Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization
Abstract
:1. Introduction
2. Results and Discussion
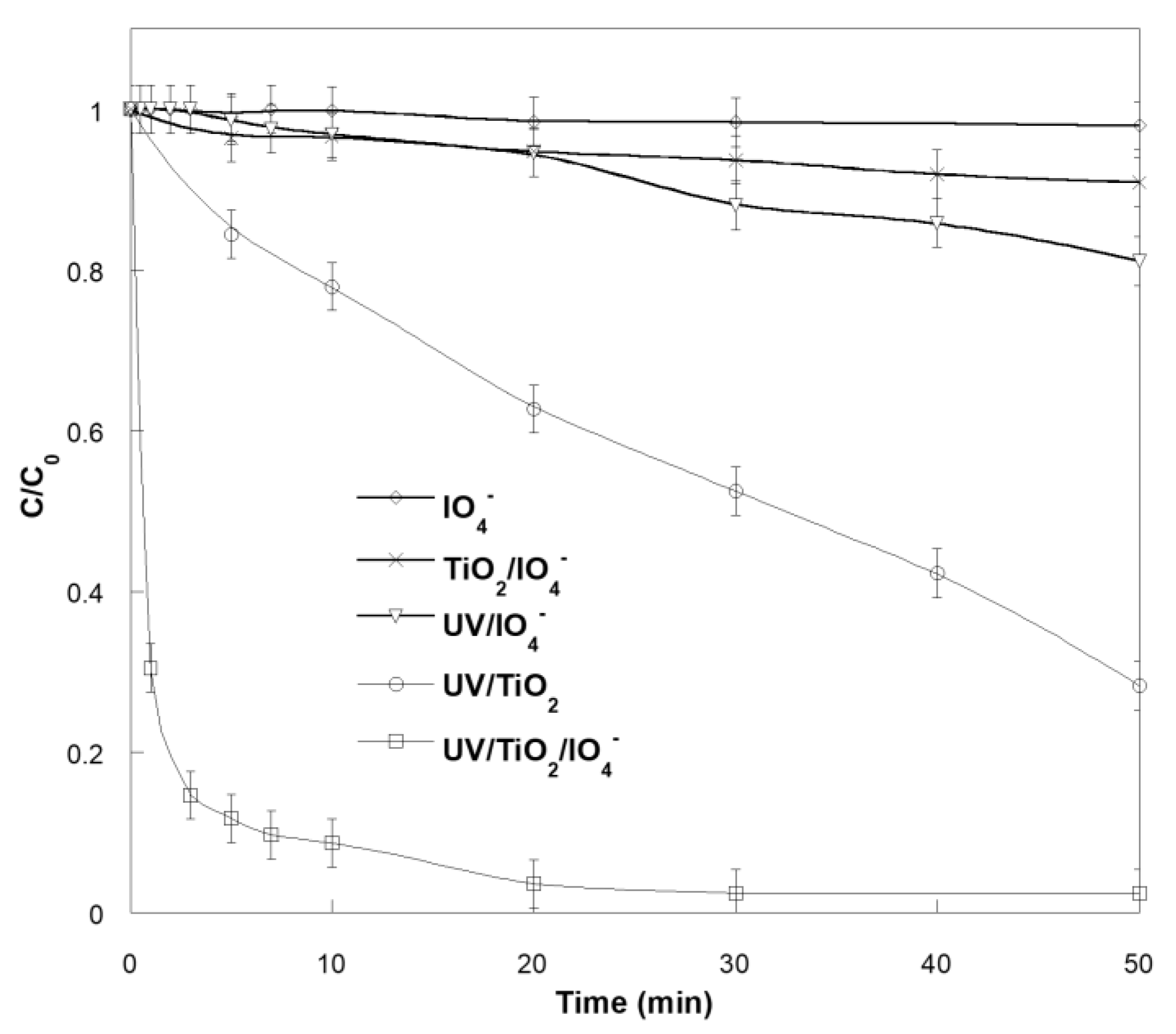
2.1. Photocatalytic Degradation of SO
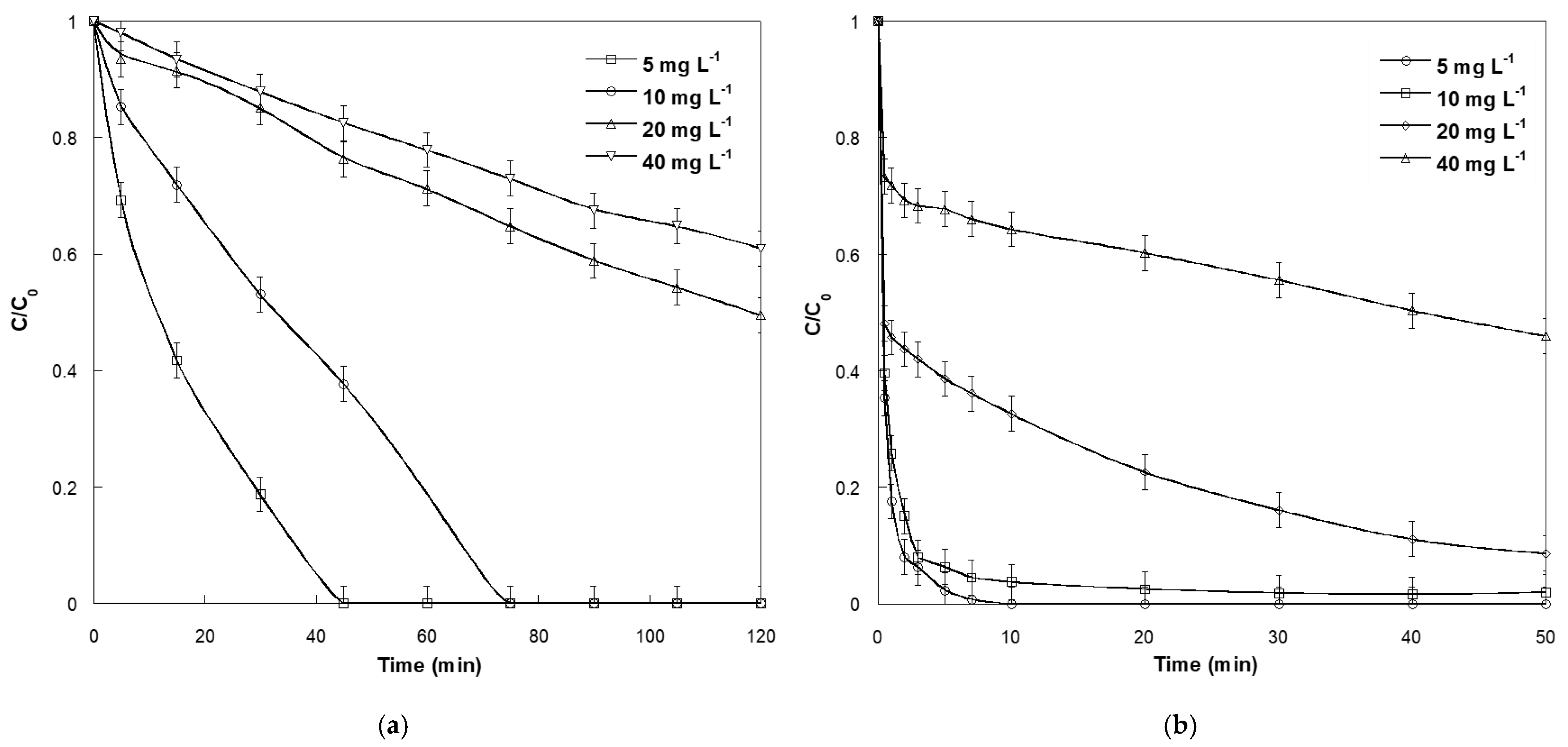
2.2. Effect of Initial SO Concentration
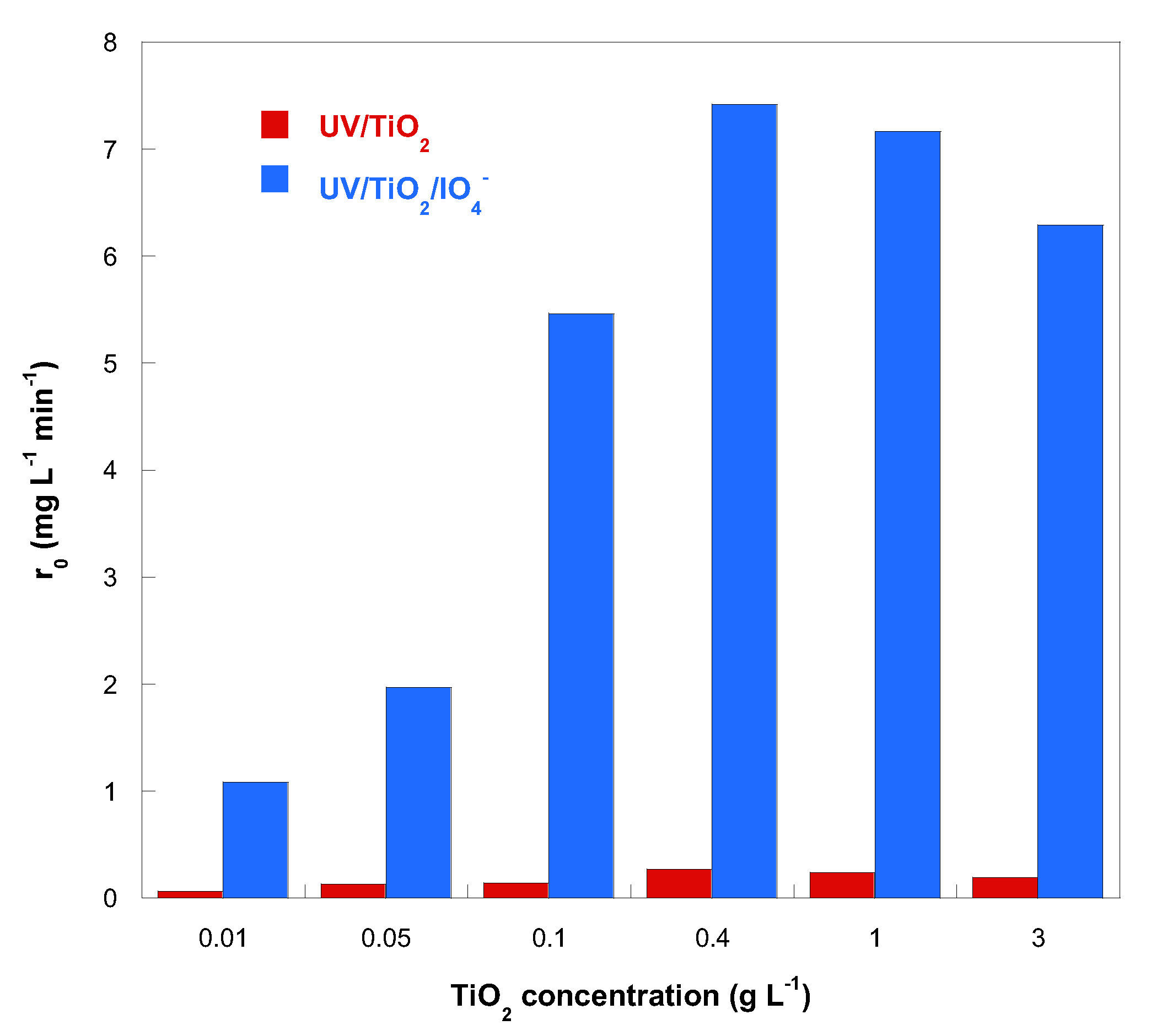
2.3. Effect of TiO2 Loading
2.4. Effect of Periodate Concentration
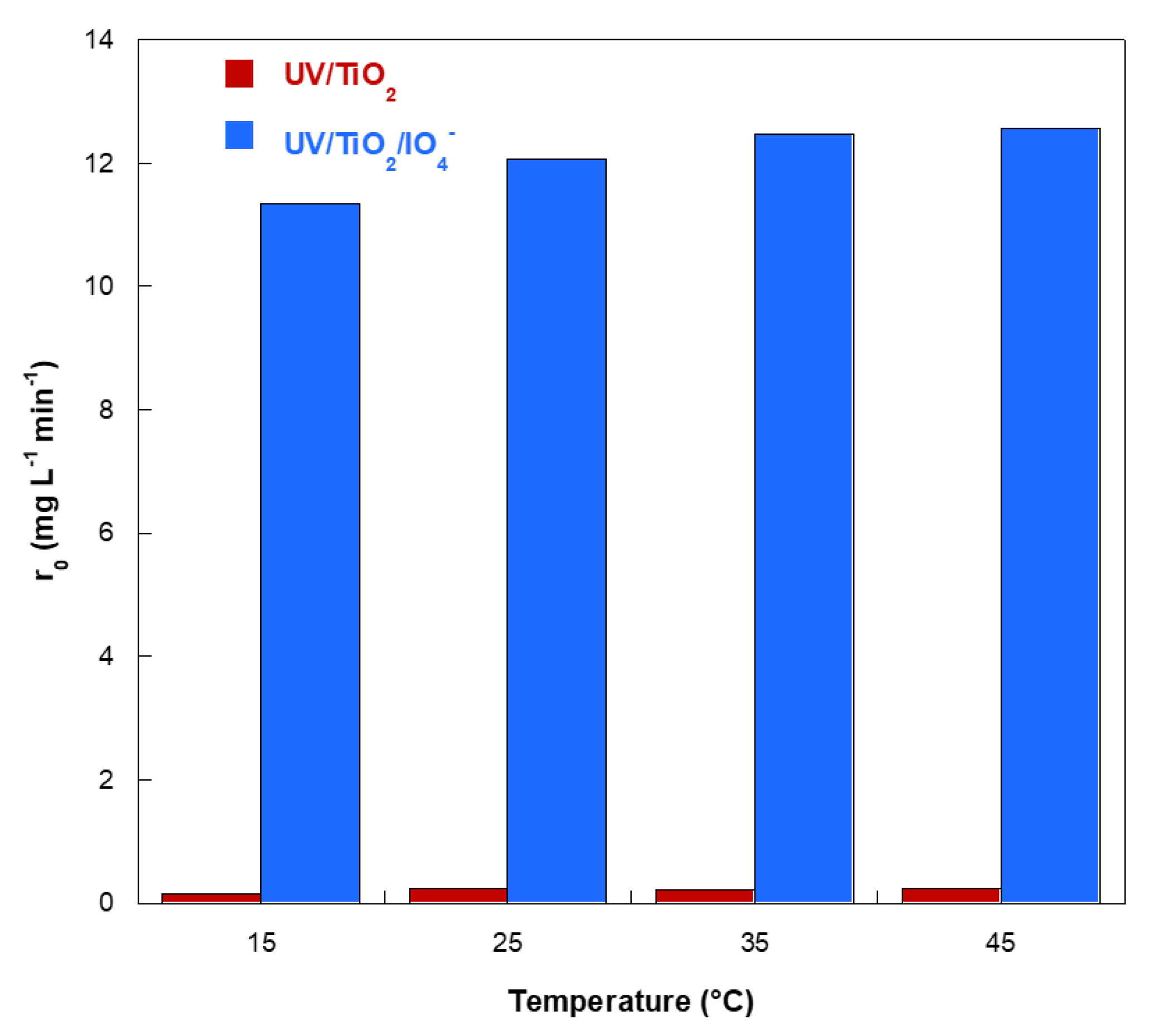
2.5. Effect of the Liquid Temperature
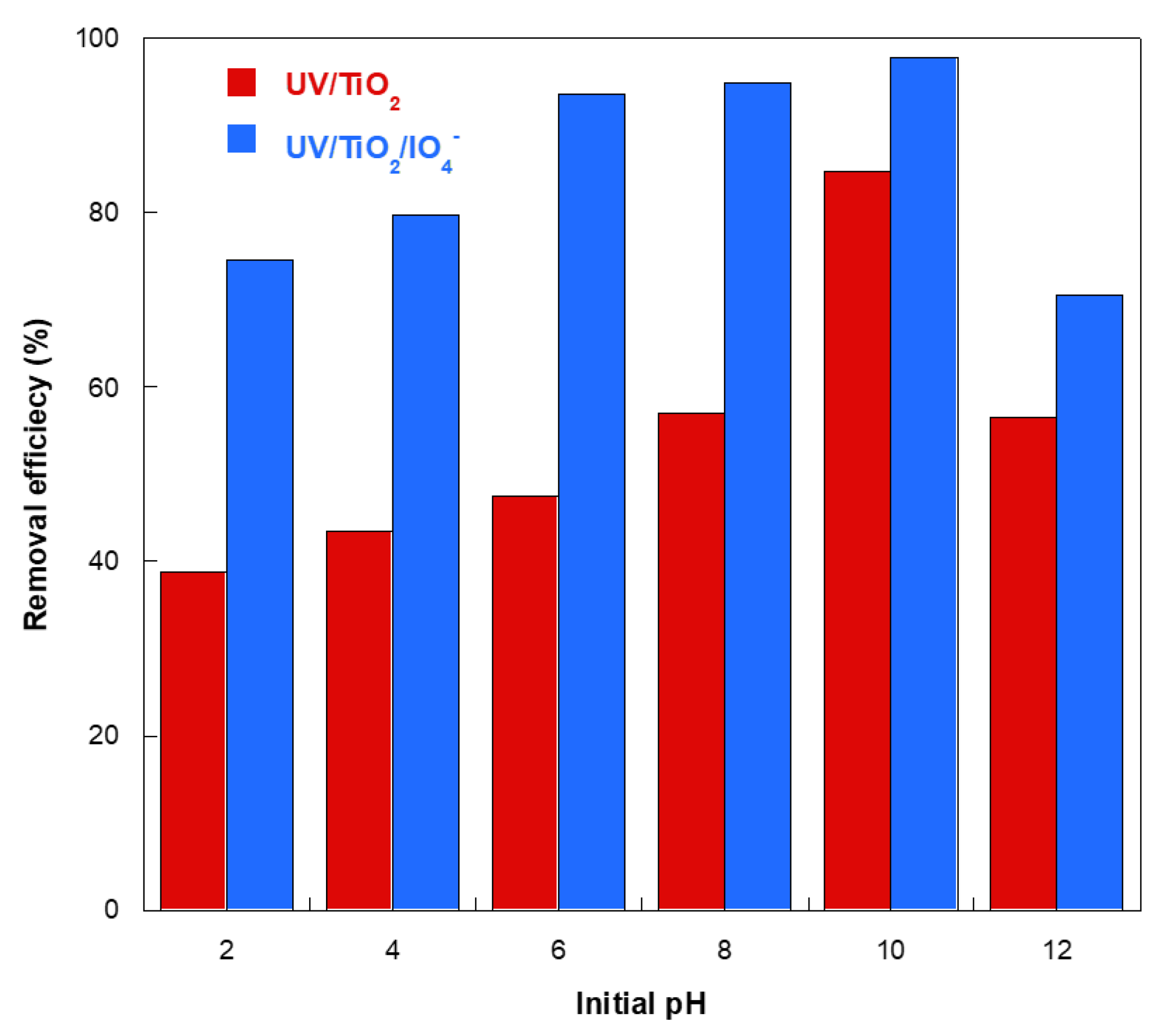
2.6. Effect of Initial Solution pH
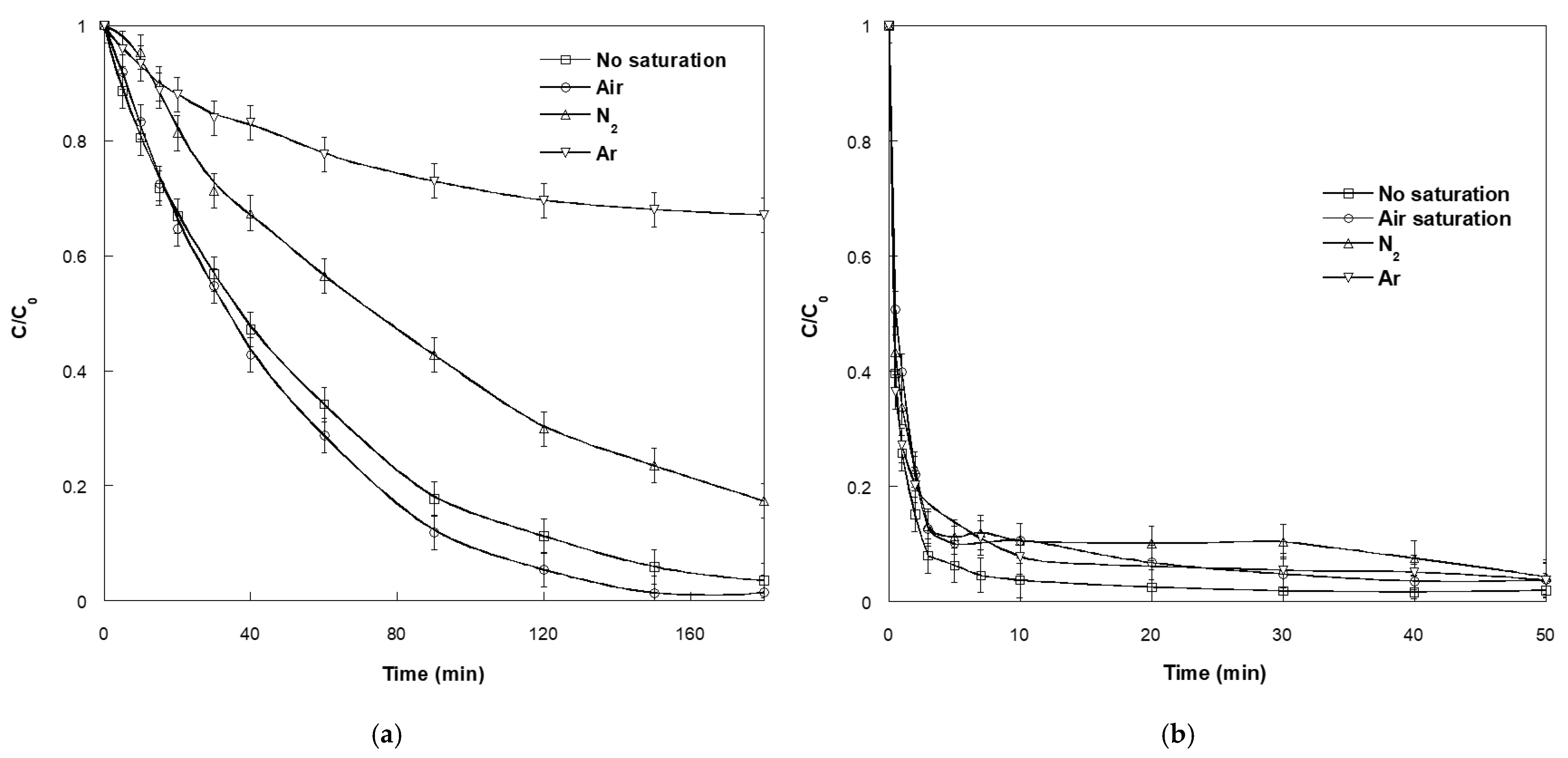
2.7. Effect of Dissolved Gases
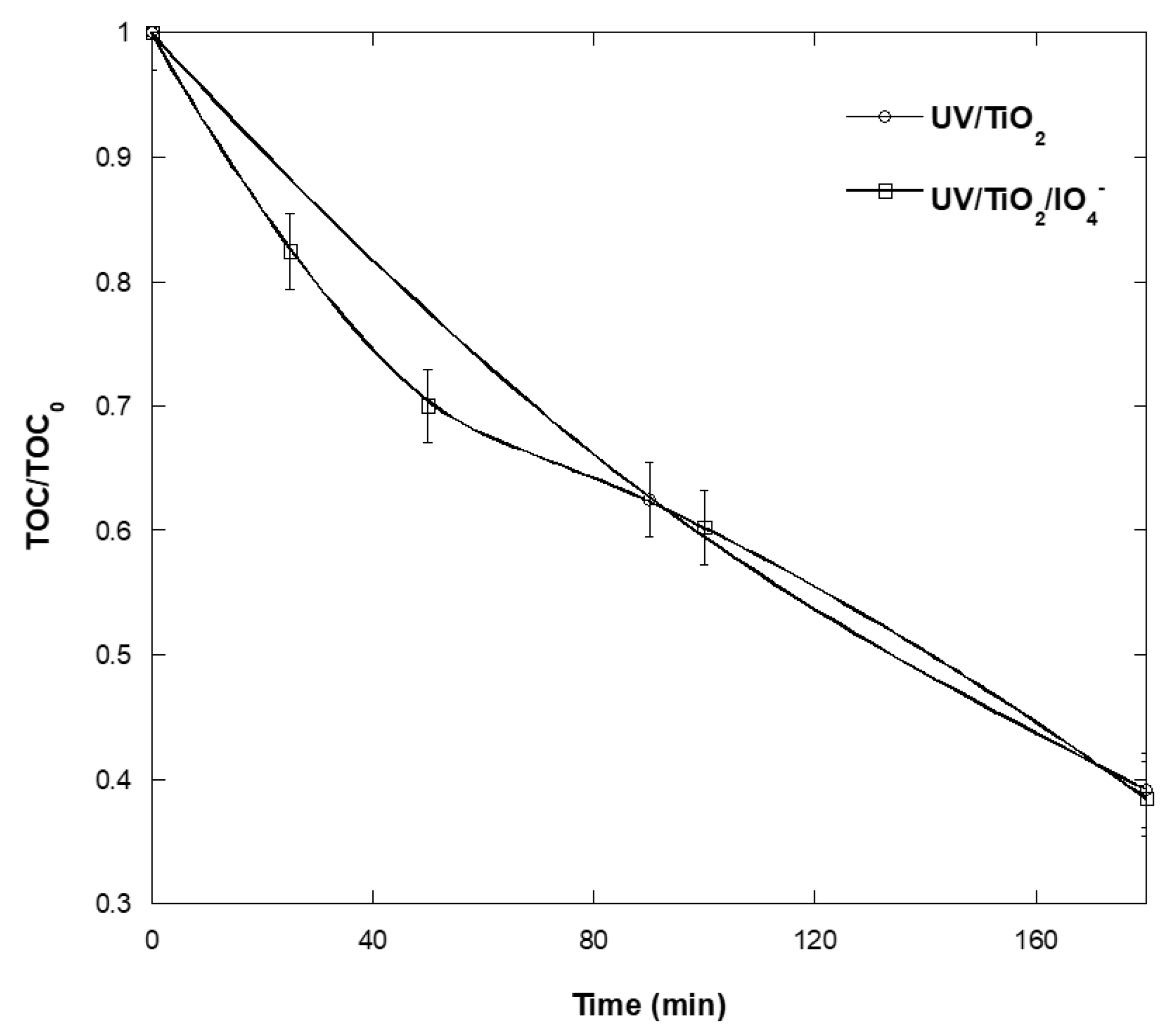
2.8. Mineralization
3. Materials and Methods
3.1. Reagents
3.2. Photocatalysis Experiments and Apparatus
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawar, R.C.; Lee, C.S. Heterogeneous Nanocomposite-Photocatalysis for Water Purification; William Andrew: Norwich, NY, USA, 2015; ISBN 9780323393133. [Google Scholar]
- Zeghioud, H.; Assadi, A.A.; Khellaf, N.; Djelal, H.; Amrane, A.; Rtimi, S. Reactive species monitoring and their contribution for removal of textile effluent with photocatalysis under UV and visible lights: Dynamics and mechanism. J. Photochem. Photobiol. A Chem. 2018, 365, 94–102. [Google Scholar] [CrossRef]
- Azzaz, A.A.; Assadi, A.A.; Jellali, S.; Bouzaza, A.; Wolbert, D.; Rtimi, S.; Bousselmi, L. Discoloration of simulated textile effluent in continuous photoreactor using immobilized titanium dioxide: Effect of zinc and sodium chloride. J. Photochem. Photobiol. A Chem. 2018, 358, 111–120. [Google Scholar] [CrossRef]
- Ogugbue, C.J.; Sawidis, T. Bioremediation and Detoxification of Synthetic Wastewater Containing Triarylmethane Dyes by Aeromonas hydrophila Isolated from Industrial Effluent. Biotechnol. Res. Int. 2011, 2011, 967925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekbala, M.R.; Soltani, S.M.; Yazdi, S.K.; Hosseini, S. Equilibrium and Kinetic Studies of Safranine Adsorption on Alkali-Treated Mango Seed Integuments. Int. J. Chem. Eng. Appl. 2012, 3, 160–166. [Google Scholar]
- Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Degradation of Safranin O by thermally activated persulfate in the presence of mineral and organic additives: Impact of environmental matrices. Desalin. Water Treat. 2017, 75, 202–212. [Google Scholar] [CrossRef]
- Sieren, B.; Baker, J.; Wang, X.; Rozzoni, S.J.; Carlson, K.; Mcbain, A.; Kerstan, D.; Allen, L.; Liao, L.; Li, Z. Sorptive Removal of Color Dye Safranin O by Fibrous Clay Minerals and Zeolites. Adv. Mater. Sci. Eng. 2020, 2020, 8845366. [Google Scholar] [CrossRef]
- Hayat, K.; Gondal, M.A.; Khaled, M.M.; Yamani, Z.H.; Ahmed, S. Laser induced photocatalytic degradation of hazardous dye (Safranin-O) using self synthesized nanocrystalline WO 3. J. Hazard. Mater. 2011, 186, 1226–1233. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Mathur, M.; Sikarwar, S. Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J. Colloid Interface Sci. 2007, 309, 464–469. [Google Scholar] [CrossRef]
- Ekka, B.; Sahu, M.K.; Patel, R.K.; Dash, P. Titania coated silica nanocomposite prepared via encapsulation method for the degradation of Safranin-O dye from aqueous solution: Optimization using statistical design. Water Resour. Ind. 2019, 22, 100071. [Google Scholar] [CrossRef] [Green Version]
- El-kemary, M.; El-shamy, H. Fluorescence modulation and photodegradation characteristics of safranin O dye in the presence of ZnS nanoparticles. J. Photochem. Photobiol. A Chem. 2009, 205, 151–155. [Google Scholar] [CrossRef]
- Abdullah, F.H.; Rauf, M.A.; Ashraf, S.S. Photolytic oxidation of Safranin-O with H2O2. Dye. Pigment. 2007, 72, 2–5. [Google Scholar] [CrossRef]
- El-kemary, M.; Abdel-moneam, Y.; Madkour, M.; El-mehasseb, I. Enhanced photocatalytic degradation of Safranin-O by heterogeneous nanoparticles for environmental applications. J. Lumin. 2011, 131, 570–576. [Google Scholar] [CrossRef]
- Choy, W.K.; Chu, W. The use of oxyhalogen in photocatalytic reaction to remove o-chloroaniline in TiO2 dispersion. Chemosphere 2007, 66, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Wu, C.-H. Decolorization of C.I. reactive red 198 in UV/oxidant and UV/TiO2/oxidant systems. React. Kinet. Mech. Catal. 2011, 104, 281–290. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, C.S. Effect of hydrogen peroxide, periodate and persulfate on photocatalysis of 2-chlorobiphenyl in aqueous TiO2 suspensions. Water Res. 1999, 33, 2031–2036. [Google Scholar] [CrossRef]
- Sadik, W.A. Effect of inorganic oxidants in photodecolourization of an azo dye. J. Photochem. Photobiol. A Chem. 2007, 191, 132–137. [Google Scholar] [CrossRef]
- Gözmen, B.; Turabik, M.; Hesenov, A. Photocatalytic degradation of Basic Red 46 and Basic Yellow 28 in single and binary mixture by UV/TiO2/periodate system. J. Hazard. Mater. 2009, 164, 1487–1495. [Google Scholar] [CrossRef]
- Yun, E.T.; Yoo, H.Y.; Kim, W.; Kim, H.E.; Kang, G.; Lee, H.; Lee, S.; Park, T.; Lee, C.; Kim, J.H.; et al. Visible-light-induced activation of periodate that mimics dye-sensitization of TiO2: Simultaneous decolorization of dyes and production of oxidizing radicals. Appl. Catal. B Environ. 2017, 203, 475–484. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, J. Application of photoactivated periodate to the decolorization of reactive dye: Reaction parameters and mechanism. J. Photochem. Photobiol. A Chem. 2004, 165, 35–41. [Google Scholar] [CrossRef]
- Guettaı, N.; Amar, H.A. Photocatalytic oxidation of methyl orange in presence of titanium dioxide in aqueous suspension. Part II: Kinetics study. Desalination 2005, 185, 439–448. [Google Scholar] [CrossRef]
- Chia, L. Kinetics and Mechanism of Photoactivated Periodate Reaction with 4-Chlorophenol in Acidic Solution. Environ. Sci. Technol. 2004, 38, 6875–6880. [Google Scholar] [CrossRef] [PubMed]
- Vulliet, E.; Chovelon, J.; Guillard, C.; Herrmann, J. Factors influencing the photocatalytic degradation of sulfonylurea herbicides by TiO2 aqueous suspension. J. Photochem. Photobiol. A Chem. 2003, 159, 71–79. [Google Scholar] [CrossRef]
- Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient photocatalytic degradation of Safranin O by integrating solar-UV/TiO2/persulfate treatment: Implication of sulfate radical in the oxidation process and effect of various water matrix components. J. Photochem. Photobiol. A Chem. 2017, 345, 80–91. [Google Scholar] [CrossRef]
- Minero, C.; Catozzo, F.; Pelizzetti, E. Role of adsorption in photocatalyzed reactions of organic molecules in aqueous titania suspensions. Langmuir 1992, 8, 481–486. [Google Scholar] [CrossRef]
- Zalazar, C.S.; Martin, C.A.; Cassano, A.E. Photocatalytic intrinsic reaction kinetics. II: Effects of oxygen concentration on the kinetics of the photocatalytic degradation of dichloroacetic acid. Chem. Eng. Sci. 2005, 60, 4311–4322. [Google Scholar] [CrossRef]
- Torres, R.A.; Nieto, J.I.; Combet, E.; Pétrier, C.; Pulgarin, C. Influence of TiO2 concentration on the synergistic effect between photocatalysis and high-frequency ultrasound for organic pollutant mineralization in water. Appl. Catal. B Environ. 2008, 80, 168–175. [Google Scholar] [CrossRef]
- Van Doorslaer, X.; Heynderickx, P.M.; Demeestere, K.; Debevere, K.; Van Langenhove, H.; Dewulf, J. Applied Catalysis B: Environmental TiO2 mediated heterogeneous photocatalytic degradation of moxifloxacin: Operational variables and scavenger study. Appl. Catal. B Environ. 2012, 112, 150–156. [Google Scholar] [CrossRef]
- Sun, H.; He, F.; Choi, W. Production of Reactive Oxygen Species by the Reaction of Periodate and Hydroxylamine for Rapid Removal of Organic Pollutants and Waterborne Bacteria. Environ. Sci. Technol. 2020, 54, 6427–6437. [Google Scholar] [CrossRef]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/Periodate (IO4−): A novel advanced oxidation technology for the degradation of refractory organic pollutants. Water Res. Technol. 2019, 5, 1113–1123. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, B.; Zhang, Z.; Song, M.; Zhao, X. Temperature effect on the photocatalytic degradation of methyl orange under UV-vis light irradiation. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2010, 25, 210–213. [Google Scholar] [CrossRef]
- Chatzitakis, A.; Berberidou, C.; Paspaltsis, I.; Kyriakou, G.; Sklaviadis, T.; Poulios, I. Photocatalytic degradation and drug activity reduction of Chloramphenicol. Water Res. 2008, 42, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Yawalkar, A.A.; Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Solar-assisted photochemical and photocatalytic degradation of phenol. J. Chem. Technol. Biotechnol. 2001, 76, 363–370. [Google Scholar] [CrossRef]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A.C.M. Photocatalytic degradation for environmental applications—A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Bahnemann, W.; Muneer, M.; Haque, M.M. Titanium dioxide-mediated photocatalysed degradation of few selected organic pollutants in aqueous suspensions. Catal. Today 2007, 124, 133–148. [Google Scholar] [CrossRef]
- Hofstadler, K.; Bauer, R.; Novallc, S.; Heisler, G. New Reactor Design for Photocatalytic Wastewater Treatment with TiO2 Immobilized on Fused-Silica Glass Fibers: Photomineralization of 4-Chlorophenol. Environ. Sci. Technol. 1994, 28, 670–674. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Insola, A.; Longo, G.; Tufano, V. Photocatalytic oxidation of 4-nitrophenol in aqueous TiO2 slurries: An experimental validation of literature kinetic models. J. Chem. Technol. Biotechnol. 2000, 75, 131–136. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Moonsiri, M.; Rangsunvigit, P.; Chavadej, S.; Gulari, E. Effects of Pt and Ag on the photocatalytic degradation of 4-chlorophenol and its by-products. Chem. Eng. J. 2004, 97, 241–248. [Google Scholar] [CrossRef]
- Tang, C.; Chen, V. The photocatalytic degradation of reactive black 5 using TiO2/UV in an annular photoreactor. Water Res. 2004, 38, 2775–2781. [Google Scholar] [CrossRef]
- Gerischer, H.; Heller, A. The Role of Oxygen in Photooxidation of Organic Molecules on Semiconductor Particles. J. Phys. Chem. 1991, 0, 5261–5267. [Google Scholar] [CrossRef]
- Mcmurray, T.A.; Dunlop, P.S.M.; Byrne, J.A. The photocatalytic degradation of atrazine on nanoparticulate TiO2films. J. Photochem. Photobiol. A Chem. 2006, 182, 43–51. [Google Scholar] [CrossRef]
- Kyoung, N.; Eun, J.; Shim, O.; Lee, H.; Kim, J.; Koun, B. The effect of dissolved oxygen on the 1,4-dioxane degradation with TiO2 and Au-TiO2 photocatalysts. J. Hazard. Mater. 2010, 177, 216–221. [Google Scholar] [CrossRef]
- Ohtaki, M.; Sato, H.; Fujii, H.; Eguchi, K. Intramolecularly selective decomposition of surfactant molecules on photocatalytic oxidative degradation over TiO2 photocatalyst. J. Mol. Catal. A Chem. 2000, 155, 121–129. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendjama, M.; Hamdaoui, O.; Ferkous, H.; Alghyamah, A. Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization. Catalysts 2022, 12, 1460. https://doi.org/10.3390/catal12111460
Bendjama M, Hamdaoui O, Ferkous H, Alghyamah A. Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization. Catalysts. 2022; 12(11):1460. https://doi.org/10.3390/catal12111460
Chicago/Turabian StyleBendjama, Meriem, Oualid Hamdaoui, Hamza Ferkous, and Abdulaziz Alghyamah. 2022. "Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization" Catalysts 12, no. 11: 1460. https://doi.org/10.3390/catal12111460
APA StyleBendjama, M., Hamdaoui, O., Ferkous, H., & Alghyamah, A. (2022). Degradation of Safranin O in Water by UV/TiO2/IO4− Process: Effect of Operating Conditions and Mineralization. Catalysts, 12(11), 1460. https://doi.org/10.3390/catal12111460





