Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate
Abstract
:1. Introduction
2. Materials and Method
2.1. Reagents
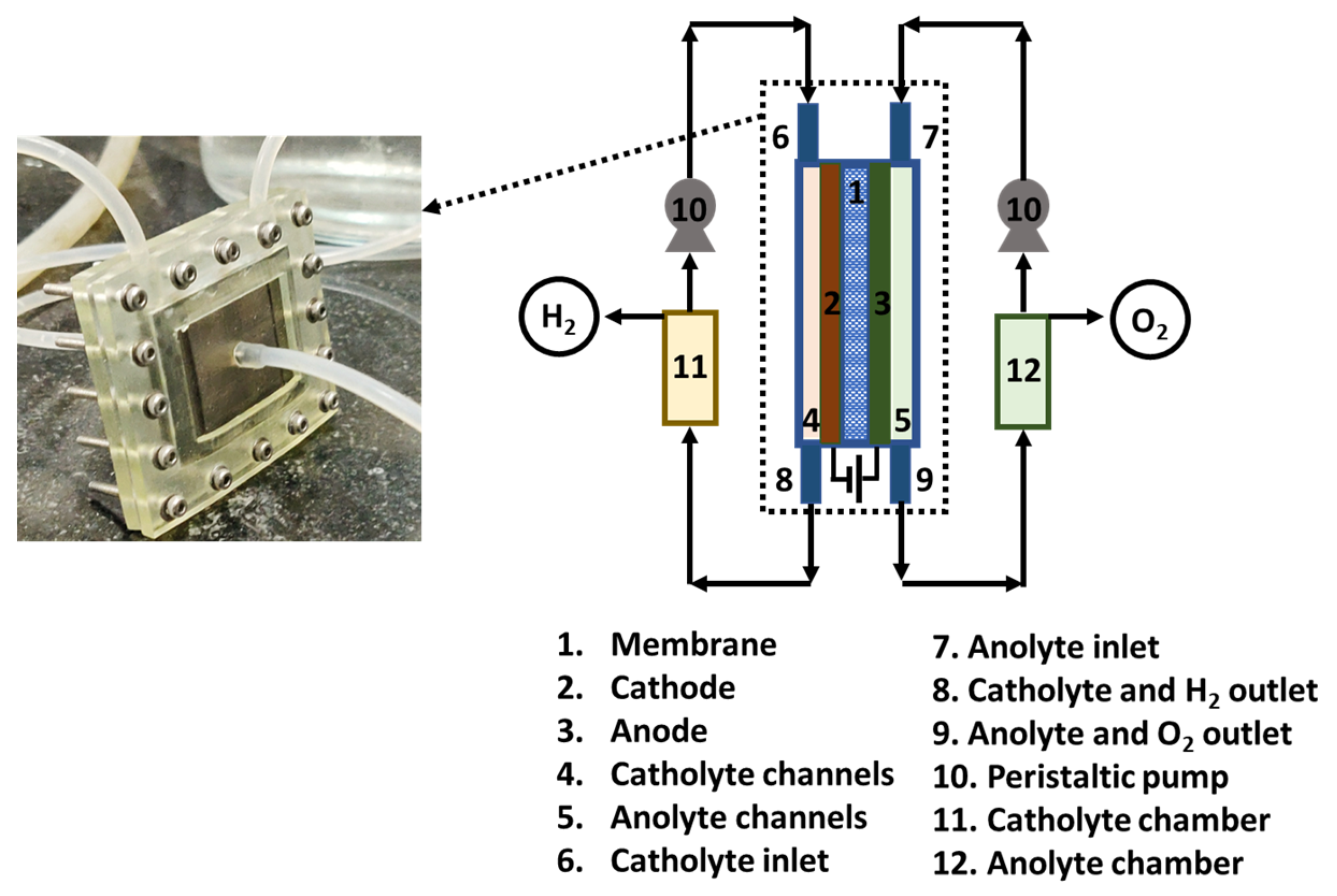
2.2. Cell Testing Measurement
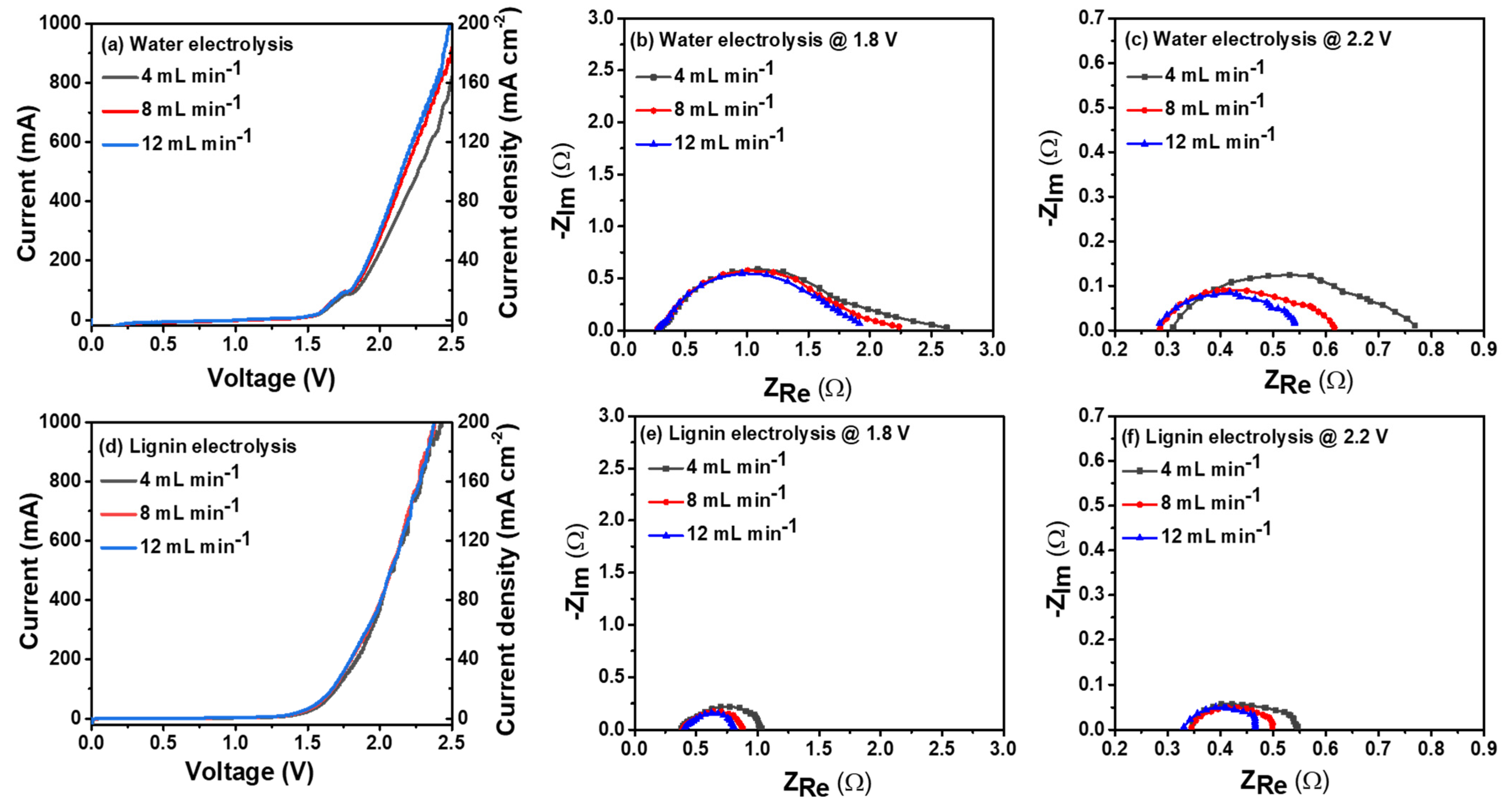
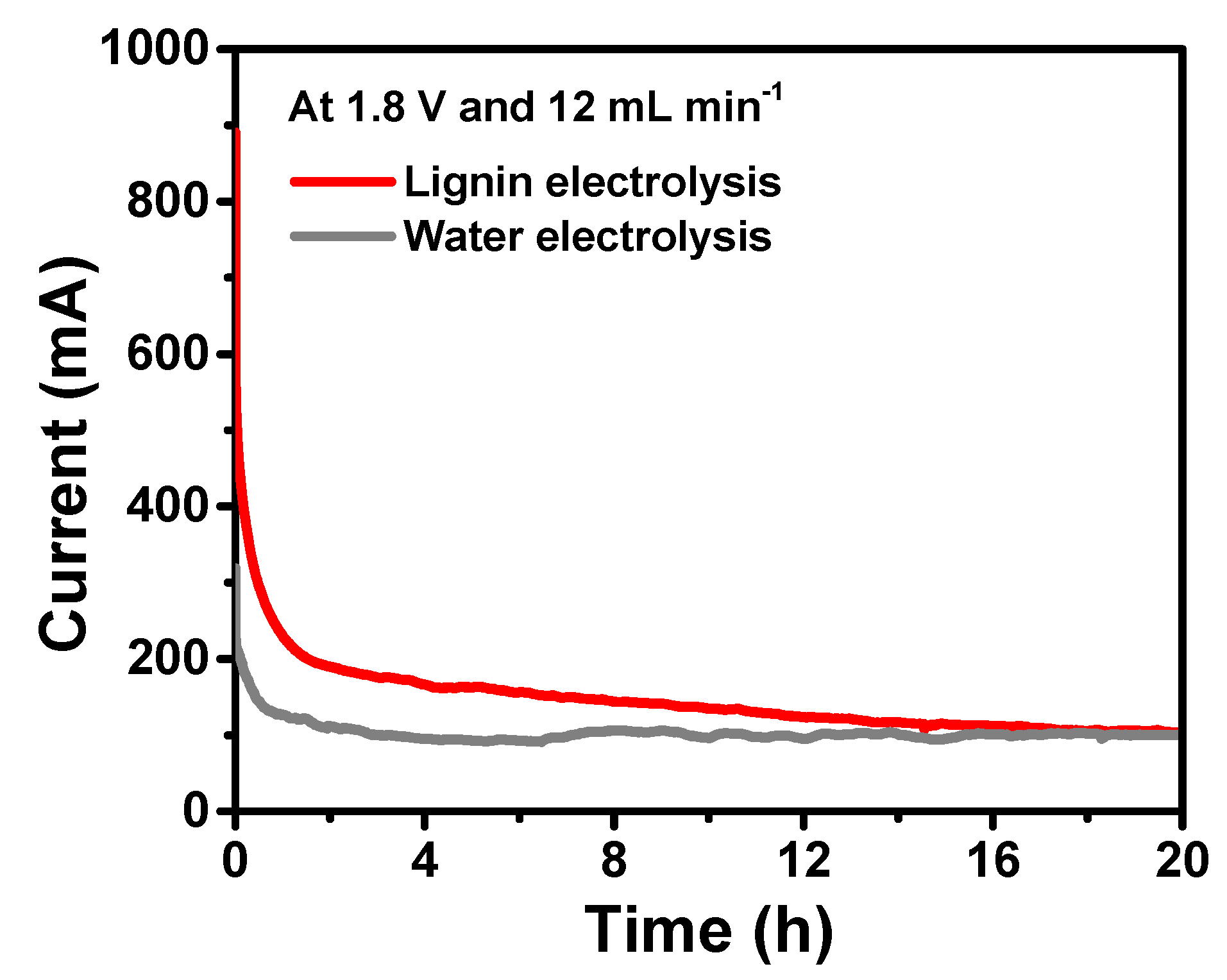
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Opportunities and Future Challenges in Hydrogen Economy for Sustainable Development. In Hydrogen Economy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 277–305. [Google Scholar]
- McCrory, C.C.L.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking Heterogeneous Electrocatalysts for the Oxygen Evolution Reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef] [PubMed]
- Lewinski, K.A.; van der Vliet, D.; Luopa, S.M. NSTF Advances for PEM Electrolysis-the Effect of Alloying on Activity of NSTF Electrolyzer Catalysts and Performance of NSTF Based PEM Electrolyzers. ECS Trans. 2015, 69, 893. [Google Scholar] [CrossRef]
- Yang, J.; Jang, M.J.; Zeng, X.; Park, Y.S.; Lee, J.; Choi, S.M.; Yin, Y. Non-Precious Electrocatalysts for Oxygen Evolution Reaction in Anion Exchange Membrane Water Electrolysis: A Mini Review. Electrochem. Commun. 2021, 131, 107118. [Google Scholar] [CrossRef]
- Ayers, K. The Potential of Proton Exchange Membrane-Based Electrolysis Technology. Curr. Opin. Electrochem. 2019, 18, 9–15. [Google Scholar] [CrossRef]
- Salehmin, M.N.I.; Husaini, T.; Goh, J.; Sulong, A.B. High-Pressure PEM Water Electrolyser: A Review on Challenges and Mitigation Strategies towards Green and Low-Cost Hydrogen Production. Energy Convers. Manag. 2022, 268, 115985. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-Abundant Catalysts for Electrochemical and Photoelectrochemical Water Splitting. Nat. Rev. Chem. 2017, 1, 3. [Google Scholar] [CrossRef]
- Li, Y.; Chen, J.; Cai, P.; Wen, Z. An Electrochemically Neutralized Energy-Assisted Low-Cost Acid-Alkaline Electrolyzer for Energy-Saving Electrolysis Hydrogen Generation. J. Mater. Chem. A 2018, 6, 4948–4954. [Google Scholar] [CrossRef]
- De, B.S.; Singh, A.; Elias, A.; Khare, N.; Basu, S. An Electrochemical Neutralization Energy-Assisted Membrane-Less Microfluidic Reactor for Water Electrolysis. Sustain. Energy Fuels 2020, 4, 6234–6244. [Google Scholar] [CrossRef]
- Lamy, C.; Guenot, B.; Cretin, M.; Pourcelly, G. A Kinetics Analysis of Methanol Oxidation under Electrolysis/Fuel Cell Working Conditions. ECS Trans. 2015, 66, 1. [Google Scholar] [CrossRef]
- Guenot, B.; Cretin, M.; Lamy, C. Clean Hydrogen Generation from the Electrocatalytic Oxidation of Methanol inside a Proton Exchange Membrane Electrolysis Cell (PEMEC): Effect of Methanol Concentration and Working Temperature. J. Appl. Electrochem. 2015, 45, 973–981. [Google Scholar] [CrossRef]
- Gutiérrez-Martín, F.; Calcerrada, A.B.; de Lucas-Consuegra, A.; Dorado, F. Hydrogen Storage for Off-Grid Power Supply Based on Solar PV and Electrochemical Reforming of Ethanol-Water Solutions. Renew. Energy 2020, 147, 639–649. [Google Scholar] [CrossRef]
- Ruiz-Lopez, E.; Amores, E.; de la Osa, A.R.; Dorado, F.; de Lucas-Consuegra, A. Electrochemical Reforming of Ethanol in a Membrane-Less Reactor Configuration. Chem. Eng. J. 2020, 379, 122289. [Google Scholar] [CrossRef]
- Simões, M.; Baranton, S.; Coutanceau, C. Electrochemical Valorisation of Glycerol. ChemSusChem 2012, 5, 2106–2124. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.G.; Choi, K.-S. Combined Biomass Valorization and Hydrogen Production in a Photoelectrochemical Cell. Nat. Chem. 2015, 7, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, A.; Garcia-Lorefice, W.E.; Gil, S.; de Lucas-Consuegra, A.; Vernoux, P. Towards a Sustainable Technology for H2 Production: Direct Lignin Electrolysis in a Continuous-Flow Polymer Electrolyte Membrane Reactor. Electrochem. Commun. 2019, 100, 43–47. [Google Scholar] [CrossRef]
- Beliaeva, K.; Grimaldos-Osorio, N.; Ruiz-López, E.; Burel, L.; Vernoux, P.; Caravaca, A. New Insights into Lignin Electrolysis on Nickel-Based Electrocatalysts: Electrochemical Performances before and after Oxygen Evolution. Int. J. Hydrogen Energy 2021, 46, 35752–35764. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Rajagopal, P. Lignin-Augmented Water Electrolysis. J. Electrochem. Soc. 1992, 139, L1. [Google Scholar] [CrossRef]
- Lalvani, S.B.; Rajagopal, P. Hydrogen Production from Lignin-Water Solution by Electrolysis. Holzforschung 1993, 47, 283–286. [Google Scholar] [CrossRef]
- Marshall, A.T.; Haverkamp, R.G. Production of Hydrogen by the Electrochemical Reforming of Glycerol–Water Solutions in a PEM Electrolysis Cell. Int. J. Hydrog. Energy 2008, 33, 4649–4654. [Google Scholar] [CrossRef]
- Yan, W.; Wang, D.; Botte, G.G. Electrochemical Decomposition of Urea with Ni-Based Catalysts. Appl. Catal. B Environ. 2012, 127, 221–226. [Google Scholar] [CrossRef]
- You, B.; Liu, X.; Liu, X.; Sun, Y. Efficient H2 Evolution Coupled with Oxidative Refining of Alcohols via a Hierarchically Porous Nickel Bifunctional Electrocatalyst. Acs Catal. 2017, 7, 4564–4570. [Google Scholar] [CrossRef]
- Arshad, F.; Haq, T.U.; Hussain, I.; Sher, F. Recent Advances in Electrocatalysts toward Alcohol-Assisted, Energy-Saving Hydrogen Production. ACS Appl. Energy Mater. 2021, 4, 8685–8701. [Google Scholar] [CrossRef]
- Movil, O.; Garlock, M.; Staser, J.A. Non-Precious Metal Nanoparticle Electrocatalysts for Electrochemical Modification of Lignin for Low-Energy and Cost-Effective Production of Hydrogen. Int. J. Hydrogen Energy 2015, 40, 4519–4530. [Google Scholar] [CrossRef]
- Ghatak, H.R. Electrolysis of Black Liquor for Hydrogen Production: Some Initial Findings. Int. J. Hydrog. Energy 2006, 31, 934–938. [Google Scholar]
- Du, X.; Liu, W.; Zhang, Z.; Mulyadi, A.; Brittain, A.; Gong, J.; Deng, Y. Low-energy Catalytic Electrolysis for Simultaneous Hydrogen Evolution and Lignin Depolymerization. ChemSusChem 2017, 10, 847–854. [Google Scholar] [CrossRef]
- Dhongde, V.R.; De, B.S.; Wasewar, K.L.; Gupta, P.; Kumar, S. Experimental Perspective for Reactive Separation of Malonic Acid Using TBP in Natural Non-Toxic Solvents. J. Ind. Eng. Chem. 2020, 91, 273–284. [Google Scholar] [CrossRef]
- De, B.S.; Wasewar, K.L.; Dhongde, V.R.; Ingle, A.A.; Mondal, H. Experimental and Modeling of Reactive Separation of Protocatechuic Acid. Chem. Eng. Res. Des. 2018, 132, 593–605. [Google Scholar] [CrossRef]
- De, B.S.; Wasewar, K.L.; Dhongde, V.; Mishra, T. A Step Forward in the Development of in Situ Product Recovery by Reactive Separation of Protocatechuic Acid. React. Chem. Eng. 2019, 4, 78–89. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K.L.; De Biswajit, S.; Kumar, S. Separation of Protocatechuic Acid Using Tri- n-Octylamine: Experimental and Mathematical Investigation. J. Chem. Eng. Data 2019, 64, 1101–1112. [Google Scholar] [CrossRef]
- Honorato, A.M.B.; Khalid, M.; Curvelo, A.A.D.S.; Varela, H.; Shahgaldi, S. Trimetallic Nanoalloy of NiFeCo Embedded in Phosphidated Nitrogen Doped Carbon Catalyst for Efficient Electro-Oxidation of Kraft Lignin. Polymers 2022, 14, 3781. [Google Scholar] [CrossRef]
- Bährle, C.; Nick, T.U.; Bennati, M.; Jeschke, G.; Vogel, F. High-Field Electron Paramagnetic Resonance and Density Functional Theory Study of Stable Organic Radicals in Lignin: Influence of the Extraction Process, Botanical Origin, and Protonation Reactions on the Radical g Tensor. J. Phys. Chem. A 2015, 119, 6475–6482. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Nandakumar, K.; Masliyah, J.H. A Model for Detachment of a Partially Wetting Drop from a Solid Surface by Shear Flow. J. Colloid Interface Sci. 1997, 190, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Segre, G.; Silberberg, A. Behaviour of Macroscopic Rigid Spheres in Poiseuille Flow Part 2. Experimental Results and Interpretation. J. Fluid Mech. 1962, 14, 136–157. [Google Scholar] [CrossRef]
- De Biswajit, S.; Cunningham, J.; Khare, N.; Luo, J.L.; Elias, A.; Basu, S. Hydrogen Generation and Utilization in a Two-Phase Flow Membraneless Microfluidic Electrolyzer-Fuel Cell Tandem Operation for Micropower Application. Appl. Energy 2022, 305, 117945. [Google Scholar]
- De Biswajit, S.; Singh, A.; Dixit, R.J.; Khare, N.; Elias, A.; Basu, S. Hydrogen Generation in Additively Manufactured Membraneless Microfluidic Electrolysis Cell: Performance Evaluation and Accelerated Stress Testing. Chem. Eng. J. 2022, 452, 139433. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, M.; De, B.S.; Singh, A.; Shahgaldi, S. Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate. Catalysts 2022, 12, 1646. https://doi.org/10.3390/catal12121646
Khalid M, De BS, Singh A, Shahgaldi S. Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate. Catalysts. 2022; 12(12):1646. https://doi.org/10.3390/catal12121646
Chicago/Turabian StyleKhalid, Mohmmad, Biswajit Samir De, Aditya Singh, and Samaneh Shahgaldi. 2022. "Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate" Catalysts 12, no. 12: 1646. https://doi.org/10.3390/catal12121646
APA StyleKhalid, M., De, B. S., Singh, A., & Shahgaldi, S. (2022). Lignin Electrolysis at Room Temperature on Nickel Foam for Hydrogen Generation: Performance Evaluation and Effect of Flow Rate. Catalysts, 12(12), 1646. https://doi.org/10.3390/catal12121646







