |
| N(2)-Co-O(1) | 90.32(12) | O(2)-Co-O(4) | 88.12(11) |
| N(2)-Co-O(2) | 90.56(11) | O(3)-Co-O(4) | 178.05(10) |
| N(2)-Co-O(3) | 85.22(12) |
| Comp. | ν (OH2) | ν (OH) | ν (C=N) | δ(OH) in Plane Bending | ν (C-O) | ν (Mn-OH2) | ν (Mn-O) | ν (Mn-N) |
|---|---|---|---|---|---|---|---|---|
| L1 | --- | 2953 (s) | 1617 (s) | 1456 (s) | 1220 (m) | --- | --- | --- |
| 7 | 3372 (br) | 2955 (s) | 1539 (s) | 1477 (m) | 1269 (m) | 835 | 603 (w) | 539 (w) |
| L2 | --- | 2953 (s) | 1615 (m) | 1460 (s) | 1265 (m) | --- | --- | --- |
| 8 | 3414 (br) | 2952 (s) | 1538 (m) | 1444 (s) | 1265 (s) | 832 | 576 (w) | 545 (w) |
 | ||||||
|---|---|---|---|---|---|---|
| Entry | Cat | Ligand | T(°C) | % Isolated Yield | TON | TOF (h−1) |
| 1 | 1 | L1-I | 80 | 13 | 661 | 41 |
| 2 | 1 | L1-I | 120 | 35 | 1758 | 109 |
| 3 | 2 | L1-Br | 80 | 12 | 600 | 38 |
| 4 | 3 | L1-NO3 | 120 | 7 | 368 | 23 |
| 5 | 4 | L4-I | 80 | 5 | 250 | 16 |
| 6 | 5 | L2-I | 80 | 26 | 1294 | 81 |
| 7 | 5 | L2-I | 120 | 33 | 1650 | 116 |
| 8 | 6 | L3-I | 80 | 20 | 1011 | 63 |
| 9 | 6 | L3-I | 120 | 37 | 1850 | 103 |
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Cat | Mol% | T(°C) | P(bar) | t(h) | % Isolated Yield | TON | TOF (h−1) |
| 1 | 7 | 0.05 | 80 | 30 | 16 | 89 | 1780 | 111 |
| 2 | 7 | 0.01 | 80 | 10 | 16 | 31 | 3092 | 193 |
| 3 | 7 | 0.01 | 120 | 30 | 0.5 | 9 | 905 | 1810 |
| 4 | 8 | 0.01 | 80 | 30 | 16 | 17 | 1714 | 107 |
| 5 | 8 | 0.01 | 120 | 30 | 16 | 48 | 4860 | 304 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viciano, M.; Muñoz, B.K.; Zangrando, E.; Godard, C.; Castillón, S.; Blanco González, M.D.; García-Ruíz, M.; Claver, C. New Co and Mn Catalysts Bearing ONO Ligands Containing Nucleophile for the Coupling of CO2 and Propylene Oxide. Catalysts 2022, 12, 1443. https://doi.org/10.3390/catal12111443
Viciano M, Muñoz BK, Zangrando E, Godard C, Castillón S, Blanco González MD, García-Ruíz M, Claver C. New Co and Mn Catalysts Bearing ONO Ligands Containing Nucleophile for the Coupling of CO2 and Propylene Oxide. Catalysts. 2022; 12(11):1443. https://doi.org/10.3390/catal12111443
Chicago/Turabian StyleViciano, Mónica, Bianca K. Muñoz, Ennio Zangrando, Cyril Godard, Sergio Castillón, Mª Dolores Blanco González, Mónica García-Ruíz, and Carmen Claver. 2022. "New Co and Mn Catalysts Bearing ONO Ligands Containing Nucleophile for the Coupling of CO2 and Propylene Oxide" Catalysts 12, no. 11: 1443. https://doi.org/10.3390/catal12111443
APA StyleViciano, M., Muñoz, B. K., Zangrando, E., Godard, C., Castillón, S., Blanco González, M. D., García-Ruíz, M., & Claver, C. (2022). New Co and Mn Catalysts Bearing ONO Ligands Containing Nucleophile for the Coupling of CO2 and Propylene Oxide. Catalysts, 12(11), 1443. https://doi.org/10.3390/catal12111443