One Bicopper Complex with Good Affinity to Nitrate for Highly Selective Electrocatalytic Nitrate Reduction to Ammonia
Abstract
:1. Introduction
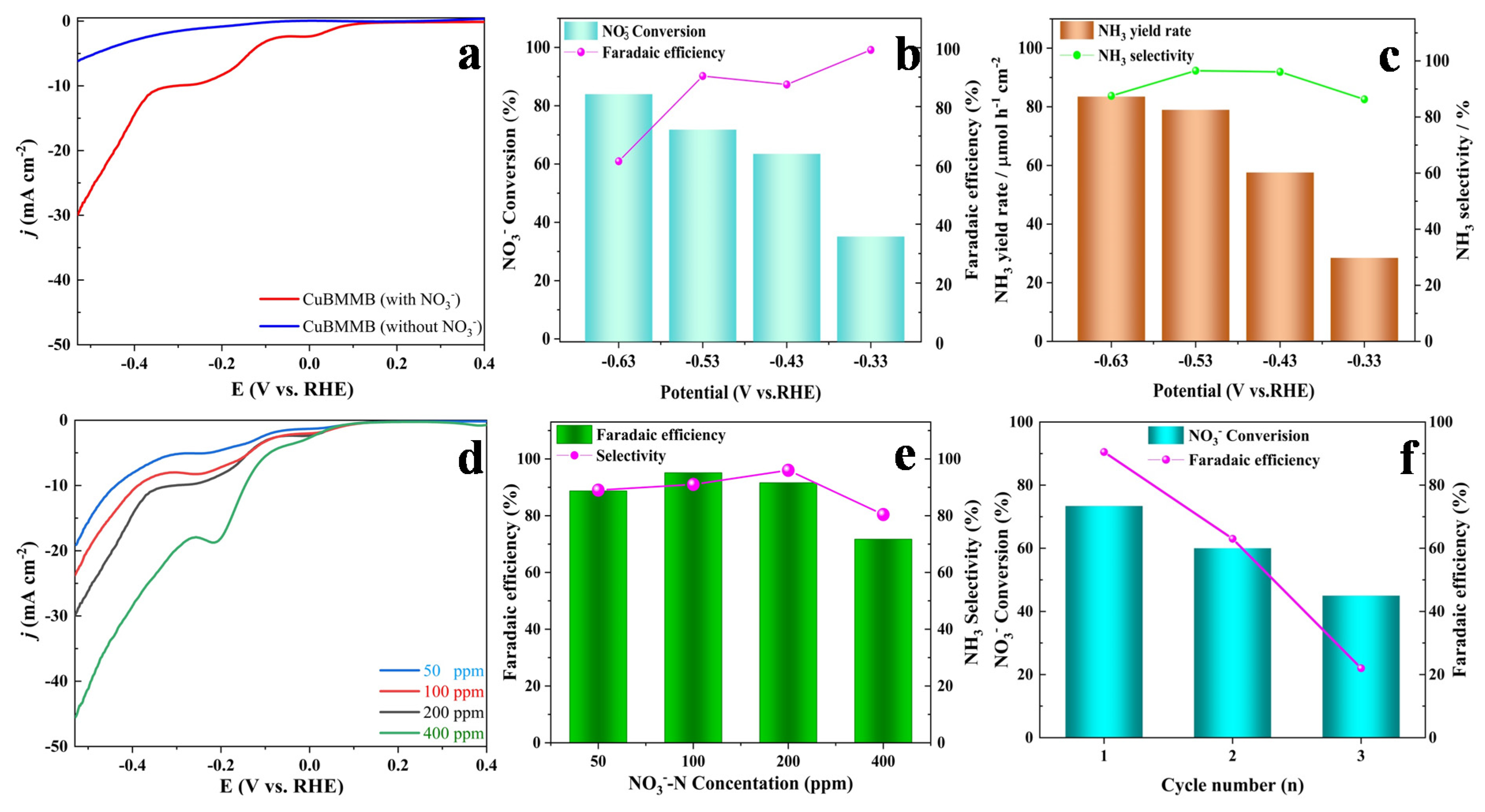
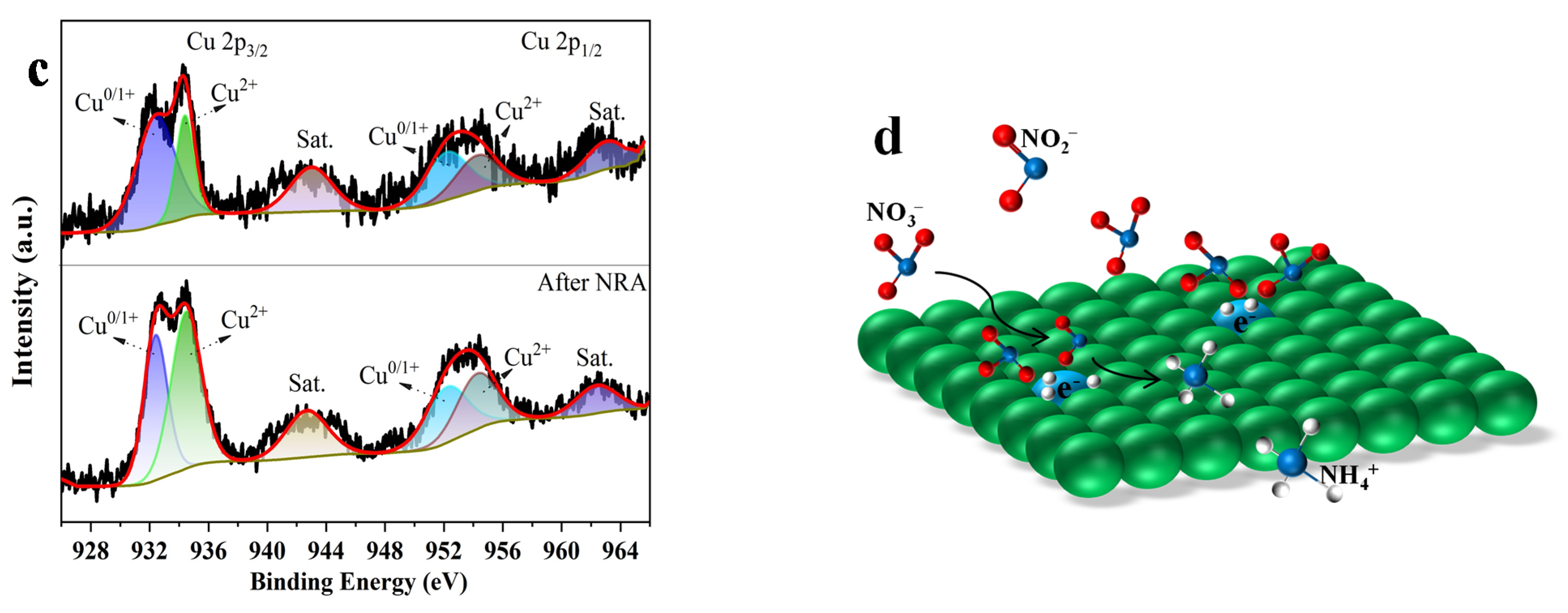
2. Results and Discussion
2.1. Synthesis of Consideration
2.2. Crystal Structure of CuBMMB
3. Materials and Methods
3.1. Synthesis of CuBMMB Crystals and Powder
3.2. Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, T.; Li, Y.; Li, L.; Zhao, Y.; Shi, S.; Jiang, R.; Ma, H. Cu Modified Pt Nanoflowers with Preferential (100) Surfaces for Selective Electroreduction of Nitrate. Catalysts 2019, 9, 536. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Wang, F.; Xiao, Q.; Yu, S.; Ji, X. Selective and Efficient Reduction of Nitrate to Gaseous Nitrogen from Drinking Water Source by UV/Oxalic Acid/Ferric Iron Systems: Effectiveness and Mechanisms. Catalysts 2022, 12, 348. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Greenberg, A.E.; Franson, M.A.H. Standard methods for the examination of water and wastewater. Am. J. Public Health Nations Health 1966, 56, 387–388. [Google Scholar]
- Sanchis, I.; Rodriguez, J.J.; Mohedano, A.F.; Diaz, E. Activity and Stability of Pd Bimetallic Catalysts for Catalytic Nitrate Reduction. Catalysts 2022, 12, 729. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Jia, R.; Zhang, C.; Zhang, B. Electrochemical Synthesis of Nitric Acid from Air and Ammonia through Waste Utilization. Natl. Sci. Rev. 2019, 6, 730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Liu, H.; Jiang, W.; Liu, W. Ordered Mesoporous nZVI/Zr-Ce-SBA-15 Catalysts Used for Nitrate Reduction: Synthesis, Optimization and Mechanism. Catalysts 2022, 12, 797. [Google Scholar] [CrossRef]
- Zou, X.; Chen, C.; Wang, C.; Zhang, Q.; Yu, Z.; Wu, H.; Zhuo, C.; Zhang, T.C. Combining electrochemical nitrate reduction and anammox for treatment of nitrate-rich wastewater: A short review. Sci. Total Environ. 2021, 800, 149645. [Google Scholar] [CrossRef]
- Kim, D.G.; Hwang, Y.H.; Shin, H.S.; Ko, S.O. Kinetics of nitrate adsorption and reduction by nano-scale zero valent iron (NZVI): Effect of ionic strength and initial pH. KSCE J. Civ. Eng. 2015, 20, 175–187. [Google Scholar] [CrossRef]
- Labarca, F.; Bórquez, R. Comparative study of nanofiltration and ion exchange for nitrate reduction in the presence of chloride and iron in groundwater. Sci. Total Environ. 2020, 723, 137809. [Google Scholar] [CrossRef]
- Sheydaei, M.; Ayoubi-Feiz, B. Nitrate reduction through the visible-light photoelectrocatalysis and photoelectrocatalysis/reverse osmosis processes: Assessment of graphene/Ag/N-TiO2 nanocomposite. J. Water Process Eng. 2021, 39, 101856. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, Y.; Ruan, X.; Yin, L. The biological denitrification coupled with chemical reduction for groundwater nitrate remediation via using SCCMs as carbon source. Chemosphere 2019, 234, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Tokazhanov, G.; Ramazanova, E.; Hamid, S.; Bae, S.; Lee, W. Advances in the catalytic reduction of nitrate by metallic catalysts for high efficiency and N2 selectivity: A review. Chem. Eng. J. 2020, 384, 123252. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward Highly Efficient and Selective Electrocatalyst for N2 Conversion to NH3 at Ambient Conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, G.F.; Ding, L.; Chen, X.; Ding, L.X.; Wang, H. Efficient Electrocatalytic N2 Fixation with MXene under Ambient Conditions. Joule 2019, 3, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Han, P.; Wei, Z.; Huang, L.; Gu, Z.; Peng, S.; Ma, J.; Zheng, G. Boron-Doped Graphene for Electrocatalytic N2 Reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Lv, C.; Yan, C.; Chen, G.; Ding, Y.; Sun, J.; Zhou, Y.; Yu, G. An Amorphous Noble-Metal-Free Electrocatalyst that Enables Nitrogen Fixation under Ambient Conditions. Angew. Chem. Int. Ed. 2018, 130, 6181–6184. [Google Scholar] [CrossRef]
- Jung, W.; Hwang, Y.J. Material strategies in the electrochemical nitrate reduction reaction to ammonia production. Mater. Chem. Front. 2021, 5, 6803–6823. [Google Scholar] [CrossRef]
- Wang, J.; Feng, T.; Chen, J.; Ramalingam, V.; Li, Z.; Kabtamu, D.M.; He, J.-H.; Fang, X. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 2021, 86, 106088. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Su, J.F.; Ruzybayev, I.; Shah, I.; Huang, C.P. The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold. Appl. Catal. B Environ. 2016, 180, 199–209. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Q.; Ding, J.; Zhai, Y. Fe–N–C single atom catalysts for the electrochemical conversion of carbon, nitrogen and oxygen elements. Mater. Rep. Energy 2022, 2, 100141. [Google Scholar] [CrossRef]
- Katsounaros, I.; Kyriacou, G. Influence of nitrate concentration on its electrochemical reduction on tin cathode: Identification of reaction intermediates. Electrochim. Acta 2008, 53, 5477–5484. [Google Scholar] [CrossRef]
- Polatides, C.; Kyriacou, G. Electrochemical reduction of nitrate ion on various cathodes-reaction kinetics on bronze cathode. J. Appl. Electrochem. 2005, 35, 421–427. [Google Scholar] [CrossRef]
- Jiang, M.; Su, J.; Song, X.; Zhang, P.; Zhu, M.; Qin, L.; Jin, Z. Interfacial Reduction Nucleation of Noble Metal Nanodots on Redox-Active Metal–Organic Frameworks for High-Efficiency Electrocatalytic Conversion of Nitrate to Ammonia. Nano Lett. 2022, 22, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, K.; Chen, L.; Wang, X.; Zhao, Q.; Liu, B.; Ye, Z. Achieving high selectivity for nitrate electrochemical reduction to ammonia over MOF-supported RuxOy clusters. J. Mater. Chem. A 2022, 10, 3963–3969. [Google Scholar] [CrossRef]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Wang, B.; Lei, F.; Li, L.; Chan, A.W.M.; et al. Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef]
- Chen, T.; Li, H.; Ma, H.; Koper, M.T. Surface Modification of Pt(100) for Electrocatalytic Nitrate Reduction to Dinitrogen in Alkaline Solution. Langmuir 2015, 31, 3277–3281. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Ahamad, N.; Uddin, S.N.; Mohamed, N. Silver modified platinum surface/H+ conducting Nafion membrane for cathodic reduction of nitrate ions. Appl. Surf. Sci. 2012, 258, 3309–3314. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.-H.; Tan, C.-S. Enhanced Nitrate-to-Ammonia Activity on Copper–Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- Li, J.; Gao, J.; Feng, T.; Zhang, H.; Liu, D.; Zhang, C.; Guo, C. Effect of supporting matrixes on performance of copper catalysts in electrochemical nitrate reduction to ammonia. J. Power Sources 2021, 511, 230463. [Google Scholar] [CrossRef]
- Dima, G.E.; De Vooys, A.C.A.; Koper, M.T.M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554–555, 15–23. [Google Scholar] [CrossRef]
- Wu, T.; Kong, X.; Tong, S.; Chen, Y.; Liu, J.; Tang, Y.; Yang, X.; Chen, Y.; Wan, P. Self-supported Cu nanosheets derived from CuCl-CuO for highly efficient electrochemical degradation of NO3−. Appl. Surf. Sci. 2019, 489, 321–329. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Braley, S.E.; Madriaga, J.P.; Smith, J.M.; Jakubikova, E. Electrocatalytic nitrate reduction with Co-based catalysts: Comparison of DIM, TIM and cyclam ligands. Dalton Trans. 2021, 50, 12324–12331. [Google Scholar] [CrossRef]
- Gao, Z.; Lai, Y.; Tao, Y.; Xiao, L.; Zhang, L.; Luo, F. Constructing Well-Defined and Robust Th-MOF-Supported Single-Site Copper for Production and Storage of Ammonia from Electroreduction of Nitrate. ACS Cent. Sci. 2021, 7, 1066–1072. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, H.; Zhang, H.; Zhang, Y.; Shi, P.; Qu, K.; Cheng, S.-B.; Wang, A.-L.; Lu, Q. Filling Mesopores of Conductive Metal–Organic Frameworks with Cu Clusters for Selective Nitrate Reduction to Ammonia. ACS Appl. Mater. Interfaces 2022, 14, 32176–32182. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.T.; Xie, M.Y.; Zhong, H.; Cao, Y. In Situ Clustering of Single-Atom Copper Precatalysts in a Metal-Organic Framework for Efficient Electrocatalytic Nitrate-to-Ammonia Reduction. ACS Catal. 2022, 12, 8698–8706. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, H.; Kim, N.K.; Oh, H.S.; Min, B.K.; Hwang, Y.J. Mixed Copper States in Anodized Cu Electrocatalyst for Stable and Selective Ethylene Production from CO2 Reduction. J. Am. Chem. Soc. 2018, 140, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Xu, J.; Hu, Y.; Huang, Y.; Song, Y. The effect of high external pressure on the structure and stability of MOF α-Mg3(HCOO)6 probed by in situ Raman and FT-IR spectroscopy. J. Mater. Chem. A 2015, 3, 11976–11984. [Google Scholar] [CrossRef]
- Zhang, D.F.; Zhang, H.; Guo, L.; Zheng, K.; Han, X.D.; Zhang, Z. Delicate control of crystallographic facet-oriented Cu2O nanocrystals and the correlated adsorption ability. J. Mater. Chem. 2009, 19, 5220–5225. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Du, F.; Gao, J.; Liu, H.; Huang, S.; Zhang, H.; Li, C.; Guo, C. Interface engineering cerium-doped copper nanocrystal for efficient electrochemical nitrate-to-ammonia production. Electrochim. Acta 2022, 411, 140095. [Google Scholar] [CrossRef]
- Xu, H.; Wu, J.; Luo, W.; Li, Q.; Zhang, W.; Yang, J. Dendritic Cell-Inspired Designed Architectures toward Highly Efficient Electrocatalysts for Nitrate Reduction Reaction. Small 2020, 16, 2001775. [Google Scholar] [CrossRef] [PubMed]
- Tolman, C.A.; Riggs, W.M.; Linn, W.J.; King, C.M.; Wendt, R.C. Electron spectroscopy for chemical analysis of nickel compounds. Inorg. Chem. 1973, 12, 2770–2778. [Google Scholar] [CrossRef]
- Nangan, S.; Ding, Y.; Alhakemy, A.Z.; Liu, Y.; Wen, Z. Hybrid alkali-acid urea-nitrate fuel cell for degrading nitrogen-rich wastewater. Appl. Catal. B: Environ. 2021, 286, 119892. [Google Scholar] [CrossRef]
- Nefedov, V.I.; Salyn, Y.V.; Leonhardt, G.; Scheibe, R. A comparison of different spectrometers and charge corrections used in X-ray photoelectron spectroscopy. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 121–124. [Google Scholar] [CrossRef]
- Du, F.; Li, J.; Wang, C.; Yao, J.; Tan, Z.; Yao, Z.; Li, C.; Guo, C. Active sites-rich layered double hydroxide for nitrate-to-ammonia production with high selectivity and stability. Chem. Eng. J. 2022, 434, 14641. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, K.-Y.; Wang, J.-J.; Fang, X.; Li, Z.-X. One Bicopper Complex with Good Affinity to Nitrate for Highly Selective Electrocatalytic Nitrate Reduction to Ammonia. Catalysts 2022, 12, 1561. https://doi.org/10.3390/catal12121561
Zeng K-Y, Wang J-J, Fang X, Li Z-X. One Bicopper Complex with Good Affinity to Nitrate for Highly Selective Electrocatalytic Nitrate Reduction to Ammonia. Catalysts. 2022; 12(12):1561. https://doi.org/10.3390/catal12121561
Chicago/Turabian StyleZeng, Kang-Yu, Jun-Jie Wang, Xiang Fang, and Zuo-Xi Li. 2022. "One Bicopper Complex with Good Affinity to Nitrate for Highly Selective Electrocatalytic Nitrate Reduction to Ammonia" Catalysts 12, no. 12: 1561. https://doi.org/10.3390/catal12121561
APA StyleZeng, K.-Y., Wang, J.-J., Fang, X., & Li, Z.-X. (2022). One Bicopper Complex with Good Affinity to Nitrate for Highly Selective Electrocatalytic Nitrate Reduction to Ammonia. Catalysts, 12(12), 1561. https://doi.org/10.3390/catal12121561





