Brief Review on High-Temperature Electrochemical Hydrogen Sensors
Abstract
1. Introduction
2. Types of Electrochemical Hydrogen Sensors
2.1. Potentiometric Hydrogen Sensors
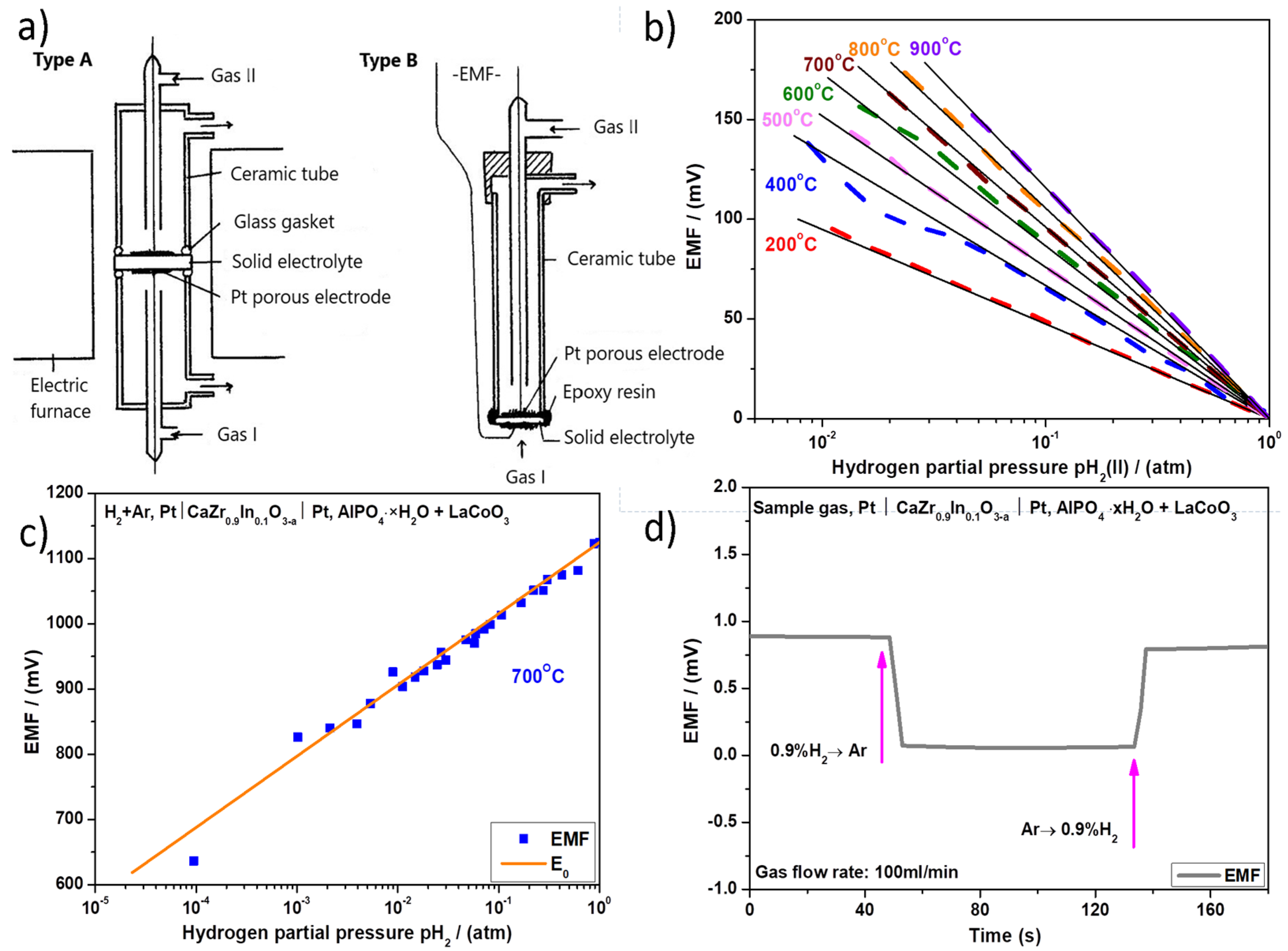
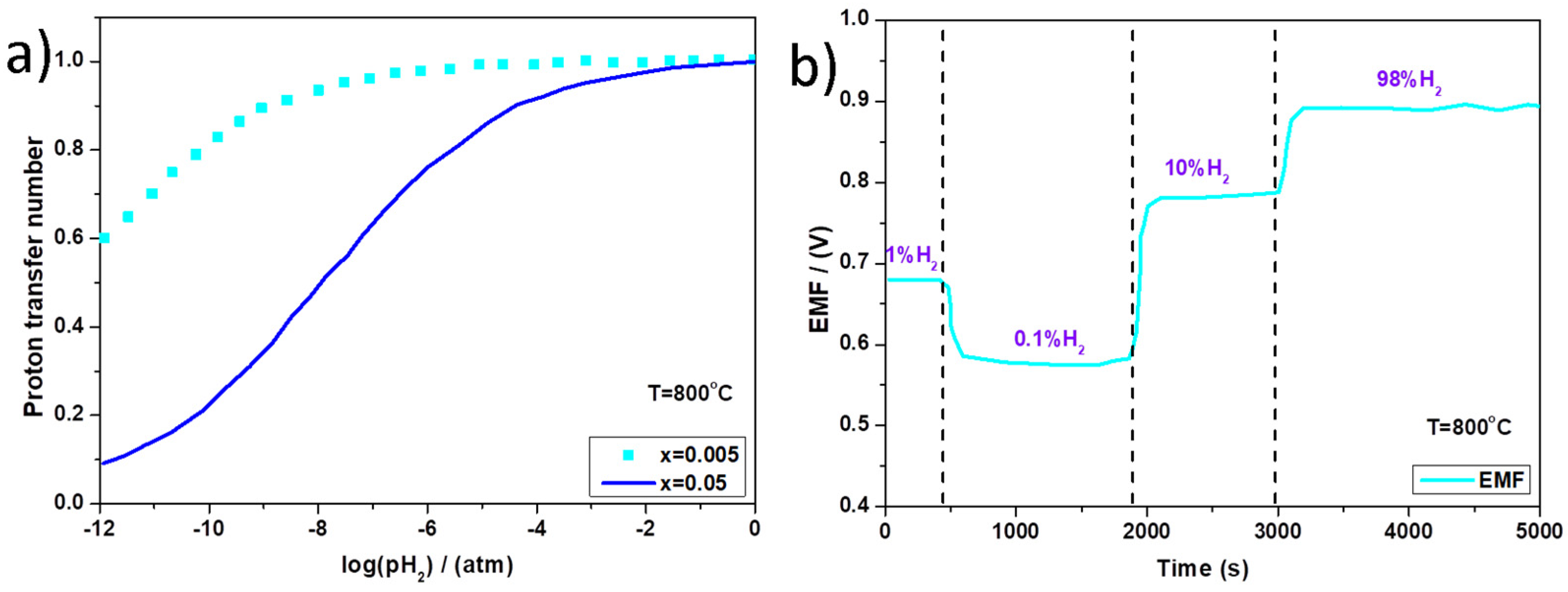
2.1.1. Equilibrium Potentiometric Sensors
2.1.2. Mixed Potential Sensors
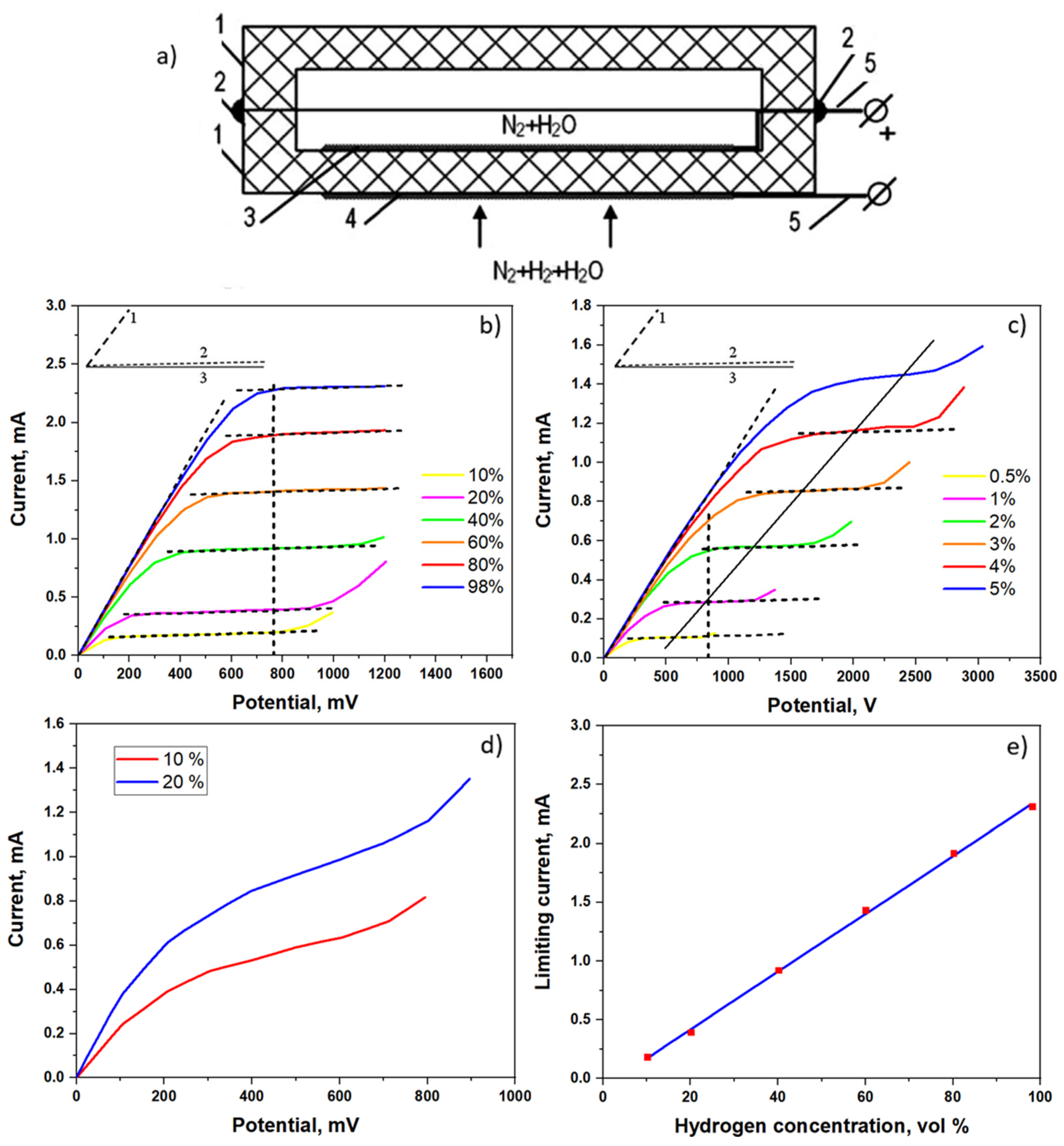
2.2. Amperometric Hydrogen Sensors
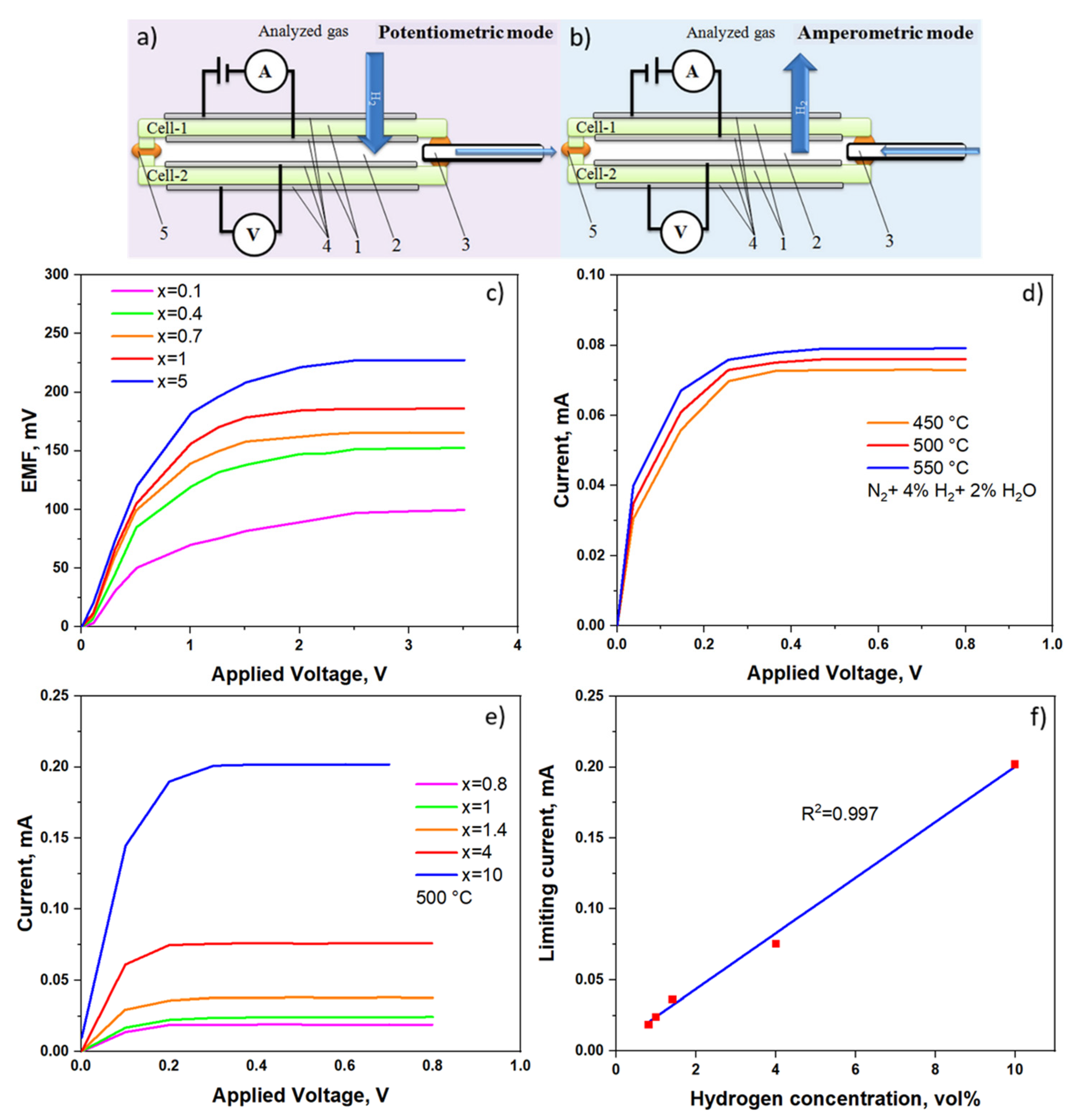
2.3. Combined Amperometric–Potentiometric H2 Sensors
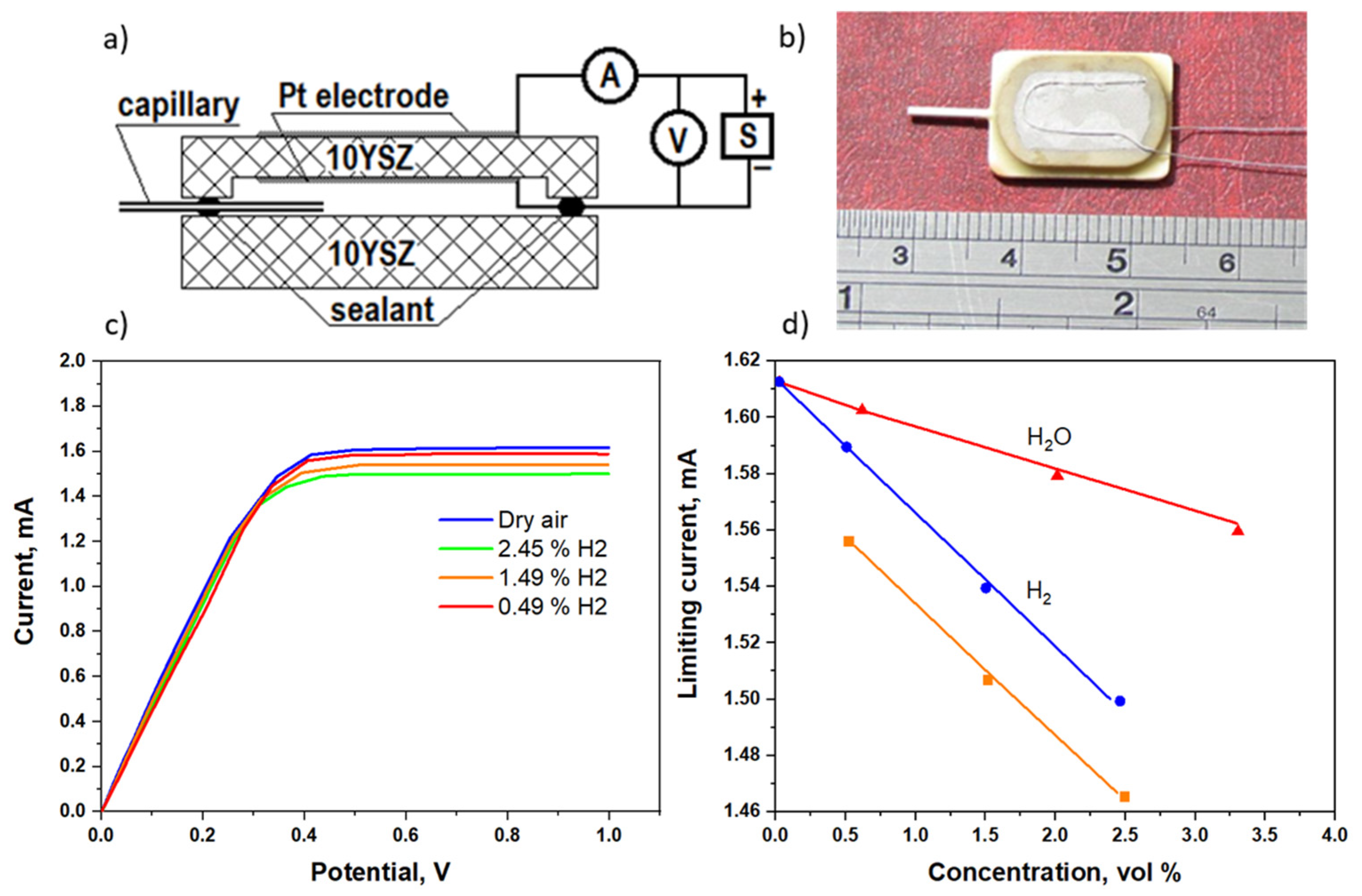
3. Amperometric Sensor for Hydrogen and Steam Detection
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qazi, U.Y. Future of hydrogen as an alternative fuel for next-generation industrial applications; Challenges and expected opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrog. Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.-A.; Pearson, P. Hydrogen and methanol: A comparison of safety, economics, efficiencies and emissions. J. Power Source 2000, 86, 548–555. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Yajima, T.; Koide, K.; Fukatsu, N.; Ohashi, T.; Iwahara, H. A new hydrogen sensor for molten aluminum. Sens. Actuators B Chem. 1993, 14, 697–699. [Google Scholar] [CrossRef]
- Schwandt, C.; Fray, D.J. Hydrogen sensing in molten aluminium using a commercial electrochemical sensor. Ionics 2000, 6, 222–229. [Google Scholar] [CrossRef]
- Serret, P.; Colominas, S.; Reyes, G.; Abellà, J. Characterization of ceramic materials for electrochemical hydrogen sensors. Fusion Eng. Des. 2011, 86, 2446–2449. [Google Scholar] [CrossRef]
- Gorbova, E.; Tzorbatzoglou, F.; Molochas, C.; Chloros, D.; Demin, A.; Tsiakaras, P. Fundamentals and Principles of Solid-State Electrochemical Sensors for High Temperature Gas Detection. Catalysts 2021, 12, 1. [Google Scholar] [CrossRef]
- Zosel, J.; Schiffel, G.; Gerlach, F.; Ahlborn, K.; Sasum, U.; Vashook, V.; Guth, U. Electrode materials for potentiometric hydrogen sensors. Solid State Ion. 2006, 177, 2301–2304. [Google Scholar] [CrossRef]
- Volkov, A.; Gorbova, E.; Vylkov, A.; Medvedev, D.; Demin, A.; Tsiakaras, P. Design and applications of potentiometric sensors based on proton-conducting ceramic materials. A brief review. Sens. Actuators B Chem. 2017, 244, 1004–1015. [Google Scholar] [CrossRef]
- Pasierb, P.; Rekas, M. Solid-state potentiometric gas sensors—Current status and future trends. J. Solid State Electrochem. 2009, 13, 3–25. [Google Scholar] [CrossRef]
- Yajima, T.; Koide, K.; Takai, H.; Fukatsu, N.; Iwahara, H. Application of hydrogen sensor using proton conductive ceramics as a solid electrolyte to aluminum casting industries. Solid State Ion. 1995, 79, 333–337. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Zhang, Z.; Wang, J. A Hydrogen Sensor Using SrCe0.95Yb0.05O3-α as Proton Conductor and YHx+YH2−z as Reference Electrode for Determining Hydrogen Pressure in Solid Steel. J. Mater. Sci. Technol. 2010, 26, 957–960. [Google Scholar] [CrossRef]
- Medvedev, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Proton-Conducting Electrolytes for Solid Oxide Fuel Cell Applications. In Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Boaro, M., Salvatore, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 574, pp. 77–118. [Google Scholar]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Medvedev, D.; Murashkina, A.; Pikalova, E.; Demin, A.; Podias, A.; Tsiakaras, P. BaCeO3: Materials development, properties and application. Prog. Mater. Sci. 2014, 60, 72–129. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Chapter 6—Sensors based on solid oxide electrolytes. In Solid Oxide-Based Electrochemical Devices. Lo Faro, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 167–215. [Google Scholar]
- Göpel, W.; Reinhardt, G.; Rösch, M. Trends in the development of solid state amperometric and potentiometric high temperature sensors. Solid State Ion. 2000, 136, 519–531. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Ogaki, K.; Nagato, H. Nernstian Hydrogen Sensor Using BaCeO3 - Based, Proton-Conducting Ceramics Operative at 200–900 °C. J. Electrochem. Soc. 1991, 138, 295–299. [Google Scholar]
- Yajima, T.; Iwahara, H.; Koide, K.; Yamamoto, K. CaZrO3-type hydrogen and steam sensors: Trial fabrication and their characteristics. Sens. Actuators B Chem. 1991, 5, 145–147. [Google Scholar] [CrossRef]
- Okuyama, Y.; Nagamine, S.; Nakajima, A.; Sakai, G.; Matsunaga, N.; Takahashi, F.; Kimata, K.; Oshima, T.; Tsuneyoshi, K. Proton-conducting oxide with redox protonation and its application to a hydrogen sensor with a self-standard electrode. RSC Adv. 2016, 6, 34019–34026. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Teng, Y.; Yi, J. Simulation of dynamic response of mixed-potential hydrogen sensors. Int. J. Hydrog. Energy 2022, 47, 33891–33902. [Google Scholar] [CrossRef]
- Vogel, A.; Baier, G.; Schüle, V. Non-Nernstian potentiometric zirconia sensors: Screening of potential working electrode materials. Sens. Actuators B Chem. 1993, 15, 147–150. [Google Scholar] [CrossRef]
- Fadeyev, G.; Kalakin, A.; Demin, A.; Volkov, A.; Brouzgou, A.; Tsiakaras, P. Electrodes for solid electrolyte sensors for the measurement of CO and H2 content in air. Int. J. Hydrog. Energy 2013, 38, 13484–13490. [Google Scholar] [CrossRef]
- Tan, Y.; Tan, T.C. Characteristics and Modeling of A Solid State Hydrogen Sensor. J. Electrochem. Soc. 1994, 141, 461–467. [Google Scholar] [CrossRef]
- Lu, G.; Miura, N.; Yamazoe, N. High-temperature hydrogen sensor based on stabilized zirconia and a metal oxide electrode. Sens. Actuators B Chem. 1996, 35, 130–135. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar]
- Yamaguchi, M.; Anggraini, S.A.; Fujio, Y.; Breedon, M.; Plashnitsa, V.V.; Miura, N. Selective hydrogen detection at high temperature by using yttria-stabilized zirconia-based sensor with coupled metal-oxide-based sensing electrodes. Electrochim. Acta 2012, 76, 152–158. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Anggraini, S.A.; Fujio, Y.; Sato, T.; Breedon, M.; Miura, N. Stabilized zirconia-based sensor utilizing SnO2-based sensing electrode with an integrated Cr2O3 catalyst layer for sensitive and selective detection of hydrogen. Int. J. Hydrog. Energy 2013, 38, 305–312. [Google Scholar] [CrossRef]
- Martin, L.P.; Glass, R.S. Hydrogen Sensor Based on YSZ Electrolyte and Tin-Doped Indium Oxide Electrode. J. Electrochem. Soc. 2005, 152, 43. [Google Scholar] [CrossRef]
- Martin, L.P.; Pham, A.Q.; Glass, R.S. Electrochemical hydrogen sensor for safety monitoring. Solid State Ion. 2004, 175, 527–530. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Brosha, E.L.; Mukundan, R.; Nelson, M.A.; Williamson, T.L.; Garzon, F.H. Development and testing of a miniaturized hydrogen safety sensor prototype. Sens. Actuators B Chem. 2010, 148, 469–477. [Google Scholar] [CrossRef]
- Tang, Z.; Li, X.; Yang, J.; Yu, J.; Wang, J.; Tang, Z. Mixed potential hydrogen sensor using ZnWO4 sensing electrode. Sens. Actuators B Chem. 2014, 195, 520–525. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tang, Z.; Tang, Z.; Yu, J.; Wang, J. Hydrogen sensing of the mixed-potential-type MnWO4/YSZ/Pt sensor. Sens. Actuators B Chem. 2015, 206, 176–180. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Tang, Z.; Wang, J.; Yu, J.; Tang, Z. Potentiometric hydrogen sensors based on yttria-stabilized zirconia electrolyte (YSZ) and CdWO4 interface. Sens. Actuators B Chem. 2016, 223, 365–371. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, J.; Jun, Y.; Tang, Z. A cobalt tungstate compound sensing electrode for hydrogen detection based upon mixed-potential type sensors. RSC Adv. 2017, 7, 2919–2925. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, X.; Yuan, L.; Yu, J. A review of high-temperature electrochemical sensors based on stabilized zirconia. Solid State Ion. 2015, 283, 91–102. [Google Scholar] [CrossRef]
- Ramaiyan, K.P.; Mukundan, R. Editors’ Choice—Review—Recent Advances in Mixed Potential Sensors. J. Electrochem. Soc. 2020, 167, 037547. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Yu, J.; Gao, X.; Jin, H.; Wang, X.; Wang, C. A limiting current oxygen sensor with La0.8Sr0.2(Ga0.8Mg0.2)1-xFexO3-δ dense diffusion barrier. J. Solid State Electrochem. 2017, 21, 1323–1328. [Google Scholar] [CrossRef]
- Liu, T.; Jin, H.; Li, L.; Yu, J. A novel method for preparing dense diffusion barrier limiting current oxygen sensor. J. Am. Ceram. Soc. 2018, 101, 1537–1543. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Zhang, H.; Yu, J.; Jin, H.; Wang, X.; Wang, C.; Gao, X. Limiting current oxygen sensors with La0.8Sr0.2Ga0.8Mg0.2O3-δ electrolyte and La0.8Sr0.2(Ga0.8Mg0.2)1-xCoxO3-δ dense diffusion barrier. Ionics 2018, 24, 827–832. [Google Scholar] [CrossRef]
- Kalyakin, A.; Fadeyev, G.; Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Application of Solid oxide proton-conducting electrolytes for amperometric analysis of hydrogen in H2+N2+H2O gas mixtures. Electrochim. Acta 2014, 141, 120–125. [Google Scholar] [CrossRef]
- Kalyakin, A.; Lyagaeva, J.; Medvedev, D.; Volkov, A.; Demin, A.; Tsiakaras, P. Characterization of proton-conducting electrolyte based on La0.9Sr0.1YO3–δ and its application in a hydrogen amperometric sensor. Sens. Actuators B Chem. 2016, 225, 446–452. [Google Scholar] [CrossRef]
- Kalyakin, A.; Volkov, A.; Lyagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Combined amperometric and potentiometric hydrogen sensors based on BaCe0.7Zr0.1Y0.2O3−δ proton-conducting ceramic. Sens. Actuators B Chem. 2016, 231, 175–182. [Google Scholar] [CrossRef]
- Yagi, H.; Ichikawa, K. Humidity sensing characteristics of a limiting current type planar oxygen sensor for high temperatures. Sens. Actuators B Chem. 1993, 13, 92–95. [Google Scholar] [CrossRef]
- Iwahara, H.; Uchida, H.; Kondo, J. Galvanic cell-type humidity sensor using high temperature-type proton conductive solid electrolyte. J. Appl. Electrochem. 1983, 13, 365–370. [Google Scholar] [CrossRef]
- Katahira, K.; Matsumoto, H.; Iwahara, H.; Koide, K.; Iwamoto, T. A solid electrolyte steam sensor with an electrochemically supplied hydrogen standard using proton-conducting oxides. Sens. Actuators B Chem. 2000, 67, 189–193. [Google Scholar] [CrossRef]
- Taniguchi, N.; Kuroha, T.; Nishimura, C.; Iijima, K. Characteristics of novel BaZr0.4Ce0.4In0.2O3 proton conducting ceramics and their application to hydrogen sensors. Solid State Ion. 2005, 176, 2979–2983. [Google Scholar] [CrossRef]
- Kalyakin, A.S.; Lyagaeva, J.G.; Chuikin, A.Y.; Volkov, A.N.; Medvedev, D.A. A high-temperature electrochemical sensor based on CaZr0.95Sc0.05O3–δ for humidity analysis in oxidation atmospheres. J. Solid State Electrochem. 2019, 23, 73–79. [Google Scholar] [CrossRef]
- Kalyakin, A.S.; Volkov, A.N.; Gorshkov, M.Y. An electrochemical sensor based on zirconia and calcium zirconate electrolytes for the inert gas humidity analysis. J. Taiwan Inst. Chem. Eng. 2020, 111, 222–227. [Google Scholar] [CrossRef]
- Kalyakin, A.S.; Danilov, N.A.; Volkov, A.N. Determining humidity of nitrogen and air atmospheres by means of a protonic ceramic sensor. J. Electroanal. Chem. 2021, 895, 115523. [Google Scholar] [CrossRef]
- Medvedev, D.; Kalyakin, A.; Volkov, A.; Demin, A.; Tsiakaras, P. Electrochemical moisture analysis by combining oxygen- and proton-conducting ceramic electrolytes. Electrochem. Commun. 2017, 76, 55–58. [Google Scholar] [CrossRef]
- Kalyakin, A.; Demin, A.K.; Gorbova, E.; Volkov, A.; Tsiakaras, P.E. Sensor Based on a Solid Oxide Electrolyte for Measuring the Water-Vapor and Hydrogen Content in Air. Catalysts 2022, 12, 1558. [Google Scholar] [CrossRef]


| Electrode Material | EMF/mV | |
|---|---|---|
| 0.02% H2 + Air | 0.02% CO + Air | |
| Ag | 1 | 0 |
| SnO2 | 127 | 66 |
| ZnO | 115 | 62 |
| Cr2O3 | 50 | 35 |
| In2O3 | 27 | 18 |
| La0.6Sr0.4MnO3 | 6 | 5 |
| La0.8Sr0.2CrO3 | 60 | 17 |
| Type | Target Gas | Temperature °C | Electrolyte | Year | Refs |
|---|---|---|---|---|---|
| potentiometric | H2 | 200–900 | BaCe0.9Nd0.1O3-α | 1991 | [23] |
| potentiometric | H2 | 600–800 | CaZr0.95Mn0.05O3-δ | 2016 | [25] |
| amperometric | H2 | 850 | CaZr0.9Sc0.1O3 | 2014 | [46] |
| amperometric | H2 | 850 | La0.95Sr0.05YO3 | 2014 | [46] |
| amperometric | H2 | 500–600 | La0.9Sr0.1YO3-δ | 2016 | [47] |
| combined | H2 | 450–550 | BaCe0.7Zr0.1Y0.2O3-δ | 2016 | [48] |
| amperometric | H2, H2O | 700 | 0.9ZrO2 + 0.1Y2O3 (YSZ) | 2022 | [57] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorbova, E.; Balkourani, G.; Molochas, C.; Sidiropoulos, D.; Brouzgou, A.; Demin, A.; Tsiakaras, P. Brief Review on High-Temperature Electrochemical Hydrogen Sensors. Catalysts 2022, 12, 1647. https://doi.org/10.3390/catal12121647
Gorbova E, Balkourani G, Molochas C, Sidiropoulos D, Brouzgou A, Demin A, Tsiakaras P. Brief Review on High-Temperature Electrochemical Hydrogen Sensors. Catalysts. 2022; 12(12):1647. https://doi.org/10.3390/catal12121647
Chicago/Turabian StyleGorbova, Elena, Georgia Balkourani, Costas Molochas, Dimitrios Sidiropoulos, Angeliki Brouzgou, Anatoly Demin, and Panagiotis Tsiakaras. 2022. "Brief Review on High-Temperature Electrochemical Hydrogen Sensors" Catalysts 12, no. 12: 1647. https://doi.org/10.3390/catal12121647
APA StyleGorbova, E., Balkourani, G., Molochas, C., Sidiropoulos, D., Brouzgou, A., Demin, A., & Tsiakaras, P. (2022). Brief Review on High-Temperature Electrochemical Hydrogen Sensors. Catalysts, 12(12), 1647. https://doi.org/10.3390/catal12121647








