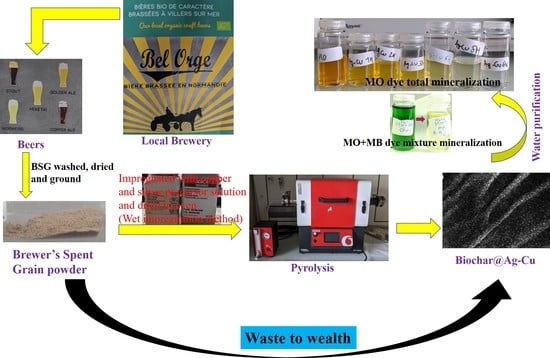
Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture
Abstract
:1. Introduction
2. Results and Discussion
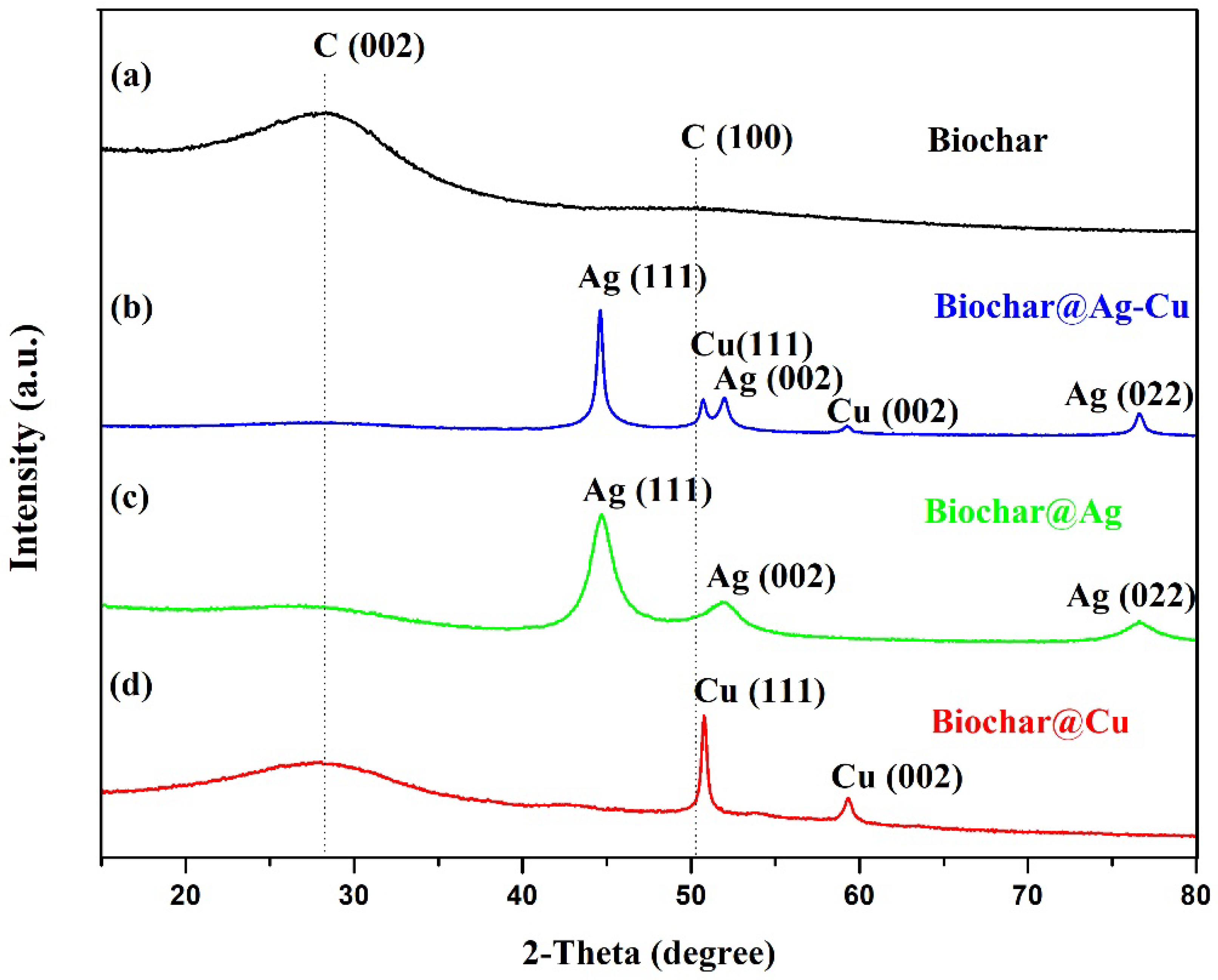
2.1. Crystallinity of the Material
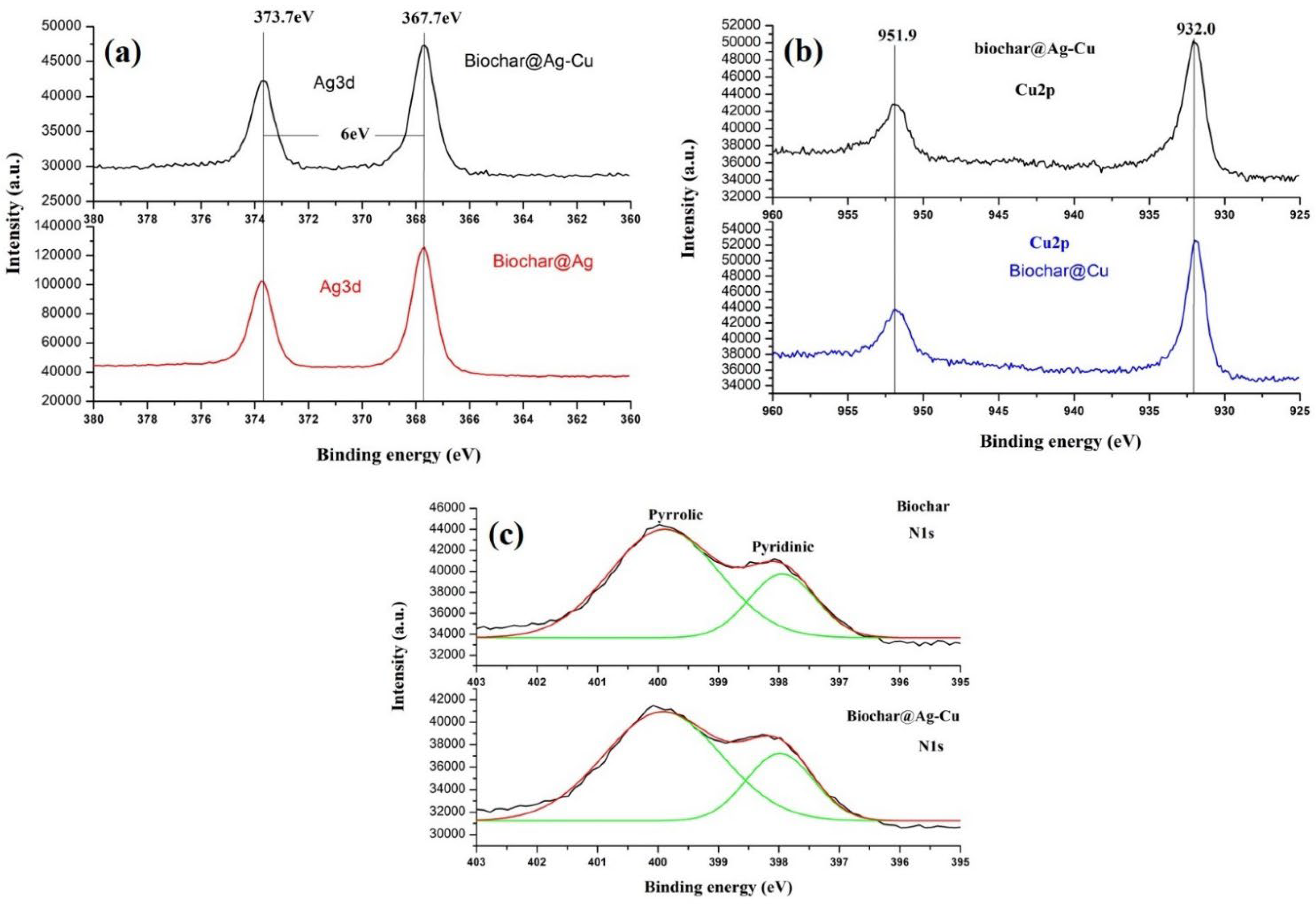
2.2. Surface Chemical Composition of the Catalyst
2.3. Surface Morphology of the Material and Bulk Composition
2.4. Study of the Degree of Graphitization
2.5. Thermal Stability
2.6. Dye Degradation
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus
3.3. Synthesis of Ag-Cu-Impregnated Biochar Catalyst
3.4. Dye Degradation Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meunier, V.; Ania, C.; Bianco, A.; Chen, Y.; Choi, G.B.; Kim, Y.A.; Koratkar, N.; Liu, C.; Tascon, J.M.D.; Terrones, M. Carbon science perspective in 2022: Current research and future challenges. Carbon N. Y. 2022, 195, 272–291. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Ugarte, D. Curling and closure of graphitic networks under electron-beam irradiation. Nature 1992, 359, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Monisha, B.; Kumar, P.S.; Gayathri, K.V. Carbon nanomaterials and its applications in pharmaceuticals: A brief review. Chemosphere 2022, 294, 133731. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.; Daverey, A.; Dutta, K.; Yata, V.K.; Arunachalam, K. Valorization of waste pine needle biomass into biosorbents for the removal of methylene blue dye from water: Kinetics, equilibrium and thermodynamics study. Environ. Technol. Innov. 2022, 25, 102200. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Fiorenza, R.; Jlassi, K.; Mekhalif, Z.; Ali, A.M.A.; Chehimi, M.M. The emerging role of biochar in the carbon materials family for hydrogen production. Chem. Eng. Res. Des. 2022, 188, 209–228. [Google Scholar] [CrossRef]
- Azan, N.A.A.M.; Sagadevan, S.; Mohamed, A.R.; Azazi, A.H.N.; Suah, F.B.M.; Kobayashi, T.; Adnan, R.; Kaus, N.H.M. Solar Light-Induced Photocatalytic Degradation of Ciprofloxacin Antibiotic Using Biochar Supported Nano Bismuth Ferrite Composite. Catalyst 2022, 12, 1269. [Google Scholar] [CrossRef]
- Ioannidi, A.A.; Vakros, J.; Frontistis, Z.; Mantzavinos, D. Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalyst 2022, 12, 1245. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O.; Onifade, D.V. Biochar from the thermochemical conversion of Orange (Citrus sinensis) peel and albedo: Product quality and potential applications. Chem. Africa 2020, 3, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Das, R.; Panda, S.N. Preparation and applications of biochar based nanocomposite: A review. J. Anal. Appl. Pyrolysis 2022, 167, 105691. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Ali, Y.A.E.H.; Ahrouch, M.; Lahcen, A.A.; Abdellaoui, Y.; Stitou, M. Recent Advances and Prospects of Biochar-based Adsorbents for Malachite Green Removal: A Comprehensive Review. Chem. Africa 2022. [Google Scholar] [CrossRef]
- Greenough, S.; Dumont, M.-J.; Prasher, S. The physicochemical properties of biochar and its applicability as a filler in rubber composites: A review. Mater. Today Commun. 2021, 29, 102912. [Google Scholar] [CrossRef]
- Aramrueang, N.; Zhang, R.; Liu, X. Application of biochar and alkalis for recovery of sour anaerobic digesters. J. Environ. Manag. 2022, 307, 114538. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M. Development of Kenaf Biochar in Engineering and Agricultural Applications. Chem. Africa 2022, 5, 1–17. [Google Scholar] [CrossRef]
- Kumar K, A.; Shobham; Panwar, J.; Gupta, S. One-pot synthesis of metal oxide-clay composite for the evaluation of dye removal studies: Taguchi optimization of parameters and environmental toxicity studies. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Shrestha, P.; Jha, M.K.; Ghimire, J.; Koirala, A.R.; Shrestha, R.M.; Sharma, R.K.; Pant, B.; Park, M.; Pant, H.R. Decoration of Zinc Oxide Nanorods into the Surface of Activated Carbon Obtained from Agricultural Waste for Effective Removal of Methylene Blue Dye. Materials 2020, 13, 5667. [Google Scholar] [CrossRef]
- Blue, M.; Orange, M. Critical Review on the Photodegradation Ability of Graphene and its Derivatives against Malachite Green, Methylene Blue, and Methyl Orange. Lett. Appl. NanoBioScience 2023, 12, 1–30. [Google Scholar] [CrossRef]
- Kotp, Y.H. Fabrication of cerium titanate cellulose fiber nanocomposite materials for the removal of methyl orange and methylene blue from polluted water by photocatalytic degradation. Environ. Sci. Pollut. Res. 2022, 29, 81583–81608. [Google Scholar] [CrossRef] [PubMed]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, N.F.A.; Him, N.R.N.; Lau, W.J. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of methylene blue on kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Benjelloun, M.; Miyah, Y.; Bouslamti, R.; Nahali, L.; Mejbar, F.; Lairini, S. The Fast-Efficient Adsorption Process of the Toxic Dye onto Shells Powders of Walnut and Peanut: Experiments, Equilibrium, Thermodynamic, and Regeneration Studies. Chem. Africa 2022, 5, 375–393. [Google Scholar] [CrossRef]
- Dabagh, A.; Bagui, A.; Abali, M.; Aziam, R.; Chiban, M.; Sinan, F.; Zerbet, M. Increasing the Adsorption Efficiency of Methylene Blue by Acid Treatment of the Plant Carpobrotus edulis. Chem. Africa 2021, 4, 585–598. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Zarin, K.; Shahzad, K.; Javed, M.S.; Jamshaid, M.; Bashir, M.A.; Shah, S.S.A.; Rehman, A.U. Enhanced adsorption removal of methyl orange from water by porous bimetallic Ni/Co MOF composite: A systematic study of adsorption kinetics. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Rigueto, C.V.T.; Alessandretti, I.; da Silva, D.H.; Rosseto, M.; Loss, R.A.; Geraldi, C.A.Q. Agroindustrial Wastes of Banana Pseudo-stem as Adsorbent of Textile Dye: Characterization, Kinetic, and Equilibrium Studies. Chem. Africa 2021, 4, 1069–1078. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Marzouki, R.; Brahmia, A.; Onwudiwe, D.C. Synthesis of AgBiS2/gC3N4 and its application in the photocatalytic reduction of Pb (II) in the matrix of methyl orange, crystal violet, and methylene blue dyes. Ceram. Int. 2022, in press. [Google Scholar] [CrossRef]
- Stanley, R.; Jebasingh, J.A.; Vidyavathy, S.M.; Stanley, P.K.; Ponmani, P.; Shekinah, M.E.; Vasanthi, J. Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik 2021, 231, 166518. [Google Scholar] [CrossRef]
- Luo, X.; Liang, C.; Hu, Y. Comparison of Different Enhanced Coagulation Methods for Azo Dye Removal from Wastewater. Sustainability 2019, 11, 4760. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Goswami, N.; Dhak, D. Photocatalytic Remediation of Industrial Dye Waste Streams Using Biochar and Metal-Biochar Hybrids: A Critical Review. Chem. Africa 2022. [Google Scholar] [CrossRef]
- Kgatle, M.; Sikhwivhilu, K.; Ndlovu, G.; Moloto, N. Degradation Kinetics of Methyl Orange Dye in Water Using Trimetallic Fe/Cu/Ag Nanoparticles. Catalysts 2021, 11, 428. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Sires, I.; Martínez-Huitle, C.A.; Brillas, E. Total mineralization of mixtures of Tartrazine, Ponceau SS and Direct Blue 71 azo dyes by solar photoelectro-Fenton in pre-pilot plant. Chemosphere 2018, 210, 1137–1144. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Krishnan, S.; Hii, Y.S.; Pan, S.; Chan, Y.S.; Acquah, C.; Danquah, M.K.; Rodrigues, J. Synthesis approach-dependent antiviral properties of silver nanoparticles and nanocomposites. J. Nanostruct. Chem. 2022, 12, 809–831. [Google Scholar] [CrossRef]
- Snoussi, Y.; Sifaoui, I.; Khalil, A.M.; Bhakta, A.K.; Semyonov, O.; Postnikov, P.S.; Michely, L.; Pires, R.; Bastide, S.; Barroso, J.E.-P.; et al. Facile synthesis of silver decorated biochar as a novel and highly active biosourced anti-kinetoplastid agent. Mater. Today Commun. 2022, 32, 104126. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Yudintceva, N.; Shevtsov, M.; Rudakova, A.; Kalganov, V.; Tolmachev, V.; Zharova, Y.; Lutakov, O.; Kraeva, L.; et al. Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition. J. Funct. Biomater. 2022, 13, 62. [Google Scholar] [CrossRef]
- Mahmood, K.; Amara, U.; Siddique, S.; Usman, M.; Peng, Q.; Khalid, M.; Hussain, A.; Ajmal, M.; Ahmad, A.; Sumrra, S.H.; et al. Green synthesis of Ag@CdO nanocomposite and their application towards brilliant green dye degradation from wastewater. J. Nanostruct. Chem. 2022, 12, 329–341. [Google Scholar] [CrossRef]
- Shahzad, K.; Hussain, S.; Nazir, M.A.; Jamshaid, M.; ur Rehman, A.; Alkorbi, A.S.; Alsaiari, R.; Alhemiary, N.A. Versatile Ag2O and ZnO nanomaterials fabricated via annealed Ag-PMOS and ZnO-PMOS: An efficient photocatalysis tool for azo dyes. J. Mol. Liq. 2022, 356, 119036. [Google Scholar] [CrossRef]
- Ameena, S.; Rajesh, N.; Anjum, S.M.; Khadri, H.; Riazunnisa, K.; Mohammed, A.; Kari, Z.A. Antioxidant, Antibacterial, and Anti-diabetic Activity of Green Synthesized Copper Nanoparticles of Cocculus hirsutus (Menispermaceae). Appl. Biochem. Biotechnol. 2022, 194, 4424–4438. [Google Scholar] [CrossRef]
- Zhu, G.; Jin, Y.; Ge, M. Simple and green method for preparing copper nanoparticles supported on carbonized cotton as a heterogeneous Fenton-like catalyst. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 647, 128978. [Google Scholar] [CrossRef]
- Omiri, J.; Snoussi, Y.; Bhakta, A.K.; Truong, S.; Ammar, S.; Khalil, A.M.; Jouini, M.; Chehimi, M.M. Citric-Acid-Assisted Preparation of Biochar Loaded with Copper / Nickel Bimetallic Nanoparticles for Dye Degradation. Colloids Interfaces 2022, 6, 18. [Google Scholar] [CrossRef]
- Arasi, K.; Arasu, S.; Raja, A.G.; Rajaram, R. Photocatalytic Degradation of Effluent water by CuO @ Ag Core—Shell Nanoparticles as Effective Catalyst under Irradiation of UV-light. Mater. Today Proc. 2022, 68, 556–563. [Google Scholar] [CrossRef]
- Tantawy, H.R.; Nada, A.A.; Baraka, A.; Elsayed, M.A. Novel synthesis of bimetallic Ag-Cu nanocatalysts for rapid oxidative and reductive degradation of anionic and cationic dyes. Appl. Surf. Sci. Adv. 2021, 3, 100056. [Google Scholar] [CrossRef]
- Passi, M.; Pal, B. Influence of Ag/Cu photodeposition on CaTiO3 photocatalytic activity for degradation of Rhodamine B dye. Korean J. Chem. Eng. 2022, 39, 942–953. [Google Scholar] [CrossRef]
- Dong, S.; Lian, X.; Chen, S.; Li, H.; Liu, E.; Xu, K. Kinetic analysis and mechanism study on the photocatalytic degradation of 2,4-dinitrophenylhydrazine over surface plasmonic Ag/Cu/TiO2 composite. React. Kinet. Mech. Catal. 2021, 134, 485–499. [Google Scholar] [CrossRef]
- Singh, E.; Mishra, R.; Kumar, A.; Shukla, S.K.; Lo, S.-L.; Kumar, S. Circular economy-based environmental management using biochar: Driving towards sustainability. Process Saf. Environ. Prot. 2022, 163, 585–600. [Google Scholar] [CrossRef]
- Bolwig, S.; Mark, M.S.; Happel, M.K.; Brekke, A. Beyond animal feed? The valorisation of brewers’ spent grain. In From Waste to Value; Taylor & Francis: Abingdon, UK, 2019; pp. 107–121. [Google Scholar]
- Sieradzka, M.; Kirczuk, C.; Kalemba-rec, I.; Mlonka-m, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Gupta, R.; Pandit, C.; Pandit, S.; Gupta, P.K.; Lahiri, D.; Agarwal, D.; Pandey, S. Potential and future prospects of biochar-based materials and their applications in removal of organic contaminants from industrial wastewater. J. Mater. Cycles Waste Manag. 2022, 24, 852–876. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of peroxymonosulfate by biochar-based catalysts and applications in the degradation of organic contaminants: A review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Snoussi, Y.; Sifaoui, I.; El Garah, M.; Khalil, A.M.; Barroso, J.E.; Jouini, M.; Ammar, S.; Lorenzo-Morales, J.; Chehimi, M.M. Green, zero-waste pathway to fabricate supported nanocatalysts and anti-kinetoplastid agents from sugarcane bagasse. Waste Manag. 2023, 155, 179–191. [Google Scholar] [CrossRef]
- Snoussi, Y.; El Garah, M.; Khalil, A.M.; Chehimi, M.M. Immobilization of biogenic silver-copper nanoparticles over arylated biochar from sugarcane bagasse: Method and catalytic performance. Appl. Organomet. Chem. 2022, 36, e6885. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; Eltaweil, A.S. Phytofabrication of bimetallic silver-copper / biochar nanocomposite for environmental and medical applications. J. Environ. Manag. 2022, 316, 115238. [Google Scholar] [CrossRef]
- Wang, L.; Mao, H.; Li, Z.; Wang, C.; Gao, D. Immobilizing Ag / Cu2O on cotton fabric to enhance visible light photocatalytic activity. New J. Chem. 2020, 44, 20759–20769. [Google Scholar] [CrossRef]
- Tian, K.; Liu, W.; Zhang, S.; Jiang, H. One-pot synthesis of a carbon supported bimetallic Cu–Ag NPs catalyst for robust catalytic hydroxylation of benzene to phenol by fast pyrolysis of biomass waste. Green Chem. 2016, 18, 5643–5650. [Google Scholar] [CrossRef]
- Al-qaradawi, S.; Salman, S.R. Photocatalytic degradation of methyl orange as a model compound. J. Photochem. Photobiol. A Chem. 2002, 148, 161–168. [Google Scholar] [CrossRef]
- Ho, L.; Alonso, A.; Eurie Forio, M.A.; Vanclooster, M.; Goethals, P.L.M. Water research in support of the Sustainable Development Goal 6: A case study in Belgium. J. Clean. Prod. 2020, 277, 124082. [Google Scholar] [CrossRef]
- Tokola, E.; Kantola, M. Annales Academiae Scientiarum Fennicae. Ser. A6 Phys. 1967, 223, 1–10. [Google Scholar]
- Vegard, L. Vega. Phys. Rev. 1922, 20, 424–432. [Google Scholar]
- Weibo, L.; Yinghong, Z.; Junqin, L. No Title. J. Alloys Compd. 1993, 191, 187–189. [Google Scholar]
- Maria Ferraria, A.; Patrícia Carapeto, A.; do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interface Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Applied Surface Science Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Hart, B.R.; Polack, R.; Kobe, B.A.; Smart, R.S.C. Analysis of mineral surface chemistry in flotation separation using imaging XPS. Miner. Eng. 2007, 20, 152–162. [Google Scholar] [CrossRef]
- Almheiri, S.; Ahmad, A.A.L.; Le Droumaguet, B.; Pires, R.; Mohamed, A.A.; Chehimi, M.M. Development of Latent Fingerprints via Aryldiazonium Tetrachloroaurate Salts on Copper Surfaces: An XPS Study. Langmuir 2020, 36, 74–83. [Google Scholar] [CrossRef]
- Kabir, S.; Artyushkova, K.; Serov, A.; Kiefer, B.; Atanassov, P. Binding energy shifts for nitrogen-containing graphene-based electrocatalysts—Experiments and DFT calculations. Surf. Interface Anal. 2016, 48, 293–300. [Google Scholar] [CrossRef]
- Saad, A.G.; Gebreil, A.; Kospa, D.A.; El-Hakam, S.A.; Ibrahim, A.A. Integrated solar seawater desalination and power generation via plasmonic sawdust-derived biochar: Waste to wealth. Desalination 2022, 535, 115824. [Google Scholar] [CrossRef]
- Yin, C.; Khan, A.; Gao, Q.; Li, Q.; Zhou, X.; Liu, X.; Xu, A.; Li, X. Synergistic activation of peroxymonosulfate for efficient aqueous p -nitrophenol degradation with Cu (II) and Ag (I) in Ag2Cu2O3. Sep. Purif. Technol. 2022, 291, 120934. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C atomic ratio as a smart linkage between pyrolytic temperatures, aromatic clusters and sorption properties of biochars derived from diverse precursory materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef] [Green Version]
- Fulong, D.; Yongrning, X. Nano-moire method. ACTA Mech. Sin. 1999, 15, 283–288. [Google Scholar] [CrossRef]
- Dou, Q.; Li, Y.; Wong, K.W.; Ng, K.M. Facile synthesis of nearly monodisperse AgCu alloy nanoparticles with synergistic effect against oxidation and electromigration. J. Mater. Res. 2019, 34, 2095–2104. [Google Scholar] [CrossRef]
- Fodjo, E.K.; Canlier, A.; Kong, C.; Yurtsever, A.; Guillaume, P.L.A.; Abe, M.; Patrice, F.T.; Tohei, T.; Sakai, A. Facile Synthesis Route of Au-Ag Nanostructures Soaked in PEG. Adv. Nanopart. 2018, 7, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Korneva, A.; Straumal, B.; Kilmametov, A.; Chulist, R.; Cios, G.; Baretzky, B.; Zięba, P. Dissolution of Ag Precipitates in the Cu–8wt.% Ag Alloy Deformed by High Pressure Torsion. Materials 2019, 12, 447. [Google Scholar] [CrossRef]
- Tran, H.N.; Tomul, F.; Ha, N.T.H.; Nguyen, D.T.; Lima, E.C.; Le, G.T.; Chang, C.-T.; Masindi, V.; Woo, S.H. Innovative spherical biochar for pharmaceutical removal from water: Insight into adsorption mechanism. J. Hazard. Mater. 2020, 394, 122255. [Google Scholar] [CrossRef]
- Mcdonald-wharry, J. 2013–2014 Survey of Chars Using Raman Spectroscopy. J. Carbon Res. 2021, 7, 63. [Google Scholar] [CrossRef]
- Inoue, J.; Yoshie, A.; Tanaka, T.; Onji, T.; Inoue, Y. Disappearance and alteration process of charcoal fragments in cumulative soils studied using Raman spectroscopy. Geoderma 2017, 285, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Pusceddu, E.; Montanaro, A.; Fioravanti, G.; Santilli, S.F.; Foscolo, P.U.; Criscuoli, I.; Raschi, A.; Miglietta, F. Comparison between Ancient and Fresh Biochar Samples, A Study on The Recalcitrance of Carbonaceous Structures During Soil Incubation. Int. J. New Technol. Res. 2017, 3, 39–46. [Google Scholar]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Gao, J. Steam Gasification of Sawdust Biochar Influenced by Chemical Speciation of Alkali and Alkaline Earth Metallic Species. Energies 2018, 11, 205. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Sun, Y.; Liang, K.; Yang, Z.; Tu, R.; Fan, X.; Cheng, S.; Yu, H.; Jiang, E.; Xu, X. Enhancing Hydrodeoxygenation of Bio-oil via Bimetallic Ni-V Catalysts Modified by Cross-Surface Migrated-Carbon from Biochar. ACS Appl. Mater. Interfaces 2021, 13, 21482–21498. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Z.; Ding, H.; Wan, Y.; Tang, Z.; Gao, J. Cost-Effective Biochar Produced from Agricultural Residues and Its Application for Preparation of High Performance Form-Stable Phase Change Material via Simple Method. Int. J. Mol. Sci. 2018, 19, 3055. [Google Scholar] [CrossRef] [Green Version]
- Riva, L.; Cardarelli, A.; Andersen, G.J.; Buø, T.V.; Barbanera, M.; Bartocci, P.; Fantozzi, F.; Nielsen, H.K. On the self-heating behavior of upgraded biochar pellets blended with pyrolysis oil: Effects of process parameters. Fuel 2020, 278, 118395. [Google Scholar] [CrossRef]
- Wang, B.; Xu, M.; Chi, C.; Wang, C.; Meng, D. Degradation of methyl orange using dielectric barrier discharge water falling film reactor. J. Adv. Oxid. Technol. 2017, 20, 20170021. [Google Scholar] [CrossRef]
- Xu, J.; Sun, M.; Zhang, C.; Wu, M.; Fu, D. Electrochemical mineralization of direct blue 71 with boron-doped diamond anodes: Factor analysis and mechanisms study. J. Environ. Chem. Eng. 2022, 10, 107031. [Google Scholar] [CrossRef]
- Yaghoot-nezhad, A.; Saebnoori, E.; Danaee, I.; Elahi, S.; Bahrami, N.; Khosravi-nikou, M.R. Evaluation of the oxidative degradation of aromatic dyes by synthesized nano ferrate (VI) as a simple and effective treatment method. J. Water Process Eng. 2022, 49, 103017. [Google Scholar] [CrossRef]
- Shahzad, K.; Najam, T.; Bashir, M.S.; Nazir, M.A.; ur Rehman, A.; Bashir, M.A.; Shah, S.S.A. Fabrication of Periodic Mesoporous Organo Silicate (PMOS) composites of Ag and ZnO: Photo-catalytic degradation of methylene blue and methyl orange. Inorg. Chem. Commun. 2021, 123, 108357. [Google Scholar] [CrossRef]
- Shan, R.; Lu, L.; Gu, J.; Zhang, Y.; Yuan, H.; Chen, Y.; Luo, B. Photocatalytic degradation of methyl orange by Ag/TiO2/ biochar composite catalysts in aqueous solutions. Mater. Sci. Semicond. Process. 2020, 114, 105088. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, X.; Li, H.; Wang, X.; Jing, X. Synthesis and visible light photocatalytic performance of HC/BiOBr/ Bi12TiO20 microspheres. Chem. Phys. Lett. 2022, 797, 139584. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Xie, X.; Wang, Z. Bifunctional MnFe2O4/ chitosan modified biochar composite for enhanced methyl orange removal based on adsorption and photo-Fenton process. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 613, 126104. [Google Scholar] [CrossRef]
- Bhakta, A.K.; Snoussi, Y.; El Garah, M.; Ammar, S.; Chehimi, M.M. Brewer’s Spent Grain Biochar: Grinding Method Matters. J. Carbon Res. 2022, 8, 46. [Google Scholar] [CrossRef]
- Wang, J.; Odinga, E.S.; Zhang, W.; Zhou, X.; Yang, B.; Waigi, M.G.; Gao, Y. Polyaromatic hydrocarbons in biochars and human health risks of food crops grown in biochar-amended soils: A synthesis study. Environ. Int. 2019, 130, 104899. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, H.; Zheng, H.; Hu, H.; Tang, Y.; Liu, X.; Wang, S. Nonprecious bimetallic Fe, Mo-embedded N-enriched porous biochar for efficient oxidation of aqueous organic contaminants. J. Hazard. Mater. 2022, 422, 126776. [Google Scholar] [CrossRef]
- Wang, R.; Huang, D.; Liu, Y.; Zhang, C.; Lai, C.; Wang, X.; Gong, X.-M.; Zeng, G.-M.; Duan, A.; Xu, P.; et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation. Chem. Eng. J. 2019, 371, 380–403. [Google Scholar] [CrossRef]
- Chu, J.; Kang, J.; Park, S.; Lee, C. Application of magnetic biochar derived from food waste in heterogeneous sono-Fenton-like process for removal of organic dyes from aqueous solution. J. Water Process Eng. 2020, 37, 101455. [Google Scholar] [CrossRef]


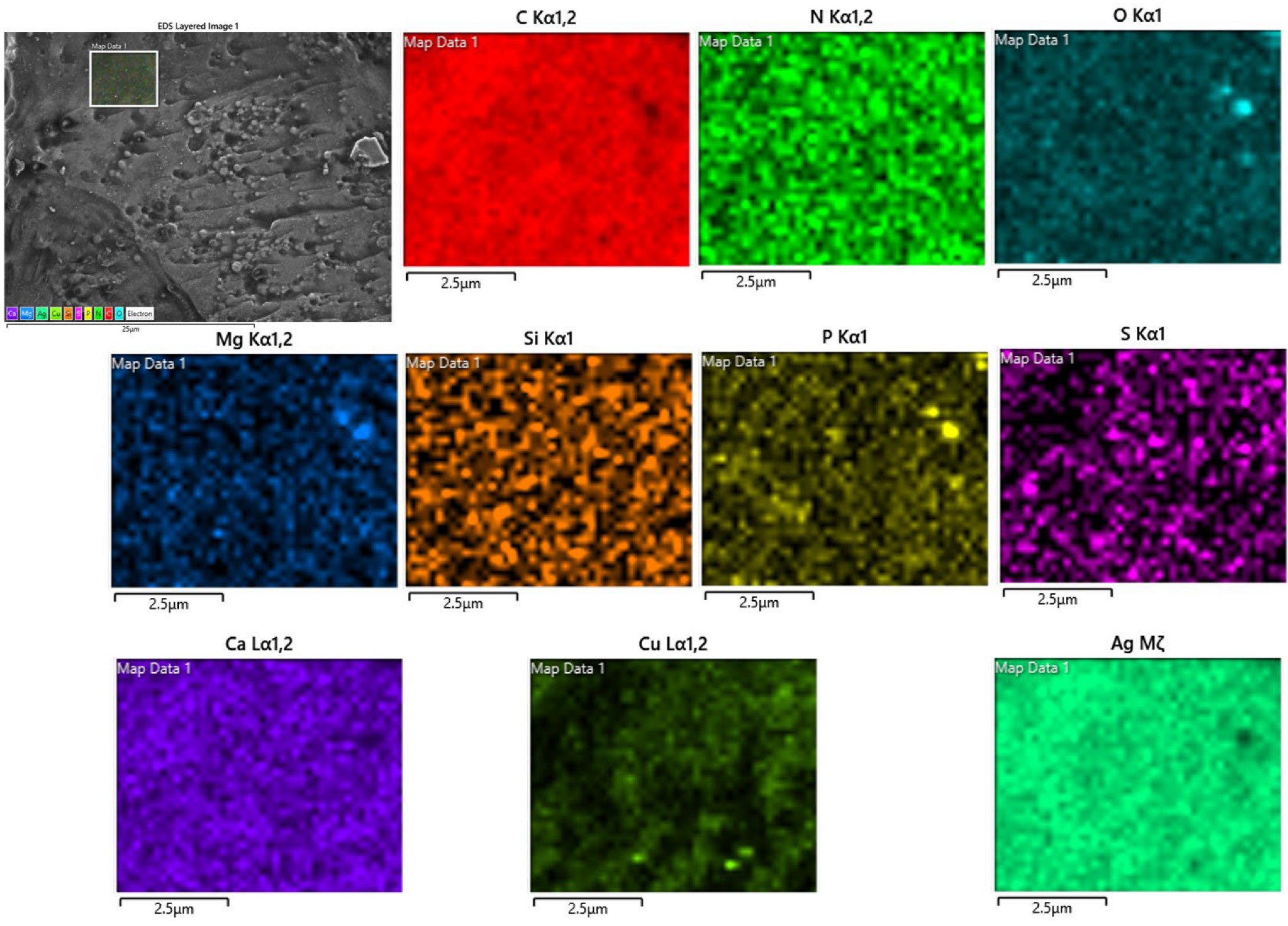
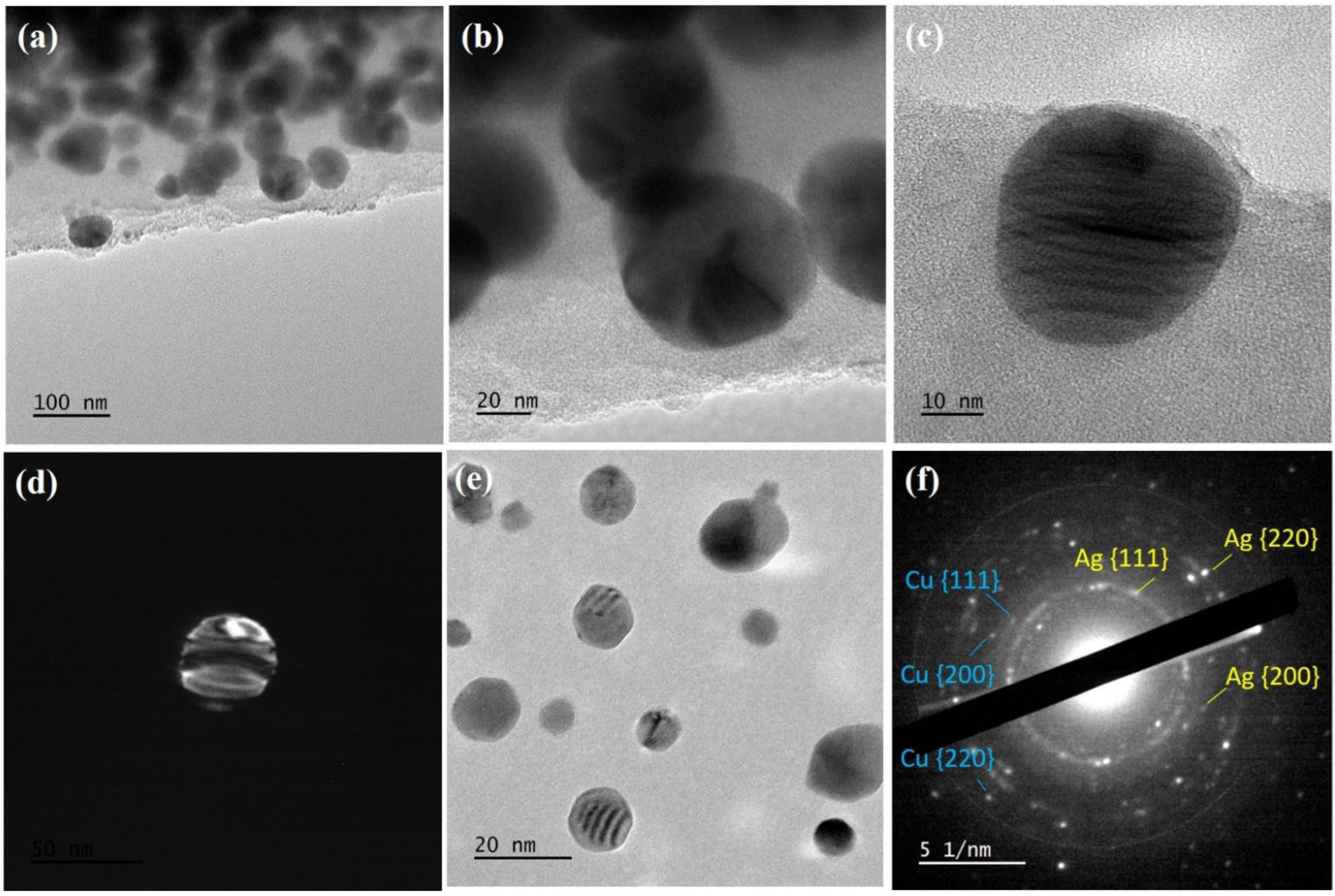
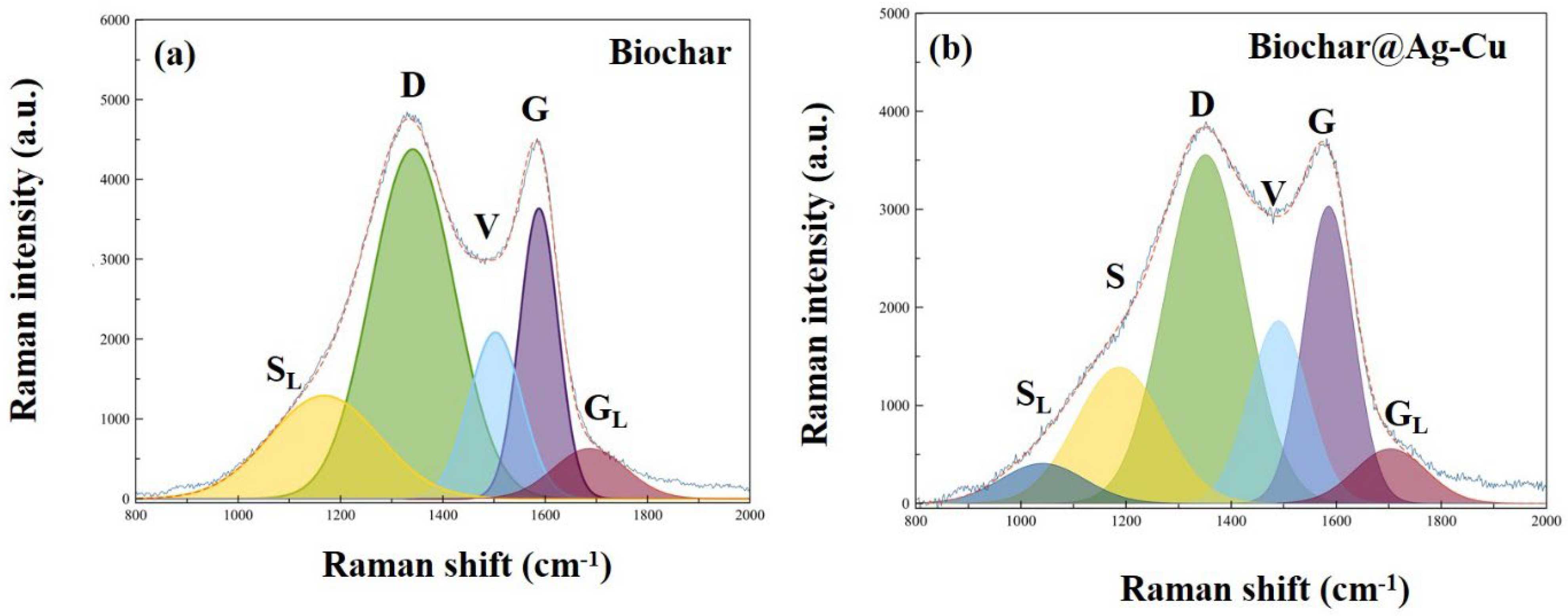

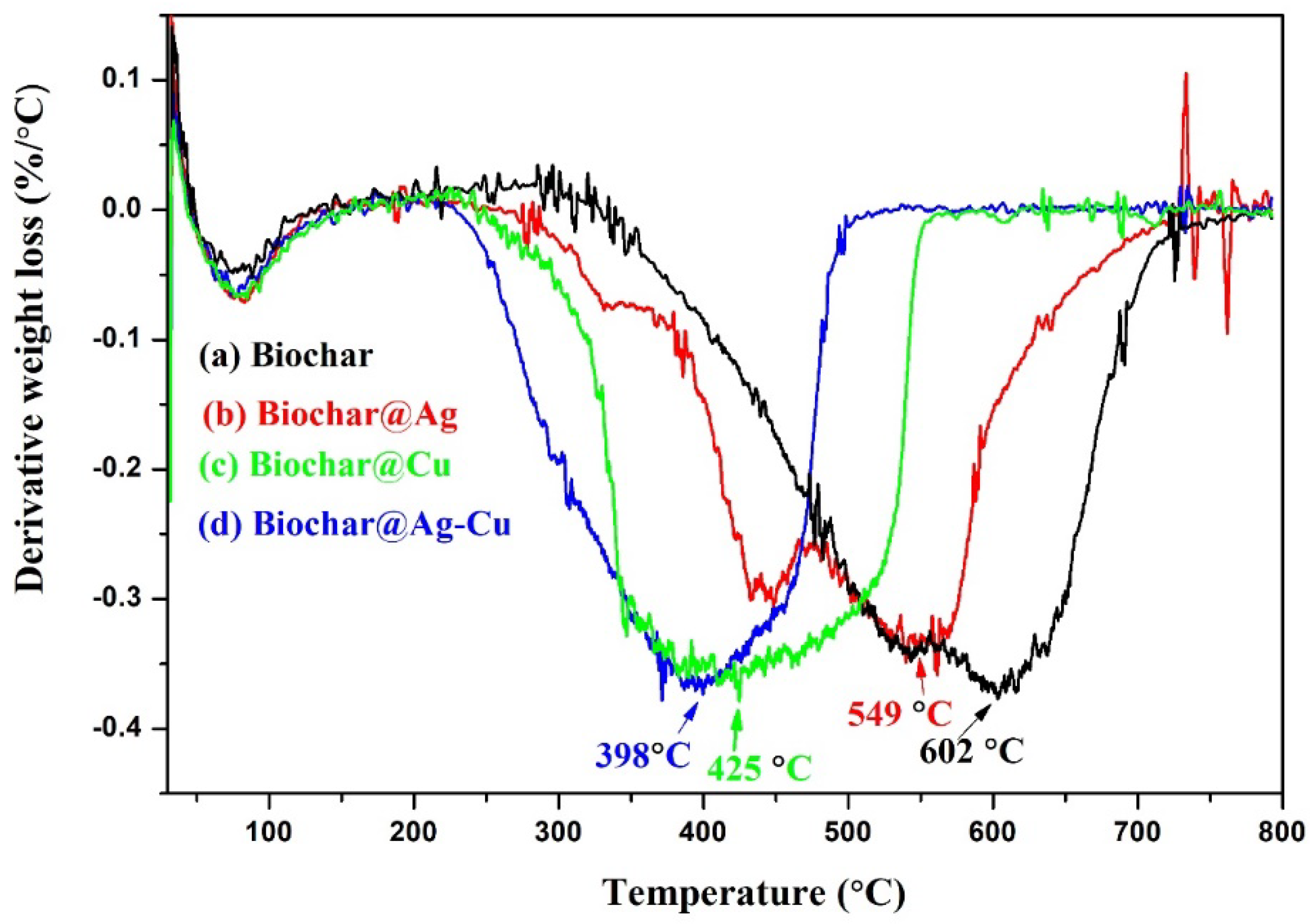

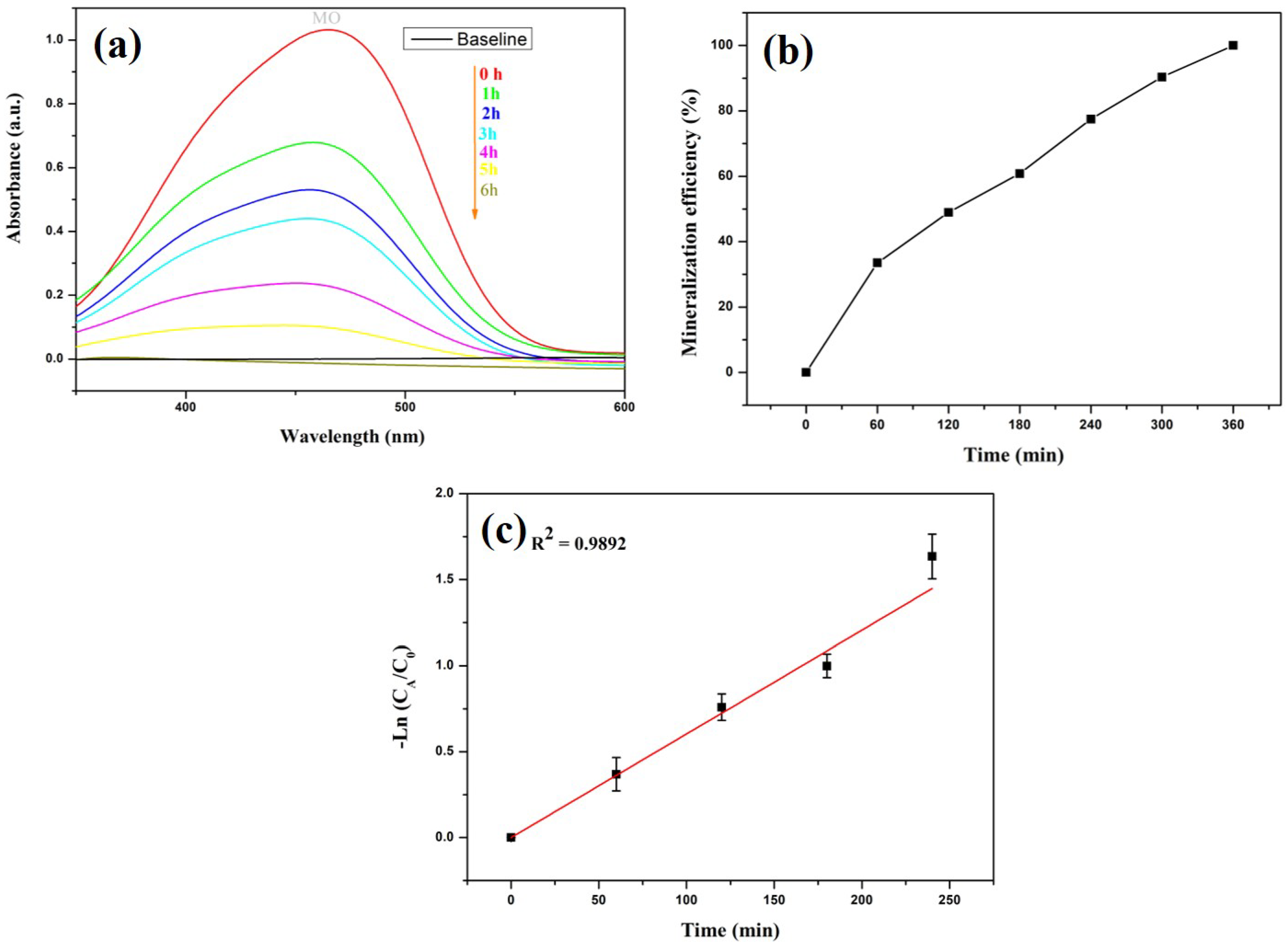

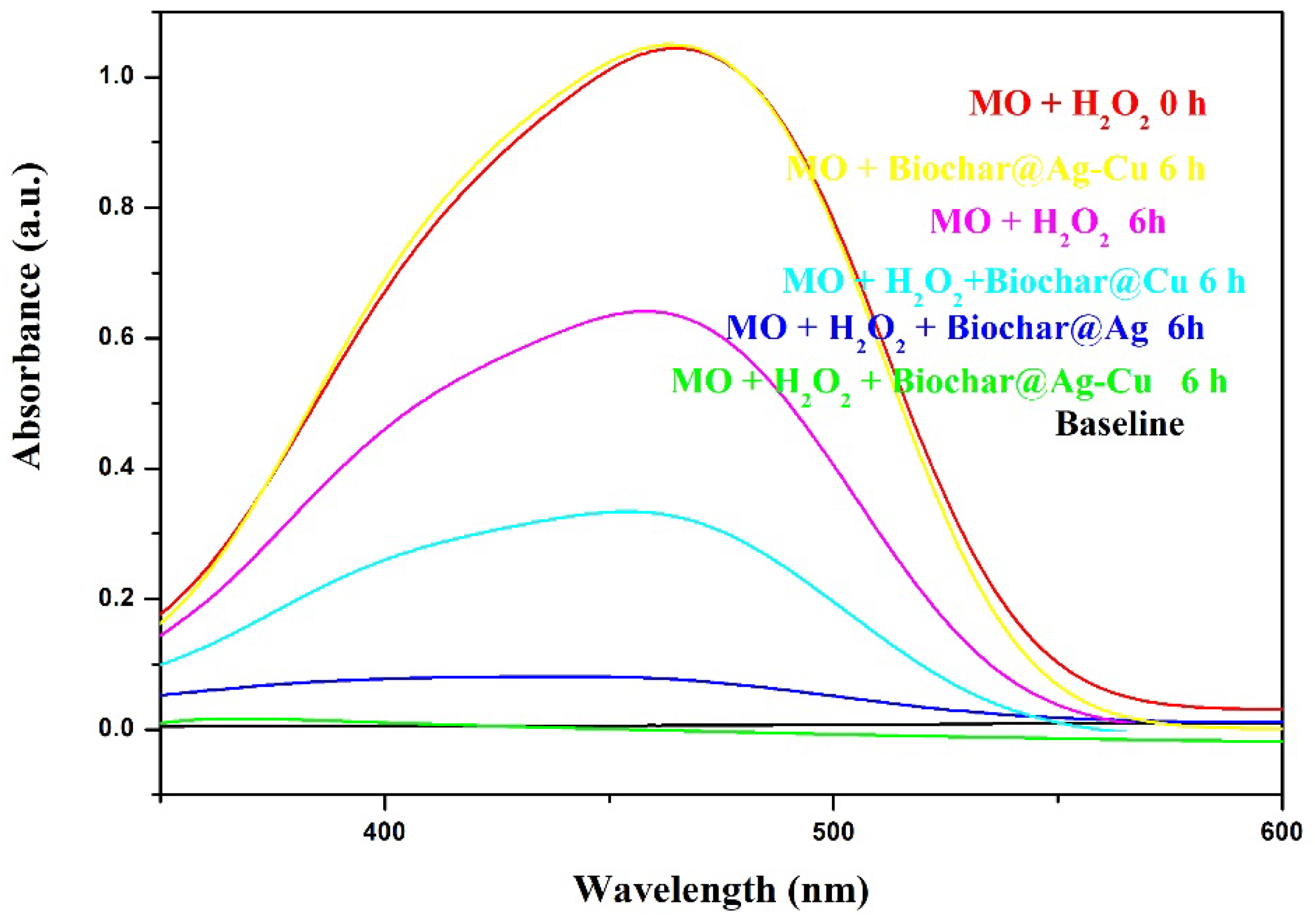

| Sample | C | O | Cu | Ag | S | N | P | Si | Ca | Mg |
|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | 78.5 | 12.6 | - | - | 3.7 | 1.6 | 2.0 | 0.8 | 0.8 | |
| Biochar@Ag | 80.3 | 10.8 | - | 1.3 | 0.1 | 4.3 | 1.0 | 1.5 | 0.4 | 0.4 |
| Biochar@Cu | 82.7 | 9.4 | 0.5 | - | 0.2 | 3.8 | 0.6 | 1.9 | 0.3 | 0.5 |
| Biochar@Ag-Cu | 81.7 | 9.8 | 0.7 | 0.4 | 0.2 | 4.5 | 1.1 | 0.6 | 0.5 | 0.5 |
| Source of Support | Composite Catalyst | Fabrication Method and Characteristics of Materials | Light Source | Mineralisation Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Periodic mesoporous Organo silicate | Ag-/ZnO-PMOS | T-80 template assisted, sonication and use of LiAlH4 as reducing agent. | Visible light | Degradation = 81% | [86] |
| Walnut shells | Ag/TiO2/biochar | Mixing, calcination, and photo-deposition (three-step process) | 500 W mercury-vapor lamp (λ = 360 nm) | 85.38% | [87] |
| Walnut shells | TiO2/ biochar | Hydrolysis method (two-step), large particles size due to agglomeration | UV irradiation | 83.23% | [88] |
| Peanut shell | HC/BiOBr/Bi12TiO2 | Hydrothermal (use of organic solvent) | 7 W-LED (λ range = 380–780 nm) | Degradation efficiency = 16.67% | [89] |
| Olive pit | B-CA@CuNi | Wet impregnation | Daylight | Degradation efficiency = 75% | [42] |
| Potato straw | MnFe2O4/chitosan/biochar | Chemical co-precipitation method (multistep process and time-consuming) | Visible LED light/H2O2 | 99.50% | [90] |
| Brewer’s spent grain | Biochar @AgCu (Ag:Cu = 1:1) | Wet impregnation (One pot) | Daylight/H2O2 | 100% | This work |
| Sample Name | Weight before Pyrolysis (g) | Weight after Pyrolysis (g) | Percentage Yield |
|---|---|---|---|
| Biochar | 3.145 | 0.896 | 28.5 |
| Biochar@Cu | 1.04 | 0.38 | 36.5 |
| Biochar@Ag | 0.98 | 0.41 | 41.8 |
| Biochar@Ag-Cu | 1.11 | 0.49 | 44.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boubkr, L.; Bhakta, A.K.; Snoussi, Y.; Moreira Da Silva, C.; Michely, L.; Jouini, M.; Ammar, S.; Chehimi, M.M. Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture. Catalysts 2022, 12, 1475. https://doi.org/10.3390/catal12111475
Boubkr L, Bhakta AK, Snoussi Y, Moreira Da Silva C, Michely L, Jouini M, Ammar S, Chehimi MM. Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture. Catalysts. 2022; 12(11):1475. https://doi.org/10.3390/catal12111475
Chicago/Turabian StyleBoubkr, Lahcen, Arvind K. Bhakta, Youssef Snoussi, Cora Moreira Da Silva, Laurent Michely, Mohamed Jouini, Souad Ammar, and Mohamed M. Chehimi. 2022. "Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture" Catalysts 12, no. 11: 1475. https://doi.org/10.3390/catal12111475
APA StyleBoubkr, L., Bhakta, A. K., Snoussi, Y., Moreira Da Silva, C., Michely, L., Jouini, M., Ammar, S., & Chehimi, M. M. (2022). Highly Active Ag-Cu Nanocrystal Catalyst-Coated Brewer’s Spent Grain Biochar for the Mineralization of Methyl Orange and Methylene Blue Dye Mixture. Catalysts, 12(11), 1475. https://doi.org/10.3390/catal12111475