Mineralization of Lipase from Thermomyces lanuginosus Immobilized on Methacrylate Beads Bearing Octadecyl Groups to Improve Enzyme Features
Abstract
1. Introduction
2. Results
2.1. Preparation of the Immobilized and Chemically Modified TLL
2.2. Mineralization of Purolite C18-TLL
2.2.1. Effect of the Mineralization on Enzyme Hydrolytic Activities
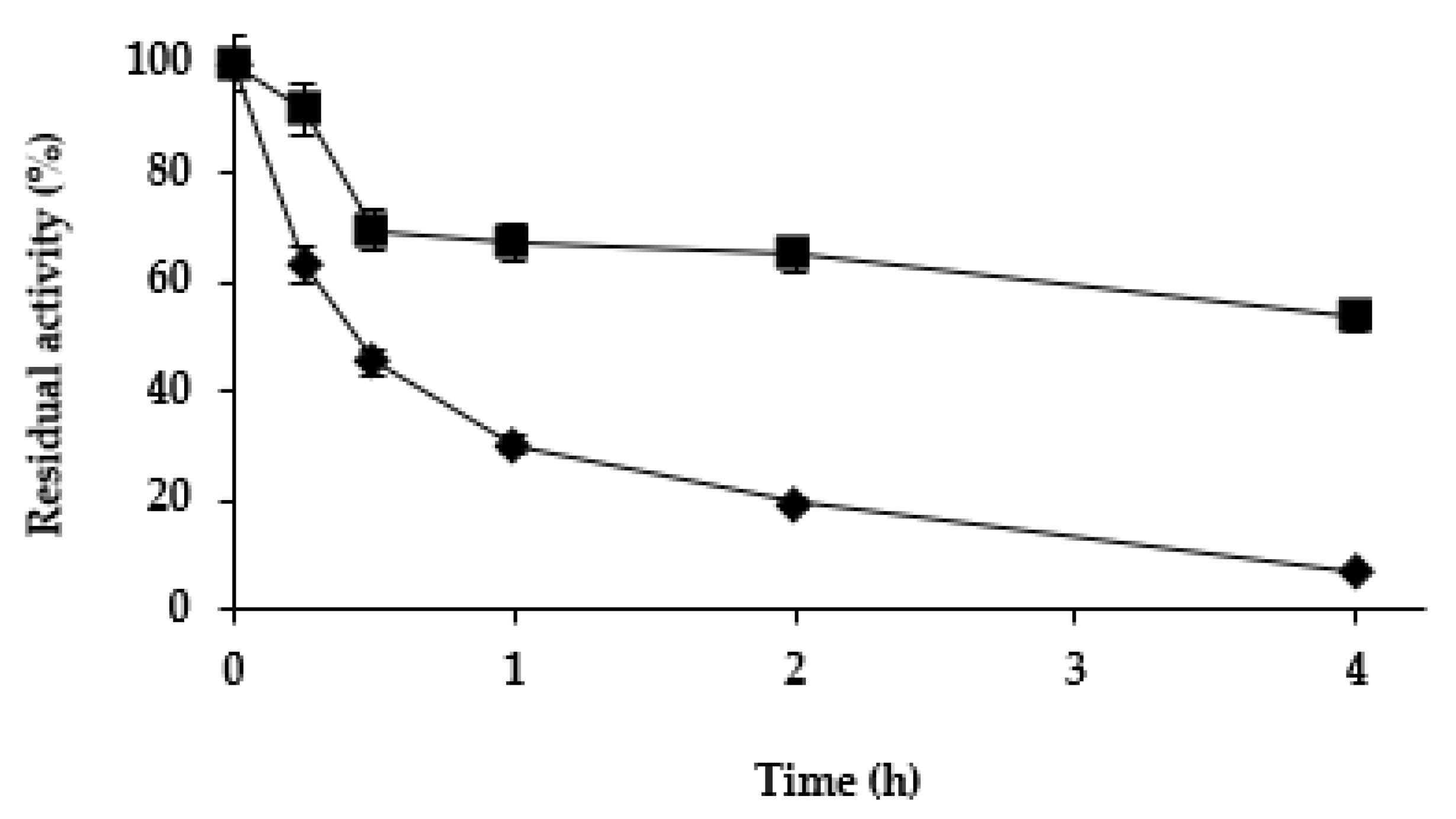
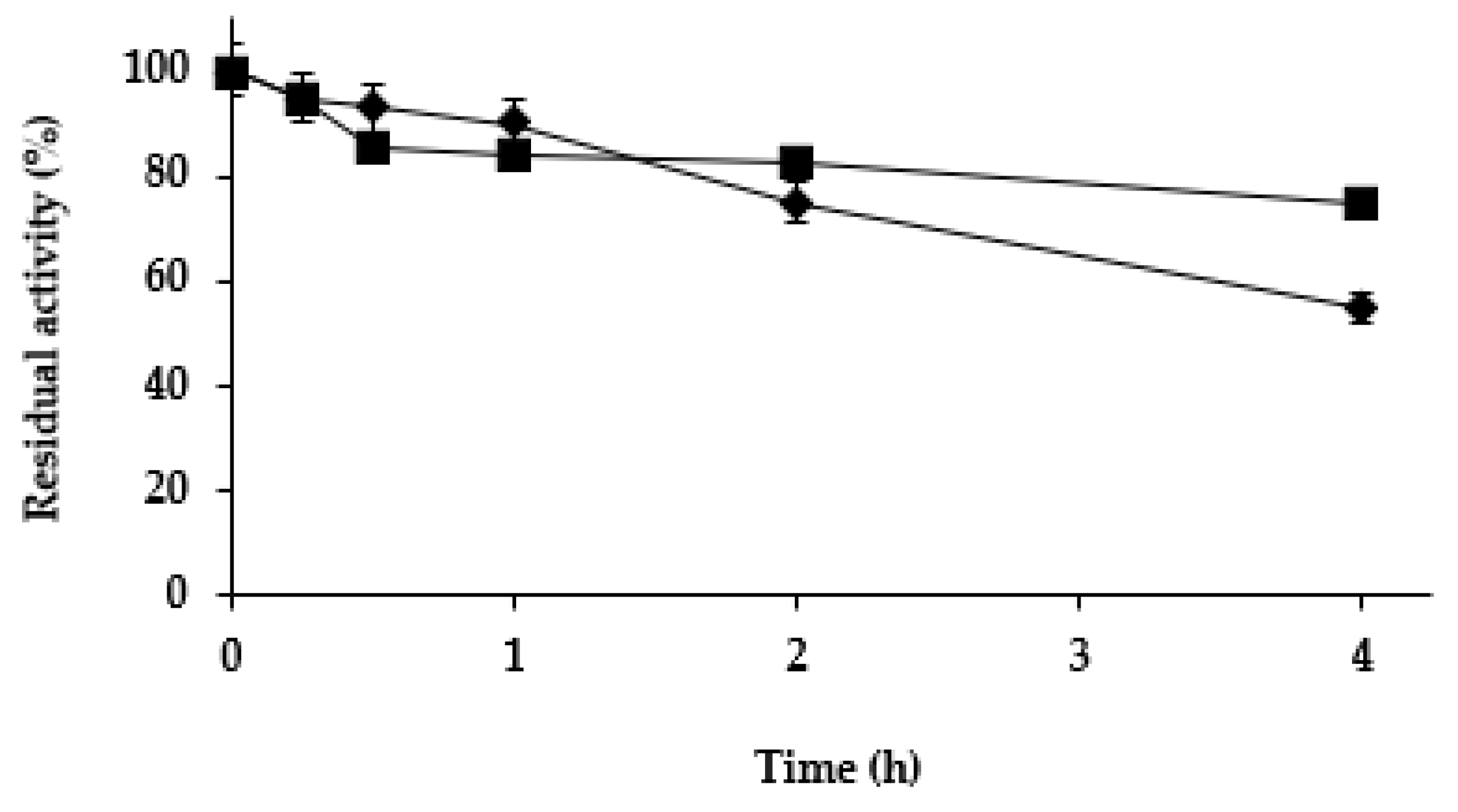
2.2.2. Effect of the Mineralization on Enzyme Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Wetting of Purolite C18 Beads
3.2.2. Immobilization of TLL
3.2.3. Modification of Immobilized Enzymes with Metal Salt/Sodium Phosphate
3.2.4. Thermal Inactivation of the Different TLL-Based Biocatalysts
3.2.5. Enzyme Hydrolytic Activity Assays
Hydrolysis of p-NPB
Hydrolysis of Triacetin
Hydrolysis of R- or S-Methyl Mandelate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernandez-Lafuente, R. Lipase from Thermomyces lanuginosus: Uses and prospects as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2010, 62, 197–212. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef]
- Guisan, J.M.; Fernandez-Lorente, G.; Rocha-Martin, J.; Moreno-Gamero, D. Enzyme immobilization strategies for the design of robust and efficient biocatalysts. Curr. Opin. Green Sustain. Chem. 2022, 35, 100593. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Enzyme immobilization and co-immobilization: Main framework, advances and some applications. Processes 2022, 10, 494. [Google Scholar] [CrossRef]
- Almeida, F.L.C.; Prata, A.S.; Forte, M.B.S. Enzyme immobilization: What have we learned in the past five years? Biofuels Bioprod. Biorefining 2022, 16, 587–608. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is enzyme immobilization a mature discipline? Some critical considerations to capitalize on the benefits of immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.; Torger, B.; Allertz, P.J.; Jähnichen, K.; Keßler, S.; Müller, M.; Simon, F.; Salchert, K.; Mäurer, H.; Pospiech, D. Multifunctional crosslinkable itaconic acid copolymers for enzyme immobilization. Eur. Polym. J. 2018, 102, 47–55. [Google Scholar] [CrossRef]
- Poliak, A.; Blumenfeld, H.; Wax, M.; Baughn, R.L.; Whitesides, G.M. Enzyme immobilization by condensation copolymerization into cross-linked polyacrylamide gels. J. Am. Chem. Soc. 1980, 102, 6324–6336. [Google Scholar] [CrossRef]
- Shakeri, F.; Ariaeenejad, S.; Ghollasi, M.; Motamedi, E. Synthesis of two novel bio-based hydrogels using sodium alginate and chitosan and their proficiency in physical immobilization of enzymes. Sci. Rep. 2022, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Alnadari, F.; Xue, Y.; Alsubhi, N.H.; Alamoudi, S.A.; Alwabli, A.S.; Al-Quwaie, D.A.; Saud Hamed, Y.; Muhammad Nasiru, M.; Ebrahim, A.A.M.; El-Saadony, M.T.; et al. Reusability of immobilized β-glucosidase on sodium alginate-coated magnetic nanoparticles and high productivity applications. J. Saudi Chem. Soc. 2022, 26, 101517. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, W.; Wang, Y.; Yan, Y. Microfluidic fabrication of tunable alginate-based microfibers for the stable immobilization of enzymes. Biotechnol. J. 2022, 17, 2200098. [Google Scholar] [CrossRef]
- Vasilescu, C.; Paul, C.; Marc, S.; Hulka, I.; Péter, F. Development of a tailored sol-gel immobilized biocatalyst for sustainable synthesis of the food aroma ester n-amyl caproate in continuous solventless system. Foods 2022, 11, 2485. [Google Scholar] [CrossRef]
- Ficanha, A.M.M.; Oro, C.E.D.; Franceschi, E.; Dallago, R.M.; Mignoni, M.L. Evaluation of different ionic liquids as additives in the immobilization of lipase CALB by sol-gel technique. Appl. Biochem. Biotechnol. 2021, 193, 2162–2181. [Google Scholar] [CrossRef]
- Fernandez Caresani, J.R.; Dallegrave, A.; dos Santos, J.H.Z. Amylases immobilization by sol–gel entrapment: Application for starch hydrolysis. J. Sol-Gel Sci. Technol. 2020, 94, 229–240. [Google Scholar] [CrossRef]
- Jegan Roy, J.; Emilia Abraham, T. Strategies in making cross-linked enzyme crystals. Chem. Rev. 2004, 104, 3705–3722. [Google Scholar] [CrossRef] [PubMed]
- Zelinski, T.; Waldmann, H. Cross-linked enzyme crystals (CLECs): Efficient and stable biocatalysts for preparative organic chemistry. Angew. Chem. (Int. Ed. English) 1997, 36, 722–724. [Google Scholar] [CrossRef]
- Staar, M.; Henke, S.; Blankenfeldt, W.; Schallmey, A. Biocatalytically active and stable cross-linked enzyme crystals of halohydrin dehalogenase HheG by protein engineering. ChemCatChem 2022, 14, e202200145. [Google Scholar] [CrossRef]
- Cao, L.; Van Rantwijk, F.; Sheldon, R.A. Cross-linked enzyme aggregates: A simple and effective method for the immobilization of penicillin acylase. Org. Lett. 2000, 2, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Schoevaart, R.; Wolbers, M.W.; Golubovic, M.; Ottens, M.; Kieboom, A.P.G.; van Rantwijk, F.; van der Wielen, L.A.M.; Sheldon, R.A. Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol. Bioeng. 2004, 87, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, C.S.; Angelotti, J.A.F.; Fernandez-Lafuente, R.; Hirata, D.B. Lipase immobilization via cross-linked enzyme aggregates: Problems and prospects—A review. Int. J. Biol. Macromol. 2022, 215, 434–449. [Google Scholar] [CrossRef]
- Kreiner, M.; Parker, M.-C. Protein-coated microcrystals for use in organic solvents: Application to oxidoreductases. Biotechnol. Lett. 2005, 27, 1571–1577. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; dos Santos, J.C.S.; Alcántara, A.R.; Fernandez-Lafuente, R. Enzyme-coated micro-crystals: An almost forgotten but very simple and elegant immobilization strategy. Catalysts 2020, 10, 891. [Google Scholar] [CrossRef]
- Kreiner, M.; Parker, M.C. High-activity biocatalysts in organic media: Solid-state buffers as the immobilisation matrix for protein-coated microcrystals. Biotechnol. Bioeng. 2004, 87, 24–33. [Google Scholar] [CrossRef]
- Gao, J.; Yin, L.; Feng, K.; Zhou, L.; Ma, L.; He, Y.; Wang, L.; Jiang, Y. Lipase immobilization through the combination of bioimprinting and cross-linked protein-coated microcrystal technology for biodiesel production. Ind. Eng. Chem. Res. 2016, 55, 11037–11043. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Ju, E.; Liu, Z.; Cao, F.; Chen, Z.; Ren, J.; Qu, X. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat. Commun. 2018, 9, 3334. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Hu, R.; Zhao, Z.; Chen, Z.; Zhang, X.; Tan, W. Noncanonical self-assembly of multifunctional DNA nanoflowers for biomedical applications. J. Am. Chem. Soc. 2013, 135, 16438–16445. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.; Betancor, L.; Fernández-Lorente, G.; Fuentes, M.; Hidalgo, A.; Guisán, J.M.; Pessela, B.C.C.; Fernández-Lafuente, R. Cross-linked aggregates of multimeric enzymes: A simple and efficient methodology to stabilize their quaternary structure. Biomacromolecules 2004, 5, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-organic frameworks with different dimensionalities: An ideal host platform for enzyme@MOF composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Li, J.; Yin, L.; Wang, Z.; Jing, Y.; Jiang, Z.; Ding, Y.; Wang, H. Enzyme-immobilized metal-organic frameworks: From preparation to application. Chem.-An Asian J. 2022, 17, e202200751. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Mohammadi-Mahani, H.; Badoei-dalfard, A.; Karami, Z. Synthesis and characterization of cross-linked lipase-metal hybrid nanoflowers on graphene oxide with increasing the enzymatic stability and reusability. Biochem. Eng. J. 2021, 172, 108038. [Google Scholar] [CrossRef]
- Escobar, S.; Velasco-Lozano, S.; Lu, C.-H.; Lin, Y.-F.; Mesa, M.; Bernal, C.; López-Gallego, F. Understanding the functional properties of bio-inorganic nanoflowers as biocatalysts by deciphering the metal-binding sites of enzymes. J. Mater. Chem. B 2017, 5, 4478–4486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, P.; Zhang, H.; Wang, H.; Li, X.; Tian, L.; Ali, N.; Ali, Z.; Zhang, Q. Preparation of lipase/Zn3(PO4)2 hybrid nanoflower and its catalytic performance as an immobilized enzyme. Chem. Eng. J. 2016, 291, 287–297. [Google Scholar] [CrossRef]
- Lee, S.W.; Cheon, S.A.; Kim, M., II; Park, T.J. Organic–inorganic hybrid nanoflowers: Types, characteristics, and future prospects. J. Nanobiotechnol. 2015, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shao, X.; Kong, D.; Li, G.; Li, Q. Immobilization of thermophilic lipase in inorganic hybrid nanoflower through biomimetic mineralization. Colloids Surf. B Biointerfaces 2021, 197, 111450. [Google Scholar] [CrossRef]
- Li, C.; Zhao, J.; Zhang, Z.; Jiang, Y.Y.; Bilal, M.; Jiang, Y.Y.; Jia, S.; Cui, J. Self-assembly of activated lipase hybrid nanoflowers with superior activity and enhanced stability. Biochem. Eng. J. 2020, 158, 107582. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Elfeky, N.M.; Wang, Y.; Zhao, D.; Zhou, H.; Wang, J.; Bao, Y. Self-assembly of lipase hybrid nanoflowers with bifunctional Ca2+ for improved activity and stability. Enzyme Microb. Technol. 2020, 132, 109408. [Google Scholar] [CrossRef]
- Xu, L.; Yu, J.; Wang, A.; Zuo, C.; Li, H.; Chen, X.; Pei, X.; Zhang, P. Efficient synthesis of vitamin A palmitate in nonaqueous medium using self-assembled lipase TLL@apatite hybrid nanoflowers by mimetic biomineralization. Green Chem. Lett. Rev. 2018, 11, 476–483. [Google Scholar] [CrossRef]
- Soni, S.; Dwivedee, B.P.; Banerjee, U.C. An ultrafast sonochemical strategy to synthesize lipase-manganese phosphate hybrid nanoflowers with promoted biocatalytic performance in the kinetic resolution of β-aryloxyalcohols. ChemNanoMat 2018, 4, 1007–1020. [Google Scholar] [CrossRef]
- Lee, H.R.; Chung, M.; Kim, M., II; Ha, S.H. Preparation of glutaraldehyde-treated lipase-inorganic hybrid nanoflowers and their catalytic performance as immobilized enzymes. Enzyme Microb. Technol. 2017, 105, 24–29. [Google Scholar] [CrossRef]
- Cui, J.; Zhao, Y.; Liu, R.; Zhong, C.; Jia, S. Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci. Rep. 2016, 6, 27928. [Google Scholar] [CrossRef]
- Gao, J.; Kong, W.; Zhou, L.; He, Y.; Ma, L.; Wang, Y.; Yin, L.; Jiang, Y. Monodisperse core-shell magnetic organosilica nanoflowers with radial wrinkle for lipase immobilization. Chem. Eng. J. 2017, 309, 70–79. [Google Scholar] [CrossRef]
- Badoei-dalfard, A.; Monemi, F.; Hassanshahian, M. One-pot synthesis and biochemical characterization of a magnetic collagenase nanoflower and evaluation of its biotechnological applications. Colloids Surf. B Biointerfaces 2022, 211, 112302. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.A.; Qamar, M.; Bilal, M.; Bharagava, R.N.; Ferreira, L.F.R.; Sher, F.; Iqbal, H.M.N. Cellulose-deconstruction potential of nano-biocatalytic systems: A strategic drive from designing to sustainable applications of immobilized cellulases. Int. J. Biol. Macromol. 2021, 185, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Yang, C.; Ma, C.; Tang, J. Enzyme-inorganic hybrid nanoflowers: Classification, synthesis, functionalization and potential applications. Chem. Eng. J. 2021, 415, 129075. [Google Scholar] [CrossRef]
- Alhayali, N.I.; Özpozan, N.K.; Dayan, S.; Özdemir, N.; Yılmaz, B.S. Catalase/Fe3O4@Cu2+ hybrid biocatalytic nanoflowers fabrication and efficiency in the reduction of organic pollutants. Polyhedron 2021, 194, 114888. [Google Scholar] [CrossRef]
- Zhaoyu, Z.; Ping, X.; Keren, S.; Weiwei, Z.; Chunmiao, H.; Peng, L. Di-functional magnetic nanoflowers: A highly efficient support for immobilizing penicillin G acylase. J. Chin. Chem. Soc. 2020, 67, 1591–1601. [Google Scholar] [CrossRef]
- Feng, N.; Zhang, H.; Li, Y.; Liu, Y.; Xu, L.; Wang, Y.; Fei, X.; Tian, J. A novel catalytic material for hydrolyzing cow’s milk allergenic proteins: Papain-Cu3(PO4)2·3H2O-magnetic nanoflowers. Food Chem. 2020, 311, 125911. [Google Scholar] [CrossRef]
- Sun, T.; Fu, M.; Xing, J.; Ge, Z. Magnetic nanoparticles encapsulated laccase nanoflowers: Evaluation of enzymatic activity and reusability for degradation of malachite green. Water Sci. Technol. 2020, 81, 29–39. [Google Scholar] [CrossRef]
- Zhang, H.; Fei, X.; Tian, J.; Li, Y.; Zhi, H.; Wang, K.; Xu, L.; Wang, Y. Synthesis and continuous catalytic application of alkaline protease nanoflowers–PVA composite hydrogel. Catal. Commun. 2018, 116, 5–9. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Z.; Wang, X.; Qiu, M.; Zhang, Z.; Wang, Z.; Cui, J.; Jia, S. Paper-based biosensor based on phenylalanine ammonia lyase hybrid nanoflowers for urinary phenylalanine measurement. Int. J. Biol. Macromol. 2021, 166, 601–610. [Google Scholar] [CrossRef]
- Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Tardioli, P.W.; Fernandez-Lafuente, R. Stabilization of immobilized lipases by treatment with metallic phosphate salts. Int. J. Biol. Macromol. 2022, 213, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Carballares, D.; Tardioli, P.W.; Rocha-Martin, J.; Fernandez-Lafuente, R. Tuning immobilized commercial lipase preparations features by simple treatment with metallic phosphate salts. Molecules 2022, 27, 4486. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Carballares, D.; Rocha-martin, J.R.; Tardioli, P.W.; Fernandez-Lafuente, R. The immobilization protocol greatly alters the effects of metal phosphate modification on the activity/stability of immobilized lipases. Int. J. Biol. Macromol. 2022, 222, 2452–2466. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.R.; Carballares, D.; Rocha-martin, J.; Tardioli, P.W.; Fernandez–Lafuente, R. Tuning immobilized enzyme features by combining solid-phase physicochemical modification and mineralization. Int. J. Mol. Sci. 2022, 23, 12808. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Bari, N.K.; Garg, A.; Sinha, S. Protein morphology drives the structure and catalytic activity of bio-inorganic hybrids. Int. J. Biol. Macromol. 2021, 176, 106–116. [Google Scholar] [CrossRef]
- Carpenter, B.P.; Talosig, A.R.; Mulvey, J.T.; Merham, J.G.; Esquivel, J.; Rose, B.; Ogata, A.F.; Fishman, D.A.; Patterson, J.P. Role of molecular modification and protein folding in the nucleation and growth of protein–metal–organic frameworks. Chem. Mater. 2022, 34, 8336–8344. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Peirce, S.; Torrestiana-Sanchez, B.; Yates, M.; Rosales-Quintero, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Evaluation of different commercial hydrophobic supports for the immobilization of lipases: Tuning their stability, activity and specificity. RSC Adv. 2016, 6, 100281–100294. [Google Scholar] [CrossRef]
- Cunha, A.G.; Besteti, M.D.; Manoel, E.A.; Da Silva, A.A.T.; Almeida, R.V.; Simas, A.B.C.; Fernandez-Lafuente, R.; Pinto, J.C.; Freire, D.M.G. Preparation of core-shell polymer supports to immobilize lipase B from Candida antarctica: Effect of the support nature on catalytic properties. J. Mol. Catal. B Enzym. 2014, 100, 59–67. [Google Scholar] [CrossRef]
- Manoel, E.A.; Pinto, M.; dos Santos, J.C.S.; Tacias-Pascacio, V.G.; Freire, D.M.G.; Pinto, J.C.; Fernandez-Lafuente, R. Design of a core–shell support to improve lipase features by immobilization. RSC Adv. 2016, 6, 62814–62824. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Pinto, M.C.C.; Robert, J.d.M.; da Silva, T.P.; Beralto, T.d.C.; Santos, J.G.F.; de Castro, R.d.P.V.; Fernandez-Lafuente, R.; Manoel, E.A.; Pinto, J.C.; et al. Pilot-scale development of core–shell polymer supports for the immobilization of recombinant lipase B from Candida antarctica and their application in the production of ethyl esters from residual fatty acids. J. Appl. Polym. Sci. 2018, 135, 46727. [Google Scholar] [CrossRef]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Torrestiana-Sánchez, B.; Dal Magro, L.; Virgen-Ortíz, J.J.; Suárez-Ruíz, F.J.; Rodrigues, R.C.; Fernandez-Lafuente, R. Comparison of acid, basic and enzymatic catalysis on the production of biodiesel after RSM optimization. Renew. Energy 2019, 135, 1–9. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Purolite LifetechTM ECR8806M-Metacrilato de Octadecil. Available online: https://www.purolite.com/ls-product/es/ecr8806m (accessed on 6 October 2022).
- Lokha, Y.; Arana-Peña, S.; Rios, N.S.; Mendez-Sanchez, C.; Gonçalves, L.R.B.B.; Lopez-Gallego, F.; Fernandez-Lafuente, R. Modulating the properties of the lipase from Thermomyces lanuginosus immobilized on octyl agarose beads by altering the immobilization conditions. Enzyme Microb. Technol. 2020, 133, 109461. [Google Scholar] [CrossRef]
- AL-Muftah, A.E.; Abu-Reesh, I.M. Effects of internal mass transfer and product inhibition on a simulated immobilized enzyme-catalyzed reactor for lactose hydrolysis. Biochem. Eng. J. 2005, 23, 139–153. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Consolati, T.; Mayr, T.; Nidetzky, B. Quantitating intraparticle O2 gradients in solid supported enzyme immobilizates: Experimental determination of their role in limiting the catalytic effectiveness of immobilized glucose oxidase. Biotechnol. Bioeng. 2013, 110, 2086–2095. [Google Scholar] [CrossRef]
- Berendsen, W.R.; Lapin, A.; Reuss, M. Investigations of reaction kinetics for immobilized enzymes-identification of parameters in the presence of diffusion limitation. Biotechnol. Prog. 2008, 22, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Bolivar, J.M.; Nidetzky, B. The microenvironment in immobilized enzymes: Methods of characterization and its role in determining enzyme performance. Molecules 2019, 24, 3460. [Google Scholar] [CrossRef] [PubMed]
- JM, G.; Alvaro, G.; CM, R.; Fernandez-Lafuente, R. Industrial design of enzymic processes catalysed by very active immobilized derivatives: Utilization of diffusional limitations (gradients of pH) as a profitable tool in enzyme engineering. Biotechnol. Appl. Biochem. 1994, 20, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.P.; Shah, M.B.; Fournier, R.L.; Varanasi, S. Generation of a pH gradient in an immobilized enzyme system. Biotechnol. Bioeng. 1993, 42, 410–420. [Google Scholar] [CrossRef]
- Chen, G.; Fournier, R.L.; Varanasi, S. A mathematical model for the generation and control of a pH gradient in an immobilized enzyme system involving acid generation. Biotechnol. Bioeng. 1998, 57, 394–408. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Martínez-Sanchez, J.A.; Arana-Peña, S.; Carballares, D.; Yates, M.; Otero, C.; Fernandez-Lafuente, R. Immobilized biocatalysts of Eversa® transform 2.0 and lipase from Thermomyces lanuginosus: Comparison of some properties and performance in biodiesel production. Catalysts 2020, 10, 738. [Google Scholar] [CrossRef]
- Lombardo, D.; Guy, O. Effect of alcohols on the hydrolysis catalyzed by human pancreatic carboxylic-ester hydrolase. Biochim. Biophys. Acta (BBA)-Enzymol. 1981, 657, 425–437. [Google Scholar] [CrossRef]
- Hernandez, K.; Garcia-Verdugo, E.; Porcar, R.; Fernandez-Lafuente, R. Hydrolysis of triacetin catalyzed by immobilized lipases: Effect of the immobilization protocol and experimental conditions on diacetin yield. Enzyme Microb. Technol. 2011, 48, 510–517. [Google Scholar] [CrossRef]
- Arana-Peña, S.; Lokha, Y.; Fernández-Lafuente, R. Immobilization on octyl-agarose beads and some catalytic features of commercial preparations of lipase a from Candida antarctica (Novocor ADL): Comparison with immobilized lipase B from Candida antarctica. Biotechnol. Prog. 2019, 35, e2735. [Google Scholar] [CrossRef]
- Paiva Souza, P.M.; Carballares, D.; Lopez-Carrobles, N.; Gonçalves, L.R.B.; Lopez-Gallego, F.; Rodrigues, S.; Fernandez-Lafuente, R. Enzyme-support interactions and inactivation conditions determine Thermomyces lanuginosus lipase inactivation pathways: Functional and florescence studies. Int. J. Biol. Macromol. 2021, 191, 79–91. [Google Scholar] [CrossRef]
- dos Santos, J.C.S.; Rueda, N.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Tuning the catalytic properties of lipases immobilized on divinylsulfone activated agarose by altering its nanoenvironment. Enzyme Microb. Technol. 2015, 77, 1–7. [Google Scholar] [CrossRef]



| Biocatalysts | Activity (U/g) | ||
|---|---|---|---|
| Substrate | |||
| Triacetin | R-Mandelate | S-Mandelate | |
| Agarose C8-TLL | 115.7 ± 5.9 | 1.3 ± 0.1 | 0.70 ± 0.05 |
| Purolite C18-TLL | 548.7 ± 25.9 | 2.2 ± 0.1 | 0.30 ± 0.02 |
| Biocatalysts | Relative Activity (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Zn₃(PO₄)₂ Modification | Cu₃(PO₄)₂ Modification | Co₃(PO₄)₂ Modification | |||||||
| Triacetin | R-Mandelate | S-Mandelate | Triacetin | R-Mandelate | S-Mandelate | Triacetin | R-Mandelate | S-Mandelate | |
| Agarose C8-TLL | 138.9 ± 6.7 | 69.2 ± 3.9 | 71.4 ± 2.8 | 100.0 ± 5.1 | 100.0 ± 3.4 | 71.4 ± 4.3 | 103.2 ± 4.2 | 69.2 ± 3.5 | 100.0 ± 5.1 |
| Purolite C18-TLL | 123.2 ± 3.7 | 114.0 ± 5.1 | 66.7 ± 2.5 | 120.2 ± 3.6 | 114.0 ± 4.4 | 33.3 ± 1.6 | 130.5 ± 3.9 | 109.1 ± 6.1 | 66.7 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guimarães, J.R.; Carballares, D.; Rocha-Martin, J.; Tardioli, P.W.; Fernandez-Lafuente, R. Mineralization of Lipase from Thermomyces lanuginosus Immobilized on Methacrylate Beads Bearing Octadecyl Groups to Improve Enzyme Features. Catalysts 2022, 12, 1552. https://doi.org/10.3390/catal12121552
Guimarães JR, Carballares D, Rocha-Martin J, Tardioli PW, Fernandez-Lafuente R. Mineralization of Lipase from Thermomyces lanuginosus Immobilized on Methacrylate Beads Bearing Octadecyl Groups to Improve Enzyme Features. Catalysts. 2022; 12(12):1552. https://doi.org/10.3390/catal12121552
Chicago/Turabian StyleGuimarães, José R., Diego Carballares, Javier Rocha-Martin, Paulo W. Tardioli, and Roberto Fernandez-Lafuente. 2022. "Mineralization of Lipase from Thermomyces lanuginosus Immobilized on Methacrylate Beads Bearing Octadecyl Groups to Improve Enzyme Features" Catalysts 12, no. 12: 1552. https://doi.org/10.3390/catal12121552
APA StyleGuimarães, J. R., Carballares, D., Rocha-Martin, J., Tardioli, P. W., & Fernandez-Lafuente, R. (2022). Mineralization of Lipase from Thermomyces lanuginosus Immobilized on Methacrylate Beads Bearing Octadecyl Groups to Improve Enzyme Features. Catalysts, 12(12), 1552. https://doi.org/10.3390/catal12121552









