Influence of the LaMn1−xFexO3 (x = 0–1) Composition on Catalytic Activities in the Reactions Involving Oxygen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
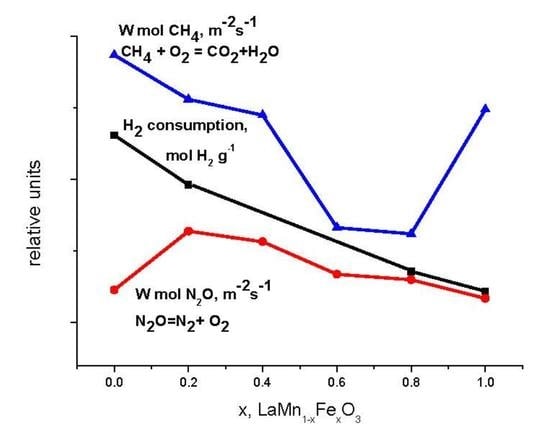
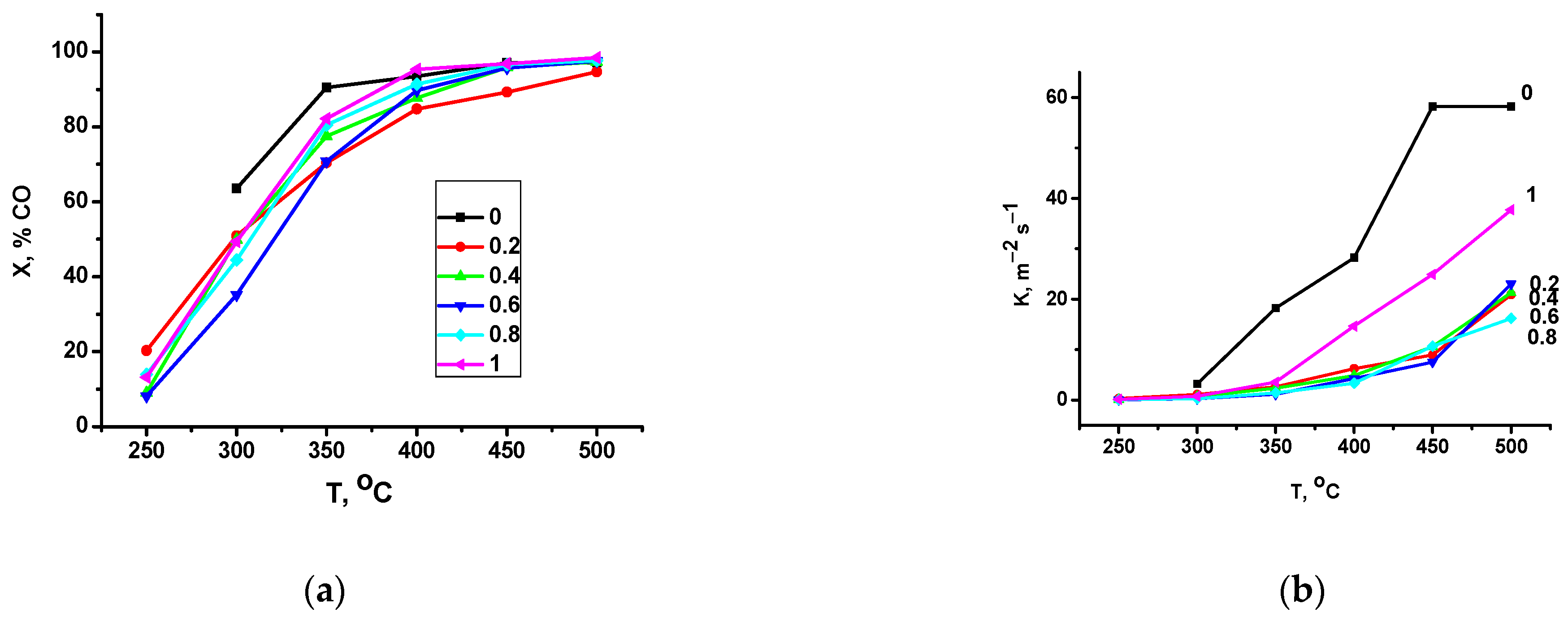
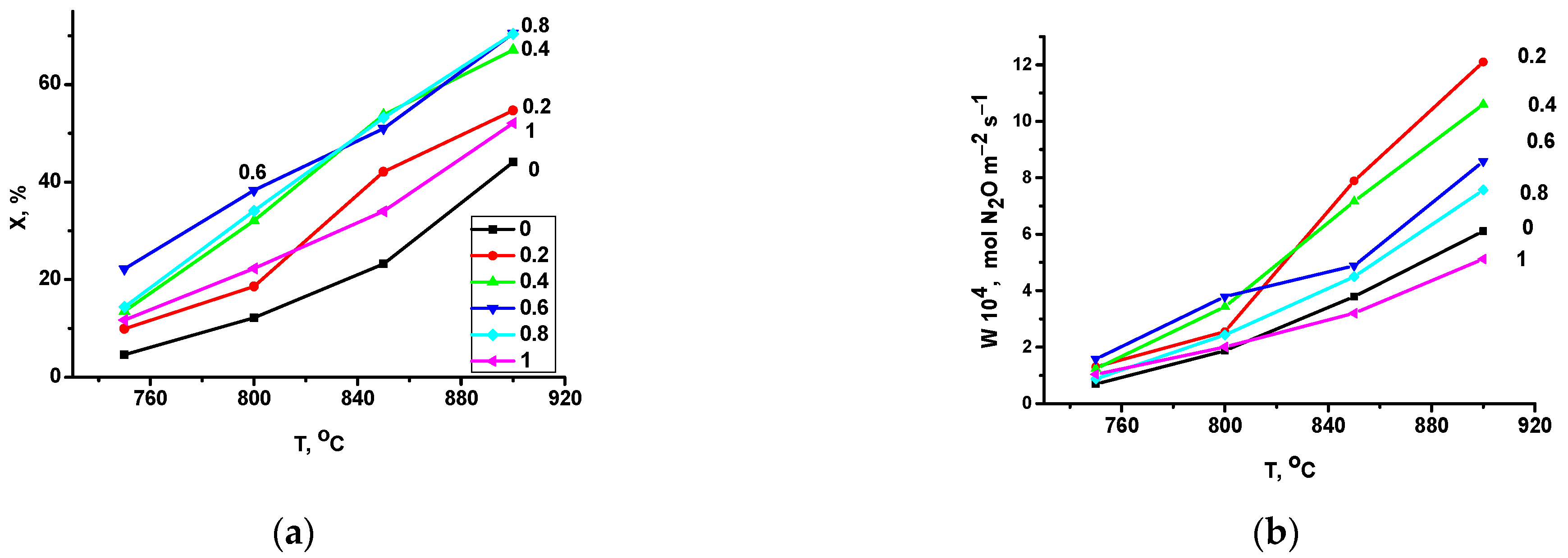
2.2. Catalytic Activity in Methane and CO Oxidation and Nitrous Oxide Decomposition
3. Materials and Methods
3.1. Preparation of the Catalysts
3.2. Characterization of the Catalysts
3.3. Catalytic Tests
4. Conclusions
- Perovskite samples of the LaMn1−xFexO3 (x = 0–1) series were prepared by the Pechini method; their physicochemical properties were studied and catalytic activity was estimated in methane and CO oxidation and nitrous oxide decomposition reactions.
- Samples with the composition x < 0.4 were shown to be the orthorhombic perovskites, whereas samples with x > 0.4 were the rhombohedral ones. Both modifications are present in the sample with x = 0.4, which indicates that the morphotropic phase transition occurs in the systems in the composition region with x~0.4. The TPR study revealed that an increase in the x value decreases the amount of weakly bound oxygen and lowers the temperature at which the reduction starts. Specific surface area values change through the maxima at x = 0.8. The XPS study demonstrated that the surface of the samples with 0 < x < 1 prepared by the Pechini method is enriched with lanthanum compounds.
- All of the intermediate samples of the series were shown to have a lower normalized per m2 catalytic activity in CO and methane oxidation reactions as compared to extreme terms of the series; therefore, LaMnO3 was more active than LaFeO3. The observed dependence of the samples’ activity with an increase in the x value cannot be attributed only to a decrease in the content of weakly bound oxygen species, which behave in a linear manner with an increase in x. An additional effect is exerted by an increase in the content of lanthanum compounds on the surface, which leads to blocking of the surface by the reaction product (CO2) that forms surface carbonates with lanthanum compounds. The higher the content of lanthanum on the surface, the more pronounced is the detected decrease in activity. It cannot be ruled out also that an additional decrease in activity of the samples with high iron content during methane oxidation is promoted by the formation of locally ordered regions and carbon on the surface of these oxides upon testing. So, the best normalized per m2 activity in methane and CO oxidation among the studied perovskites was revealed for LaMnO3.
- It was shown that all of the intermediate samples of the series, on the contrary, are more active in the high-temperature decomposition of nitrous oxide than the extreme terms of the series; the maximum normalized catalytic activity was observed for the sample with x = 0.2. A decrease in activity with the growth of x in the series with x = 0.2–1 correlates with the content of weakly bound oxygen species in the samples. Lanthanum manganite may fall out of the correlation due to the difference in the rate-determining stage of the process in the case of this oxide. Therefore, the best normalized per m2 activity in high-temperature N2O decomposition was revealed for LaMn0.2Fe0.8O3.
- Different dependences of activities on the catalyst’s composition or amount of weakly bound oxygen species in the samples obtained in the study for the tested reactions, which are implemented with participation of the catalyst oxygen, are caused by the effect of characteristic features of the reaction mechanisms and the preparation method.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Tang, Y.; Yin, Y.; Gong, W.; Song, J.; Ma, Y.; Ruan, M.; Xu, H.; Chen, D. Research progress in enhanced catalytic oxidation of VOCs by modified La-based perovskite catalyst. Chem. Ind. Eng. Progress 2021, 40, 1425–1437. [Google Scholar]
- Huang, H.F.; Sun, Z.; Lu, H.F.; Shen, L.Q.; Chen, B.V.; Ust, Y. Perovskite catalysts for methane combustion: Applications, design, effects for reactivity and partial oxidation. Energy Res. 2019, 43, 7755–7789. [Google Scholar]
- Russo, N.; Mescia, D.; Fino, D.; Saracco, G.; Specchia, V. N2O decomposition over perovskite catalysts. Ind. Eng. Chem. Res. 2007, 46, 4226–4231. [Google Scholar] [CrossRef]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Isupova, L.A.; Zolotarskii, I.A.; Bobrova, L.N.; Noskov, A.S.; Parmon, V.N.; Brushtein, E.A.; Telyatnikova, T.V.; Chernyshev, V.I.; Lunin, V.V. Oxide catalysts for ammonia oxidation in nitric acid production: Properties and perspectives. Appl. Catal. A Gen. 2000, 204, 59–87. [Google Scholar] [CrossRef]
- Wang, X.; Wu, T.; Zachariah, M.R. Doped Perovskites to Evaluate the Relationship between Fuel–Oxidizer Thermite Ignition and Bond Energy, Electronegativity, and Oxygen Vacancy. J. Phys. Chem. C 2017, 121, 147–152. [Google Scholar] [CrossRef]
- Yakovleva, I.S.; Isupova, L.A.; Rogov, V.A.; Sadykov, V.A. Forms of oxygen in La1−xCaxMnO3+δ (x = 0–1) perovskites and their reactivities in oxidation reactions. Kinet. Catal. 2008, 49, 261–270. [Google Scholar] [CrossRef]
- Miao, F.; Wang, F.; Mao, D.; Guo, X.; Yu, J.; Huang, H.; Lu, G. Effect of different reaction conditions on catalytic activity of La(Mn, Fe)O3+λ catalyst for methane combustion. Mater. Res. Express 2019, 6, 055001. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Sadovskaya, E.M.; Isupova, L.A. Influence of the mobility of oxygen on the reactivity of La1−x Srx MnO3 perovskites in methane oxidation. Kinet. Catal. 2011, 52, 401–408. [Google Scholar] [CrossRef]
- Zheng, S.; Hua, Q.; Gu, W.; Liu, B. Catalytic oxidation of CO on LaMn1−xFexO3 perovskites solid solution. J. Mol. Catal. A Chem. 2014, 391, 7–11. [Google Scholar] [CrossRef]
- Isupova, L.A.; Tsybulya, S.V.; Kryukova, G.N.; Alikina, G.M.; Boldyreva, N.N.; Yakovleva, I.S.; Ivanov, V.P.; Sadykov, V.A. Real structure and catalytic activity of La1−xCaxMnO3 perovskites. Solid State Ion. 2001, 141–142, 417–425. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Wołcyrz, M.; Horyn, R.; Bourée, F.; Bukowska, E. Structural defects in LaMnO3 phase studied by neutron diffraction. J. Alloys Compd. 2003, 353, 170–174. [Google Scholar] [CrossRef]
- Mizusaki, J.; Sasamoto, T.; Cannon, W.R.; Bowen, H.K. Electronic Conductivity, Seebeck Coefficient, and Defect Structure of LaFeO3. J. Am. Ceram. Soc. 2006, 65, 363–368. [Google Scholar] [CrossRef]
- Tarjomannejad, A.; Farzi, A.; Gómez, M.J.I.; Niaei, A.; Salari, D.; Albaladejo-Fuentes, V. Catalytic Reduction of NO by CO over LaMn1−xFexO3 and La0.8A0.2Mn0.3Fe0.7O3 (A = Sr, Cs, Ba, Ce) Perovskite Catalysts. Catal. Lett. 2016, 146, 2330–2340. [Google Scholar] [CrossRef]
- Magalhaes, F.; Moura, F.C.C.; Ardisson, J.D.; Lago, R.M. LaMn1−xFexO3 and LaMn0.1−xFe0.90MoxO3 Perovskites: Synthesis, Characterization and Catalytic Activity in H2O2 Reactions. Mater. Res. 2008, 11, 307–312. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, J.; Zhao, Z.; Xu, J.; Jiang, G. The synthesis and catalytic performances of three-dimensionally ordered macroporous perovskite-type LaMn1−xFexO3 complex oxide catalysts with different pore diameters for diesel soot combustion. Catal. Today 2012, 191, 146–153. [Google Scholar] [CrossRef]
- Zhou, Z.; Pederson, L.R.; Cai, Q.; Jang, J.; Scarfino, B.J.; Kim, M.; Yelon, W.B.; James, W.J.; Anderson, H.U.; Wang., C. Structural and magnetic properties of LaMn1−xFexO3 (0 < x < 1.0). J. Appl. Phys. 2006, 99, 08M918. [Google Scholar]
- Isupova, L.A.; Gerasimov, E.Y.; Zaikovskii, V.I.; Tsybulya, S.V. Effect of the Reaction Medium on the Structure of the La1−xCaxMnO3 (x = 0–1) Solid Solutions Prepared by the Pechini Method. Kinet. Catal. 2011, 52, 104–110. [Google Scholar] [CrossRef]
- Sedykh, V.; Shekhtman, V.S.; Zverkova, I.I.; Dubovitskii, A.V.; Kulakov, V.I. Reversibility of structure phase transitions in LaMnO3+δ manganite under heat treatment. Phys. C 2006, 433, 189–194. [Google Scholar] [CrossRef]
- Belysheva, D.N.; Sinelshchikova, O.Y.; Tyurnina, N.G.; Tyurnina, Z.G.; Sviridov, S.I.; Tumarkin, A.V.; Zlygostov, M.V.; Ugolkov, V.L. Glycine–Nitrate Synthesis of Solid Solutions of Barium–Strontium Metatitanate. Phys. Solid State 2019, 61, 2371. [Google Scholar] [CrossRef]
- Mengli, L.; Xiaolong, Y.; Liping, T.; Xumao, X.; Sili, R.; Hu, B.H. Catalysts for Catalytic Decomposition of Nitrous Oxide. Prog. Chem. 2012, 9, 1801–1817. [Google Scholar]
- Konsolakis, M. Recent Advances on Nitrous Oxide (N2O) Decomposition over Non-Noble-Metal Oxide Catalysts: Catalytic Performance, Mechanistic Considerations, and Surface Chemistry Aspects. ACS Catal. 2015, 5, 6397–6421. [Google Scholar] [CrossRef]
- Ivanov, D.V.; Pinaeva, L.G.; Sadovskaya, E.M.; Isupova, L.A. Isotopic transient kinetic study of N2O decomposition on LaMnO3+δ. J. Mol. Catal. A Chem. 2016, 412, 34–38. [Google Scholar] [CrossRef]
- Tsybulya, S.V.; Cherepanova, S.V.; Soloviova, L.P. Polycrystal software package for IBM/PC. J. Struct. Chem. 1996, 37, 332–334. [Google Scholar] [CrossRef]
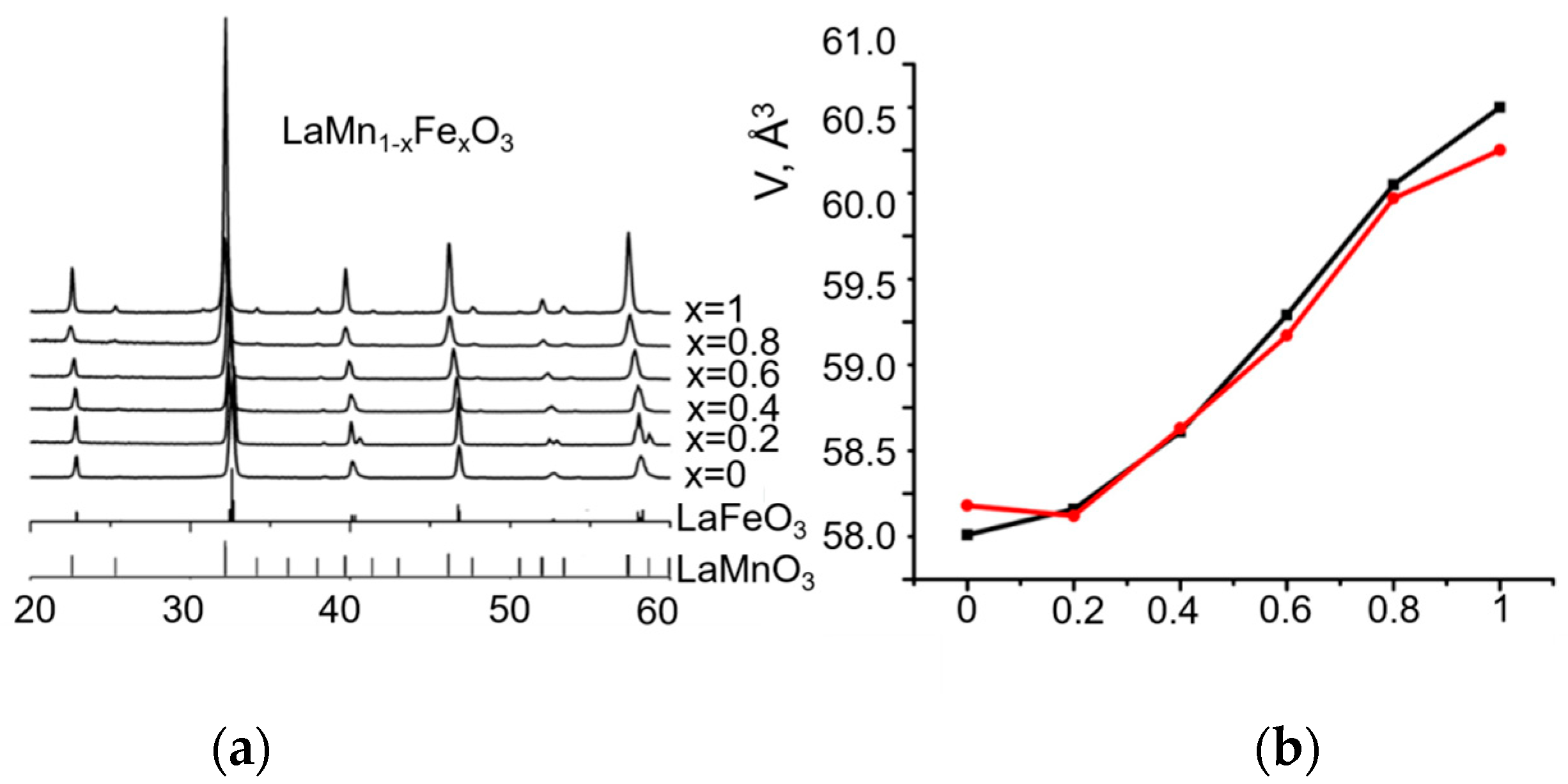

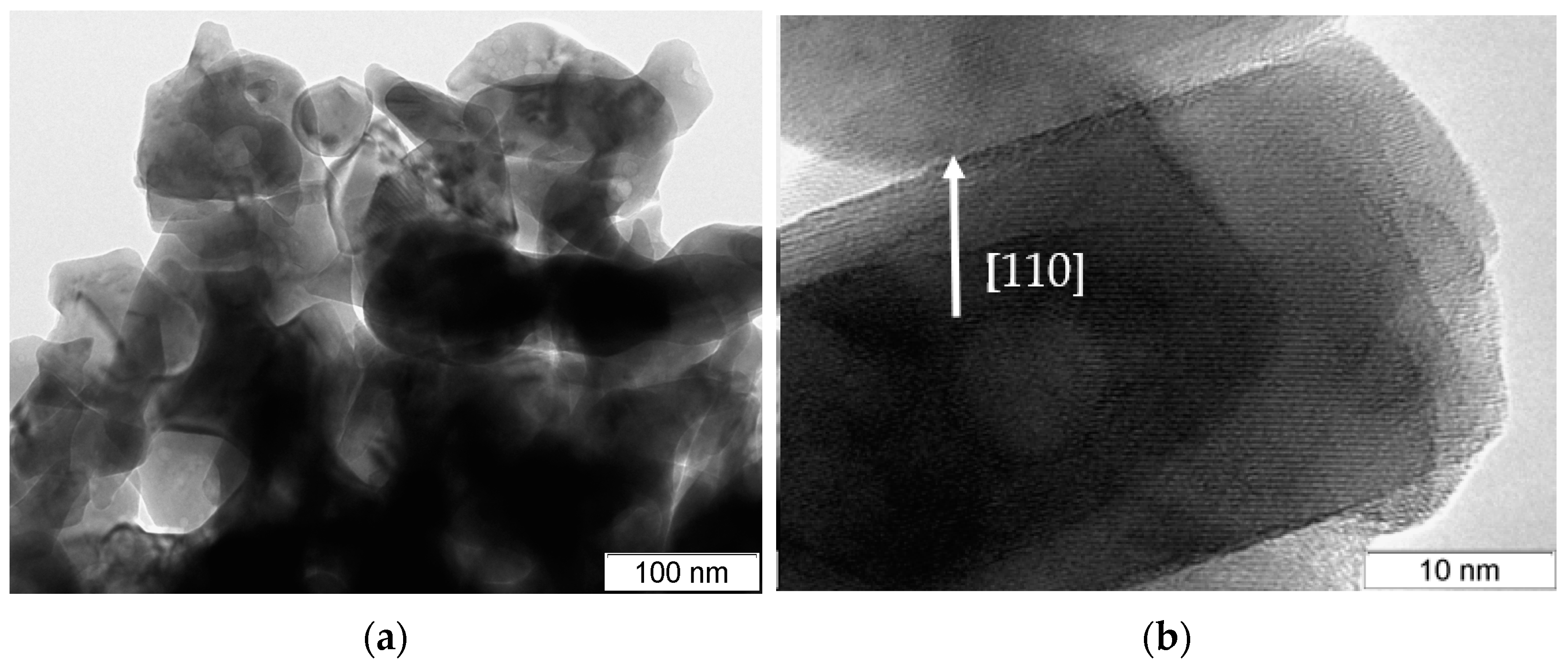
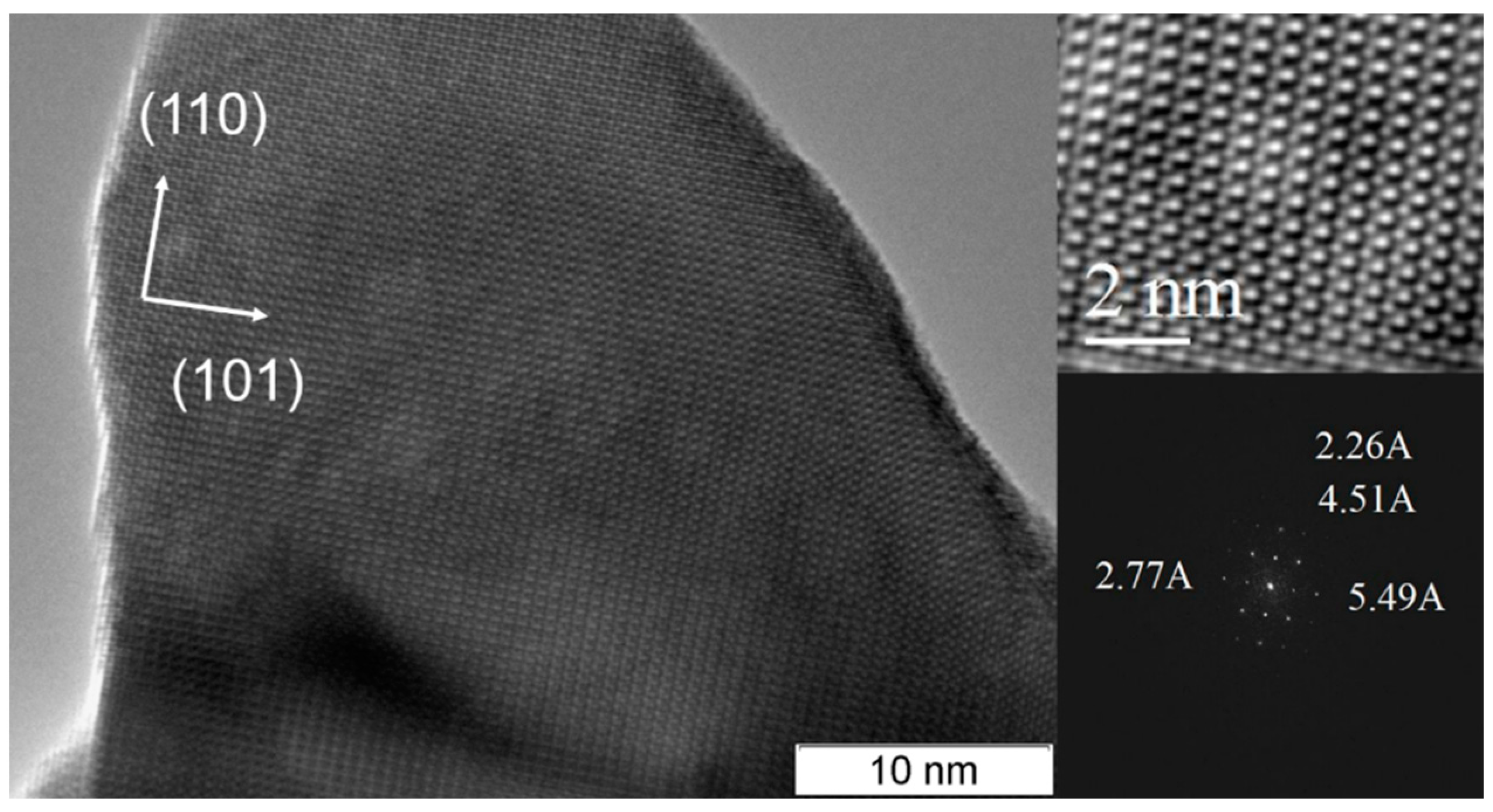


| Sample | Space Group | Unit Cell Parameters before Testing | Unit Cell Parameters after Testing | D (Å) before Testing | D (Å) after Testing | Δd/d∙104 before Testing | Δd/d∙104 after Testing | Ssp, m2/g |
|---|---|---|---|---|---|---|---|---|
| LaMnO3 | Pnmb * | a = 5.491 (8) b = 7.774 (4) c = 5.489 (9) | a = b = 5.526 (3) c = 13.324 (8) | 660 | 660 | 3.9 | 4.5 | 3.2 |
| LaMn0.8Fe0.2O3 | R-3c | a = b = 5.514 (1) c = 13.310 (1) | a = b = 5.513 (1) c = 13.307 (1) | >1000 | - | - | 4.3 | |
| LaMn0.6Fe0.4O3 | R-3c | a = b = 5.521 (1) c = 13.352 (4) | a = b = 5.517 (1) c = 13.310 (2) | - | - | - | - | 6.0 |
| Pnma | a = 5.486 (1) b = 7.769 (1) c = 5.524 (1) | a = 5.486 (1) b = 7.771 (1) c = 5.525 (1) | 950 | 860 | 1.8 | 5.9 | ||
| LaMn0.4Fe0.6O3 | Pnma | a = 5.512 (1) b = 7.805 (1) c = 5.536 (1) | a = 5.502 (1) b = 7.812 (1) c = 5.530 (1) | 920 | 920 | 7.4 | 11.0 | 8.4 |
| LaMn0.2Fe0.8O3 | Pnma | a = 5.543 (1) b = 7.832 (1) c = 5.556 (1) | a = 5.540 (1) b = 7.829 (1) c = 5.554 (1) | 750 | 650 | 11.0 | 10.0 | 10.0 |
| LaFeO3 | Pnma | a = 5.563 (1) b = 7.860 (1) c = 5.560 (1) | a = 5.555 (2) b = 7.852 (2) c = 5.549 (1) | 900 | 1000 | 10.0 | 20.0 | 6.1 |
| Oxide | Mn2p3/2 | Fe2p3/2 | La3d5/2 | O1s | [Fe]/[La] | [Mn]/[La] | [Fe]/[Mn] | [O]/[La] | [C]/[La] | [Fe+Mn]/[La] |
|---|---|---|---|---|---|---|---|---|---|---|
| LaMn0.8Fe0.2O3 | 642.29 | 710.95 | 834.26 | 529.51 531.27 533.33 | 0.06 | 0.51 | 0.13 | 3.11 | 1.53 | 0.57 |
| LaMn0.6Fe0.4O3 | 642.30 | 710.91 | 834.29 | 529.57 531.56 533.62 | 0.14 | 0.39 | 0.35 | 2.92 | 1.33 | 0.53 |
| LaMn0.4Fe0.6O3 | 642.31 | 710.85 | 834.48 | 529.96 532.15 533.92 | 0.17 | 0.28 | 0.62 | 3.14 | 3.13 | 0.45 |
| LaMn0.2Fe0.8O3 | 642.35 | 710.79 | 834.37 | 529.85 532.07 533.75 | 0.23 | 0.15 | 1.51 | 3.20 | 3.12 | 0.38 |
| Sample | Adsorption in the Range of 40–900 °C, mol H2/g | Adsorption in the Range of 40–550 °C, mol H2/g | Temperature of Adsorption Maxima in the Range of 40–550 °C |
|---|---|---|---|
| LaMnO3 | 34.4 × 10–4 | 13.1 × 10−4 | 520 |
| LaMn0.8Fe0.2O3 | 18.3 × 10–4 | 9.64 × 10−4 | 413 |
| LaMn0.2Fe0.8O3 | 6.71 × 10–4 | 3.58 × 10−4 | 382 |
| LaFeO3 | 5.8 × 10–4 | 2.2 × 10−4 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isupova, L.; Rogov, V.; Gerasimov, E.; Prosvirin, I. Influence of the LaMn1−xFexO3 (x = 0–1) Composition on Catalytic Activities in the Reactions Involving Oxygen. Catalysts 2022, 12, 1563. https://doi.org/10.3390/catal12121563
Isupova L, Rogov V, Gerasimov E, Prosvirin I. Influence of the LaMn1−xFexO3 (x = 0–1) Composition on Catalytic Activities in the Reactions Involving Oxygen. Catalysts. 2022; 12(12):1563. https://doi.org/10.3390/catal12121563
Chicago/Turabian StyleIsupova, Lyubov, Vladimir Rogov, Evgenii Gerasimov, and Igor Prosvirin. 2022. "Influence of the LaMn1−xFexO3 (x = 0–1) Composition on Catalytic Activities in the Reactions Involving Oxygen" Catalysts 12, no. 12: 1563. https://doi.org/10.3390/catal12121563
APA StyleIsupova, L., Rogov, V., Gerasimov, E., & Prosvirin, I. (2022). Influence of the LaMn1−xFexO3 (x = 0–1) Composition on Catalytic Activities in the Reactions Involving Oxygen. Catalysts, 12(12), 1563. https://doi.org/10.3390/catal12121563