Catalytic Combustion of Propane over Ce-Doped Lanthanum Borate Loaded with Various 3d Transition Metals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.2. Variations over Surface Chemistry
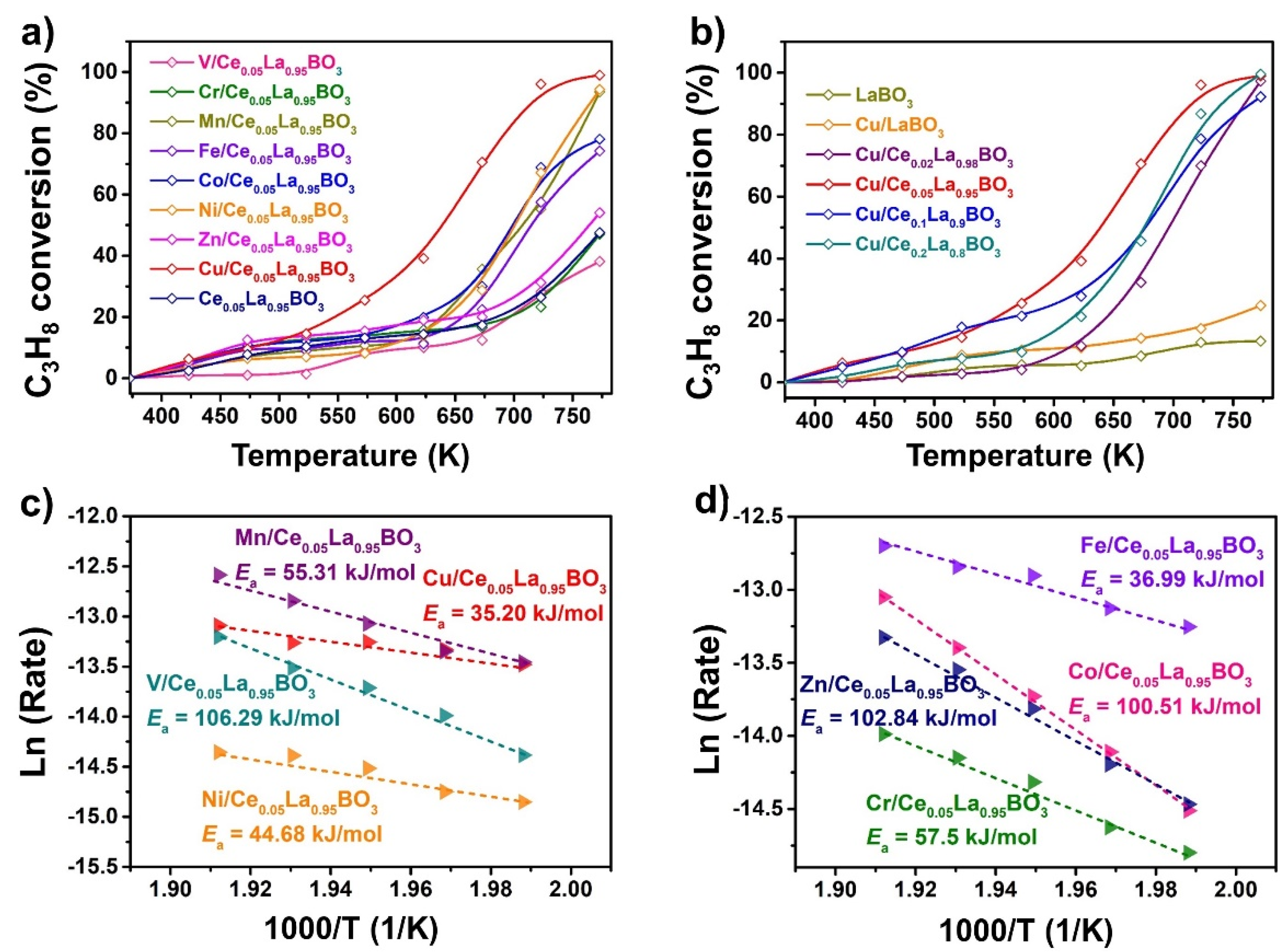
2.3. Catalyst Performance
3. Materials and Methods
3.1. Catalyst Synthesis
3.1.1. Preparation of Ce0.05La0.95BO3 Support
3.1.2. Preparation of Ce0.05La0.95BO3 Supported with 1 wt% Metal Oxide
3.2. Catalyst Characterization
3.3. Catalytic Evaluations and Kinetic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- He, C.; Cheng, J.; Zhang, X.; Douthwaite, M.; Pattisson, S.; Hao, Z. Recent advances in the catalytic oxidation of volatile organic compounds: A review based on pollutant sorts and sources. Chem. Rev. 2019, 119, 4471–4568. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.C. The changing face of urban air pollution. Science 2018, 359, 744–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padilla, O.; Munera, J.; Gallego, J.; Santamaria, A. Approach to the Characterization of Monolithic Catalysts Based on La Perovskite-like Oxides and Their Application for VOC Oxidation under Simulated Indoor Environment Conditions. Catalysts 2022, 12, 168. [Google Scholar] [CrossRef]
- Jian, Y.; Tian, M.; He, C.; Xiong, J.; Jiang, Z.; Jin, H.; Zheng, L.; Albilali, R.; Shi, J.W. Efficient propane low-temperature destruction by Co3O4 crystal facets engineering: Unveiling the decisive role of lattice and oxygen defects and surface acid-base pairs. Appl. Catal. B 2021, 283, 119657. [Google Scholar] [CrossRef]
- Liao, Z.; Zha, K.; Sun, W.; Huang, Z.; Xu, H.; Shen, W. Catalytic Combustion of Propane over Pt-Mo/ZSM-5 Catalyst: The Promotional Effects of Molybdenum. Catalysts 2020, 10, 1377. [Google Scholar] [CrossRef]
- Dong, T.; Liu, W.; Ma, M.; Peng, H.; Yang, S.; Tao, J.; He, C.; Wang, L.; An, T. Hierarchical zeolite enveloping Pd-CeO2 nanowires: An efficient adsorption/catalysis bifunctional catalyst for low temperature propane total degradation. Chem. Eng. J. 2020, 393, 124717. [Google Scholar] [CrossRef]
- He, Y.; Song, Y.; Cullen, D.A.; Laursen, S. Selective and stable non-noble-metal intermetallic compound catalyst for the direct dehydrogenation of propane to propylene. J. Am. Chem. Soc. 2018, 140, 14010–14014. [Google Scholar] [CrossRef]
- Song, Y.; He, Y.; Laursen, S. Controlling Selectivity and Stability in the Hydrocarbon Wet-Reforming Reaction Using Well-Defined Ni+ Ga Intermetallic Compound Catalysts. ACS Catal. 2020, 10, 8968–8980. [Google Scholar] [CrossRef]
- He, Y.; Song, Y.; Laursen, S. The origin of the special surface and catalytic chemistry of Ga-rich Ni3Ga in the direct dehydrogenation of ethane. ACS Catal. 2019, 9, 10464–10468. [Google Scholar] [CrossRef]
- Van der Vaart, D.R.; Marchand, E.G.; Bagely-Pride, A. Thermal and catalytic incineration of volatile organic compounds. Crit. Rev. Environ. Sci. Technol. 1994, 24, 203–236. [Google Scholar] [CrossRef]
- Chen, C.; Huang, Y.; Hung, S.; Chen, C.; Lin, C.; Yang, H. Hydrophobic deep eutectic solvents as attractive media for low-concentration hydrophobic VOC capture. Chem. Eng. J. 2021, 424, 130420. [Google Scholar] [CrossRef]
- Jiang, X.; Lis, B.M.; Purdy, S.C.; Paladugu, S.; Fung, V.; Quan, W.; Bao, Z.; Yang, W.; He, Y.; Sumpter, B.G.; et al. CO2-Assisted Oxidative Dehydrogenation of Propane over VOx/In2O3 Catalysts: Interplay between Redox Property and Acid-Base Interactions. ACS Catal. 2022, 12, 11239–11252. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, X.; Purdy, S.C.; He, Y.; Huang, Z.; You, R.; Wei, Z.; Meyer III, H.M.; Yang, J.; Pan, Y.; et al. Multiple Promotional Effects of Vanadium Oxide on Boron Nitride for Oxidative Dehydrogenation of Propane. JACS Au 2022, 2, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xu, X.; Chen, L.; Guo, J.; Wang, J. A novel cryogenic condensation system based on heat-driven refrigerator without power input for volatile organic compounds recovery. Energy Convers. Manag. 2021, 238, 114157. [Google Scholar] [CrossRef]
- Dong, F.; Han, W.; Guo, Y.; Han, W.; Tang, Z. CeCoOx-MNS catalyst derived from three-dimensional mesh nanosheet Co-based metal–organic frameworks for highly efficient catalytic combustion of VOCs. Chem. Eng. J. 2021, 405, 126948. [Google Scholar] [CrossRef]
- Qiu, Y.; Ye, N.; Situ, D.; Zuo, S.; Wang, X. Study of catalytic combustion of chlorobenzene and temperature programmed reactions over CrCeOx/AlFe pillared clay catalysts. Materials 2019, 12, 728. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Li, X.; Sun, C.; Huang, Z.; Xu, H.; Shen, W. Insights into the pyrolysis processes of Ce-MOFs for preparing highly active catalysts of toluene combustion. Catalysts 2019, 9, 682. [Google Scholar] [CrossRef] [Green Version]
- Chai, G.; Zhang, W.; Liotta, L.F.; Li, M.; Guo, Y.; Giroir-Fendler, A. Total oxidation of propane over Co3O4-based catalysts: Elucidating the influence of Zr dopant. Appl. Catal. B 2021, 298, 120606. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Z.; Wang, D.; Wang, C.; Yu, J.; Wu, G.; Zhang, Q.; Li, X.; Zhang, C.; Zhao, X. Three-dimensionally ordered macroporous MnSmOx composite oxides for propane combustion: Modification effect of Sm dopant. Catal. Today 2021, 376, 211–221. [Google Scholar] [CrossRef]
- Cai, T.; Liu, Z.; Yuan, J.; Xu, P.; Zhao, K.; Tong, Q.; Lu, W.; He, D. The structural evolution of MnOx with calcination temperature and their catalytic performance for propane total oxidation. Appl. Surf. Sci. 2021, 565, 150596. [Google Scholar] [CrossRef]
- Zhang, W.; Descorme, C.; Valverde, J.L.; Giroir-Fendler, A. Cu-Co mixed oxide catalysts for the total oxidation of toluene and propane. Catal. Today 2022, 384, 238–245. [Google Scholar] [CrossRef]
- Liao, W.; Zhao, P.; Cen, B.; Jia, A.; Lu, J.; Luo, M. Co–Cr–O mixed oxides for low–temperature total oxidation of propane: Structural effects, kinetics, and spectroscopic investigation. Chin. J. Catal. 2020, 41, 442–453. [Google Scholar] [CrossRef]
- Hao, Y.; Chen, S.; Wu, L.; Chen, R.; Sun, P.; Chen, T. Hierarchically porous silica supported ceria and platinum nanoparticles for catalytic combustion of toluene. J. Alloys Compd. 2021, 867, 159030. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, Q.; Lin, B.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, L.; Zhan, W. Superior catalytic activity of a Pd catalyst in methane combustion by fine-tuning the phase of ceria-zirconia support. Appl. Catal. B 2020, 266, 118631. [Google Scholar] [CrossRef]
- Zhan, W.; Guo, Y.; Gong, X.; Guo, Y.; Wang, Y.; Lu, G. Current status and perspectives of rare earth catalytic materials and catalysis. Chin. J. Catal. 2014, 35, 1238–1250. [Google Scholar] [CrossRef]
- Ye, N.; Li, Y.; Yang, Z.; Zheng, J.; Zuo, S. Rare earth modified kaolin-based Cr2O3 catalysts for catalytic combustion of chlorobenzene. Appl. Catal. A 2019, 579, 44–51. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Sun, Y.; Wang, W.; Shao, Z. Perovskite oxides in catalytic combustion of volatile organic compounds: Recent advances and future prospects. Energy Environ. Mater. 2022, 5, 751–776. [Google Scholar] [CrossRef]
- Luo, Y.; Zuo, J.; Feng, X.; Qian, Q.; Zheng, Y.; Lin, D.; Huang, B.; Chen, Q. Good interaction between well dispersed Pt and LaCoO3 nanorods achieved rapid Co3+/Co2+ redox cycle for total propane oxidation. Chem. Eng. J. 2019, 357, 395–403. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, F.; Niu, X.; Zhu, Y. Preparation of Pd supported on La (Sr)-Mn-O perovskite by microwave irradiation method and its catalytic performances for the methane combustion. Sci. Rep. 2016, 6, 19511. [Google Scholar] [CrossRef] [Green Version]
- Zang, M.; Zhao, C.; Wang, Y.; Chen, S. A review of recent advances in catalytic combustion of VOCs on perovskite-type catalysts. J. Saudi Chem. Soc. 2019, 23, 645–654. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, K.; Wang, C.; Liu, X.; Wu, G.; Wang, Z.; Wang, D. LaMnO3 perovskites via a facile nickel substitution strategy for boosting propane combustion performance. Ceram. Int. 2020, 46, 6652–6662. [Google Scholar] [CrossRef]
- Dai, L.; Lu, X.B.; Chu, G.H.; He, C.H.; Zhan, W.C.; Zhou, G.J. Surface tuning of LaCoO3 perovskite by acid etching to enhance its catalytic performance. Rare Met. 2021, 40, 555–562. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Liu, Z.; Liang, C. Insight into the effect of cobalt substitution on the catalytic performance of LaMnO3 perovskites for total oxidation of propane. J. Phys. Chem. A 2020, 124, 14646–14657. [Google Scholar] [CrossRef]
- Lu, Y.; Dai, Q.; Wang, X. Catalytic combustion of chlorobenzene on modified LaMnO3 catalysts. Catal. Commun. 2014, 54, 114–117. [Google Scholar] [CrossRef]
- Byron, C.; Bai, S.; Celik, G.; Ferrandon, M.S.; Liu, C.; Ni, C.; Mehdad, A.; Delferro, M.; Lobo, R.; Teplyakov, A.V. Role of boron in enhancing the catalytic performance of supported platinum catalysts for the nonoxidative dehydrogenation of n-butane. ACS Catal. 2019, 10, 1500–1510. [Google Scholar] [CrossRef]
- Zhou, H.; Yi, X.; Hui, Y.; Wang, L.; Chen, W.; Qin, Y.; Wang, M.; Ma, J.; Chu, X.; Wang, Y.; et al. Isolated boron in zeolite for oxidative dehydrogenation of propane. Science 2021, 372, 76–80. [Google Scholar] [CrossRef]
- Park, S.; Chang, S. Catalytic Dearomatization of N-Heteroarenes with Silicon and Boron Compounds. Angew. Chem. Int. Ed. 2017, 56, 7720–7738. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, X.; Dang, Y.; Ouyang, P.; Zhang, H.; Jiang, G.; Dong, F.; Yang, T.; Suib, S.L.; He, Y. Fe doped aluminoborate PKU-1 catalysts for the ketalization of glycerol to solketal: Unveiling the effects of iron composition and boron. Chin. Chem. Lett. 2022, 33, 1346–1352. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, X.; He, S.; Zhang, H.; Jiang, G.; He, Y.; Liu, Y.; Dong, F.; Zhang, X.; Suib, S.L. Understanding how the crystalline nature of Fe-promoted alumino-borate PKU-1 catalyst affects Glycerin ketalization: The systematic control of surface composition and acidity. Appl. Catal. A 2022, 118945. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Zhang, J.; Ding, X.; Qi, S.; Tang, C. Preparation and characterization of lanthanum borate nanowires. Mater. Lett. 2007, 61, 1596–1600. [Google Scholar] [CrossRef]
- Sari, S.; Senberber, F.T.; Yildirim, M.; Kipcak, A.S.; Yuksel, S.A.; Derun, E.M. Lanthanum borate synthesis via the solid-state method from a La2O3 precursor: Electrical and optical properties. Mater. Chem. Phys. 2017, 200, 196–203. [Google Scholar] [CrossRef]
- Górecka, S.; Pacultová, K.; Smýkalová, A.; Fridrichová, D.; Górecki, K.; Rokicińska, A.; Kuśtrowskic, P.; Žebrákd, R.; Obalová, L. Role of the Cu content and Ce activating effect on catalytic performance of Cu-Mg-Al and Ce/Cu-Mg-Al oxides in ammonia selective catalytic oxidation. Appl. Surf. Sci. 2022, 573, 151540. [Google Scholar] [CrossRef]
- Boehlhoff, R.; Bambauer, H.U.; Hoffmann, W. Die Kristallstruktur von Hoch-LaBO3. Z. Kristallogr. Cryst.-Mater. 1971, 133, 386–395. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Song, F.; Zhang, S.; Zhong, Q. The effect of CuO loading on different method prepared CeO2 catalyst for toluene oxidationn. Sci. Total Environ. 2020, 712, 135635. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Li, C.; Zhang, X.; Zeng, F.; Lin, H.; Su, Z. Optical and mechanical properties of NaCl: Ce3+ crystal grown by the Czochralski method. J. Mater. Sci. Mater. Electron. 2020, 31, 13070–13077. [Google Scholar] [CrossRef]
- Dai, Y.; Yu, J.; Zhang, Z.; Cheng, C.; Tan, P.; Shao, Z.; Ni, M. Interfacial La diffusion in the CeO2/LaFeO3 hybrid for enhanced oxygen evolution activity. ACS Appl. Mater. Interfaces 2021, 13, 2799–2806. [Google Scholar] [CrossRef]
- Zeng, Y.; Haw, K.G.; Wang, Z.; Wang, Y.; Zhang, S.; Hongmanorom, P.; Zhong, Q.; Kawi, S. Double redox process to synthesize CuO–CeO2 catalysts with strong Cu–Ce interaction for efficient toluene oxidation. J. Hazard. Mater. 2021, 404, 124088. [Google Scholar] [CrossRef]
- Arshad, M.F.; El Kasmi, A.; Waqas, M.; Tian, Z.Y. Insight into one-step synthesis of active amorphous La-Co thin films for catalytic oxidation of CO. Appl. Energy Combust. Sci. 2021, 5, 100021. [Google Scholar] [CrossRef]
- Charak, I.; Manhas, M.; Bedyal, A.K.; Singh, S.; Srivastava, A.; Swart, H.C.; Kumar, V. Structural and spectral studies of highly pure red-emitting Ca3B2O6: Eu3+ phosphors for white light emitting diodes. J. Alloys Compd. 2021, 869, 159363. [Google Scholar] [CrossRef]
- Zhou, F.; Xu, D.; Shi, M.; Bi, Y. Investigation on microstructure and its transformation mechanisms of B2O3-SiO2-Al2O3-CaO brazing flux system. High Temp. Mater. Process. 2020, 39, 88–95. [Google Scholar] [CrossRef]
- Yao, G.; Wu, L.; Lv, T.; Li, J.; Huang, Y.; Dong, K.; Li, X. The effect of CuO modification for a TiO2 nanotube confined CeO2 catalyst on the catalytic combustion of butane. Open Chem. 2018, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.; Zhu, W.; Dai, B.; Chao, Y.; Li, C.; Li, H.; Zhang, M.; Jiang, W.; Li, H. Copper nanoparticles advance electron mobility of graphene-like boron nitride for enhanced aerobic oxidative desulfurization. Chem. Eng. J. 2016, 301, 123–131. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Jo, Y.; Lee, S.; Seo, Y.; Ahn, B.; Kim, G.; Jang, G.; Park, J.; Ryua, B.; et al. Air-stable, surface-oxide free Cu nanoparticles for highly conductive Cu ink and their application to printed graphene transistors. J. Mater. Chem. C 2013, 1, 2704–2710. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Wang, J.; Wei, Y.; Liu, X.; Yu, F.; Ji, J. Effect of La-Fe/Si-MCM-41 catalysts and CaO additive on catalytic cracking of soybean oil for biofuel with low aromatics. J. Anal. Appl. Pyrolysis 2019, 143, 104693. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Yang, J.; Zhao, J.; Yan, L.; Song, H.; Chou, L. Insight into the selective oxidation of isobutene to methacrolein over Ce-accelerated Mo-Bi-Fe-Co-KO catalyst. Mol. Catal. 2022, 527, 112401. [Google Scholar] [CrossRef]
- Yan, D.; Mo, S.; Sun, Y.; Ren, Q.; Feng, Z.; Chen, P.; Wu, J.; Fu, M.; Ye, D. Morphology-activity correlation of electrospun CeO2 for toluene catalytic combustion. Chemosphere 2020, 247, 125860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Zheng, J.; Zuo, S. Effect of Particle Size and Crystal Surface of CeO2 on the Catalytic Combustion of Benzene. Materials 2020, 13, 5768. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.; Kim, M.; Lee, J.; Lee, E.; Jung, C.; Choung, J.; Kim, C.; Lee, K. Effects of La incorporation in catalytic activity of Ag/La-CeO2 catalysts for soot oxidation. J. Hazard. Mater. 2021, 414, 125523. [Google Scholar] [CrossRef]
- Song, B.; Li, C.; Du, X.; Li, S.; Zhang, Y.; Lyu, Y.; Zhou, Q. Superior performance of Cu-Ce binary oxides for toluene catalytic oxidation: Cu-Ce synergistic effect and reaction pathways. Fuel 2021, 306, 121654. [Google Scholar] [CrossRef]
- Li, G.; He, K.; Zhang, F.; Jiang, G.; Zhao, Z.; Zhang, Z.; Cheng, J.; Hao, Z. Defect enhanced CoMnNiOx catalysts derived from spent ternary lithium-ion batteries for low-temperature propane oxidation. Appl. Catal. B 2022, 309, 121231. [Google Scholar] [CrossRef]
- Zeng, K.; Wang, Y.; Huang, C.; Liu, H.; Liu, X.; Wang, Z.; Yu, J.; Zhang, C. Catalytic combustion of propane over MnNbOx composite oxides: The promotional role of niobium. Ind. Eng. Chem. Res. 2021, 60, 6111–6120. [Google Scholar] [CrossRef]
- Liu, C.; He, L.; Wang, X.; Chen, J.; Lu, J.; Luo, M.F. Tailoring Co3O4 active species to promote propane combustion over Co3O4/ZSM-5 catalyst. Mol. Catal. 2022, 524, 112297. [Google Scholar] [CrossRef]
- Han, Q.; Jin, S.; Wang, J.; Wang, J.; Sun, P.; Zhou, Y.; Wang, X.; Zhang, R.; Qiao, W.; Ling, L.; et al. Insights to sulfur-resistant mechanisms of reduced graphene oxide supported MnOx-CeOy catalysts for low-temperature NH3-SCR. J. Phys. Chem. Solids 2022, 167, 110782. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Jiao, Y.; Wang, L.; Wang, J.; Chen, Y.; Zhu, Q.; Li, X. Role of acidity in catalytic cracking of n-decane over supported Pt-based catalysts. Appl. Surf. Sci. 2020, 507, 145113. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Q.; Zhao, Y.; Yang, J.; Xu, Y.; Zhang, J. Enhancement of CeO2 modified commercial SCR catalyst for synergistic mercury removal from coal combustion flue gas. RSC Adv. 2020, 10, 25325–25338. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wang, L.; Hu, Y.; Ning, P.; Ma, Y.; Tan, L. Catalytic oxidation and hydrolysis of HCN over LaxCuy/TiO2 catalysts at low temperatures. Microporous Mesoporous Mater. 2019, 282, 260–268. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Wang, J.; Li, Z.; Xu, J. Catalytic combustion of methane over a highly active and stable NiO/CeO2 catalyst. Front. Chem. Sci. Eng. 2020, 14, 534–545. [Google Scholar] [CrossRef]
- Guan, Y.; Zhou, Y.; Jiang, C.; Xu, X.; Yang, Z.; Zhang, J.; Fan, X.; Jiao, Y. Catalytic combustion of volatile organic compounds (VOCs) over structured Co3O4 nano-flowers on silicalite-1/SiC foam catalysts. Microporous Mesoporous Mater. 2021, 323, 111173. [Google Scholar] [CrossRef]
- Yang, P.; Li, J.; Cheng, Z.; Zuo, S. Promoting effects of Ce and Pt addition on the destructive performances of V2O5/γ-Al2O3 for catalytic combustion of benzene. Appl. Catal. A 2017, 542, 38–46. [Google Scholar] [CrossRef]
- Mi, R.; Li, D.; Hu, Z.; Yang, R.T. Morphology effects of CeO2 nanomaterials on the catalytic combustion of toluene: A combined kinetics and diffuse reflectance infrared fourier transform spectroscopy study. ACS Catal. 2021, 11, 7876–7889. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Aylón, E.; Dejoz, A.M.; Vázquez, I.; Agouram, S.; Davies, T.E.; Taylor, S.H. Promoting the activity and selectivity of high surface area Ni-Ce-O mixed oxides by gold deposition for VOC catalytic combustion. Chem. Eng. J. 2011, 175, 271–278. [Google Scholar] [CrossRef]
- Luo, Y.; Zuo, J.; Lin, D.; Qian, Q.; Zheng, Y.; Feng, X.; Huang, B.; Chen, Q. Anchoring Pt on surface/bulk of LaCoO3 nanotubes via one step of coaxial electrospinning for efficient total propane oxidation. Mol. Catal. 2019, 475, 110504. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Lv, L.; Li, D.; Yue, X.; Wang, S. Insights into the behaviors of the catalytic combustion of propane over spinel catalysts. Catal. Lett. 2020, 150, 3617–3625. [Google Scholar] [CrossRef]
- Tasca, J.E.; Lavat, A.E.; González, M.G. Double perovskites La2MMnO6 as catalyst for propane combustion. J. Asian Ceram. Soc. 2017, 5, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Abbas-Ghaleb, R.; Garbowski, E.; Kaddouri, A.; Gelin, P. Al18B4O33 aluminium borate: A new efficient support for palladium in the high temperature catalytic combustion of methane. Catal. Today 2006, 117, 514–517. [Google Scholar] [CrossRef]
- Hinokuma, S.; Matsuki, S.; Kawabata, Y.; Shimanoe, H.; Kiritoshi, S.; Machida, M. Copper oxides supported on aluminum oxide borates for catalytic ammonia combustion. J. Phys. Chem. C. 2016, 120, 24734–24742. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, S.; You, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Lu, G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane. Appl. Catal. B 2018, 225, 110–120. [Google Scholar] [CrossRef]
- Ronda-Lloret, M.; Rico-Francés, S.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. CuOx/CeO2 catalyst derived from metal organic framework for reverse water-gas shift reaction. Appl. Catal. A 2018, 562, 28–36. [Google Scholar] [CrossRef]
- Wang, W.; Yu, W.; Du, P.; Xu, H.; Jin, Z.; Si, R.; Ma, C.; Shi, S.; Jia, C.; Yan, C. Crystal plane effect of ceria on supported copper oxide cluster catalyst for CO oxidation: Importance of metal–support interaction. ACS Catal. 2017, 7, 1313–1329. [Google Scholar] [CrossRef]
- Yuan, E.; Zhou, M.; Shi, G.; Jian, P.; Hou, X. Ultralow-loading single-atom cobalt on graphitic carbon nitrogen with robust Co-N pairs for aerobic cyclohexane oxidation. Nano Res. 2022, 15, 8791–8803. [Google Scholar] [CrossRef]
- Zheng, K.; Zhou, Z.; Wang, Y.; Xin, Z.; Zhao, Z.; Zhang, J.; Bo, T.; Lin, T.; Zhang, B.; Shao, L. Ultralow loading of nanostructured Mn species onto two-dimensional Co3O4 nanosheets for selective catalytic reduction of NOx with NH3. Catal. Sci. Technol. 2020, 10, 3450–3457. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Qiao, B.; Pan, X.; Wang, C.; Cao, L.; Li, L.; Lin, J.; Wang, X. Enhanced stability of Pt/Al2O3 modified by Zn promoter for catalytic dehydrogenation of ethane. J. Energy Chem. 2020, 51, 14–20. [Google Scholar] [CrossRef]
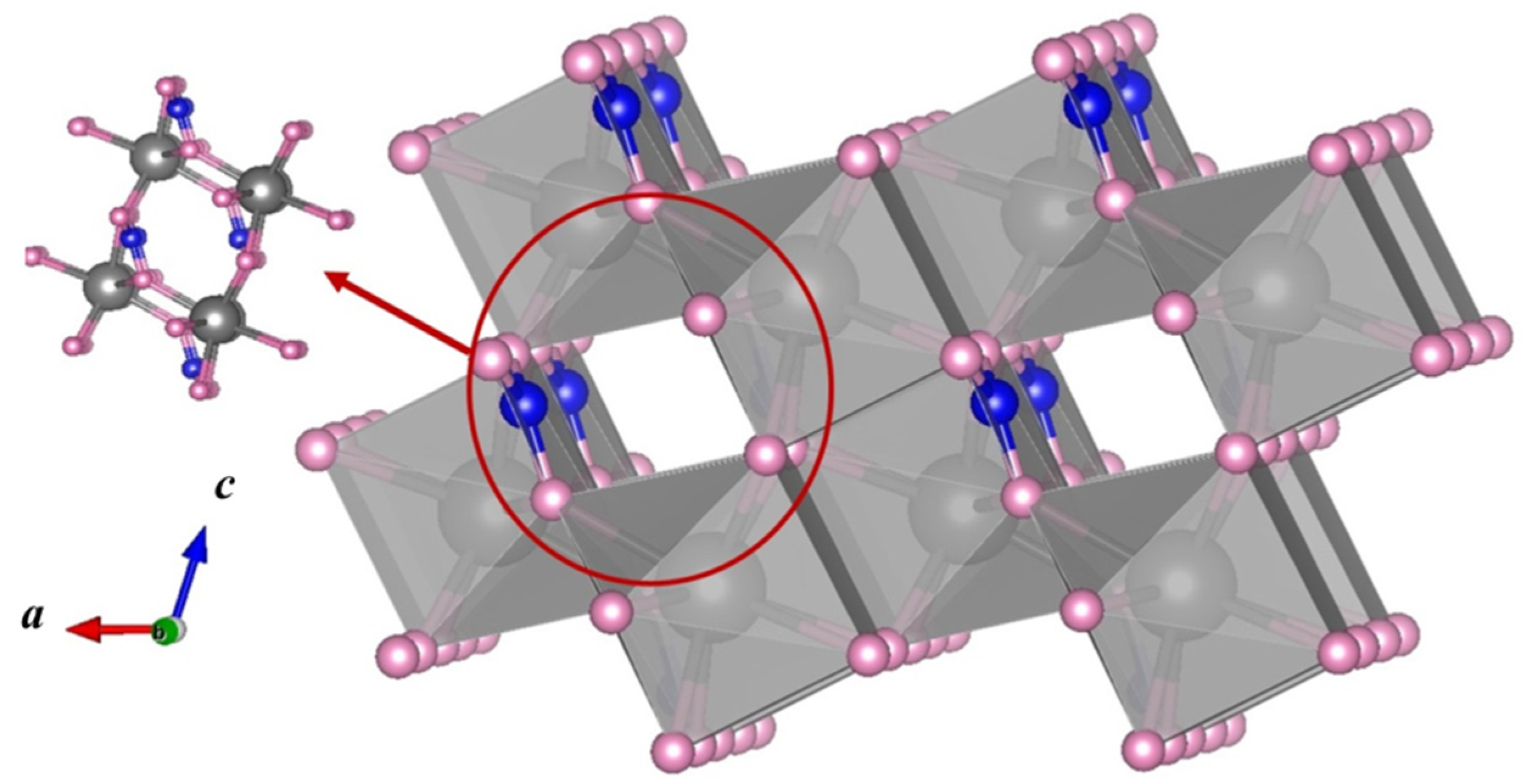


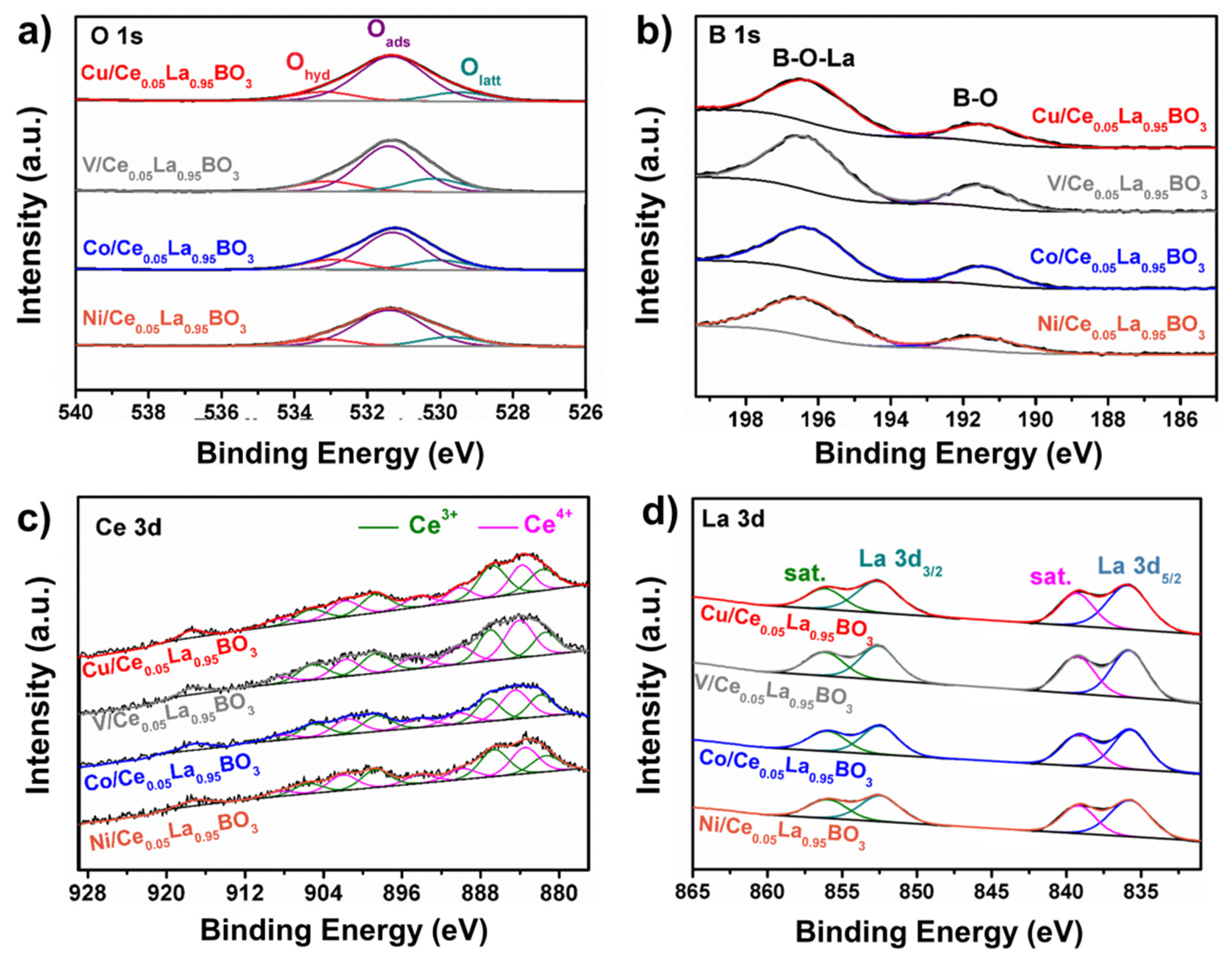
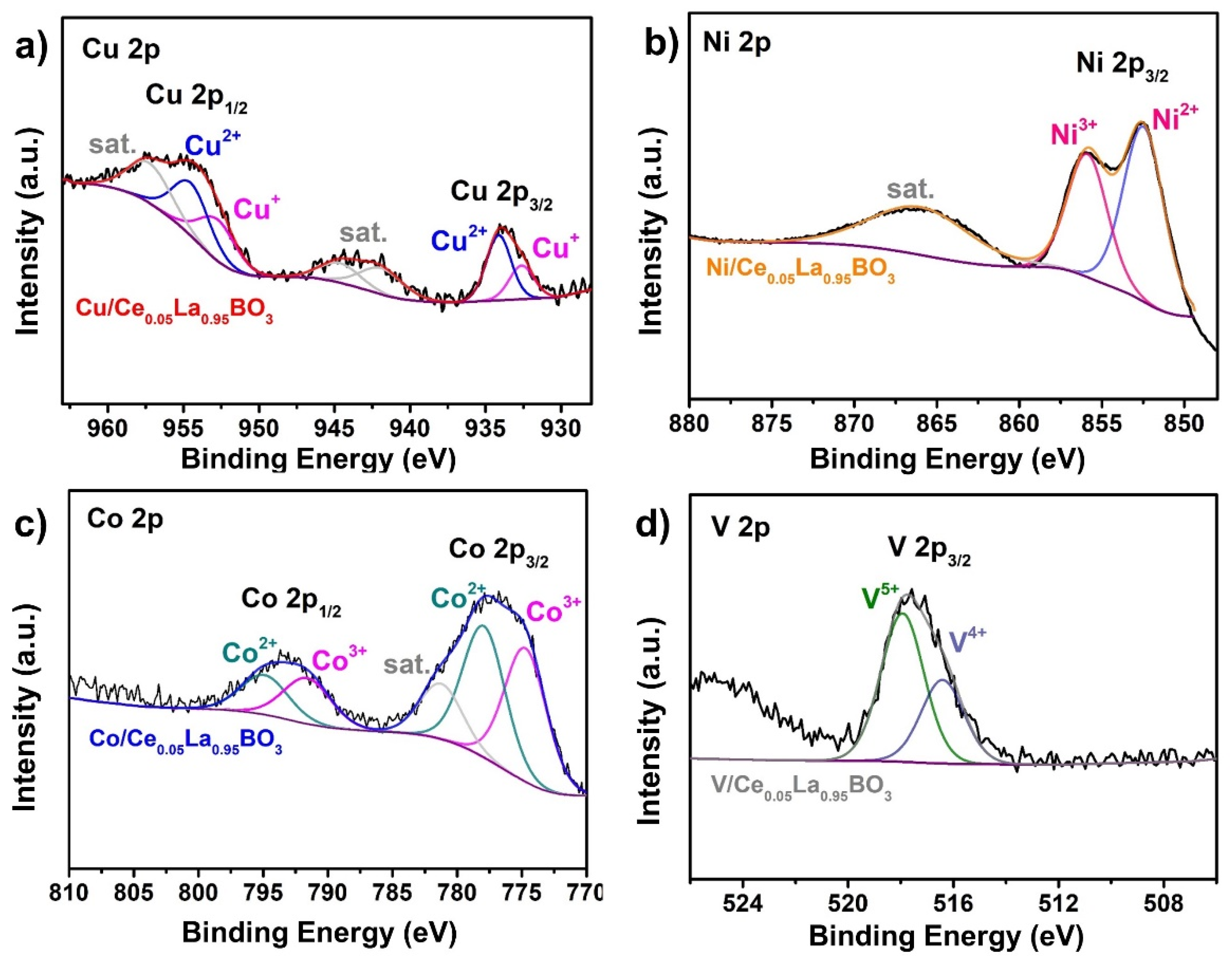


| Sample | Atomic Ratios (%) | ||||
|---|---|---|---|---|---|
| Olatt/(Olatt + Oads + Ohyd) | Oads/(Olatt + Oads + Ohyd) | Ohyd/(Olatt + Oads + Ohyd) | Ce3+/(Ce3+ + Ce4+) | Ce4+/(Ce3+ + Ce4+) | |
| Cu/Ce0.05La0.95BO3 | 12.9 | 74.1 | 13.0 | 52.6 | 47.4 |
| Ni/Ce0.05La0.95BO3 | 16.3 | 71.8 | 11.9 | 49.7 | 50.3 |
| Co/Ce0.05La0.95BO3 | 16.7 | 65.7 | 17.6 | 47.0 | 53.0 |
| V/Ce0.05La0.95BO3 | 19.2 | 66.0 | 14.8 | 45.9 | 54.1 |
| Sample | Acid Amount (mmol·g−1) |
|---|---|
| V/Ce0.05La0.95BO3 | 0.040 |
| Cr/Ce0.05La0.95BO3 | 0.097 |
| Mn/Ce0.05La0.95BO3 | 0.108 |
| Fe/Ce0.05La0.95BO3 | 0.103 |
| Co/Ce0.05La0.95BO3 | 0.063 |
| Ni/Ce0.05La0.95BO3 | 0.213 |
| Zn/Ce0.05La0.95BO3 | 0.088 |
| Cu/Ce0.05La0.95BO3 | 0.241 |
| Ce0.05La0.95BO3 | 0.089 |
| LaBO3 | 0.037 |
| Catalysts | T10(K) | T50(K) | T90(K) |
|---|---|---|---|
| V/Ce0.05La0.95BO3 | 604 | --- | --- |
| Cr/Ce0.05La0.95BO3 | 473 | --- | --- |
| Mn/Ce0.05La0.95BO3 | 555 | 708 | 769 |
| Fe/Ce0.05La0.95BO3 | 523 | 715 | --- |
| Co/Ce0.05La0.95BO3 | 475 | 700 | --- |
| Ni/Ce0.05La0.95BO3 | 589 | 702 | 765 |
| Zn/Ce0.05La0.95BO3 | 456 | 764 | --- |
| Cu/Ce0.05La0.95BO3 | 473 | 641 | 718 |
| Ce0.05La0.95BO3 | 515 | --- | --- |
| LaBO3 | 691 | --- | --- |
| Sample | Deactivation Rate (h−1) |
|---|---|
| V/Ce0.05La0.95BO3 | 0.046 |
| Co/Ce0.05La0.95BO3 | 0.052 |
| Ni/Ce0.05La0.95BO3 | 0.013 |
| Cu/Ce0.05La0.95BO3 | 0.013 |
| Ce0.05La0.95BO3 | 0.021 |
| LaBO3 | 0.611 |
| Catalyst | T50(K) | Reaction Temperature (K) | Selectivity to CO2 (%) | Ref. |
|---|---|---|---|---|
| Cu/Ce0.05La0.95BO3 | 641 | 673 | 91 | This work |
| NiCeOx (Ni/Ce = 1:1) | 528 | 501 | 87.8 | [71] |
| Pt/LaCoO3 | 599 | 723 | 97 | [72] |
| CuAl2O4 | 796 | -- | -- | [73] |
| CuFe2O4 | 698 | -- | -- | [73] |
| LaCuMnO6-CIT | 753 | -- | -- | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Gong, X.; Wang, F.; Wei, X.; Dang, Y.; Wu, Y.; Zhang, X. Catalytic Combustion of Propane over Ce-Doped Lanthanum Borate Loaded with Various 3d Transition Metals. Catalysts 2022, 12, 1632. https://doi.org/10.3390/catal12121632
Wang W, Gong X, Wang F, Wei X, Dang Y, Wu Y, Zhang X. Catalytic Combustion of Propane over Ce-Doped Lanthanum Borate Loaded with Various 3d Transition Metals. Catalysts. 2022; 12(12):1632. https://doi.org/10.3390/catal12121632
Chicago/Turabian StyleWang, Weilu, Xudong Gong, Fang Wang, Xinyi Wei, Yanliu Dang, Yun Wu, and Xianming Zhang. 2022. "Catalytic Combustion of Propane over Ce-Doped Lanthanum Borate Loaded with Various 3d Transition Metals" Catalysts 12, no. 12: 1632. https://doi.org/10.3390/catal12121632





