Abstract
In this study, a sequential reaction using selected metal oxides, followed by ZSM-5-based catalysts, was employed to demonstrate a promising route for enhancing light olefin production in the catalytic cracking of naphtha. The rationale for the reaction is based on the induction of alkenes into hydrocarbon feeds prior to cracking. The optimum olefin induction was achieved by carefully optimizing the dehydrogenation active sites Mo/Al2O3 catalyst. The formed alkenes have a lower activation energy for C-H/C-C bond breaking compared to alkanes. This could accelerate the formation of carbenium ions, thus promoting the conversion of n-octane to produce light olefins. Detailed product distribution and DFT calculation indicated a remarkable increase in ethylene and propylene production in the final product through a modified reaction pathway. Compared with the common metal-promoted zeolite catalysts, the new route could avoid the block of zeolite channels and corresponding decreased catalytic cracking activity. The feasibility of the proposed route was confirmed with different ratios of dehydrogenation catalyst to the reactant. The highest yields of ethylene and propylene reached 13.22% and 33.12% with ratios of Mo/Al2O3 and ZSM-5-based catalyst to n-octane both 10:1 at 600 °C. Stability tests showed that the catalytic activity of the double-bed system was stable over 10 cycles.
1. Introduction
Light olefins, such as ethylene and propylene, are important basic materials in the chemical industry. Because of the increased consumption of their downstream derivatives, the demand for them is growing tremendously each year, especially for propylene [,].
Traditionally, steam thermal cracking has been the main source of light olefins; however, this method involves high energy consumption and large CO2 emissions. Moreover, ethylene dominates in cracking products, and propylene is produced as a byproduct, and thus the process is unable to satisfy the demand of the current light olefin market. These limitations have urged substantial interest in the development of alternatives for propylene production. The dehydrogenation of propane has recently gained much attention as an alternative to producing propylene []. The CrOx-Al2O3 and platinum-based catalyst systems have been used for industrial dehydrogenation processes. However, major challenges associated with these catalytic systems are the improvement of process economics and olefin selectivity [].
Catalytic cracking of naphtha has been considered one of the most promising technologies for energy conservation [,,]. This process should favor the production of propylene rather than ethylene because the transformation proceeds mainly via carbenium ion chemistry. However, the ethylene and propylene productivities achieved using these technologies and catalysts still cannot fulfill the gap between the supply and the demand [,,]. Continuous effort should be devoted to developing methods of further increasing light olefin production.
Dehydrogenation cracking, which combines catalytic dehydrogenation and cracking reactions, is a promising approach to reducing energy consumption and carbon emission for light olefin production from naphtha [,]. The incorporation of transition metals (Zn, Co, Cu, and Ni) into ZSM-5 results in the formation of a bifunctional catalyst in which the metal and acid sites can act simultaneously to improve the yields of light olefins. This is because transition metals are generally used as active components for hydrogenation or dehydrogenation. Metal oxides interact with the hydrogen atoms of alkanes and promote the dissociation of C-H bonds and the subsequent formation of hydrogen and olefins [,,,]. The activation energy for C-H/C-C bond breaking is lower for the catalytic cracking of alkenes than that for alkanes [,]. However, the improvement is limited because they can only function with little influence on the surface chemistry of the catalyst. Furthermore, the increased amount of metal particles or clusters may cause serious aggregation. Their block of zeolite channels caused a decrease in the catalytic cracking activity.
The influence of the reaction pathways is vital in the catalytic cracking of naphtha. The purpose of this paper is to propose a novel reaction route to increase light olefin production. This sequential reaction was conducted using carefully optimized dehydrogenation metal oxides, followed by ZSM-5-based catalysts in the form of two layers. The introduced metal oxides were expected to induce alkene formation in the hydrocarbon feed after dehydrogenation. Therefore, n-octane could be activated by dehydrogenation sites before further cracking over acidic sites in the ZSM-5-based catalyst. The formation of olefin can accelerate the formation of a carbenium ion and suppress the alkane intermediates, thus promoting the conversion of n-octane to produce light olefins. Catalytic cracking of n-octane was used as the model reaction in this study. A remarkable increase in ethylene and propylene productivity was achieved with the developed reaction route. Stability was also demonstrated in the long-term tests.
2. Results and Discussion
2.1. Characterization of Catalysts
Figure 1 displays the XRD patterns of all the catalysts employed in this study. Peaks corresponding to the cubic crystalline phases of bare γ-Al2O3 appeared at 2θ values of 37°, 45°, and 67° according to JCPDS card no. 10-0425. The Mo/Al2O3 sample exhibited two weak diffraction peaks at 2θ values of 45° and 67°, which were attributed to crystalline γ-Al2O3. The small diffraction peaks at 2θ values of 23° and 25.7°, assignable to the (110) and (040) planes, respectively, indicate the presence of an orthorhombic phase of MoO3 (JCPDS card no. 35-0609). The weak diffraction peaks at 23° and 25.7° indicate that molybdenum oxide was well dispersed over the support [,]. The XRD pattern of the ZSM-5-based catalyst showed intense peaks corresponding to ZSM-5 in 2θ ranges of 7°–9° and 22°–25° [,].
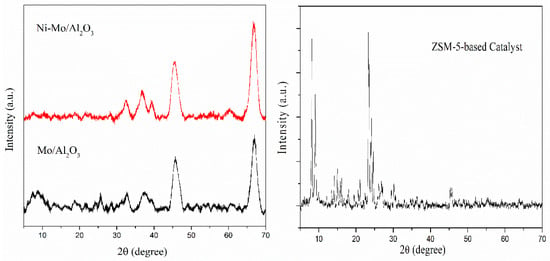
Figure 1.
XRD patterns of Mo/Al2O3, Ni-Mo/Al2O3, and the ZSM-5-based catalyst.
Figure 2I,II shows the N2 adsorption/desorption isotherms and pore-size distribution of the catalysts. All the synthesized samples showed similar isotherms, consisting of monolayer adsorption, multilayer adsorption, and capillary condensation (Figure 2I). The shape of the adsorption/desorption curves suggested that the synthesized Mo/Al2O3 and Ni-Mo/Al2O3 catalysts exhibited type IV adsorption isotherms, which were unequivocally attributed to mesoporous solids. The H2-type hysteresis loop of the isotherms indicated the existence of bottleneck-like mesopores. Pore size analysis (Figure 2II) of the synthesized catalysts indicated a unimodal pore size distribution in the range of 20–100 Å. The calculated average pore diameter of the samples was 10 nm, which was within the standard range of 2–50 nm for mesoporous materials, according to the IUPAC classification. The presence of mesopores and the absence of micropores improved the diffusion (mass transport) of the reactants and products across the active sites.
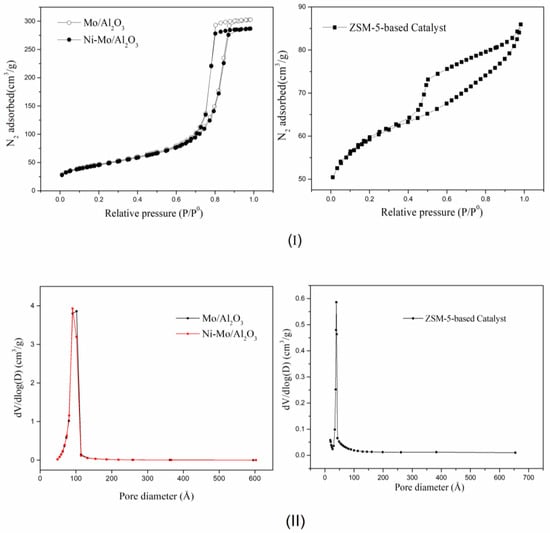
Figure 2.
(I) N2 adsorption-desorption isotherms for Mo/Al2O3, Ni-Mo/Al2O3, and ZSM-5-based catalysts. (II) Pore size distribution of the catalysts.
The acid-base characterization of the catalyst demonstrates its essential role in achieving the desired catalyst performance in the dehydrogenation and cracking reactions. NH3-TPD was used to evaluate the strength and availability of the acid sites for adsorption on the catalyst. The complete set of the NH3-TPD traces is presented in Figure 3 to demonstrate the acidic properties of all the employed catalysts. The peak temperature of Mo/Al2O3 and Ni-Mo/Al2O3 was almost the same, centered at 270 ℃. This corresponded to the desorption of chemisorbed ammonia on the weak to moderate acidic sites of the catalyst. In the alumina-supported catalysts, the γ-alumina support not only provides the required porosity and surface area for the dispersion of the active catalyst components but also contains acidic sites that take part in some of the reactions.
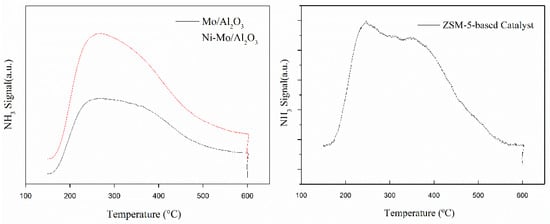
Figure 3.
NH3-TPD of Mo/Al2O3, Ni-Mo/Al2O3, and ZSM-5-based catalyst.
The total acidity of the catalysts was related to the area under the curve. The desorption peak at a low temperature (T ≤ 300 °C) corresponds to weak acid sites, and the desorption peak at a medium temperature (T = 300−450 °C) corresponds to medium−strong acid sites. The distribution of the amount of acid with different strengths of the catalysts was obtained by integrating the peak area of the catalysts at the corresponding temperatures. From Table 1, it can be seen that the total amount of acid of Ni-Mo/Al2O3 was 86.5 μmol/g, which was higher than that of Mo/Al2O3. The amounts of weak acid were 33.9 and 35.3, respectively. The trend in the change in the strength of medium−strong acid is almost consistent with that of the total amount of acid. The amount of medium−strong acid was 51.2 μmol/g with Ni-Mo/Al2O3 and 22.3 μmol/g with Mo/Al2O3. Two desorption peaks were observed in the rich acidic region for the ZSM-5-based catalysts, corresponding to ammonia desorption from the weak and intermediate acid sites. The amounts of weak and medium-strong acids were 90.5 and 45.2 μmol/g, respectively.

Table 1.
Textural properties and acid distribution of the catalysts.
The textural characteristics of the materials are listed in Table 1. Table 1 indicates that the specific surface area of Mo/Al2O3 was 168 m2/g, which decreased slightly to 163 m2/g in Ni-Mo/Al2O3. The total pore volume of Ni-Mo/Al2O3 was 0.44 cm3/g, which was also slightly smaller than that of Mo/Al2O3 (0.47 cm3/g). This was attributed to the pore blockage caused by the addition of nickel oxide. The average pore diameter of the synthesized catalyst decreased after the introduction of nickel oxide, and this is related to the blockage of small pores. The Mo/Al2O3 catalyst had a relatively high surface area (168 m2/g), large pore diameter (11 nm), and relatively large pore volume (0.47 cm3/g). All these properties are indicative of a well-formed mesoporous material that can enhance the dispersion of active metals on a support. The micropore volume of the ZSM-5-based catalyst was 0.08 cm3/g and the total pore volume was 0.13 cm3/g. The micropore volume was not obtained from Mo/Al2O3 and Ni-Mo/Al2O3 catalysts, which was in excellent agreement with the results acquired by N2 adsorption.
2.2. Dehydrogenation Performance of Mo/Al2O3 and Ni-Mo/Al2O3
Identifying an appropriate dehydrogenation catalyst is essential for the on-purpose production of olefins. The dehydrogenation performance of Mo/Al2O3 and Ni-Mo/Al2O3 was investigated using an ACE equipment to identify a suitable dehydrogenation catalyst. The product distribution at various reaction temperatures is presented in Table 2.

Table 2.
The product distribution of Mo/Al2O3 and Ni-Mo/Al2O3.
For the n-octane feedstock with a molecular mass of 114, the H2 yield was predicted to be approximately 1.75% when one hydrogen molecule was abstracted and 5.26% when aromatization occurred. The increase in H2 concentration suggests successful dehydrogenation of alkanes. The H2 yield increased with increasing reaction temperature. The H2 yield of 1.75% at 550 ℃ for Ni-Mo/Al2O3 indicates that one H2 molecule was abstracted from n-octane. When the reaction temperatures were 600 and 650 ℃, the H2 yields were 6.28% and 10.05%, respectively, indicating aromatization and severe coking. The coke yield was consistent with the trend in H2 production because of the carbon–hydrogen equilibrium.
The dehydrogenation reaction in the presence of Mo/Al2O3 was much milder than that in the presence of Ni-Mo/Al2O3. The H2 production was very low between 500 and 550 ℃, with 0.09% and 0.40% yields, respectively. Between 600 and 650 ℃, one H2 molecule was abstracted from n-octane. To establish the structure–activity relationship, the reaction rates were evaluated with low n-octane conversion (<10%), so as to minimize the effect of mass transport. The reaction rate of Mo/Al2O3 at 650 ℃ was 2.01 mmoln-octaneh−1gCat−1, which was higher than that at 500 and 550 ℃. Mass balance of these reactions ranged between 98 and 102%. There are several reports on the use of Mo/Al2O3 and Ni-Mo/Al2O3 for the dehydrogenation of saturated hydrocarbons [,]. When the dehydrogenation activity is weak, the conversion of light hydrocarbon is low; however, when the dehydrogenation activity is strong, an aromatization and severe coking reaction will happen. A high proportion of olefins was found in the liquid product because of the successful dehydrogenation. Metal oxides interact with the hydrogen atoms of alkanes and promote the dissociation of C-H bonds and the formation of hydrogen and olefins [,]. It is worth mentioning that the olefins produced were unstable, often considered intermediate products, and easily cracked into smaller molecules [].
2.3. Proposed Reaction Pathway and DFT Computations
The proposed reaction pathways of n-octane over a single cracking catalyst (Path I), dehydrogenation, and cracking catalysts (Path II) are shown in Figure 4. The ability of solid acids to protolyze non-activated C-H and C-C bonds was first suggested by Haag and Dessau [,]. In the monomolecular mechanism, n-octane first obtains protons from the acid sites of the zeolite, resulting in the formation of carbonium ions [,].
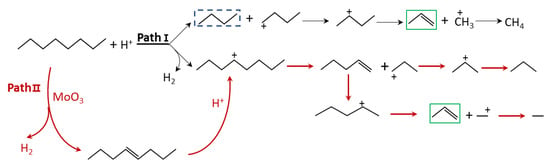
Figure 4.
The schematic diagram for the proposed reaction pathways of n-octane over cracking catalyst (Path Ⅰ), dehydrogenation, and cracking catalysts (Path Ⅱ).
As shown in Path I, from the DFT calculation results, the protolysis of the C-C bond results in butane and butyl carbonium, octonium, and H2. The corresponding energy barrier is 240 and 291 kJ/mol, respectively. It indicated that Brønsted active site trend to react with the C-C bond in n-octane, forming the corresponding small molecule alkanes and carbonium ions. The resulting n-butyl carbonium is easily transformed into more stable sec-butyl carbonium. The energy barrier is 60 kJ/mol. The following (classical) bimolecular mechanism involves hydride transfer between an n-octane and an adsorbed sec-butyl carbonium ion, forming butane and sec-octonium. The formed carbenium ions can undergo a number of reactions. The nature and strength of the acid sites of the catalyst influence the extent to which these reactions occur. The dominant reactions of the carbenium ions include β-scission, isomerization, hydrogen transfer, and dehydrogenation. The β-scission of sec-butyl carbonium forms propylene and methyl carbonium. This step has an activation enthalpy of 218 kcal/mol. Hydride transfer between methyl carbonium and n-octane forms methane and octonium. This step has an activation enthalpy of 170 kcal/mol. The associated activation barriers of the initial step are relatively high, and trends to form carbenium and alkane intermediates. It is also difficult for this formed alkane to further crack on the Brønsted active sites. Therefore, the initial step is the rate-determining step.
Transition metal oxides such as MoO3 are generally used as active components for hydrogenation or dehydrogenation. The activation of n-octane on the (010) surface of MoO3 requires about 140~150 kJ/mol, which is much lower than that required for protolysis [,,]. The presence of MoO3 was proposed to activate the n-octane molecule and promote the initiation step. After activation, octene is induced in the feed. The octene could further react with active Brønsted sites, forming octonium. DFT calculations indicated that the energy barrier of zeolite to protolyze octene is 110 kJ/mol, which is much lower than that required for the case of n-octane. The octonium can undergo β-scission forming pentene and propyl carbonium. The formed pentene can further react with the Brønsted active sites. The red lines in Path II (Figure 2) indicate that if the reaction pathways could be modulated to convert n-octane to octene via dehydrogenation, the energy barrier would decrease. The formation of olefins can accelerate the formation of a carbenium ion and suppress the alkane intermediates, thus promoting the conversion of n-octane to produce light olefins.
2.4. The Feasibility of Sequential Dehydrogenation and Cracking Reactions
The textural characteristics and detailed product distribution are presented to better understand the catalyst activities. The results indicate that Mo/Al2O3 was the optimum catalyst for promoting the production of light olefins under the chosen reaction conditions. We designed a progressive double-bed catalyst system based on a dehydrogenation cracking mechanism (Figure 5). In the first stage, Mo/Al2O3 was utilized as the dehydrogenation site. Hydrocarbon feeds containing adequate amounts of olefins proceed to the second bed (containing a ZSM-5-based catalyst), where a cracking reaction occurs. ZSM-5 containing acid sites is a good choice to demonstrate this new route [,].
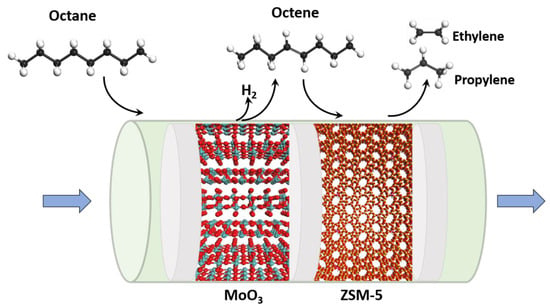
Figure 5.
Proposed reaction path comprising dehydrogenation and cracking reactions in a double-bed catalyst system.
The catalytic performance of the sequential reactions involving Mo/Al2O3 and ZSM-5-based catalysts was tested in a fixed-bed reactor. Figure 6 summarizes the catalytic performance after the introduction of the Mo/Al2O3 catalyst in the set of reactions. n-Octane conversion and the yields of ethylene, propylene, and H2 with increasing metal oxide/n-octane ratio are displayed. The reactions catalyzed by a Mo/Al2O3 or ZSM-5-based catalyst alone (denoted as MOX and Z, respectively, in Figure 6) were tested as reference reactions. A blank test corresponding to thermal cracking was also presented, denoted as Blank in Figure 6.
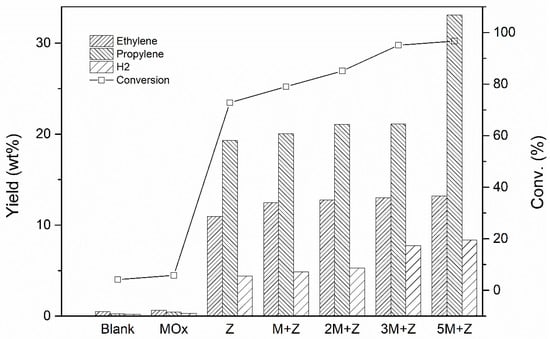
Figure 6.
n-Octane conversion and yields of ethylene, propylene, and H2 in a sequential reaction using metal oxides and ZSM-5-based catalysts. M + Z, 2M + Z, 3M + Z and 5M + Z indicate increasing metal oxide/n-octane ratios of 2:1, 4:1, 6:1 and 10:1 and constant ZSM-5-based catalyst/n-octane ratio of 10:1.
Due to the great enhancement of conversion level, light olefins had higher yields. The yield of ethylene and propylene is 10.9%, and 19.3% over single ZSM-5 catalysts at WHSV = 360 h−1, which is much higher than that of thermal cracking. The double-bed reaction using the Mo/Al2O3 and ZSM-5 catalysts (metal oxide/n-octane ratios of 2:1, denoted as M + Z) gave 12.5% and 20.0% yields of ethylene and propylene at WHSV = 300 h−1, respectively. The conversion increased from 72.8% to 79.0%. The olefin content increases significantly after coupling the Mo/Al2O3 and ZSM-5-based catalysts. This result suggests a prominent synergetic effect between the dehydrogenation and cracking of active sites, in contrast to that in the ZSM-5 reference reaction. The feasibility of the proposed route was further confirmed when the amount of dehydrogenation catalysts was increased—the Mo/Al2O3 to n-octane ratio was changed to 4:1, 6:1, and 10:1 (denoted as 2M + Z, M + Z and 5M + Z in Figure 6) at space velocities (WHSV) of 257, 225 and 180 h−1. The slower flow velocity caused by lower WHSV results in a longer contact time between n-octane and the catalysts. As a result, a larger portion of n-octane performed the dehydrogenation and cracking reactions on the catalyst surface. Because higher amounts of olefins were induced by the Mo/Al2O3 catalyst, the yields of ethylene and propylene increased to 12.78% and 21.07% for 2M + Z, 13.01% and 21.12% for 3M + Z, 13.22% and 33.12% for 5M + Z, respectively, in the product feed. As stated earlier, the influx of adequate amounts of olefins is advantageous for the production of carbenium ions and olefins over the acid sites of zeolites. This was successfully achieved via this route. Substantial increases of ethylene and propylene were obtained by coupling the Mo/Al2O3 and ZSM-5 catalysts.
n-Octane conversion and selectivities of ethylene and propylene in the set of reactions were presented in Table 3. The results demonstrated that both the conversion and selectivity of ethylene and propylene improved after MoO3/Al2O3 introduction. The results obtained for this double-bed system compare favorably with that of the Chunyi Li group []. In their study, the conversion of n-heptane increased from 51% to 59% by replacing 20% of the HZSM-5 catalyst with V2O5/Al2O3. The contacting sequence with the two different catalysts greatly influenced product distribution. n-heptane could be activated by lattice oxygen provided by oxidized vanadia before further cracking over acidic sites in the HZSM-5 catalyst.

Table 3.
n-Octane conversion and selectivities of ethylene and propylene in the sequential reactions and reference reactions.
Compared with the transition metal-promoted zeolites, the double bed system facilitated a modified reaction route. The transition metal-promoted zeolites can act as bifunctional catalysts containing the acid sites in zeolite frameworks and the dehydrogenation centers created by metal species. However, the overloading of metal species on zeolite may cause the serious aggregation blocking of zeolite pores, resulting in the decrease of the catalytic activities. For the double-bed system, the distance of dehydrogenation and cracking active site was beneficial for improving the selectivity to the desired product.
2.5. Evaluation of Double-Bed Reaction Performance and Stability
Lastly, we demonstrate that the double-bed system is capable of maintaining desirable properties (n-octane conversion, light olefin selectivity) in long-term tests. The reactions were performed in a pulse injection manner in the fixed-bed reactor. Figure 7 gives key reaction characteristics for multiple n-octane catalytic dehydrogenation cracking cycles. This route preserves its high selectivity towards light olefins over 10 cycles. The slight decline in light olefin generation over five cycles may be explained by the coke formation. The 10th cycle still shows high light olefin formation. It is thus a stable and very promising route for n-octane catalytic dehydrogenation cracking reaction.
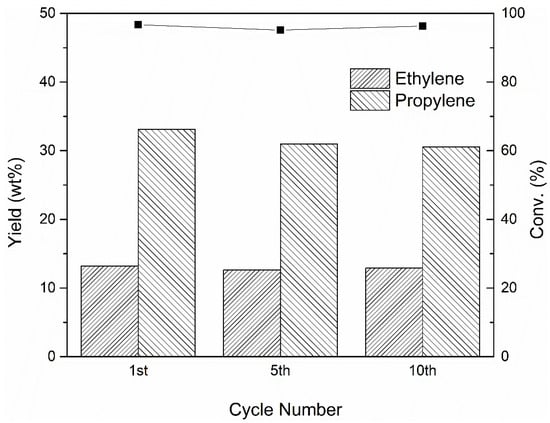
Figure 7.
n-Octane conversion and yields of ethylene and propylene over 10 cycles.
3. Materials and Methods
3.1. Materials
The commercial ZSM-5-based catalysts were obtained from Sinopec Catalyst Co., Ltd. (Beijing, China), with 30 wt% of HZSM-5 zeolite (Si/Al ratio = 21) as an active component. γ-Al2O3, (NH4)6Mo7O24·4H2O, and Ni(NO3)2·6H2O were purchased from Aladdin Reagent Co. Ltd. (Shanghai, China).
The Mo/Al2O3 and Ni-Mo/Al2O3 catalysts were prepared using an incipient wetness impregnation technique. The Ni and Mo contents were 2.91 and 1.59 wt%, respectively, corresponding to a Ni:Mo mole ratio of 3:1. Typically, desired amounts of ammonium molybdate tetrahydrate and nickel nitrate hexahydrate were dissolved in deionized water to match the total pore volume of the support. Subsequently, support was added to the solution. The mixture was continuously stirred for 3 h to achieve homogeneous mixing. The resultant paste was dried at 120 °C for 6 h and then calcined at 550 °C for 6 h under an air stream.
The catalysts above were pressed, crushed, sieved to 0.4–0.8 mm, and dried at 80 ℃ overnight before catalytic tests.
3.2. Catalytic Activity Test
The catalytic cracking of n-octane was carried out in the ACE-model unit R+ (Kayser Corporation). ACE tests were conducted at atmospheric pressure with a catalyst mass of 9.0 g and a catalyst-to-oil mass ratio of 10. The feed was introduced by a pump at a rate of 9 mL/min. The cracking temperature ranged from 500 ℃ to 650 ℃ and the WHSV was 4 h−1. After the reaction, the remaining hydrocarbons were stripped from the catalyst bed, and the reactor was purged with flowing N2 (140 mL/min). During the cracking and stripping modes, the liquid product was collected in a glass receiver that was located at the end of the reactor outlet, and the temperature was maintained at −15 °C. The gas-phase products were collected in a gas receiver using the water displacement method. The dry gases (H2 and C1–C2) and LPG (C3–C4) in the gaseous products were analyzed using an HP 5880A gas chromatograph. The coke content was determined using a CO and CO2 analyzer at the flue gas outlet.
As the evaluation units require different operating conditions, the sequential reaction with the double-bed catalyst system was performed on a microreactor unit with two reaction zones. The performances were evaluated by pulse injection. Typically, the mass of n-octane was 2 μL. The ZSM-5-based catalyst/n-octane ratio was 10:1 and the metal oxides/n-octane ratio was variable from 2:1 to 10:1 to demonstrate the feasibility of the novel approach. Cracking reactions were conducted at 600 °C, and catalysts were filled in the constant temperature zone of the reactor. The residence time is 28 ms. The first bed was separated from the second one using quartz wool. The gas products were analyzed using an online gas chromatograph (GC) equipped with two columns.
The conversion of n-octane and the selectivity of the products were used to determine the activities of the prepared catalysts, calculated as follows:
Conversion = [1 − (C8H18)out/(C8H18)in] × 100%
Selectivity = m/[(C8H18)in − (C8H18)out] × 100%
Here, (C8H18)in is the feed mass, (C8H18)out is the n-octane mass in the product, and m is the product mass.
Yield = Conversion × Selectivity
3.3. Characterization
XRD (X-ray diffraction) characterization was carried out on a Philips X’Pert powder diffractometer using Cu Kα radiation (λ = 154.05 pm) in the 2θ range from 5° to 70°. It was operated at 40 kV and 40 mA, with a scanning speed of 10°/min. Nitrogen physisorption isotherms were obtained using a Micromeritics ASAP 2400 volumetric adsorption system. The pore size distribution and surface area were calculated from the adsorption isotherms using the Barrett–Joyner–Halenda method and the–Brunauer–Emmett Teller equation. The micropore volume was calculated from the t-plot curve. The total acidity of the different samples was measured by NH3-TPD using a Micromeritics Autochem II (ASAP 2920) chemisorption system. The acquired TPD curve was then deconvoluted by a method developed by C. Costa et al. [].
3.4. Computational Studies
DFT calculations were performed using the DMol3 software developed by Accelrys Inc. The geometries of the metal oxide and zeolite cluster models used in this work were obtained from the framework structure obtained from Material Studio 2019. Relativistic effects were taken into account using an all-electron scalar relativistic method. All calculations were performed using the DNP basis set. The convergence criteria (energy, force, and displacement) were set at 0.05 kJ/mol, 1012 N, and 5 × 10−13 m, respectively.
4. Conclusions
In summary, we successfully demonstrated a feasible route to increase light olefin production in the catalytic cracking of naphtha, using n-octane as a model compound. This novel route involves sequential dehydrogenation and cracking reactions in a single reactor with two reaction zones. The Mo/Al2O3 dehydrogenation catalyst bed placed before the ZSM-5-based catalyst bed induced alkenes into the hydrocarbon feed. The formation of alkenes can accelerate the formation of a carbenium ion and suppress the alkane intermediates, thus promoting the conversion of n-octane to produce light olefins. The yields of ethylene and propylene increased to 12.78% and 21.07% with a Mo/Al2O3:n-octane ratio of 4:1 and to 13.22% and 33.12% with a Mo/Al2O3:n-octane ratio of 10:1, respectively, in the products. Stability tests showed that the catalytic activity of the double-bed system was stable over 10 cycles. This study opens a new avenue for optimizing catalytic cracking reactions of naphtha. Further investigations to optimize and develop this novel route are currently underway.
Author Contributions
Conceptualization, X.Z., J.G. and L.L.; investigation, X.Z.; resources, X.Z. and J.G.; writing—original draft preparation, X.Z., J.G., X.W. and L.L.; writing—review and editing, X.Z.; supervision, J.G.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SINOPEC (Grant ST20030-9-002).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was performed with the financial support of SINOPEC. Lixin Wang is gratefully acknowledged for DFT calculation and valuable discussion. The authors are grateful for experimental help from Yueqin Zhang and Renjun Kan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, Z.; Huang, Y.; Ma, H.; Qian, W.; Zhang, H.; Ying, W. Syngas-to-olefins over MOF-derived ZnZrOx and SAPO-34 bifunctional catalysts. Catal. Commun. 2021, 152, 106292. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Zhang, P.; Duan, A. FeHZSM-5 molecular sieves—Highly active catalysts for catalytic cracking of isobutane to produce ethylene and propylene. Catal. Commun. 2006, 7, 199–203. [Google Scholar] [CrossRef]
- Balogun, M.L.; Adamu, S.; Bakare, I.A.; Ba-Shammakh, M.S.; Hossain, M.M. CO2Assisted Oxidative Dehydrogenation of Propane to Propylene over Fluidizable MoO3/La2O3-γAl2O3Catalysts. J. CO2 Util. 2020, 42, 101329. [Google Scholar] [CrossRef]
- Dixit, M.; Kostetskyy, P.; Mpourmpakis, G. Structure-Activity Relationships in Alkane Dehydrogenation on γ-Al2O3: Site-Dependent Reactions. ACS Catal. 2018, 8, 11570–11578. [Google Scholar] [CrossRef]
- Kubo, K.; Iida, H.; Namba, S.; Igarashi, A. Selective formation of light olefins by catalytic cracking of naphtha components over ZSM-5 zeolite catalysts. J. Jpn. Pet. Inst. 2018, 61, 10–19. [Google Scholar] [CrossRef]
- Ajumobi, O.O.; Muraza, O.; Bakare, I.A.; al Amer, A.M. Iron- and Cobalt-Doped Ceria-Zirconia Nanocomposites for Catalytic Cracking of Naphtha with Regenerative Capability. Energy Fuels 2017, 31, 12612–12623. [Google Scholar] [CrossRef]
- Wattanapaphawong, P.; Reubroycharoen, P.; Mimura, N.; Sato, O.; Yamaguchi, A. Effect of carbon number on the production of propylene and ethylene by catalytic cracking of straight-chain alkanes over phosphorus-modified ZSM-5. Fuel Process. Technol. 2020, 202, 106367. [Google Scholar] [CrossRef]
- Al-Khattaf, S.S.; Ali, S.A. Catalytic Cracking of Arab Super Light Crude Oil to Light Olefins: An Experimental and Kinetic Study. Energy Fuels 2018, 32, 2234–2244. [Google Scholar] [CrossRef]
- Jung, J.S.; Kim, T.J.; Seo, G. Catalytic cracking of n-octane over zeolites with different pore structures and acidities. Korean J. Chem. Eng. 2004, 21, 777–781. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, X.; Li, C. Recent Progress in Commercial and Novel Catalysts for Catalytic Dehydrogenation of Light Alkanes. Chem. Rec. 2020, 20, 604–616. [Google Scholar] [CrossRef]
- Phadke, N.M.; Mansoor, E.; Head-Gordon, M.; Bell, A.T. Mechanism and kinetics of light alkane dehydrogenation and cracking over isolated Ga species in Ga/H-MFI. ACS Catal. 2021, 11, 2062–2075. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Xiao, Z.; Li, G.; Wang, L. Understanding deep dehydrogenation and cracking of: N -butane on Ni(111) by a DFT study. Phys. Chem. Chem. Phys. 2020, 22, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Q.; Long, H.; Sun, L.; Murayama, T.; Qi, C. Insights into Au Nanoparticle Size and Chemical State of Au/ZSM-5 Catalyst for Catalytic Cracking of n-Octane to Increase Propylene Production. J. Phys. Chem. C 2021, 125, 16013–16023. [Google Scholar] [CrossRef]
- Tsiatouras, V.A.; Katranas, T.K.; Triantafillidis, C.S.; Vlessidis, A.G.; Paulidou, E.G.; Evmiridis, N.P. Dehydrogenation of propane over various chromium-modified MFI-type zeolite catalysts. Stud. Surf. Sci. Catal. 2002, 142, 839–846. [Google Scholar] [CrossRef]
- Kotrel, S.; Knözinger, H.; Gates, B.C. The Haag-Dessau mechanism of protolytic cracking of alkanes. Microporous Mesoporous Mater. 2000, 35–36, 11–20. [Google Scholar] [CrossRef]
- Cnudde, P.; de Wispelaere, K.; Vanduyfhuys, L.; Demuynck, R.; van der Mynsbrugge, J.; Waroquier, M.; van Speybroeck, V. How Chain Length and Branching Influence the Alkene Cracking Reactivity on H-ZSM-5. ACS Catal. 2018, 8, 9579–9595. [Google Scholar] [CrossRef]
- Wang, T.; Cui, X.; Winther, K.T.; Abild-Pedersen, F.; Bligaard, T.; Nørskov, J.K. Theory-Aided Discovery of Metallic Catalysts for Selective Propane Dehydrogenation to Propylene. ACS Catal. 2021, 11, 6290–6297. [Google Scholar] [CrossRef]
- Zahara, Z.; Krisnandi, Y.K.; Wibowo, W.; Nurani, D.A.; Rahayu, D.U.C.; Haerudin, H. Synthesis and characterization of hierarchical ZSM-5 zeolite using various templates as cracking catalysts. AIP Conf. Proc. 2018, 2023, 020088. [Google Scholar] [CrossRef]
- Feng, R.; Yan, X.; Hu, X.; Yan, Z.; Lin, J.; Li, Z.; Hou, K.; Rood, M.J. Surface dealumination of micro-sized ZSM-5 for improving propylene selectivity and catalyst lifetime in methanol to propylene (MTP) reaction. Catal. Commun. 2018, 109, 1–5. [Google Scholar] [CrossRef]
- Wang, M.; He, M.; Fang, Y.; Baeyens, J.; Tan, T. The Ni-Mo/Γ-Al2O3 catalyzed hydrodeoxygenation of FAME to aviation fuel. Catal. Commun. 2017, 100, 237–241. [Google Scholar] [CrossRef]
- Qian, Y.; Liang, S.; Wang, T.; Wang, Z.; Xie, W.; Xu, X. Enhancement of pyrolysis gasoline hydrogenation over Zn- and Mo-promoted Ni/γ-Al2O3 catalysts. Catal. Commun. 2011, 12, 851–853. [Google Scholar] [CrossRef]
- Abdelgaid, M.; Dean, J.; Mpourmpakis, G. Improving alkane dehydrogenation activity on γ-Al2O3through Ga doping. Catal. Sci. Technol. 2020, 10, 7194–7202. [Google Scholar] [CrossRef]
- Kissin, Y.V. Chemical Mechanisms of Catalytic Cracking over Solid Acidic Catalysts: Alkanes and Alkenes. Catal. Rev. Sci. Eng. 2001, 43, 85–146. [Google Scholar] [CrossRef]
- Sommer, J.; Jost, R.; Hachoumy, M. Activation of small alkanes on strong solid acids: Mechanistic approaches. Catal. Today 1997, 38, 309–319. [Google Scholar] [CrossRef]
- Boronat, M.; Corma, A. Are carbenium and carbonium ions reaction intermediates in zeolite-catalyzed reactions? Appl. Catal. A Gen. 2008, 336, 2–10. [Google Scholar] [CrossRef]
- Babitz, S.M.; Williams, B.A.; Miller, J.T.; Snurr, R.Q.; Haag, W.O.; Kung, H.H. Monomolecular cracking of n-hexane on Y, MOR, and ZSM-5 zeolites. Appl. Catal. A Gen. 1999, 179, 71–86. [Google Scholar] [CrossRef]
- Tsilomelekis, G.; Christodoulakis, A.; Boghosian, S. Support effects on structure and activity of molybdenum oxide catalysts for the oxidative dehydrogenation of ethane. Catal. Today 2007, 127, 139–147. [Google Scholar] [CrossRef]
- Liao, C.C.; Chang, C.C.; Choi, Y.M.; Tsai, M.K. Ethane oxidative dehydrogenation mechanism on MoO3(010) surface: A first-principle study using on-site Coulomb correction. Surf. Sci. 2018, 674, 45–50. [Google Scholar] [CrossRef]
- Martin, R.; Kim, M.; Asthagiri, A.; Weaver, J.F. Alkane Activation and Oxidation on Late-Transition-Metal Oxides: Challenges and Opportunities. ACS Catal. 2021, 11, 4682–4703. [Google Scholar] [CrossRef]
- Lü, Q.; Lin, X.; Wang, L.; Gao, J.; Bao, X. On-stream stability enhancement of HZSM-5 based fluid catalytic cracking naphtha hydro-upgrading catalyst via magnesium modification. Catal. Commun. 2016, 83, 31–34. [Google Scholar] [CrossRef]
- Altwasser, S.; Welker, C.; Traa, Y.; Weitkamp, J. Catalytic cracking of n-octane on small-pore zeolites. Microporous Mesoporous Mater. 2005, 83, 345–356. [Google Scholar] [CrossRef]
- Hu, X.; Xu, T.; Li, C.; Yang, C. Catalytic cracking of n-heptane under activation of lattice oxygen in a circulating fluidized bed unit. Chem. Eng. J. 2011, 172, 410–417. [Google Scholar] [CrossRef]
- Costa, C.; Lopes, J.M.; Lemos, F.; Ramoa, F. Activity–acidity relationship in zeolite Y Part 2. Determination of the acid strength distribution by temperature programmed desorption of ammonia. J. Mol. Catal. A Chem. 1999, 144, 221–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).