CO2 Electroreduction on Carbon-Based Electrodes Functionalized with Molecular Organometallic Complexes—A Mini Review
Abstract
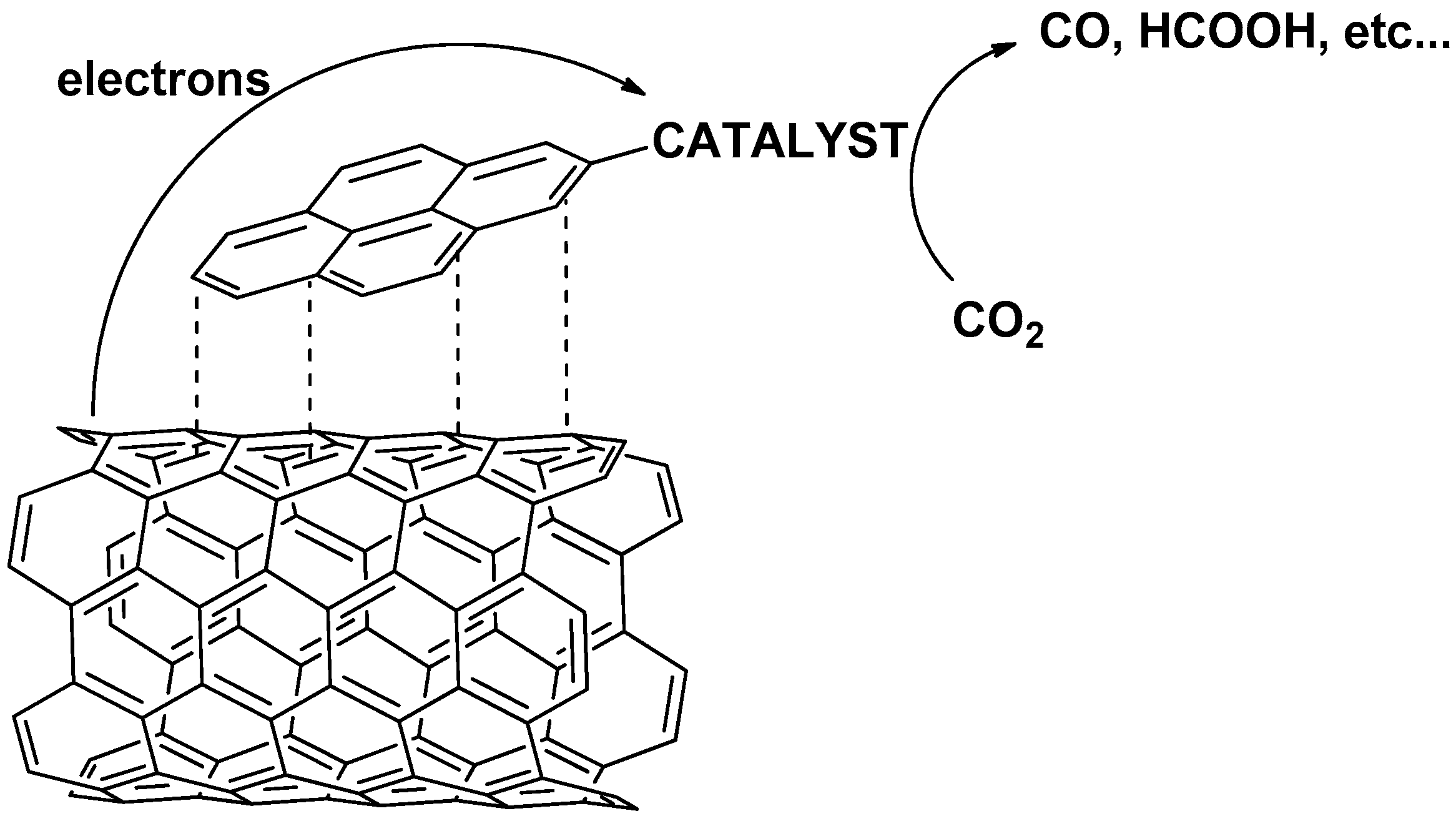
:1. Introduction
2. Anchoring Strategies
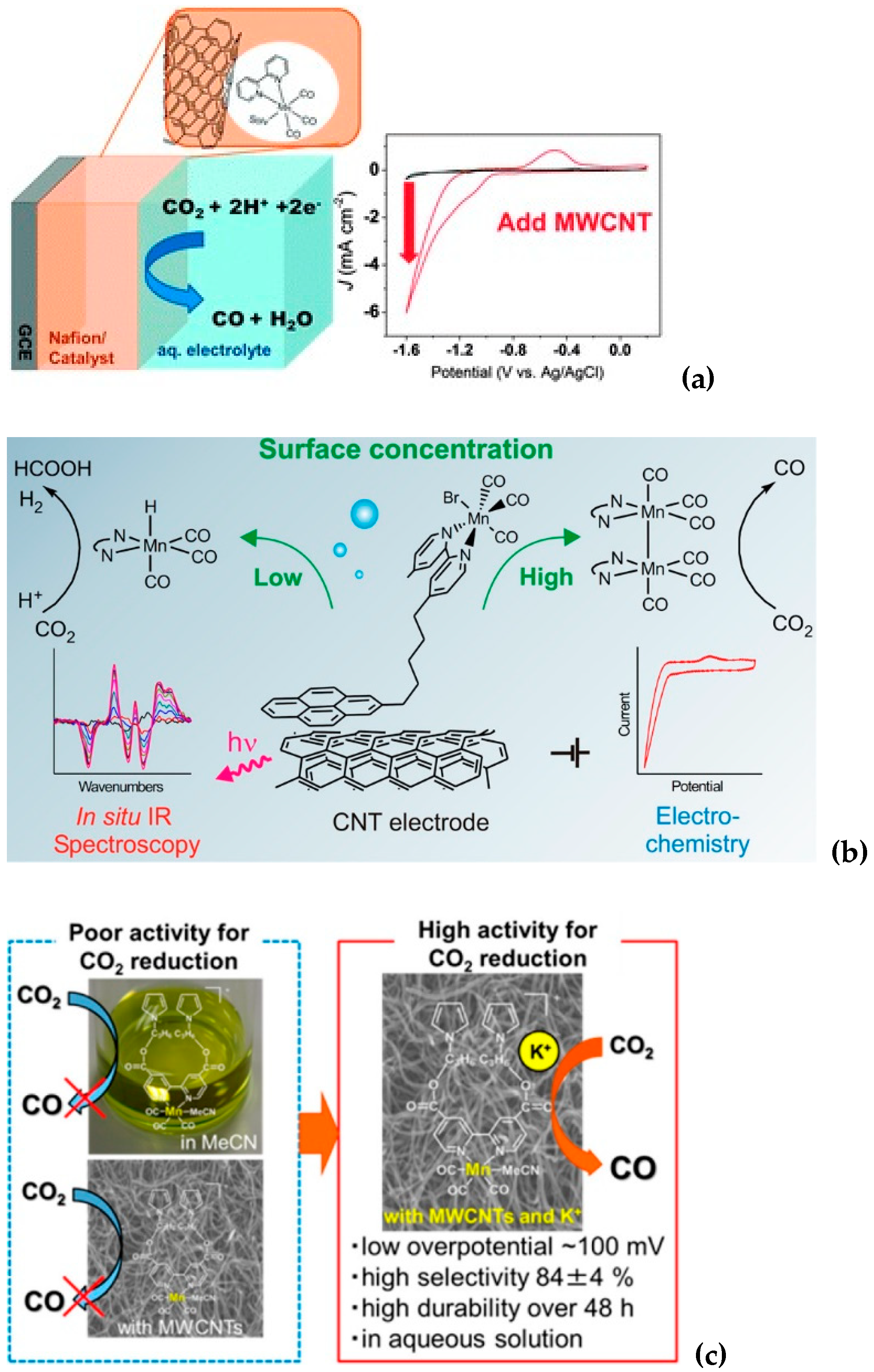
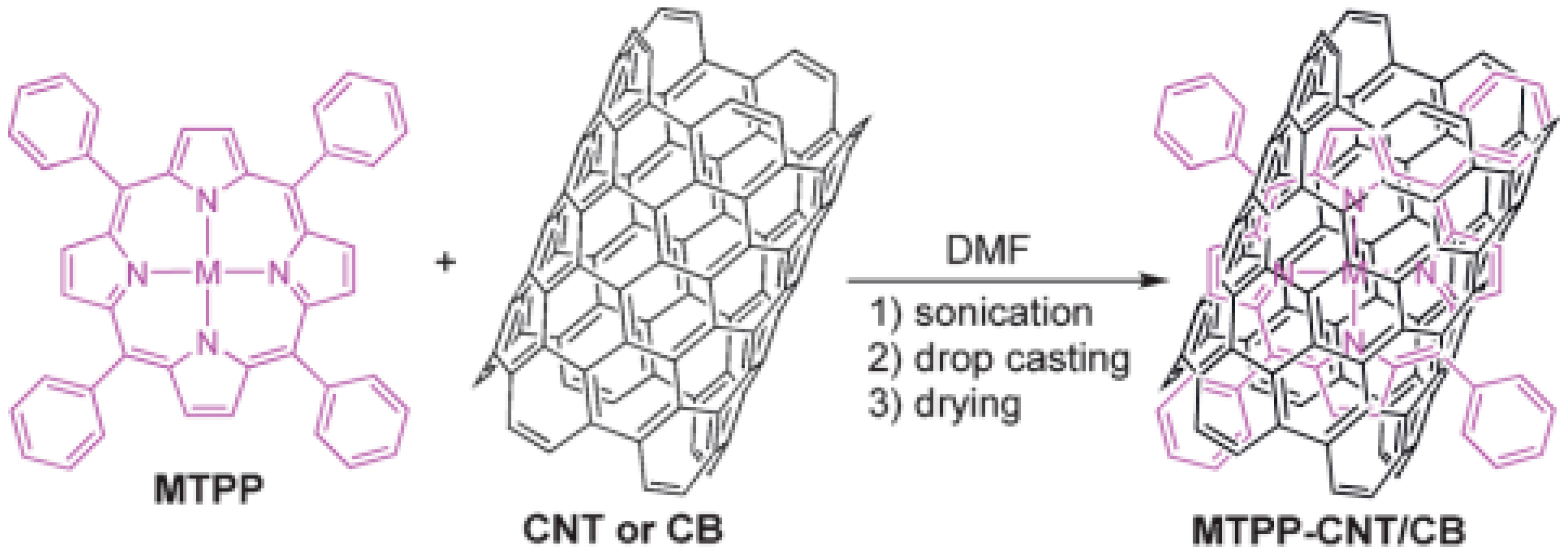
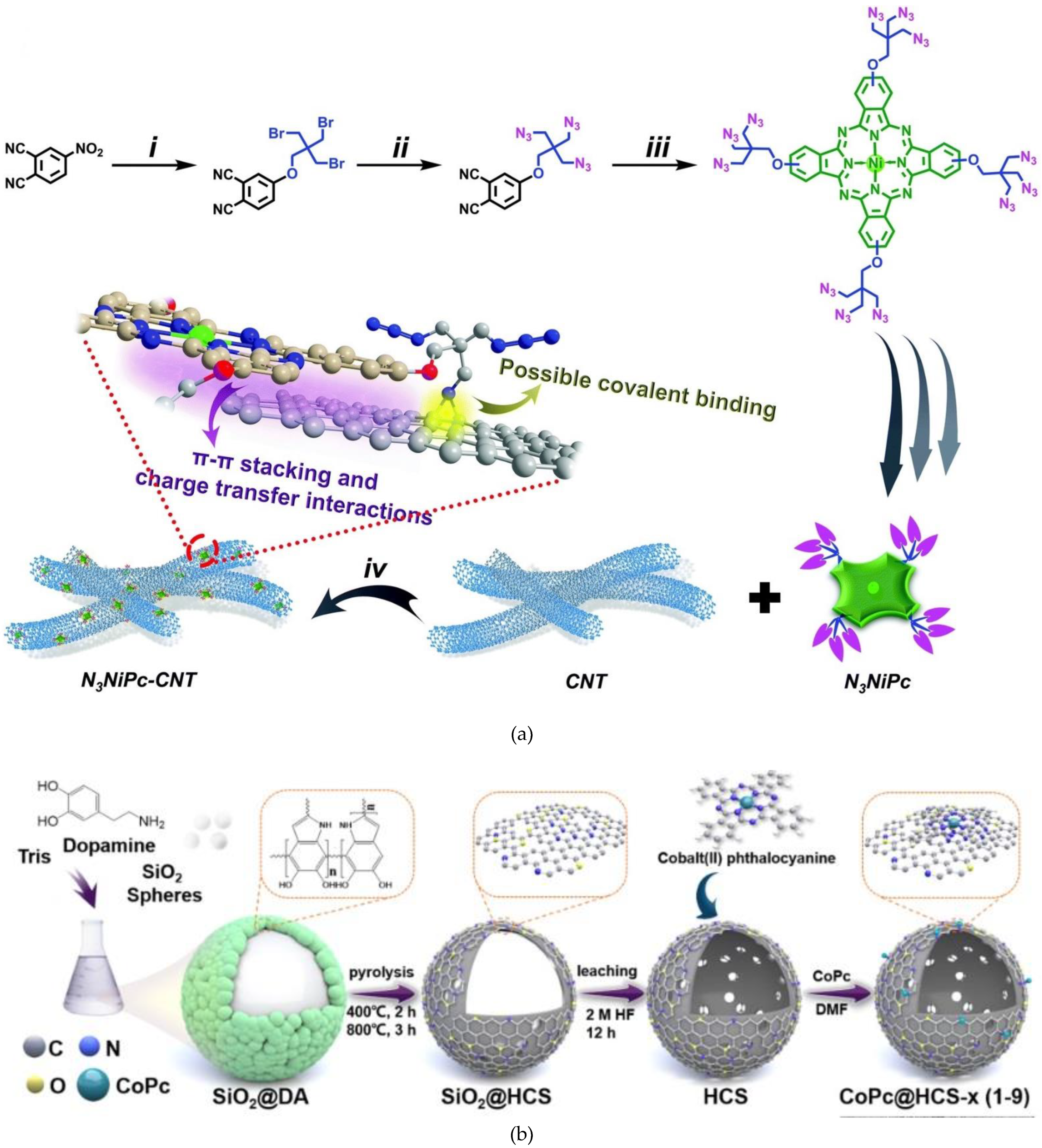
2.1. Non-Covalent Bonding
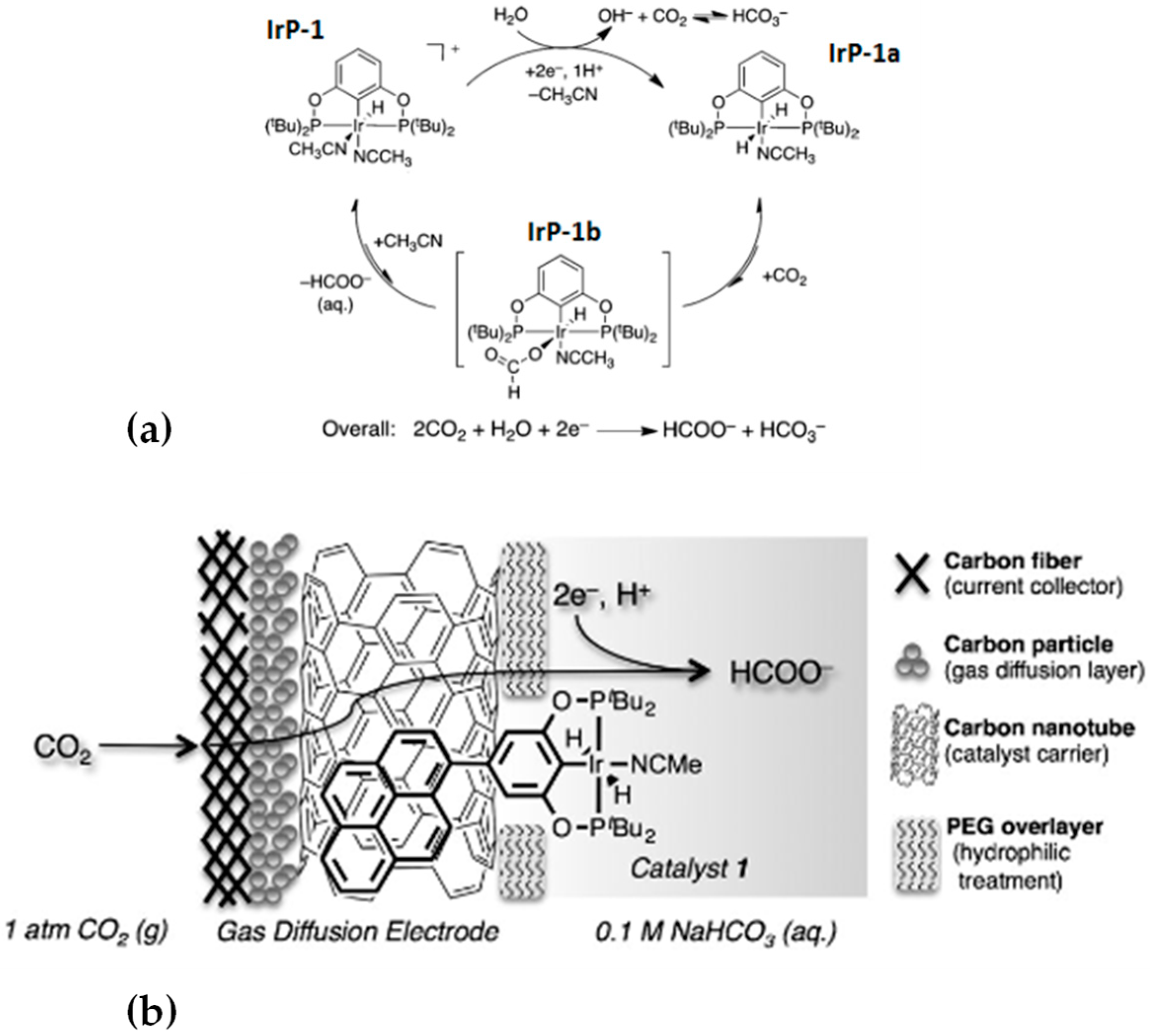
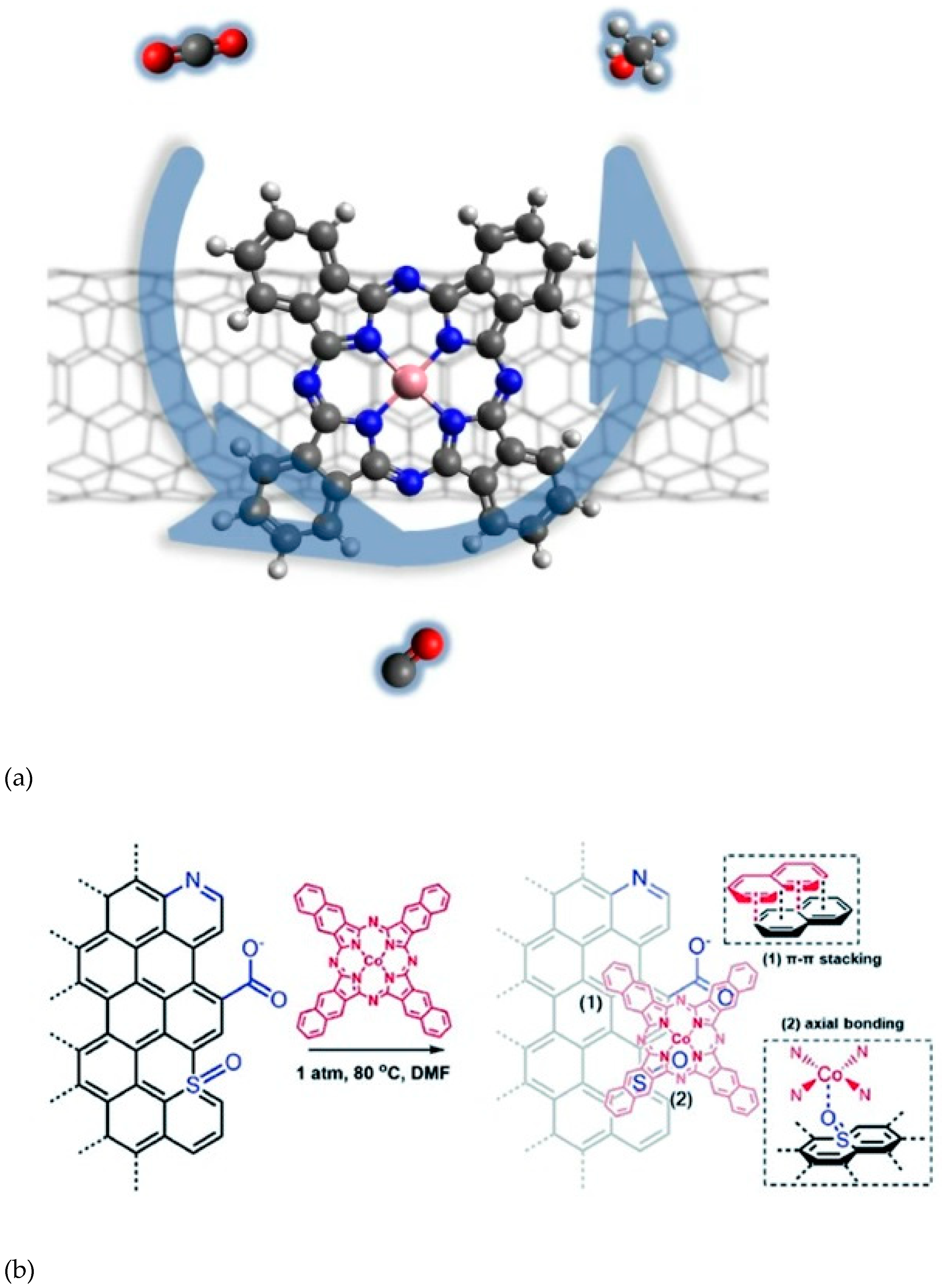
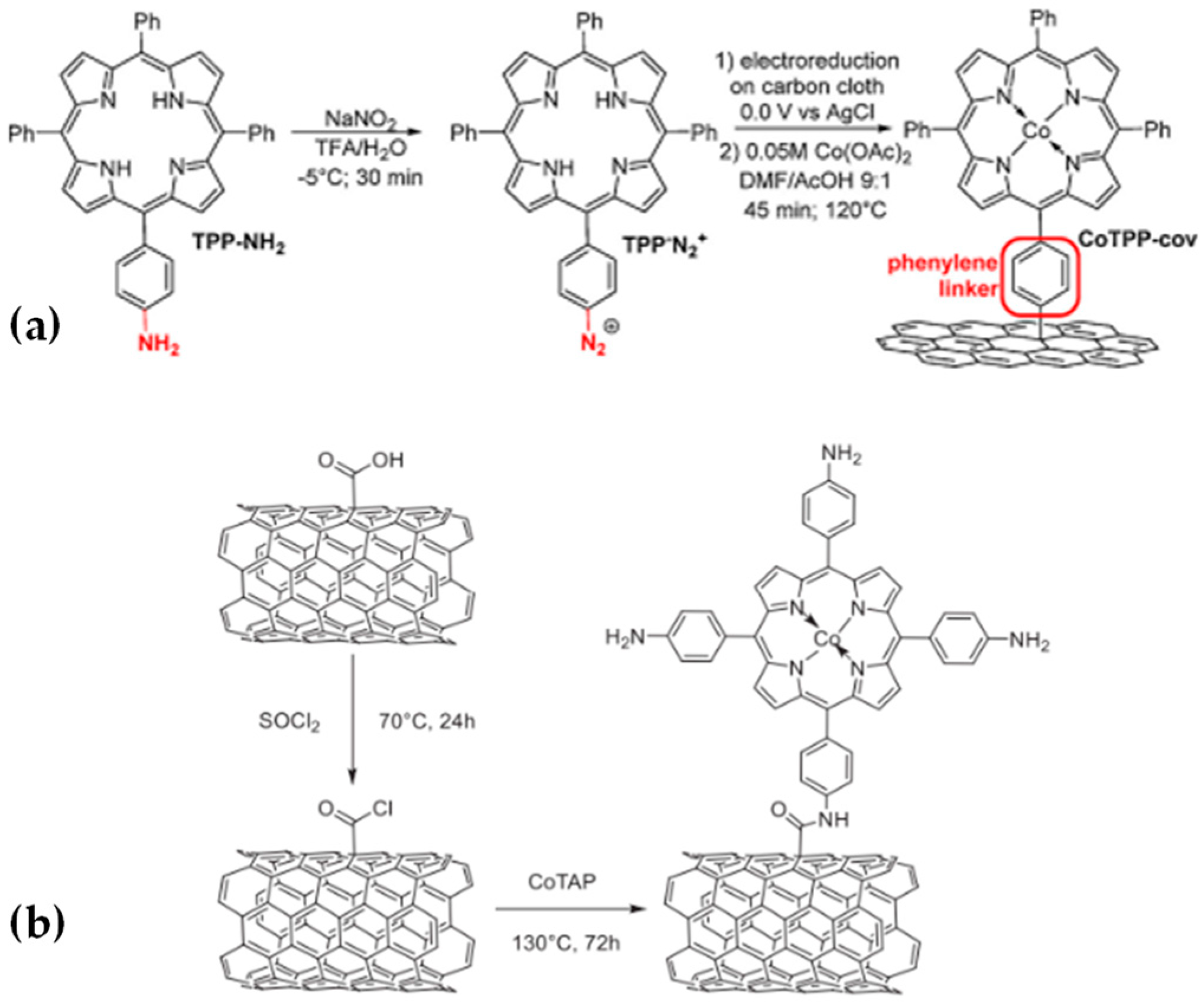
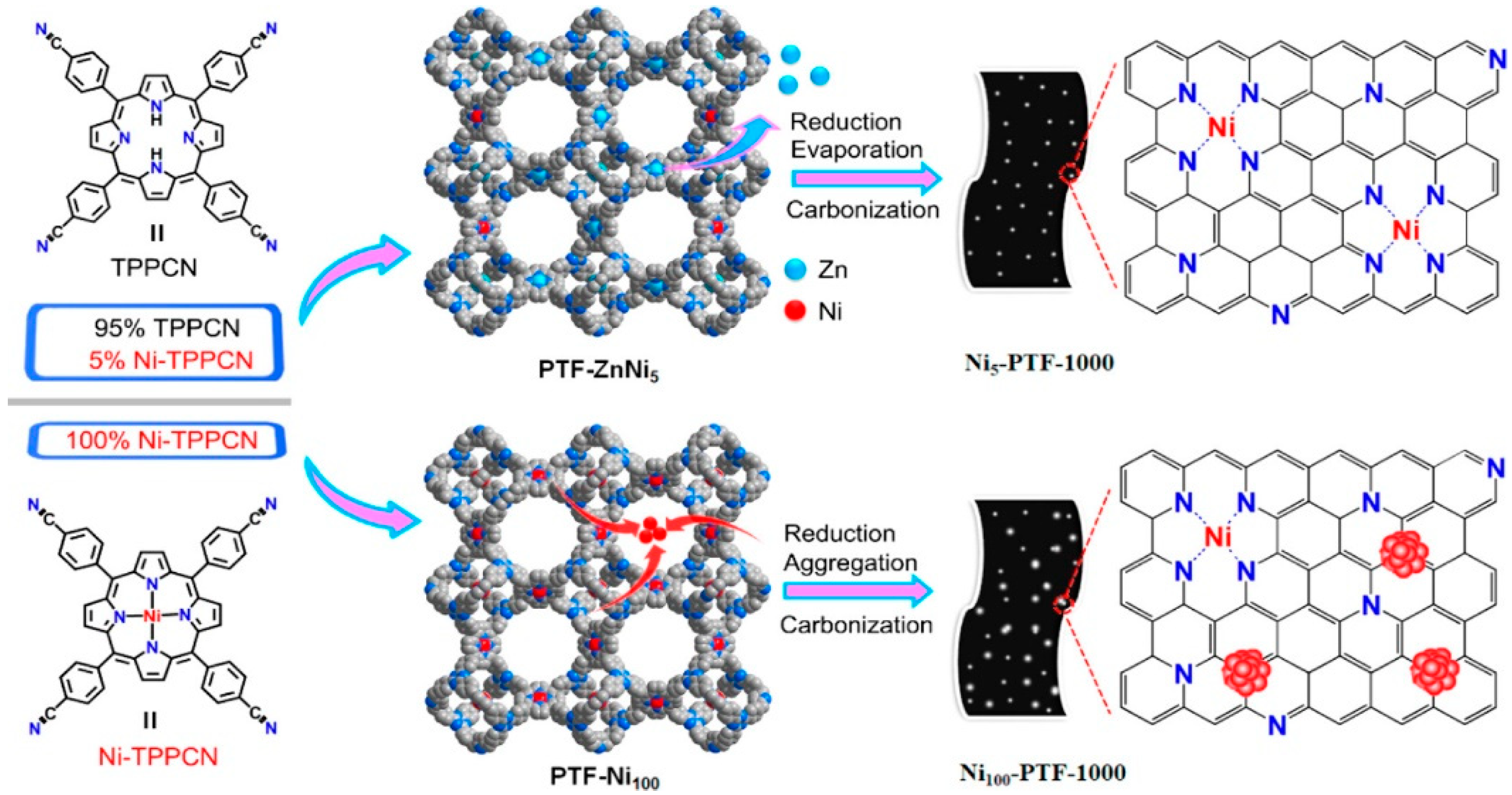
2.2. Covalent Bonding
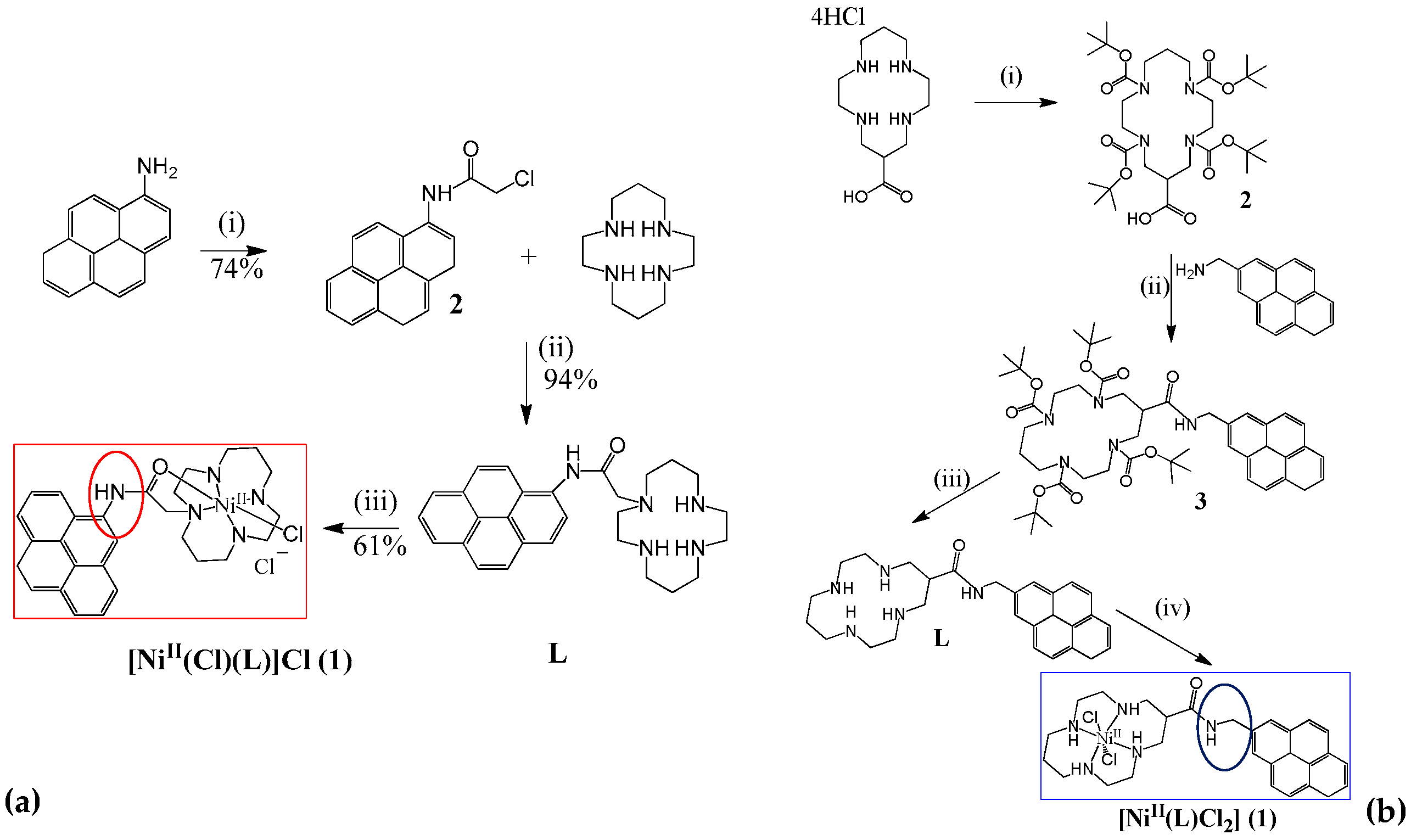
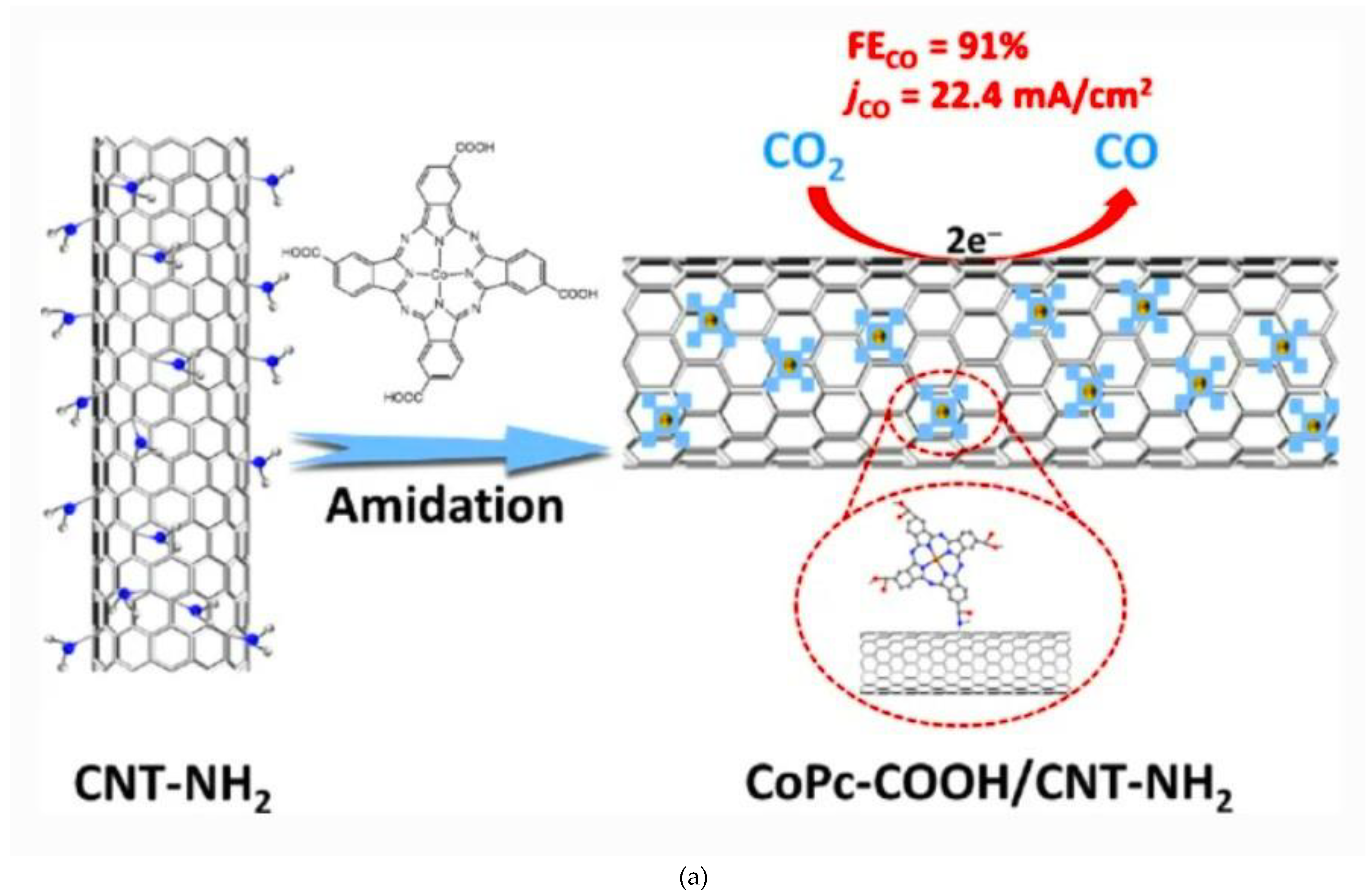
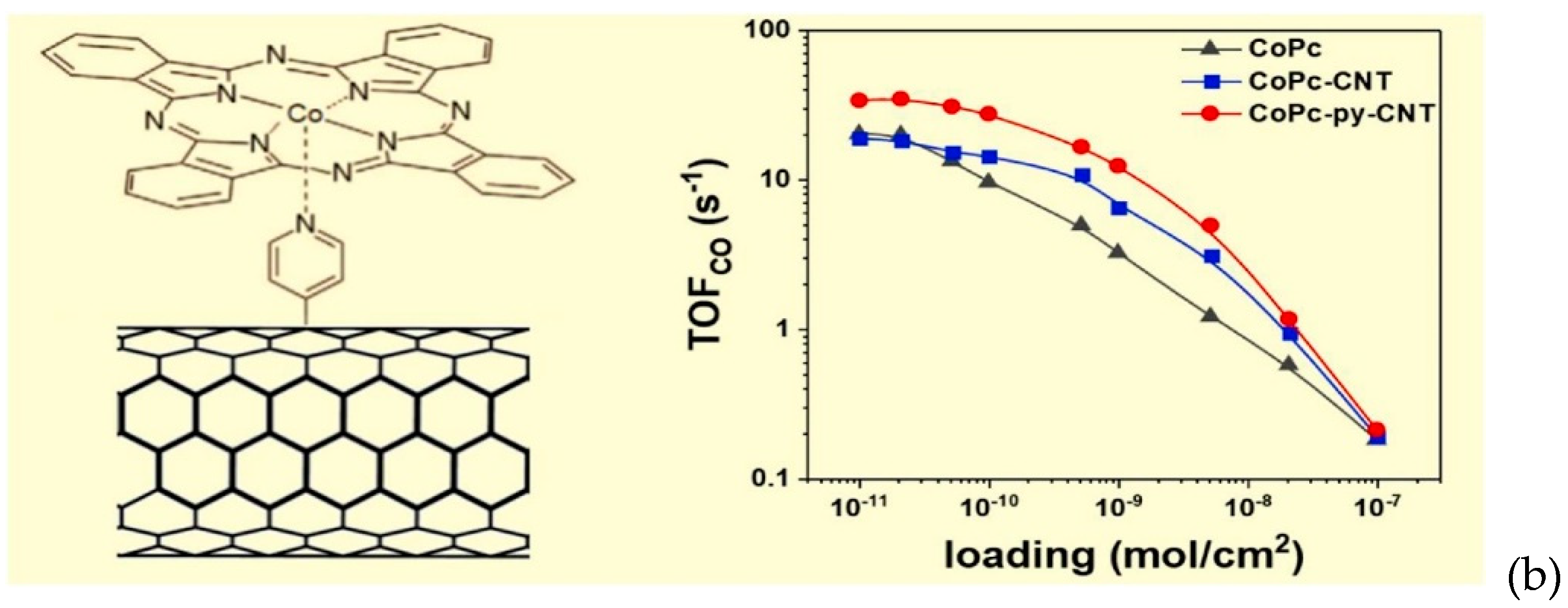
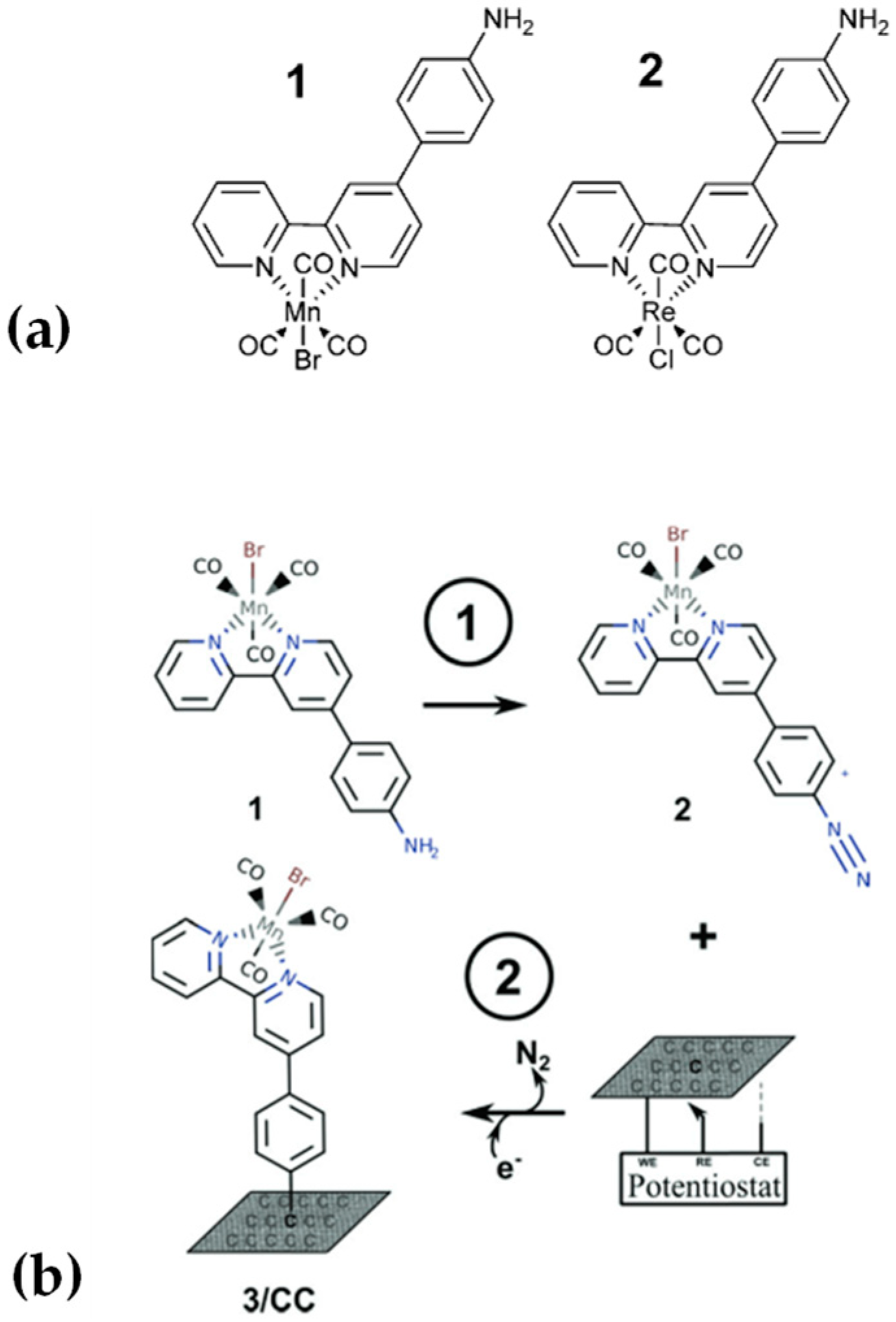
2.2.1. Formation of Chemical Bonds
2.2.2. Electropolymerization
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Perazio, A.; Lowe, G.; Gobetto, R.; Bonin, J.; Robert, M. Light-driven catalytic conversion of CO2 with heterogenized molecular catalysts based on fourth period transition metals. Coord. Chem. Rev. 2021, 443, 214018. [Google Scholar] [CrossRef]
- Franco, F.; Rettenmaier, C.; Sang Jeon, H.; Cuenya, B.R. Transition metal-based catalysts for the electrochemical CO2 reduction: From atoms and molecules to nanostructured materials. Chem. Soc. Rev. 2020, 49, 6884–6946. [Google Scholar] [CrossRef]
- Franco, F.; Fernández, S.; Lloret-Fillol, J. Advances in the electrochemical catalytic reduction of CO2 with metal complexes. Curr. Opin. Electrochem. 2019, 15, 109–117. [Google Scholar] [CrossRef]
- Dalle, K.E.; Warnan, J.; Leung, J.J.; Reuillard, B.; Karmel, I.S.; Reisner, E. Electro- and Solar-Driven Fuel Synthesis with First Row Transition Metal Complexes. Chem. Rev. 2019, 119, 2752–2875. [Google Scholar] [CrossRef]
- Elgrishi, N.; Chambers, M.B.; Wang, X.; Fontecave, M. Molecular polypyridine-based metal complexes as catalysts for the reduction of CO2. Chem. Soc. Rev. 2017, 46, 761–796. [Google Scholar] [CrossRef] [Green Version]
- Takeda, H.; Cometto, C.; Ishitani, O.; Robert, M. Electrons, Photons, Protons and Earth-Abundant Metal Complexes for Molecular Catalysis of CO2 Reduction. ACS Catal. 2017, 7, 70–88. [Google Scholar] [CrossRef]
- Rotundo, L.; Gobetto, R.; Nervi, C. Electrochemical CO2 reduction with earth-abundant metal catalysts. Curr. Opin. Green Sust. Chem. 2021, 31, 100509. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Rivera, S.; Zhou, Y.; Vlachos, D.G.; Jiao, F. Mechanistic Insights into the Electrochemical Reduction of CO2 to CO on Nanostructured Ag Surfaces. ACS Catal. 2015, 5, 4293–4299. [Google Scholar] [CrossRef]
- Marshall-Roth, T.; Libretto, N.J.; Wrobel, A.T.; Anderton, K.J.; Pegis, M.L.; Ricke, N.D.; Voorhis, T.V.; Miller, J.T.; Surendranath, Y. A pyridinic Fe-N4 macrocycle models the active sites in Fe/N-doped carbon electrocatalysts. Nat. Commun. 2020, 11, 5283. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 Electroreduction on N,P-Co-doped Carbon Aerogels. Angew. Chem. Int. Ed. 2020, 59, 11123–11129. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Y.; Zhang, X.; Zheng, H.; Wang, X.; Liang, Y. Revealing the hidden performance of metal phthalocyanines for CO2 reduction electrocatalysis by hybridization with carbon nanotubes. Nano Res. 2019, 12, 2330–2334. [Google Scholar] [CrossRef]
- Manbeck, G.F.; Fujita, E. A review of iron and cobalt porphyrins, phthalocyanines and related complexes for electrochemical and photochemical reduction of carbon dioxide. J. Porphyr. Phthalocyanines 2015, 19, 45–64. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, T.; Wang, R.; Sun, C.; Zhang, N.; Gao, R.; Song, Y. Heat-treated copper phthalocyanine on carbon toward electrochemical CO2 conversion into ethylene boosted by oxygen reduction. Chem. Commun. 2022, 58, 12192–12195. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, P.; Li, A.; Wei, B.; Si, K.; Wei, Y.; Wang, X.; Zhu, G.; Chen, Q.; Gu, X.; et al. Electrochemical CO2 reduction to ethylene by ultrathin CuO nanoplate arrays. Nat. Commun. 2022, 13, 1877. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Torbensen, K.; Salvatore, D.; Ren, S.; Joulie, D.; Dumoulin, F.; Mendoza, D.; Lassalle-Kaiser, B.; Isci, U.; Berlinguette, C.P.; et al. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 2019, 10, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco, F.; Cometto, C.; Vallana, F.F.; Sordello, F.; Priola, E.; Minero, C.; Nervi, C.; Gobetto, R. A local proton source in a [Mn(bpy-R)(CO)3Br]-type redox catalyst enables CO2 reduction even in the absence of Brønsted acids. Chem. Commun. 2014, 50, 14670–14673. [Google Scholar] [CrossRef] [PubMed]
- Franco, F.; Cometto, C.; Nencini, L.; Barolo, C.; Sordello, F.; Minero, C.; Fiedler, J.; Robert, M.; Gobetto, R.; Nervi, C. Local Proton Source in Electrocatalytic CO2 Reduction with [Mn(bpy-R)(CO)3 Br] Complexes. Chem. Eur. J. 2017, 23, 4782–4793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rønne, M.H.; Cho, D.; Madsen, M.R.; Jakobsen, J.B.; Eom, S.; Escoudé, É.; Hammershøj, H.C.D.; Nielsen, D.U.; Pedersen, S.U.; Baik, M.-H.; et al. Ligand-Controlled Product Selectivity in Electrochemical Carbon Dioxide Reduction Using Manganese Bipyridine Catalysts. J. Am. Chem. Soc. 2020, 142, 4265–4275. [Google Scholar] [CrossRef]
- Madsen, M.R.; Rønne, M.H.; Heuschen, M.; Golo, D.; Ahlquist, M.S.G.; Skrydstrup, T.; Pedersen, S.U.; Daasbjerg, K. Promoting Selective Generation of Formic Acid from CO2 Using Mn(bpy)(CO)Br as Electrocatalyst and Triethylamine/Isopropanol as Additives. J. Am. Chem. Soc. 2021, 143, 20491–20500. [Google Scholar] [CrossRef]
- Stuardi, F.M.; Tiozzo, A.; Rotundo, L.; Leclaire, J.; Gobetto, R.; Nervi, C. Efficient Electrochemical Reduction of CO2 to Formate in Methanol Solutions by Mn-Functionalized Electrodes in the Presence of Amines. Chem. Eur. J. 2022, 28, e202104377. [Google Scholar] [CrossRef]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef]
- Fujita, E.; Grills, D.C.; Manbeck, G.F.; Polyansky, D.E. Understanding the Role of Inter- and Intramolecular Promoters in Electro- and Photochemical CO2 Reduction Using Mn, Re, and Ru Catalysts. Acc. Chem. Res. 2022, 55, 616–628. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef]
- Sun, L.; Reddu, V.; Fisher, A.C.; Wang, X. Electrocatalytic reduction of carbon dioxide: Opportunities with heterogeneous molecular catalysts. Energy Environ. Sci. 2020, 13, 374–403. [Google Scholar] [CrossRef]
- Feng, D.-M.; Zhu, Y.-P.; Chen, P.; Ma, T.-Y. Recent Advances in Transition-Metal-Mediated Electrocatalytic CO2 Reduction: From Homogeneous to Heterogeneous Systems. Catalysts 2017, 7, 373. [Google Scholar] [CrossRef] [Green Version]
- Bullock, R.M.; Das, A.K.; Appel, A.M. Surface Immobilization of Molecular Electrocatalysts for Energy Conversion. Chemistry 2017, 23, 7626–7641. [Google Scholar] [CrossRef]
- Sun, C.; Gobetto, R.; Nervi, C. Recent advances in catalytic CO2 reduction by organometal complexes anchored on modified electrodes. New J. Chem 2016, 40, 5656–5661. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Electrocatalytic reduction of carbon dioxide mediated by Re(bipy)(CO)3Cl (bipy = 2,2′-bipyridine). J. Chem. Soc. Chem. Commun. 1984, 328–330. [Google Scholar] [CrossRef]
- Hawecker, J.; Lehn, J.-M.; Ziessel, R. Photochemical and Electrochemical Reduction of Carbon Dioxide to Carbon Monoxide Mediated by (2,2’-Bipyridine) tricarbonylchlororhenium(I) and Related Complexes as Homogeneous Catalysts. Helv. Chim. Acta 1986, 69, 1990–2012. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Gupta, A.; Warren, J.J.; Brunschwig, B.S.; Gray, H.B. Noncovalent immobilization of electrocatalysts on carbon electrodes for fuel production. J. Am. Chem. Soc. 2013, 135, 18288–18291. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.; Gallagher, J.R.; Miller, J.T.; Surendranath, Y. Graphite-Conjugated Rhenium Catalysts for Carbon Dioxide Reduction. J. Am. Chem. Soc. 2016, 138, 1820–1823. [Google Scholar] [CrossRef] [Green Version]
- Guyot, M.; Lalloz, M.-N.; Aguirre-Araque, J.S.; Rogez, G.; Costentin, C.; Chardon-Noblat, S. Rhenium Carbonyl Molecular Catalysts for CO2 Electroreduction: Effects on Catalysis of Bipyridine Substituents Mimicking Anchorage Functions to Modify Electrodes. Inorg. Chem. 2022, 61, 16072–16080. [Google Scholar] [CrossRef]
- Bourrez, M.; Molton, F.; Chardon-Noblat, S.; Deronzier, A. [Mn(bipyridyl)(CO)3Br]: An Abundant Metal Carbonyl Complex as Efficient Electrocatalyst for CO2 Reduction. Angew. Chem. Int. Ed. 2011, 50, 9903–9906. [Google Scholar] [CrossRef]
- Sampson, M.D.; Kubiak, C.P. Manganese Electrocatalysts with Bulky Bipyridine Ligands: Utilizing Lewis Acids To Promote Carbon Dioxide Reduction at Low Overpotentials. J. Am. Chem. Soc. 2016, 138, 1386–1393. [Google Scholar] [CrossRef]
- Riplinger, C.; Sampson, M.D.; Ritzmann, A.M.; Kubiak, C.P.; Carter, E.A. Mechanistic Contrasts between Manganese and Rhenium Bipyridine Electrocatalysts for the Reduction of Carbon Dioxide. J. Am. Chem. Soc. 2014, 136, 16285–16298. [Google Scholar] [CrossRef]
- Smieja, J.M.; Sampson, M.D.; Grice, K.A.; Benson, E.E.; Froehlich, J.D.; Kubiak, C.P. Manganese as a Substitute for Rhenium in CO2 Reduction Catalysts: The Importance of Acids. Inorg. Chem. 2013, 52, 2484–2491. [Google Scholar] [CrossRef]
- Keith, J.A.; Grice, K.A.; Kubiak, C.P.; Carter, E.A. Elucidation of the Selectivity of Proton-Dependent Electrocatalytic CO2 Reduction by fac-Re(bpy)(CO)3Cl. J. Am. Chem. Soc. 2013, 135, 15823–15829. [Google Scholar] [CrossRef]
- Grills, D.C.; Ertem, M.Z.; McKinnon, M.; Ngo, K.T.; Rochford, J. Mechanistic aspects of CO2 reduction catalysis with manganese-based molecular catalysts. Coord. Chem. Rev. 2018, 374, 173–217. [Google Scholar] [CrossRef]
- Siritanaratkul, B.; Eagle, C.; Cowan, A.J. Manganese Carbonyl Complexes as Selective Electrocatalysts for CO2 Reduction in Water and Organic Solvents. Acc. Chem. Res. 2022, 55, 955–965. [Google Scholar] [CrossRef]
- Rønne, M.H.; Madsen, M.R.; Skrydstrup, T.; Pedersen, S.U.; Daasbjerg, K. Mechanistic Elucidation of Dimer Formation and Strategies for Its Suppression in Electrochemical Reduction of fac-Mn(bpy)(CO)3Br. ChemElectroChem 2021, 8, 2108–2114. [Google Scholar] [CrossRef]
- Walsh, J.J.; Neri, G.; Smith, C.L.; Cowan, A.J. Electrocatalytic CO2 reduction with a membrane supported manganese catalyst in aqueous solution. Chem. Commun. 2014, 50, 12698–12701. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Smith, C.L.; Neri, G.; Whitehead, G.F.S.; Robertson, C.M.; Cowan, A.J. Improving the efficiency of electrochemical CO2 reduction using immobilized manganese complexes. Faraday Discuss. 2015, 183, 147–160. [Google Scholar] [CrossRef] [Green Version]
- Walsh, J.J.; Forster, M.; Smith, C.L.; Neri, G.; Potter, R.J.; Cowan, A.J. Directing the mechanism of CO2 reduction by a Mn catalyst through surface immobilization. Phys. Chem. Chem. Phys. 2018, 20, 6811–6816. [Google Scholar] [CrossRef]
- Reuillard, B.; Ly, K.H.; Rosser, T.E.; Kuehnel, M.F.; Zebger, I.; Reisner, E. Tuning Product Selectivity for Aqueous CO2 Reduction with a Mn(bipyridine)-pyrene Catalyst Immobilized on a Carbon Nanotube Electrode. J. Am. Chem. Soc. 2017, 139, 14425–14435. [Google Scholar] [CrossRef]
- Sato, S.; Saita, K.; Sekizawa, K.; Maeda, S.; Morikawa, T. Low-Energy Electrocatalytic CO2 Reduction in Water over Mn-Complex Catalyst Electrode Aided by a Nanocarbon Support and K+ Cations. ACS Catal. 2018, 8, 4452–4458. [Google Scholar] [CrossRef]
- Kang, P.; Zhang, S.; Meyer, T.J.; Brookhart, M. Rapid Selective Electrocatalytic Reduction of Carbon Dioxide to Formate by an Iridium Pincer Catalyst Immobilized on Carbon Nanotube Electrodes. Angew. Chem. Int. Ed. 2014, 53, 8709–8713. [Google Scholar] [CrossRef]
- Kang, P.; Cheng, C.; Chen, Z.; Schauer, C.K.; Meyer, T.J.; Brookhart, M. Selective electrocatalytic reduction of CO2 to formate by water-stable iridium dihydride pincer complexes. J. Am. Chem. Soc. 2012, 134, 5500–5503. [Google Scholar] [CrossRef]
- Cao, L.; Sun, C.; Sun, N.; Meng, L.; Chen, D. Theoretical mechanism studies on the electrocatalytic reduction of CO2 to formate by water-stable iridium dihydride pincer complex. Dalton Trans. 2013, 42, 5755–5763. [Google Scholar] [CrossRef]
- Ahn, S.T.; Bielinski, E.A.; Lane, E.M.; Chen, Y.; Bernskoetter, W.H.; Hazari, N.; Palmore, G.T.R. Enhanced CO2 electroreduction efficiency through secondary coordination effects on a pincer iridium catalyst. Chem. Commun. 2015, 51, 5947–5950. [Google Scholar] [CrossRef]
- Kang, P.; Meyer, T.J.; Brookhart, M. Selective electrocatalytic reduction of carbon dioxide to formate by a water-soluble iridium pincer catalyst. Chem. Sci. 2013, 4, 3497–3502. [Google Scholar] [CrossRef]
- Beley, M.; Collin, J.-P.; Ruppert, R.; Sauvage, J.-P. Nickel(II)-cyclam: An extremely selective electrocatalyst for reduction of CO2 in water. J. Chem. Soc. Chem. Commun. 1984, 1315–1316. [Google Scholar] [CrossRef]
- Beley, M.; Collin, J.P.; Ruppert, R.; Sauvage, J.P. Electrocatalytic reduction of carbon dioxide by nickel cyclam2+ in water: Study of the factors affecting the efficiency and the selectivity of the process. J. Am. Chem. Soc. 1986, 108, 7461–7467. [Google Scholar] [CrossRef] [PubMed]
- Collin, J.P.; Jouaiti, A.; Sauvage, J.P. Electrocatalytic properties of (tetraazacyclotetradecane)nickel2+ and Ni2(biscyclam)4+ with respect to carbon dioxide and water reduction. Inorg. Chem. 1988, 27, 1986–1990. [Google Scholar] [CrossRef]
- Balazs, G.B.; Anson, F.C. Effects of CO on the electrocatalytic activity of Ni (cyclam)2+ toward the reduction of CO2. J. Electroanal. Chem. 1993, 361, 149–157. [Google Scholar] [CrossRef]
- Zhanaidarova, A.; Moore, C.E.; Gembicky, M.; Kubiak, C.P. Covalent attachment of [Ni(alkynyl-cyclam)]2+catalysts to the glassy carbon electrodes. Chem. Commun. 2018, 54, 4116–4119. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, S.; Huan, N.T.; Forte, J.; Grammatico, D.; Zanna, S.; Su, B.-L.; Li, Y.; Fontecave, M. Functionalization of Carbon Nanotubes with Nickel Cyclam for the Electrochemical Reduction of CO2. ChemSusChem 2020, 13, 6449–6456. [Google Scholar] [CrossRef]
- Greenwell, F.; Neri, G.; Piercy, V.; Cowan, A.J. Noncovalent immobilization of a nickel cyclam catalyst on carbon electrodes for CO2 reduction using aqueous electrolyte. Electrochim. Acta 2021, 392, 139015. [Google Scholar] [CrossRef]
- Pugliese, S.; Huan, N.T.; Solé-Daura, A.; Li, Y.; Rivera de la Cruz, J.-G.; Forte, J.; Zanna, S.; Krief, A.; Su, B.-L.; Fontecave, M. CO2 Electroreduction in Water with a Heterogenized C-Substituted Nickel Cyclam Catalyst. Inorg. Chem. 2022, 61, 15841–15852. [Google Scholar] [CrossRef]
- Corbin, N.; Zeng, J.; Williams, K.; Manthiram, K. Heterogeneous molecular catalysts for electrocatalytic CO2 reduction. Nano Res. 2019, 12, 2093–2125. [Google Scholar] [CrossRef]
- Huai, M.; Yin, Z.; Wei, F.; Wang, G.; Xiao, L.; Lu, J.; Zhuang, L. Electrochemical CO2 reduction on heterogeneous cobalt phthalocyanine catalysts with different carbon supports. Chem. Phys. Lett. 2020, 754, 137655. [Google Scholar] [CrossRef]
- Abdinejad, M.; Tang, K.; Dao, C.; Saedy, S.; Burdyny, T. Immobilization strategies for porphyrin-based molecular catalysts for the electroreduction of CO2. J. Mater. Chem. A 2022, 10, 7626–7636. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Hiratsuka, K.; Sasaki, H.; Toshima, S. Electrocatalytic behavior of metal porphyrins in the reduction of carbon dioxide. Chem. Lett. 1979, 8, 305–308. [Google Scholar] [CrossRef]
- Boutin, E.; Merakeb, L.; Ma, B.; Boudy, B.; Wang, M.; Bonin, J.; Anxolabehere-Mallart, E.; Robert, M. Molecular catalysis of CO2 reduction: Recent advances and perspectives in electrochemical and light-driven processes with selected Fe, Ni and Co aza macrocyclic and polypyridine complexes. Chem. Soc. Rev. 2020, 49, 5772–5809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Z.; Wu, B.-Y.; Li, Q.; Lu, L.-L.; Shi, W.; Cheng, P. Design strategies and mechanism studies of CO2 electroreduction catalysts based on coordination chemistry. Coord. Chem. Rev. 2020, 422, 213436. [Google Scholar] [CrossRef]
- Costentin, C.; Savéant, J.-M. Towards an intelligent design of molecular electrocatalysts. Nat. Chem. Rev. 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Saveant, J.-M. Current Issues in Molecular Catalysis Illustrated by Iron Porphyrins as Catalysts of the CO2-to-CO Electrochemical Conversion. Acc. Chem. Res. 2015, 48, 2996–3006. [Google Scholar] [CrossRef]
- Costentin, C.; Saveant, J.-M. Homogeneous Molecular Catalysis of Electrochemical Reactions: Catalyst Benchmarking and Optimization Strategies. J. Am. Chem. Soc. 2017, 139, 8245–8250. [Google Scholar] [CrossRef]
- Azcarate, I.; Costentin, C.; Robert, M.; Saveant, J.-M. Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion. J. Am. Chem. Soc. 2016, 138, 16639–16644. [Google Scholar] [CrossRef]
- Martin, D.J.; Mayer, J.M. Oriented Electrostatic Effects on O2 and CO2 Reduction by a Polycationic Iron Porphyrin. J. Am. Chem. Soc. 2021, 143, 11423–11434. [Google Scholar] [CrossRef]
- Martin, D.J.; Mercado, B.Q.; Mayer, J.M. All Four Atropisomers of Iron Tetra(o-N,N,N-trimethylanilinium)porphyrin in Both the Ferric and Ferrous States. Inorg. Chem. 2021, 60, 5240–5251. [Google Scholar] [CrossRef] [PubMed]
- Maurin, A.; Robert, M. Noncovalent Immobilization of a Molecular Iron-Based Electrocatalyst on Carbon Electrodes for Selective, Efficient CO2-to-CO Conversion in Water. J. Am. Chem. Soc. 2016, 138, 2492–2495. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-M.; Rønne, M.H.; Pedersen, S.U.; Skrydstrup, T.; Daasbjerg, K. Enhanced Catalytic Activity of Cobalt Porphyrin in CO2 Electroreduction upon Immobilization on Carbon Materials. Angew. Chem. Int. Ed. 2017, 56, 6468–6472. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Hu, X.-M.; Daasbjerg, K.; Ahlquist, M.S.G. Understanding the Enhanced Catalytic CO2 Reduction upon Adhering Cobalt Porphyrin to Carbon Nanotubes and the Inverse Loading Effect. Organometallics 2020, 39, 1634–1641. [Google Scholar] [CrossRef]
- Wang, J.; Huang, X.; Xi, S.; Lee, J.-M.; Wang, C.; Du, Y.; Wang, X. Linkage Effect in the Heterogenization of Cobalt Complexes by Doped Graphene for Electrocatalytic CO2 Reduction. Angew. Chem. Int. Ed. 2019, 58, 13532–13539. [Google Scholar] [CrossRef]
- BaQais, A.; Ait Ahsaine, H. β-Cobalt phthalocyanine sono-immobilized on carbon cloth for efficient electrochemical reduction of CO2-to-CO. Mater. Lett. 2022, 324, 132614. [Google Scholar] [CrossRef]
- Abdinejad, M.; Wilm, L.F.B.; Dielmann, F.; Kraatz, H.B. Electroreduction of CO2 Catalyzed by Nickel Imidazolin-2-ylidenamino-Porphyrins in Both Heterogeneous and Homogeneous Molecular Systems. ACS Sustain. Chem. Eng. 2021, 9, 521–530. [Google Scholar] [CrossRef]
- Abdinejad, M.; Dao, C.; Zhang, X.-A.; Kraatz, H.B. Enhanced electrocatalytic activity of iron amino porphyrins using a flow cell for reduction of CO2 to CO. J. Energy Chem. 2021, 58, 162–169. [Google Scholar] [CrossRef]
- Xu, H.; Cai, H.; Cui, L.; Yu, L.; Gao, R.; Shi, C. Molecular modulating of cobalt phthalocyanines on aminofunctionalized carbon nanotubes for enhanced electrocatalytic CO2 conversion. Nano Res. 2022, in press. [Google Scholar] [CrossRef]
- Yao, S.A.; Ruther, R.E.; Zhang, L.; Franking, R.A.; Hamers, R.J.; Berry, J.F. Covalent attachment of catalyst molecules to conductive diamond: CO2 reduction using “smart” electrodes. J. Am. Chem. Soc. 2012, 134, 15632–15635. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Guo, R.; Xu, J.; Fang, X.; Han, Y.-F. Cobalt phthalocyanine coordinated to pyridine-functionalized carbon nanotubes with enhanced CO2 electroreduction. Appl. Catal. B 2019, 251, 112–118. [Google Scholar] [CrossRef]
- Liu, Y.; McCrory, C.C.L. Modulating the mechanism of electrocatalytic CO2 reduction by cobalt phthalocyanine through polymer coordination and encapsulation. Nat. Commun. 2019, 10, 1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.-D.; Han, S.-G.; Cao, C.; Wei, W.; Li, X.; Chen, B.; Wu, X.-T.; Zhu, Q.-L. Bifunctional single-molecular heterojunction enables completely selective CO2-to-CO conversion integrated with oxidative 3D nano-polymerization. Energy Environ. Sci. 2021, 14, 1544–1552. [Google Scholar] [CrossRef]
- Gong, S.; Wang, W.; Xiao, X.; Liu, J.; Wu, C.; Lv, X. Elucidating influence of the existence formation of anchored cobalt phthalocyanine on electrocatalytic CO2-to-CO conversion. Nano Energy 2021, 84, 105904. [Google Scholar] [CrossRef]
- Maurin, A.; Robert, M. Catalytic CO2-to-CO conversion in water by covalently functionalized carbon nanotubes with a molecular iron catalyst. Chem. Commun. 2016, 52, 12084–12087. [Google Scholar] [CrossRef]
- Zhu, M.; Chen, J.; Huang, L.; Ye, R.; Xu, J.; Han, Y.-F. Covalently Grafting Cobalt Porphyrin onto Carbon Nanotubes for Efficient CO2 Electroreduction. Angew. Chem. Int. Ed. 2019, 58, 6595–6599. [Google Scholar] [CrossRef]
- Marianov, A.N.; Jiang, Y. Covalent ligation of Co molecular catalyst to carbon cloth for efficient electroreduction of CO2 in water. Appl. Catal. B 2019, 244, 881–888. [Google Scholar] [CrossRef]
- Gu, S.; Marianov, A.N.; Jiang, Y. Covalent grafting of cobalt aminoporphyrin-based electrocatalyst onto carbon nanotubes for excellent activity in CO2 reduction. Appl. Catal. B 2022, 300, 120750. [Google Scholar] [CrossRef]
- Sun, C.; Rotundo, L.; Garino, C.; Nencini, L.; Yoon, S.S.; Gobetto, R.; Nervi, C. Electrochemical CO2 Reduction at Glassy Carbon Electrodes Functionalized by Mn I and Re I Organometallic Complexes. ChemPhysChem 2017, 18, 3219–3229. [Google Scholar] [CrossRef] [Green Version]
- Rotundo, L.; Filippi, J.; Gobetto, R.; Miller, H.A.; Rocca, R.; Nervi, C.; Vizza, F. Electrochemical CO2 reduction in water at carbon cloth electrodes functionalized with a fac-Mn(apbpy)(CO)3Br complex. Chem. Commun. 2019, 55, 775–777. [Google Scholar] [CrossRef]
- Filippi, J.; Rotundo, L.; Gobetto, R.; Miller, H.A.; Nervi, C.; Lavacchi, A.; Vizza, F. Turning manganese into gold: Efficient electrochemical CO2 reduction by a fac-Mn(apbpy)(CO)3Br complex in a gas–liquid interface flow cell. Chem. Eng. J. 2021, 416, 129050. [Google Scholar] [CrossRef]
- Jakobsen, J.B.; Rønne, M.H.; Daasbjerg, K.; Skrydstrup, T. Are Amines the Holy Grail for Facilitating CO2 Reduction? Angew. Chem. Int. Ed. 2021, 60, 9174–9179. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, T.R.; Margerum, L.D.; Westmoreland, T.D.; Vining, W.J.; Murray, R.W.; Meyer, T.J. Electrocatalytic reduction of CO2 at a chemically modified electrode. J. Chem. Soc. Chem. Commun. 1985, 1416–1417. [Google Scholar] [CrossRef]
- O’Toole, T.R.; Sullivan, B.P.; Bruce, M.R.-M.; Margerum, L.D.; Murray, R.W.; Meyer, T.J. Electrocatalytic reduction of CO2 by a complex of rhenium in thin polymeric films. J. Electroanal. Chem. Interfacial Electrochem. 1989, 259, 217–239. [Google Scholar] [CrossRef]
- Guadalupe, A.R.; Usifer, D.A.; Potts, K.T.; Hurrell, H.C.; Mogstad, A.E.; Abruna, H.D. Novel chemical pathways and charge-transport dynamics of electrodes modified with electropolymerized layers of [Co(v-terpy)2]2+. J. Am. Chem. Soc. 1988, 110, 3462–3466. [Google Scholar] [CrossRef]
- Denisevich, P.; Abruna, H.D.; Leidner, C.R.; Meyer, T.J.; Murray, R.W. Electropolymerization of vinylpyridine and vinylbipyridine complexes of iron and ruthenium: Homopolymers, copolymers, reactive polymers. Inorg. Chem. 1982, 21, 2153–2161. [Google Scholar] [CrossRef]
- Calvert, J.M.; Schmehl, R.H.; Sullivan, B.P.; Facci, J.S.; Meyer, T.J.; Murray, R.W. Synthetic and mechanistic investigations of the reductive electrochemical polymerization of vinyl-containing complexes of iron(II), ruthenium(II), and osmium(II). Inorg. Chem. 1983, 22, 2151–2162. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Oppelt, K.; Ulbricht, C.; Egbe, D.A.M.; Neugebauer, H.; Knoer, G.; Sariciftci, N.S. Electrocatalytic and photocatalytic reduction of carbon dioxide to carbon monoxide using the alkynyl-substituted rhenium(I) complex (5,5’-bisphenylethynyl-2,2’-bipyridyl)Re(CO)3Cl. J. Organomet. Chem. 2012, 716, 19–25. [Google Scholar] [CrossRef]
- Portenkirchner, E.; Gasiorowski, J.; Oppelt, K.; Schlager, S.; Schwarzinger, C.; Neugebauer, H.; Knör, G.; Sariciftci, N.S. Electrocatalytic Reduction of Carbon Dioxide to Carbon Monoxide by a Polymerized Film of an Alkynyl-Substituted Rhenium(I) Complex. ChemCatChem 2013, 5, 1790–1796. [Google Scholar] [CrossRef] [Green Version]
- Sun, C.; Prosperini, S.; Quagliotto, P.; Viscardi, G.; Yoon, S.S.; Gobetto, R.; Nervi, C. Electrocatalytic reduction of CO2 by thiophene-substituted rhenium(I) complexes and by their polymerized films. Dalton Trans. 2016, 45, 14678–14688. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, J.; Xie, Z.-L.; Huang, Y.-B.; Cao, R. Spatial Sites Separation Strategy to Fabricate Atomically Isolated Nickel Catalysts for Efficient CO2 Electroreduction. ACS Mater. Lett. 2021, 3, 454–461. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Jones, R.A.; Holliday, B.J. Recent advances in the functional applications of conducting metallopolymers. Coord. Chem. Rev. 2018, 377, 237–258. [Google Scholar] [CrossRef]
- Whittell, G.R.; Hager, M.D.; Schubert, U.S.; Manners, I. Functional soft materials from metallopolymers and metallosupramolecular polymers. Nat. Mater. 2011, 10, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Elmas, S.; Macdonald, T.J.; Skinner, W.; Andersson, M.; Nann, T. Copper Metallopolymer Catalyst for the Electrocatalytic Hydrogen Evolution Reaction (HER). Polymers 2019, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.; Olean-Oliveira, A.; Anastácio, F.; David-Parra, D.; Cardoso, C. Electrocatalytic Reduction of CO2 in Water by a Palladium-Containing Metallopolymer. Nanomaterials 2022, 12, 1193. [Google Scholar] [CrossRef] [PubMed]
- Moura de Salles Pupo, M.; Kortlever, R. Electrolyte Effects on the Electrochemical Reduction of CO2. ChemPhysChem 2019, 20, 2926–2935. [Google Scholar] [CrossRef]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. ISBN 978-0-387-49489-0. [Google Scholar]



| Support/Catalyst | E/(V) | Faradaic Efficiencies | TON/TOF | J/(mAcm−2) | Time (min) | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| Graphite/Re | −2.3 vs. Fc+/0 | FECO = 70% | TONCO = 58 | n.s. * | 85 | MeCN/0.1 M TBAPF6 | [31] |
| GCC/Rephen | −2.16 vs. Fc+/0 | FECO = 96% | TONCO = 12,000 1 | jCO = 1.0 | 120 | MeCN/0.1 M TBAPF6 | [32] |
| Mn/Nafion/CNT | −1.4 V vs. Ag/AgCl | FECO = 22% | TONCO = 101 | jCO = 1.79 | 240 | 30 mM Na2HPO4 + 30 mM NaH2PO4) | [42] |
| CNT/Mn | −1.1 vs. SHE | FECO = 34 ± 4% | TONCO = 1000 | jCO = 5 and 1.5 in the first hour, then 0.5 | 480 | 0.5 M KHCO3 | [45] |
| MWCNT/Mn | −0.39 vs. RHE | FECO = 84 ± 4% | TONCO = 722 (after 24 h) | jCO = 2.6 to 2.0 | 2880 | 0.1 M K2B4O7 + 0.2 M K2SO4 | [46] |
| GDE coated with CNT/ Ir-pincer | −1.4 vs. NHE | FEformate = 83% | TONformate = 54,000 TOFformate = 15 s−1 | jformate = 15 | 60 | 0.1 M NaHCO3 | [47] |
| MWCNT on GDL/ Ni cyclam (N-functionalized) | −2.54 vs. Fc+/0 | FECO = 92% | TONCO = 61,460 TOFCO = 4.27 s−1 | jCO = 6 | 240 | MeCN/0.1 M TBAPF6 + 1%water | [57] |
| GDE/[Ni(CycPy)]2+ | −1.4 vs. Ag/AgCl | FECO = from 50% after 20 min to 20% after 140 min | TOFCO = 55 h−1 | jCO = 0.6 | 150 | 0.5 M KHCO3 | [58] |
| MWCNT on GDL/ Ni cyclam (C-functionalized) | −0.8 vs. RHE | FECO = 90% | TONCO = 248 after 60 min | jCO = 6 to 2.5 | 240 | 0.1 M KHCO3 | [59] |
| CNT on GC/ Ironporphyrin | −0.59 vs. RHE | FECO = 93% | TONCO = 432 TOFCO = 144 h−1 | jCO = 0.186 | 180 | 0.5 M NaHCO3 | [72] |
| CNT/FeTPPNH2 | −0.8 vs. RHE | FECO = 79% | TOFCO = 0.05 s−1 | jCO = 12.9 | 150 | 0.1 M KHCO3 | [78] |
| CNT/CoTPP | −0.8 vs. RHE | FECO = 70% | TOFCO = 2.75 s−1 | jCO = 0.9 | 240 | 0.5 M NaHCO3 | [73] |
| CNT/CoPcNH2 | −1.00 vs. RHE | FEMeOH = 28% | TOFMeOH = 0.88 s−1 TONMeOH = 38,000 1 | jMeOH = 10 | 720 | 0.1 M KHCO3 | [15] |
| Doped graphene/NapCo | −0.8 vs. RHE | FECO = 97% | TOFCO = 0.45 s−1 | jCO = 2.5 | 150 | 0.1 M KHCO3 | [75] |
| Support/Catalyst | E/(V) | Faradaic Efficiencies | TON/TOF | J/(mAcm−2) | Time/h | Electrolyte | Ref. |
|---|---|---|---|---|---|---|---|
| CNT-NH2/CoPc through click chemistry | −0.88 vs. RHE | FECO = 91% | *1 TOFCO = 9606 h−1 | jCO = 22.4 | 48 | 0.5 M KHCO3 | [79] |
| Pyridine functionalized CNT/CoPc | −0.63 vs. RHE | FECO > 98% | TOFCO = 34.5 s−1 | n.s. | 12 | 0.1 M NaHCO3 | [81] |
| Hollow Carbon Spheres-6/CoPc | −0.82 vs. RHE | FECO = 96% | TONCO ~753,864 TOFCO = 21 s−1 | jCO = 20.47 | 10 | 0.5 M KHCO3 | [84] |
| CNT-NH2/FeTPP-COOH through amidation | −1.06 vs. SHE | FECO = 80% | TONCO = 750 TOFCO = 178 h−1 | n.s. | 3 | 0.5 M NaHCO3 | [85] |
| CNT-OH/CoPPCl | −0.65 vs. RHE | FECO = 90% | TONCO ~ 60,000 TOFCO = 1.37 s−1 | jCO = 25.1 | 12 | 0.5 M NaHCO3 | [86] |
| Carbon Cloth/CoTAP through in situ diazonium reduction | −1.05 vs. NHE | FECO = 67% (= 81% is maintained for 8 h) | TONCO = 3,900,000 TOFCO = 8.3 s−1 | n.s. | 24 | 0.5 M KHCO3 | [87] |
| CNT/CoTAP | −1.095 vs. NHE | FECO ~ 100% | *2 TONCO ~ 60,000 intrinsic TOFCO = 36.6 s−1 | jCO = 25.4 | 24 | 0.5 M KHCO3 | [88] |
| Glassy Carbon/Mnbpy | −1.75 vs. Fc+/0 | FECO = 75% | TONCO = 360 | jCO = 0.2 | 1 | MeCN/0.1 M TBAPF6 +4%v H2O | [89] |
| CC/Mnbpy | −1.35 vs. Ag/AgCl | FECO = 60% | TONCO = 33,200 | jCO = 1 | 10 | 0.1 M KHCO3 | [90] |
| GDL/Mnbpy | −0.67 vs. RHE | FECO = 76% FEformate = 10% | TONCO = 145,286 TONformate = 19,252 | jCO = 4.0 | 16 | 0.1 M KHCO3 | [91] |
| CC/Mnbpy | −1.75 vs. Fc+/0 | FEformate = 66% FECO = 5% | TONformate = 28,000 TONCO = 3000 | Jtot = 1 | 22 | MeCN/0.1 M TBAPF6 + 1 mM PMDETA | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rotundo, L.; Barbero, A.; Nervi, C.; Gobetto, R. CO2 Electroreduction on Carbon-Based Electrodes Functionalized with Molecular Organometallic Complexes—A Mini Review. Catalysts 2022, 12, 1448. https://doi.org/10.3390/catal12111448
Rotundo L, Barbero A, Nervi C, Gobetto R. CO2 Electroreduction on Carbon-Based Electrodes Functionalized with Molecular Organometallic Complexes—A Mini Review. Catalysts. 2022; 12(11):1448. https://doi.org/10.3390/catal12111448
Chicago/Turabian StyleRotundo, Laura, Alice Barbero, Carlo Nervi, and Roberto Gobetto. 2022. "CO2 Electroreduction on Carbon-Based Electrodes Functionalized with Molecular Organometallic Complexes—A Mini Review" Catalysts 12, no. 11: 1448. https://doi.org/10.3390/catal12111448
APA StyleRotundo, L., Barbero, A., Nervi, C., & Gobetto, R. (2022). CO2 Electroreduction on Carbon-Based Electrodes Functionalized with Molecular Organometallic Complexes—A Mini Review. Catalysts, 12(11), 1448. https://doi.org/10.3390/catal12111448







