Metal-Organic Framework-Derived Atomically Dispersed Co-N-C Electrocatalyst for Efficient Oxygen Reduction Reaction
Abstract
1. Introduction
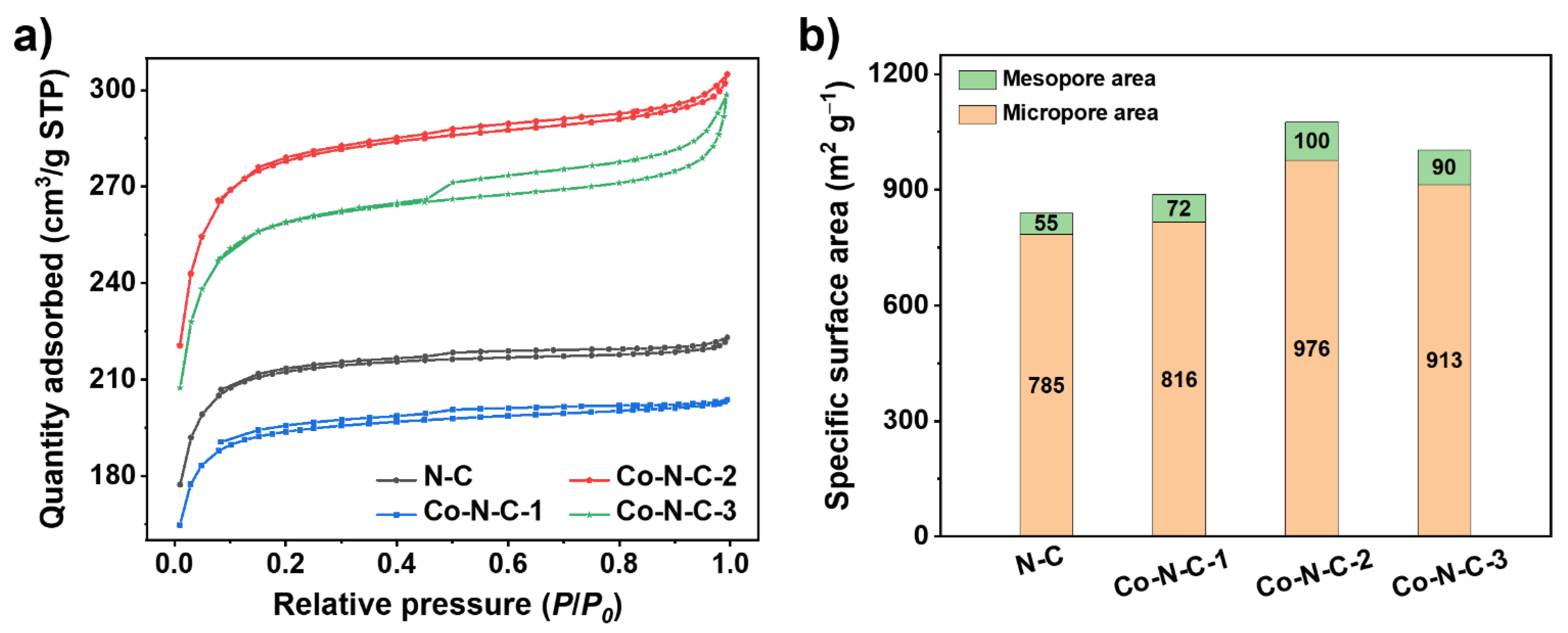
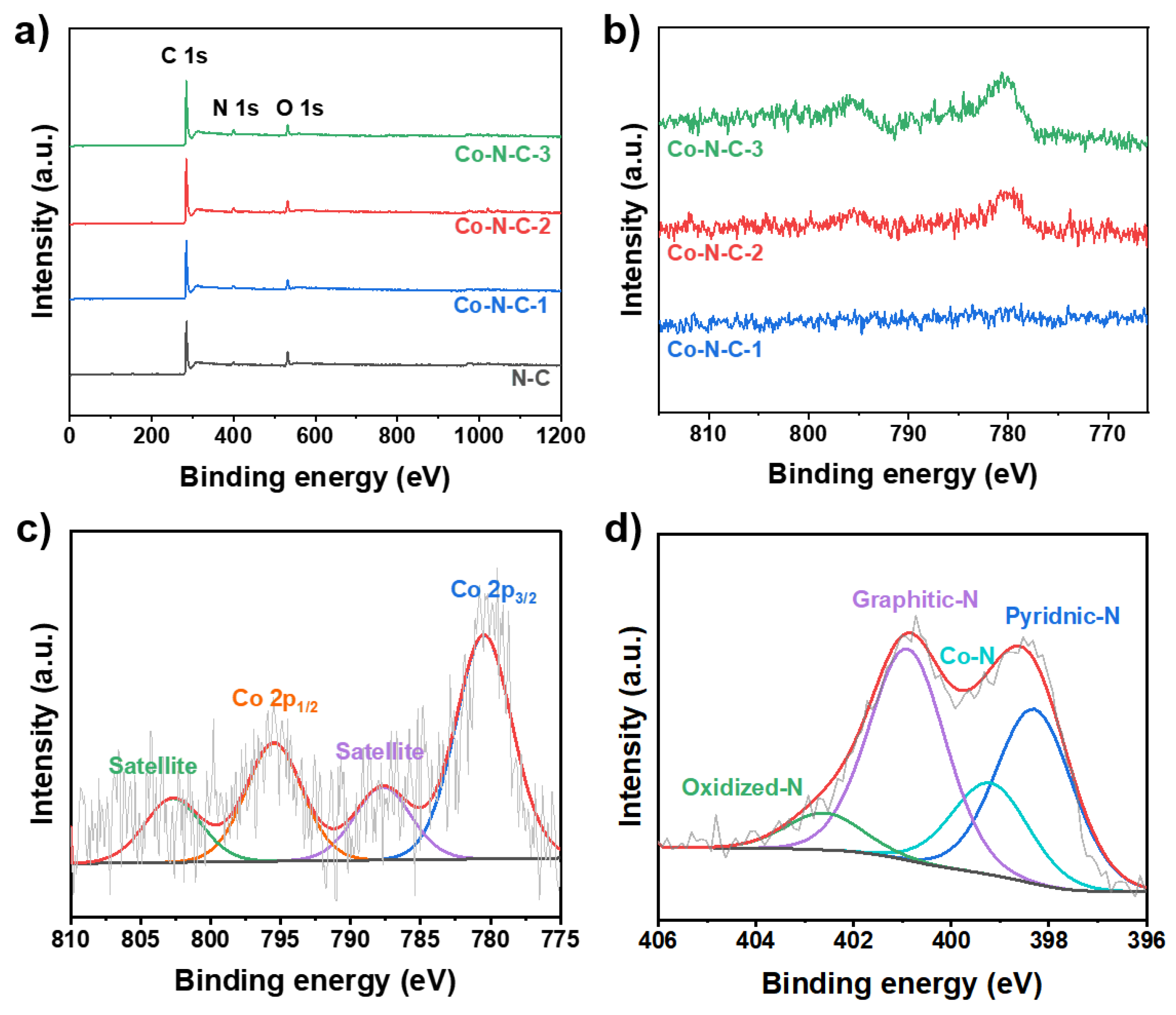
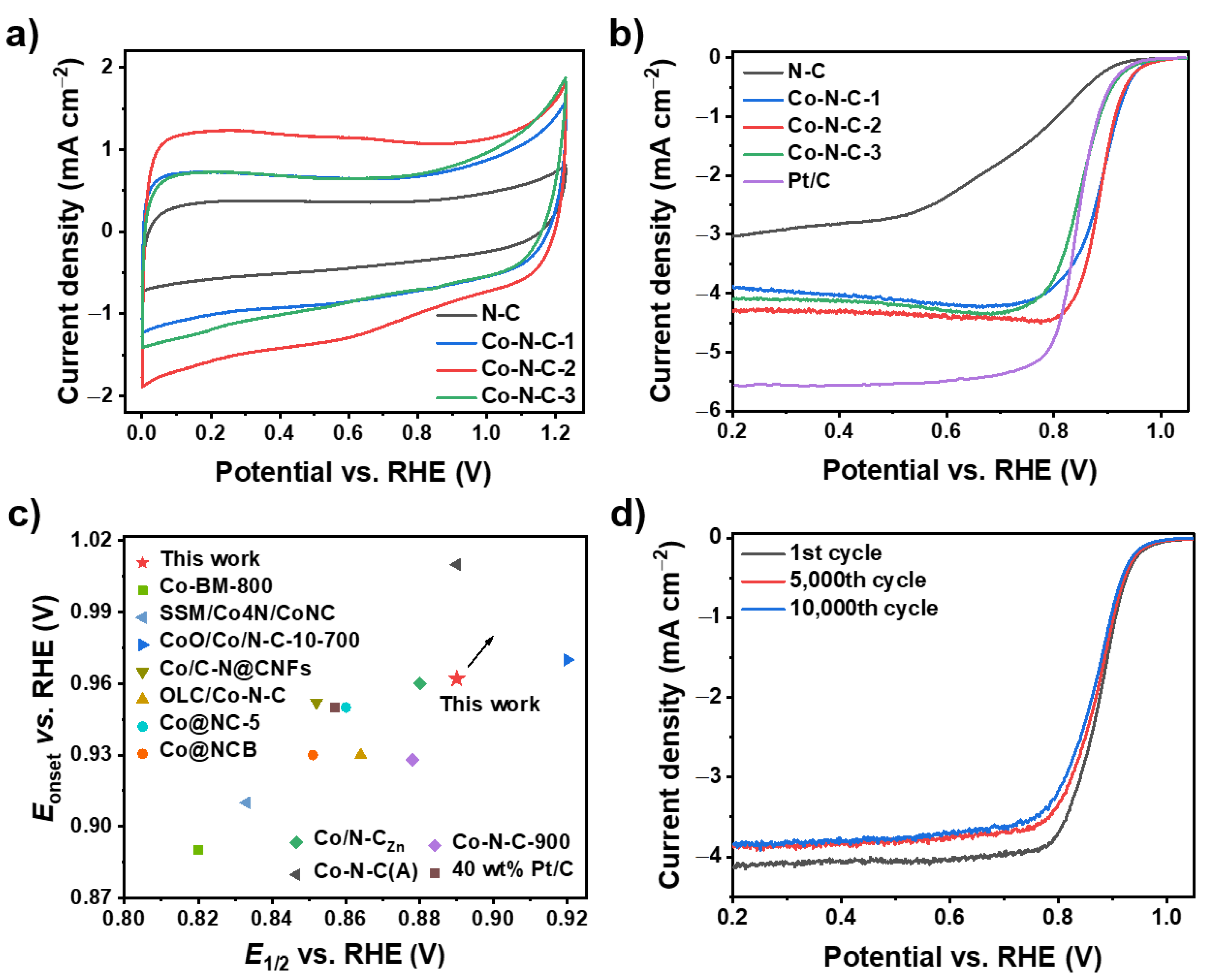
2. Results and Discussion
3. Materials and Methods
3.1. Material Synthesis
3.2. Physicochemical Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchison, P.; Rice, P.S.; Warburton, R.E.; Raugei, S.; Hammes-Schiffer, S. Multilevel Computational Studies Reveal the Importance of Axial Ligand for Oxygen Reduction Reaction on Fe-N-C Materials. J. Am. Chem. Soc. 2022, 144, 16524–16534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, X.; Xin, S.; Chen, S.; Bao, C.; Xu, W.; Xue, J.; Hui, B.; Zhang, J.; She, X.; et al. Enhanced Oxygen Reduction Reaction for Zn-Air Battery At Defective Carbon Fibers Derived From Seaweed Polysaccharide. Appl. Catal. B Environ. 2022, 301, 120785. [Google Scholar] [CrossRef]
- Xie, X.Y.; Shang, L.; Xiong, X.Y.; Shi, R.; Zhang, T.R. Fe Single-Atom Catalysts on MOF-5 Derived Carbon for Efficient Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102688. [Google Scholar] [CrossRef]
- Han, N.; Feng, S.; Guo, W.; Mora, O.M.; Zhao, X.; Zhang, W.; Xie, S.; Zhou, Z.; Liu, Z.; Liu, Q.; et al. Rational Design of Ruddlesden–Popper Perovskite Electrocatalyst for Oxygen Reduction to Hydrogen Peroxide. SusMat 2022, 2, 456–465. [Google Scholar] [CrossRef]
- Ruan, M.B.; Liu, J.; Song, P.; Xu, W.L. Meta-Analysis of Commercial Pt/C Measurements for Oxygen Reduction Reactions via Data Mining. Chin. J. Catal. 2022, 43, 116–121. [Google Scholar] [CrossRef]
- Lim, J.; Jung, C.; Hong, D.; Bak, J.; Shin, J.; Kim, M.; Song, D.; Lee, C.; Lim, J.; Lee, H.; et al. Atomically Ordered Pt3Mn Intermetallic Electrocatalysts for the Oxygen Reduction Reaction in Fuel Cells. J. Mater. Chem. A 2022, 10, 7399–7408. [Google Scholar] [CrossRef]
- Huang, S.Y.; Shan, A.X.; Wang, R.M. Low Pt Alloyed Nanostructures for Fuel Cells Catalysts. Catalysts 2018, 8, 538. [Google Scholar] [CrossRef]
- Peng, J.H.; Tao, P.; Song, C.Y.; Shang, W.; Deng, T.; Wu, J.B. Structural Evolution of Pt-based Oxygen Reduction Reaction Electrocatalysts. Chin. J. Catal. 2022, 43, 47–58. [Google Scholar] [CrossRef]
- Costa de Oliveira, M.A.; D’Epifanio, A.; Ohnuki, H.; Mecheri, B. Platinum Group Metal-Free Catalysts for Oxygen Reduction Reaction: Applications in Microbial Fuel Cells. Catalysts 2020, 10, 475. [Google Scholar] [CrossRef]
- Luo, E.; Chu, Y.; Liu, J.; Shi, Z.; Zhu, S.; Gong, L.; Ge, J.; Choi, C.H.; Liu, C.; Xing, W. Pyrolyzed M-Nx Catalysts for Oxygen Reduction Reaction: Progress and Prospects. Energ. Environ. Sci. 2021, 14, 2158–2185. [Google Scholar] [CrossRef]
- Cruz-Martinez, H.; Guerra-Cabrera, W.; Flores-Rojas, E.; Ruiz-Villalobos, D.; Rojas-Chavez, H.; Pena-Castaneda, Y.A.; Medina, D.I. Pt-Free Metal Nanocatalysts for the Oxygen Reduction Reaction Combining Experiment and Theory: An Overview. Molecules 2021, 26, 6689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Yang, H.B.; Liu, B. Coordination Engineering of Single-Atom Catalysts for the Oxygen Reduction Reaction: A Review. Adv. Energy Mater. 2021, 11, 2002473. [Google Scholar] [CrossRef]
- Cepitis, R.; Kongi, N.; Grozovski, V.; Ivanistsev, V.; Lust, E. Multifunctional Electrocatalysis on Single-Site Metal Catalysts: A Computational Perspective. Catalysts 2021, 11, 1165. [Google Scholar] [CrossRef]
- Shen, S.Q.; Sun, Y.Y.; Sun, H.; Pang, Y.P.; Xia, S.X.; Chen, T.Q.; Zheng, S.Y.; Yuan, T. Research Progress in ZIF-8 Derived Single Atomic Catalysts for Oxygen Reduction Reaction. Catalysts 2022, 12, 525. [Google Scholar] [CrossRef]
- Lv, H.W.; Guo, W.X.; Chen, M.; Zhou, H.; Wu, Y. Rational Construction of Thermally Stable Single Atom Catalysts: From Atomic Structure to Practical Applications. Chin. J. Catal. 2022, 43, 71–91. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Li, Y. Atom-Level Interfacial Synergy of Single-Atom Site Catalysts for Electrocatalysis. J. Energy Chem. 2022, 65, 103–115. [Google Scholar] [CrossRef]
- Wang, Y.; Su, H.; He, Y.; Li, L.; Zhu, S.; Shen, H.; Xie, P.; Fu, X.; Zhou, G.; Feng, C.; et al. Advanced Electrocatalysts with Single-Metal-Atom Active Sites. Chem. Rev. 2020, 120, 12217–12314. [Google Scholar] [CrossRef]
- He, Y.; Liu, S.; Priest, C.; Shi, Q.; Wu, G. Atomically Dispersed Metal-Nitrogen-Carbon Catalysts for Fuel Cells: Advances in Catalyst Design, Electrode Performance, and Durability Improvement. Chem. Soc. Rev. 2020, 49, 3484–3524. [Google Scholar] [CrossRef]
- Gao, C.; Mu, S.; Yan, R.; Chen, F.; Ma, T.; Cao, S.; Li, S.; Ma, L.; Wang, Y.; Cheng, C. Recent Advances in ZIF-Derived Atomic Metal-N-C Electrocatalysts for Oxygen Reduction Reaction: Synthetic Strategies, Active Centers, and Stabilities. Small 2022, 18, 2105409. [Google Scholar] [CrossRef]
- Li, J.C.; Maurya, S.; Kim, Y.S.; Li, T.; Wang, L.G.; Shi, Q.R.; Liu, D.; Feng, S.; Lin, Y.H.; Shao, M.H. Stabilizing Single-Atom Iron Electrocatalysts for Oxygen Reduction via Ceria Confining and Trapping. ACS Catal. 2020, 10, 2452–2458. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Song, H.Q.; Yu, J.K.; Chang, J.W.; Waterhouse, G.I.N.; Tang, Z.Y.; Yang, B.; Lu, S.Y. Carbon Dots-Derived Carbon Nanoflowers Decorated with Cobalt Single Atoms and Nanoparticles as Efficient Electrocatalysts for Oxygen Reduction. Chin. J. Catal. 2022, 43, 2443–2452. [Google Scholar] [CrossRef]
- Yang, L.; Xu, H.; Liu, H.; Zeng, X.; Cheng, D.; Huang, Y.; Zheng, L.; Cao, R.; Cao, D. Oxygen-Reconstituted Active Species of Single-Atom Cu Catalysts for Oxygen Reduction Reaction. Research 2020, 2020, 7593023. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.P.; Zhang, Y.P.; Tang, L.B.; Liu, C.Y.; Gao, X.H.; Sun, M.H.; Zheng, J.C.; Ling, M.; Liang, C.D.; Lin, Z. Platinum Single-Atom and Cluster Anchored on Functionalized MWCNTs with Ultrahigh Mass Efficiency for Electrocatalytic Hydrogen Evolution. Nano Energy 2019, 63, 103849. [Google Scholar] [CrossRef]
- Xu, D.; Wang, S.; Wu, B.; Zhang, B.; Qin, Y.; Huo, C.; Huang, L.; Wen, X.; Yang, Y.; Li, Y. Highly Dispersed Single-Atom Pt and Pt Clusters in the Fe-Modified KL Zeolite with Enhanced Selectivity for n-Heptane Aromatization. ACS Appl. Mater. Interfaces 2019, 11, 29858–29867. [Google Scholar] [CrossRef]
- Hu, C.; Song, E.; Wang, M.; Chen, W.; Huang, F.; Feng, Z.; Liu, J.; Wang, J. Partial-Single-Atom, Partial-Nanoparticle Composites Enhance Water Dissociation for Hydrogen Evolution. Adv. Sci. 2021, 8, 2001881. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, Y.; Tang, C.; Chen, Y.; Qian, B.; Hu, Z.; Chang, Y.C.; Pao, C.W.; Lin, Q.; Kazemi, S.A.; et al. High-Efficiency Electrosynthesis of Hydrogen Peroxide from Oxygen Reduction Enabled by a Tungsten Single Atom Catalyst with Unique Terdentate N1O2 Coordination. Adv. Funct. Mater. 2021, 32, 2110224. [Google Scholar] [CrossRef]
- Zhang, H.G.; Chung, H.T.; Cullen, D.A.; Wagner, S.; Kramm, U.I.; More, K.L.; Zelenay, P.; Wu, G. High-Performance Fuel Cell Cathodes Exclusively Containing Atomically Dispersed Iron Active Sites. Energ. Environ. Sci. 2019, 12, 2548–2558. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Tobbala, D.E.; El-Bahy, S.M.; Nassan, M.A.; Salama, R.S. The Role of Phosphotungstic Acid in Enhancing the Catalytic Performance of UiO-66 (Zr) and its Applications as an Efficient Solid Acid Catalyst for Coumarins and Dihydropyrimidinones Synthesis. Catal. Commun. 2022, 169, 106479. [Google Scholar] [CrossRef]
- Altass, H.M.; Ahmed, S.A.; Salama, R.S.; Moussa, Z.; Jassas, R.S.; Alsantali, R.I.; Al-Rooqi, M.M.; Ibrahim, A.A.; Khder, M.A.; Morad, M.; et al. Low Temperature CO Oxidation Over Highly Active Gold Nanoparticles Supported on Reduced Graphene Oxide@Mg-BTC Nanocomposite. Catal. Lett. 2022. [Google Scholar] [CrossRef]
- Al-Thabaiti, S.A.; Mostafa, M.M.M.; Ahmed, A.I.; Salama, R.S. Synthesis of Copper/Chromium Metal Organic Frameworks—Derivatives as an Advanced Electrode Material for High-Performance Supercapacitors. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Li, Z.Q.; Gao, R.; Feng, M.; Deng, Y.P.; Xiao, D.J.; Zheng, Y.; Zhao, Z.; Luo, D.; Liu, Y.L.; Zhang, Z.; et al. Modulating Metal-Organic Frameworks as Advanced Oxygen Electrocatalysts. Adv. Energy Mater. 2021, 11, 2003291. [Google Scholar] [CrossRef]
- Song, Z.X.; Zhang, L.; Doyle-Davis, K.; Fu, X.Z.; Luo, J.L.; Sun, X.L. Recent Advances in MOF-Derived Single Atom Catalysts for Electrochemical Applications. Adv. Energy Mater. 2020, 10, 2001561. [Google Scholar] [CrossRef]
- Sun, W.; Du, L.; Tan, Q.; Zhou, J.; Hu, Y.; Du, C.; Gao, Y.; Yin, G. Engineering of Nitrogen Coordinated Single Cobalt Atom Moieties for Oxygen Electroreduction. ACS Appl. Mater. Interfaces 2019, 11, 41258–41266. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption Of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Meng, H.; Liu, Y.; Liu, H.; Pei, S.; Yuan, X.; Li, H.; Zhang, Y. ZIF67@MFC-Derived Co/N-C@CNFs Interconnected Frameworks with Graphitic Carbon-Encapsulated Co Nanoparticles as Highly Stable and Efficient Electrocatalysts for Oxygen Reduction Reactions. ACS Appl. Mater. Interfaces 2020, 12, 41580–41589. [Google Scholar] [CrossRef]
- Zhao, S.L.; Yang, J.; Han, M.; Wang, X.M.; Lin, Y.; Yang, R.; Xu, D.D.; Shi, N.E.; Wang, Q.; Yang, M.J.; et al. Synergistically Enhanced Oxygen Reduction Electrocatalysis by Atomically Dispersed and Nanoscaled Co Species in Three-Dimensional Mesoporous Co, N-codoped Carbon Nanosheets Network. Appl. Catal. B Environ. 2020, 260, 118207. [Google Scholar] [CrossRef]
- Yue, C.; Zhang, N.; Zhu, Z.; Chen, P.; Meng, F.; Liu, X.; Wei, X.; Liu, J. Multi-Strategy Architecture of High-Efficiency Electrocatalysts for Underwater Zn-H2O2 Batteries with Superior Power Density of 442 mW cm−2. Small 2022, 18, 2106532. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, P.P.; Zhou, Q.; Li, B.; Wei, Y.G.; Gong, T.; Huang, N.; Lv, X.W.; Fang, L.; Sun, X.H. Enhanced Oxygen Reduction and Evolution in N-Doped Carbon Anchored with Co Nanoparticles for Rechargeable Zn-Air Batteries. Appl. Surf. Sci. 2021, 542, 148700. [Google Scholar] [CrossRef]
- Cheng, X.; Yang, J.; Yan, W.; Han, Y.; Qu, X.; Yin, S.; Chen, C.; Ji, R.; Li, Y.; Li, G.; et al. Nano-Geometric Deformation and Synergistic Co Nanoparticles-Co-N4 Composite Sites for Proton Exchange Membrane Fuel Cells. Energy Environ. Sci. 2021, 14, 5958–5967. [Google Scholar] [CrossRef]
- Dai, Y.X.; Xin, W.L.; Xu, L.H.; Li, J.; Li, Y.X.; Li, J.; Cosnier, S.; Zhang, X.J.; Marks, R.S.; Shan, D. 2-Methylimidazole-tuned “4-Self” strategy based on benzimidazole-5-carboxylate for boosting oxygen reduction electrocatalysis. Appl. Surf. Sci. 2022, 591, 153066. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, S.; Wang, Y.; Yu, J.; Dai, Y.; Wang, J.; Sun, X.; Liu, K.; Ni, M. In Situ Anchoring Co-N-C Nanoparticles on Co4N Nanosheets toward Ultrastable Flexible Self-Supported Bifunctional Oxygen Electrocatalyst Enables Recyclable Zn-Air Batteries Over 10000 Cycles and Fast Charging. Small 2022, 18, 2105887. [Google Scholar] [CrossRef] [PubMed]
- Du, H.M.; Ding, F.F.; Gu, L.; Zhao, J.S.; Zhang, X.X.; Qu, K.G.; Li, Y.W.; Lan, T.Y.; Li, J.; Zhang, Y.; et al. CoO/Co/N-C Nanoparticles Embedded in Carbon as Mediate for Oxygen Reduction Electrocatalysts. J. Alloy. Compd. 2021, 885, 161174. [Google Scholar] [CrossRef]
- Liang, Z.; Kong, N.; Yang, C.; Zhang, W.; Zheng, H.; Lin, H.; Cao, R. Highly Curved Nanostructure-Coated Co, N-Doped Carbon Materials for Oxygen Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 12759–12764. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.Y.; Xiao, Y.Y.; Xi, W.H.; Lin, X.F.; Gao, B.F.; Chen, Y.L.; Zheng, Y.; Lin, B.Z. Cobalt-Nanoparticle Impregnated Nitrogen-Doped Porous Carbon Derived from Schiff-Base Polymer as Excellent Bifunctional Oxygen Electrocatalysts for Rechargeable Zinc-Air Batteries. J. Power Sources 2021, 490, 229570. [Google Scholar] [CrossRef]
- Zhong, H.; Shi, C.; Li, J.; Yu, R.; Yu, Q.; Liu, H.; Yao, Y.; Wu, J.; Zhou, L.; Mai, L. Cobalt Decorated Nitrogen-Doped Carbon Bowls as Efficient Electrocatalysts for the Oxygen Reduction Reaction. Chem. Commun. 2020, 56, 4488–4491. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Hu, Y.; Wang, Y.; Lin, X.; Hu, K.; Lin, X.; Xie, G.; Liu, X.; Reddy, K.M.; Yuan, Q.; et al. MOF Structure Engineering to Synthesize CoNC Catalyst with Richer Accessible Active Sites for Enhanced Oxygen Reduction. Small 2021, 17, 2104684. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Xu, B.Z.; Fu, S.; Wan, G.; Yang, C.; Yao, S.; Song, J.; Zhou, H.; Du, D.; et al. Hierarchically Porous M-N-C (M = Co and Fe) Single-Atom Electrocatalysts with Robust MNx Active Moieties Enable Enhanced ORR Performance. Adv. Energy Mater. 2018, 8, 1801956. [Google Scholar] [CrossRef]
- Leenders, S.H.; Becker, R.; Kumpulainen, T.; de Bruin, B.; Sawada, T.; Kato, T.; Fujita, M.; Reek, J.N. Selective Co-Encapsulation Inside an M6L4 Cage. Chem. Eur. J. 2016, 22, 15468–15474. [Google Scholar] [CrossRef]
- Kawano, M.; Haneda, T.; Hashizume, D.; Izumi, F.; Fujita, M. A Selective Instant Synthesis of a Coordination Network and Its Ab Initio Powder Structure Determination. Angew. Chem. Int. Ed. 2008, 47, 1269–1271. [Google Scholar] [CrossRef]
- Xiang, X.; Zhang, X.R.; Yan, B.W.; Wang, K.; Wang, Y.Q.; Lyu, D.D.; Xi, S.B.; Tian, Z.Q.; Shen, P.K. Atomic Iron Coordinated by Nitrogen Doped Carbon Nanoparticles Synthesized Via a Synchronous Complexation-Polymerization Strategy as Efficient Oxygen Reduction Reaction Electrocatalysts for Zinc-Air Battery and Fuel Cell Application. Chem. Eng. J. 2022, 440, 135721. [Google Scholar] [CrossRef]
- Hong, Y.; Li, L.; Huang, B.; Tang, X.; Zhai, W.; Hu, T.; Yuan, K.; Chen, Y. Deciphering the Precursor–Performance Relationship of Single-Atom Iron Oxygen Electroreduction Catalysts via Isomer Engineering. Small 2022, 18, 2106122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, X.; Tang, Z.; Peng, W.; Zhang, J.; Tong, Y.; Li, J.; Zhang, J. Facile Synthesis of Hemin-based Fe-N-C Catalyst by MgAl-LDH Confinement Effect for Oxygen Reduction Reaction. Appl. Surf. Sci. 2022, 573, 151505. [Google Scholar] [CrossRef]
- Wan, X.H.; Guo, X.M.; Duan, M.T.; Shi, J.; Liu, S.J.; Zhang, J.H.; Liu, Y.J.; Zheng, X.J.; Kong, Q.H. Ultrafine CoO Nanoparticles and Co-N-C Lamellae Supported on Mesoporous Carbon for Efficient Electrocatalysis of Oxygen Reduction in Zinc-Air Batteries. Electrochim. Acta 2021, 394, 139135. [Google Scholar] [CrossRef]
- Zhou, W.H.; Li, Y.; Zheng, L.C.; Liu, J.; Tang, R.R.; Shi, K.J.; Zhang, Y.Y. Three-Dimensional MOF-Derived Co and N Co-Doped Porous Carbon Bifunctional Catalyst for the Zn–Air Battery. CrystEngComm 2021, 23, 4930. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Xu, D.W.; Wang, Y.F.; Li, X.H.; Chen, Z.B.; Li, K.; Li, Z.F.; Zheng, L.R.; Zuo, X. Single Atoms Anchored on Cobalt-Based Catalysts Derived from Hydrogels Containing Phthalocyanine toward the Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2020, 8, 8338–8347. [Google Scholar] [CrossRef]
- Ha, Y.; Fei, B.; Yan, X.X.; Xu, H.B.; Chen, Z.L.; Shi, L.X.; Fu, M.S.; Xu, W.; Wu, R.B. Atomically Dispersed Co-Pyridinic N-C for Superior Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10, 2002592. [Google Scholar] [CrossRef]
- Yi, J.D.; Xu, R.; Chai, G.L.; Zhang, T.; Zang, K.T.; Nan, B.; Lin, H.; Liang, Y.L.; Lv, J.Q.; Luo, J.; et al. Cobalt Single-Atoms Anchored on Porphyrinic Triazine-based Frameworks as Bifunctional Electrocatalysts for Oxygen Reduction and Hydrogen Evolution Reactions. J Mater. Chem. A 2019, 7, 1252–1259. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-Derived Co@N-C Bifunctional Catalysts for Highly Efficient Zn-Air Batteries and Water Splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, D.; Liao, L.; Li, M.; Yin, Y. Metal-Organic Framework-Derived Atomically Dispersed Co-N-C Electrocatalyst for Efficient Oxygen Reduction Reaction. Catalysts 2022, 12, 1462. https://doi.org/10.3390/catal12111462
Ge D, Liao L, Li M, Yin Y. Metal-Organic Framework-Derived Atomically Dispersed Co-N-C Electrocatalyst for Efficient Oxygen Reduction Reaction. Catalysts. 2022; 12(11):1462. https://doi.org/10.3390/catal12111462
Chicago/Turabian StyleGe, Dongqi, Longfei Liao, Mingyu Li, and Yongli Yin. 2022. "Metal-Organic Framework-Derived Atomically Dispersed Co-N-C Electrocatalyst for Efficient Oxygen Reduction Reaction" Catalysts 12, no. 11: 1462. https://doi.org/10.3390/catal12111462
APA StyleGe, D., Liao, L., Li, M., & Yin, Y. (2022). Metal-Organic Framework-Derived Atomically Dispersed Co-N-C Electrocatalyst for Efficient Oxygen Reduction Reaction. Catalysts, 12(11), 1462. https://doi.org/10.3390/catal12111462






