Sulfur-Resistant CeO2-Supported Pt Catalyst for Waste-to-Hydrogen: Effect of Catalyst Synthesis Method
Abstract
:1. Introduction
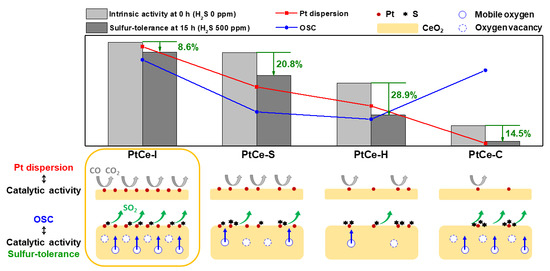
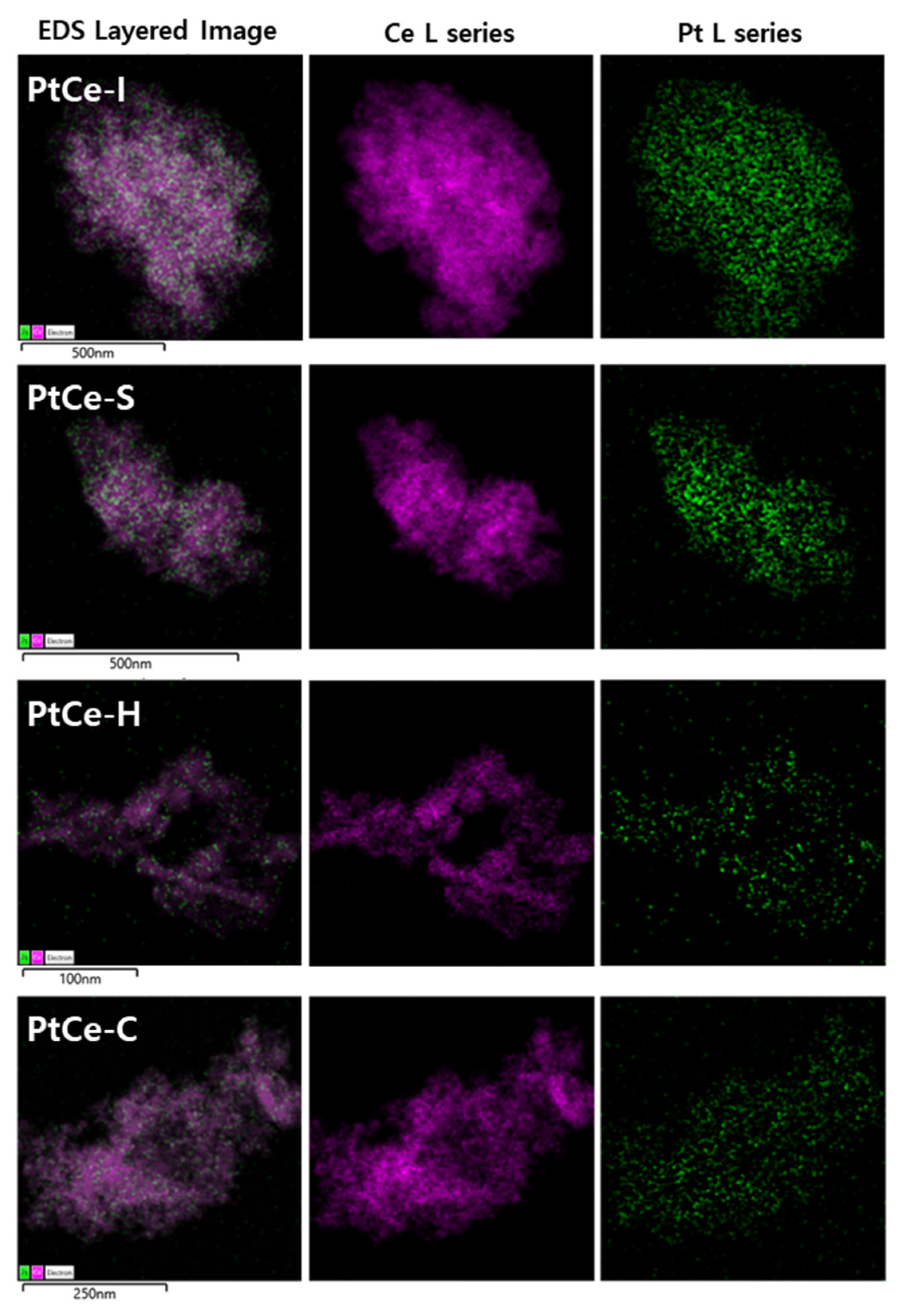
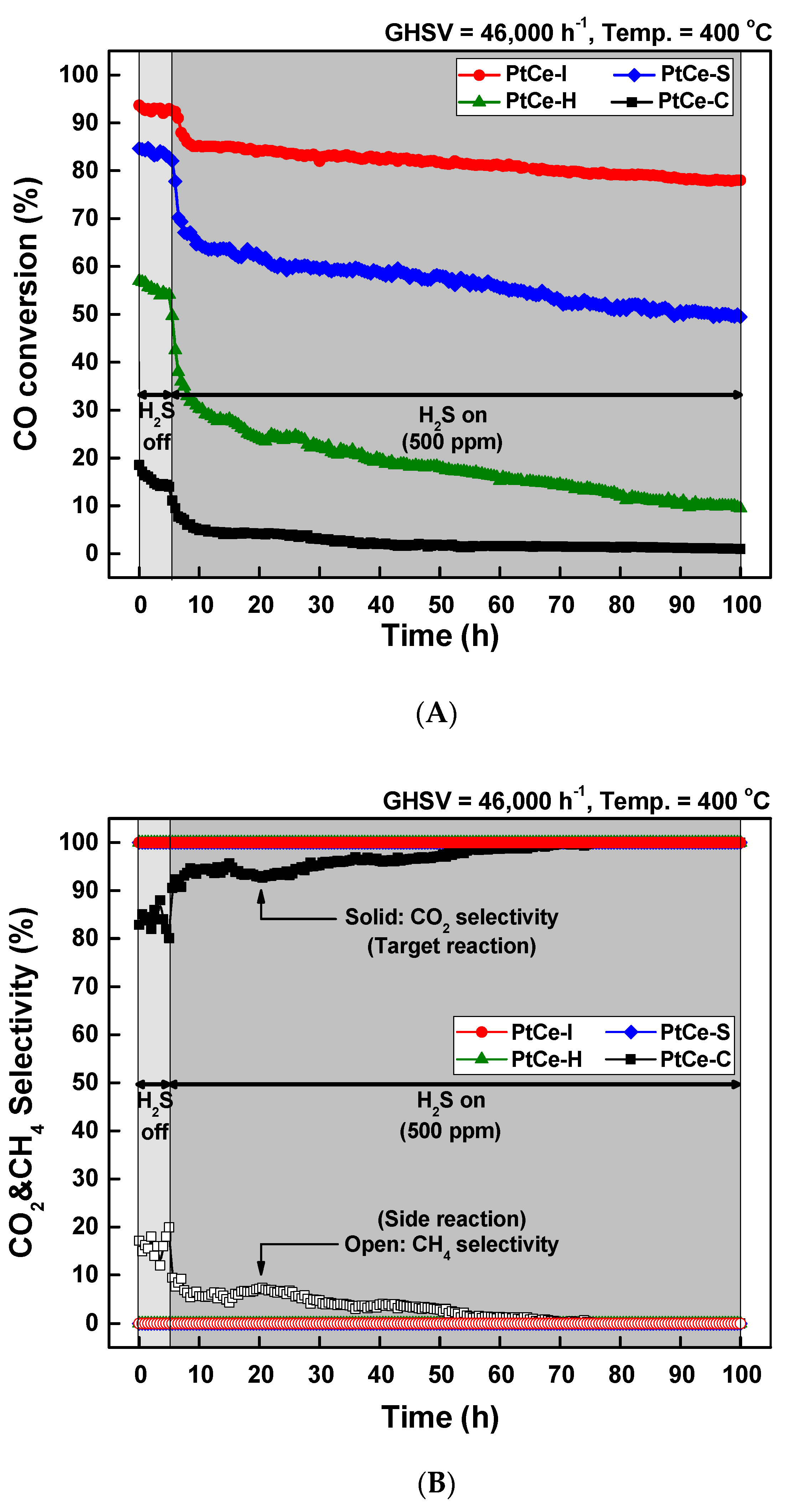
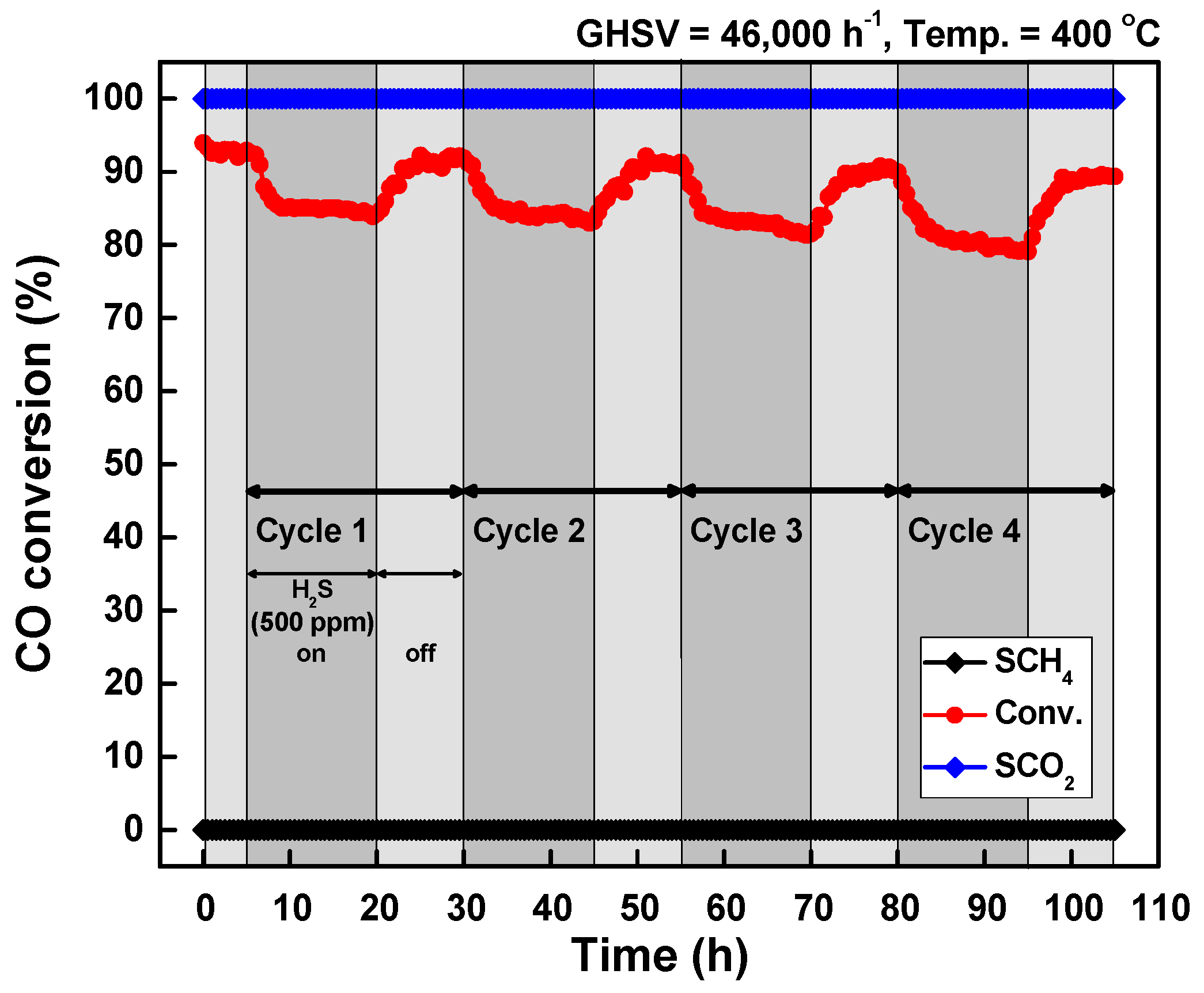
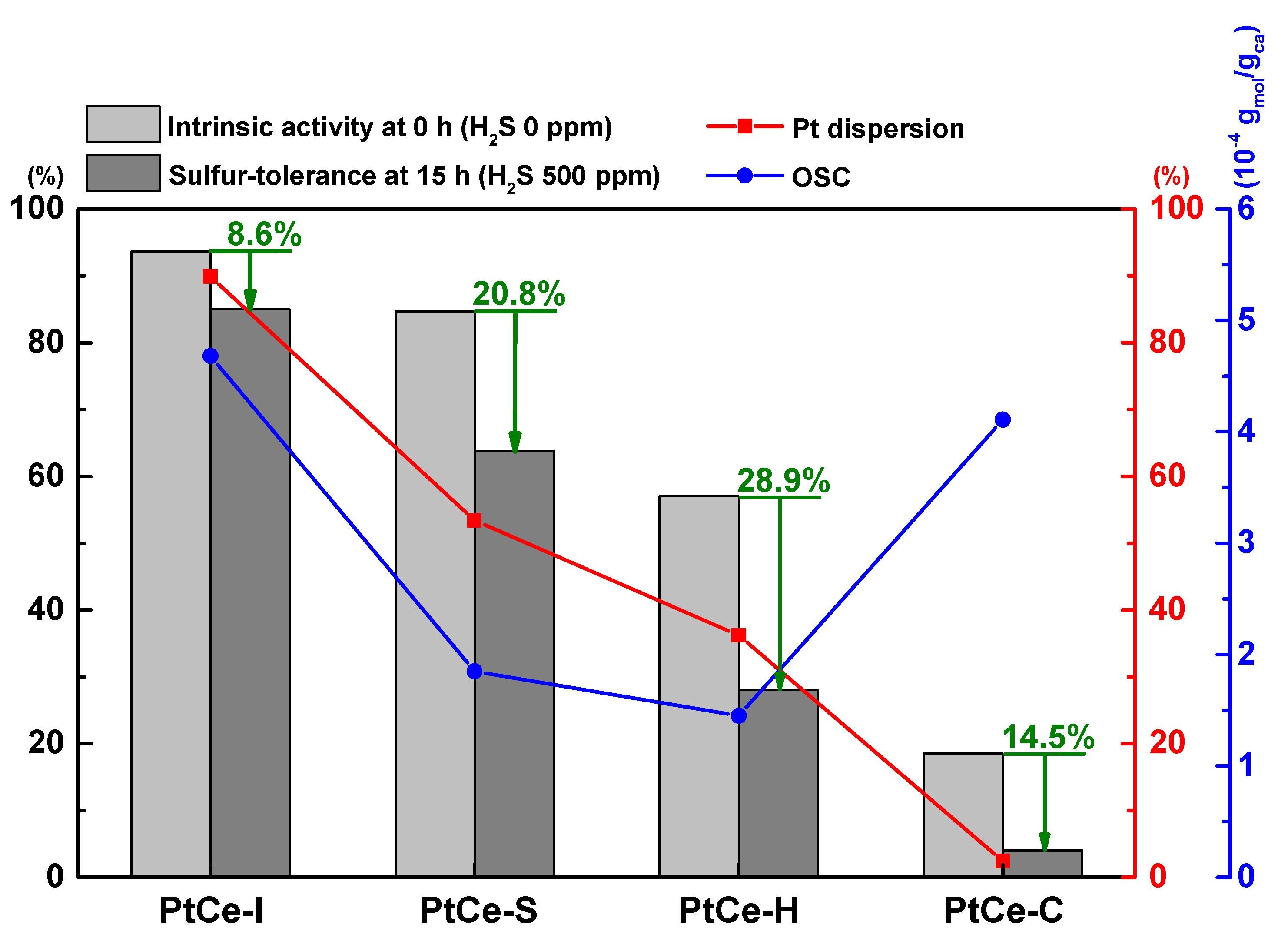
2. Results and Discussion
3. Materials and Methods
3.1. Catalyst Synthesis
3.1.1. Incipient Wetness Impregnation Method
3.1.2. Sol-Gel Method
3.1.3. Hydrothermal Method
3.1.4. Co-Precipitation Method
3.2. Catalytic Reaction
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Ozturk, M.; Dincer, I. An Integrated System for Clean Hydrogen Production from Municipal Solid Wastes. Int. J. Hydrogen Energy 2021, 46, 6251–6261. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Wang, B.; Chen, C. Hydrogen and Syngas Production from Municipal Solid Waste (MSW) Gasification via Reusing CO2. Appl. Therm. Eng. 2018, 144, 242–247. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lee, K.; Hyun Ko, C.; Roh, H.S. Optimization of Nano-Catalysts for Application in Compact Reformers. Chem. Eng. J. 2022, 431, 134299. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Zheng, Y.; Tian, J.; Jing, T.; Chang, J.S.; Ho, S.H. Recent Advances in Hydrogen Production by Thermo-Catalytic Conversion of Biomass. Int. J. Hydrogen Energy 2019, 44, 14266–14278. [Google Scholar] [CrossRef]
- Su, W.; Cai, C.; Liu, P.; Lin, W.; Liang, B.; Zhang, H.; Ma, Z.; Ma, H.; Xing, Y.; Liu, W. Supercritical Water Gasification of Food Waste: Effect of Parameters on Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 14744–14755. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, D.; Wang, S.; Zhang, R.; Wu, Z.; Meng, H.; Chen, L.; Wang, T.; Guo, Y. Hydrogen-Rich Syngas Produced from Co-Gasification of Municipal Solid Waste and Wheat Straw in an Oxygen-Enriched Air Fluidized Bed. Int. J. Hydrogen Energy 2021, 46, 18051–18063. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. A Review on Technological Options of Waste to Energy for Effective Management of Municipal Solid Waste. Waste Manag. 2017, 69, 407–422. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lim, D.; Lee, B.; Upadhyay, M.; Brigljević, B.; Roh, H.S.; Lim, H. Comparative Techno-Economic Analysis of Methanol Production via Carbon Dioxide Reforming of Landfill Gas Using a Highly Active and Stable Nickel-Based Catalyst. Energy Convers. Manag. 2022, 259, 115585. [Google Scholar] [CrossRef]
- Park, S.W.; Lee, J.S.; Yang, W.S.; Alam, M.T.; Seo, Y.C. A Comparative Study of the Gasification of Solid Refuse Fuel in Downdraft Fixed Bed and Bubbling Fluidized Bed Reactors. Waste Biomass Valorization 2020, 11, 2345–2356. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Jeon, K.W.; Na, H.S.; Kim, H.M.; Lee, Y.L.; Roh, H.S.; Jeong, D.W. Design and Scale-up of a Cr-Free Fe-Al-Cu Catalyst for Hydrogen Production from Waste-Derived Synthesis Gas. Appl. Catal. B 2019, 249, 72–81. [Google Scholar] [CrossRef]
- Renkel, M.F.; Lümmen, N. Supplying Hydrogen Vehicles and Ferries in Western Norway with Locally Produced Hydrogen from Municipal Solid Waste. Int. J. Hydrogen Energy 2018, 43, 2585–2600. [Google Scholar] [CrossRef]
- Jeong, D.W.; Jang, W.J.; Shim, J.O.; Roh, H.S. High Temperature Water–Gas Shift without Pre-Reduction over Spinel Ferrite Catalysts Synthesized by Glycine Assisted Sol–Gel Combustion Method. Int. J. Hydrogen Energy 2016, 41, 3870–3876. [Google Scholar] [CrossRef]
- Indrawan, N.; Mohammad, S.; Kumar, A.; Huhnke, R.L. Modeling Low Temperature Plasma Gasification of Municipal Solid Waste. Environ. Technol. Innov. 2019, 15, 100412. [Google Scholar] [CrossRef]
- Dou, X.; Veksha, A.; Chan, W.P.; Oh, W.D.; Liang, Y.N.; Teoh, F.; Mohamed, D.K.B.; Giannis, A.; Lisak, G.; Lim, T.T. Poisoning Effects of H2S and HCl on the Naphthalene Steam Reforming and Water-Gas Shift Activities of Ni and Fe Catalysts. Fuel 2019, 241, 1008–1018. [Google Scholar] [CrossRef]
- Ngo, T.N.L.T.; Chiang, K.Y.; Liu, C.F.; Chang, Y.H.; Wan, H.P. Hydrogen Production Enhancement Using Hot Gas Cleaning System Combined with Prepared Ni-Based Catalyst in Biomass Gasification. Int. J. Hydrogen Energy 2021, 46, 11269–11283. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, K.J.; Hong, G.R.; Ahn, S.Y.; Kim, B.J.; Shim, J.O.; Roh, H.S. Highly Sulfur Tolerant and Regenerable Pt/CeO2 Catalyst for Waste to Energy. Renew. Energy 2021, 178, 334–343. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, K.J.; Hong, G.R.; Ahn, S.Y.; Kim, B.J.; Park, H.R.; Yun, S.J.; Bae, J.W.; Jeon, B.H.; Roh, H.S. Sulfur-Tolerant Pt/CeO2 Catalyst with Enhanced Oxygen Storage Capacity by Controlling the Pt Content for the Waste-to-Hydrogen Processes. ACS Sustain. Chem. Eng. 2021, 9, 15287–15293. [Google Scholar] [CrossRef]
- Lee, Y.L.; Mnoyan, A.; Na, H.S.; Ahn, S.Y.; Kim, K.J.; Shim, J.O.; Lee, K.; Roh, H.S. Comparison of the Effects of the Catalyst Preparation Method and CeO2 morphology on the Catalytic Activity of Pt/CeO2 catalysts for the Water-Gas Shift Reaction. Catal. Sci. Technol. 2020, 10, 6299–6308. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Ramos-Fernández, E.V.; Sepúlveda-Escribano, A. Effect of the CeO2 Synthesis Method on the Behaviour of Pt/CeO2 Catalysis for the Water-Gas Shift Reaction. Int. J. Hydrogen Energy 2019, 44, 21837–21846. [Google Scholar] [CrossRef]
- Happel, M.; Mysliveček, J.; Johánek, V.; Dvořák, F.; Stetsovych, O.; Lykhach, Y.; Matolín, V.; Libuda, J. Adsorption Sites, Metal-Support Interactions, and Oxygen Spillover Identified by Vibrational Spectroscopy of Adsorbed CO: A Model Study on Pt/Ceria Catalysts. J. Catal. 2012, 289, 118–126. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, J.; Wang, H.; Li, H.; Wang, J.; Shen, M. Effect of Active Oxygen on the Performance of Pt/CeO2 Catalysts for CO Oxidation. J. Rare Earths 2018, 36, 257–264. [Google Scholar] [CrossRef]
- Puigdollers, A.R.; Schlexer, P.; Tosoni, S.; Pacchioni, G. Increasing Oxide Reducibility: The Role of Metal/Oxide Interfaces in the Formation of Oxygen Vacancies. ACS Catal. 2017, 7, 6493–6513. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Lee, E.; Kim, D.H. Inhibition of Water-Gas Shift Reaction Activity of Oxide-Supported Pt Catalyst by H2 and CO2. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Park, Y.M.; Son, M.; Park, M.J.; Bae, J.W. Effects of Pt Precursors on Pt/CeO2 to Water-Gas Shift (WGS) Reaction Activity with Langmuir-Hinshelwood Model-Based Kinetics. Int. J. Hydrogen Energy 2020, 45, 26953–26966. [Google Scholar] [CrossRef]
- Li, Y.; Kottwitz, M.; Vincent, J.L.; Enright, M.J.; Liu, Z.; Zhang, L.; Huang, J.; Senanayake, S.D.; Yang, W.C.D.; Crozier, P.A.; et al. Dynamic Structure of Active Sites in Ceria-Supported Pt Catalysts for the Water Gas Shift Reaction. Nat. Commun. 2021, 12, 914. [Google Scholar] [CrossRef]
- Lee, J.; Shin, D.; Lee, E.; Li, C.; Kim, J.M.; Han, J.W.; Kim, D.H. Alleviating Inhibitory Effect of H2 on Low-Temperature Water-Gas Shift Reaction Activity of Pt/CeO2 Catalyst by Forming CeO2 Nano-Patches on Pt Nano-Particles. Appl. Catal. B 2022, 305, 121038. [Google Scholar] [CrossRef]
- Zhang, R.; Lu, K.; Zong, L.; Tong, S.; Wang, X.; Zhou, J.; Lu, Z.H.; Feng, G. Control Synthesis of CeO2 Nanomaterials Supported Gold for Catalytic Oxidation of Carbon Monoxide. Mol. Catal. 2017, 442, 173–180. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, Y.L.; Na, H.S.; Ahn, S.Y.; Shim, J.O.; Jeon, B.H.; Roh, H.S. Efficient Waste to Energy Conversion Based on Co-CeO2 Catalyzed Water-Gas Shift. Catalysts 2020, 10, 420. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, Y. Influence of the Preparation Method on the Performance of CeO2-MoO3 Catalyst for the Selective Catalytic Reduction of NO with NH3. React. Kinet. Mech. Catal. 2014, 112, 27–36. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, B.J.; Park, H.R.; Ahn, S.Y.; Kim, K.J.; Roh, H.S. Customized Ni-MgO-Al2O3catalyst for Carbon Dioxide Reforming of Coke Oven Gas: Optimization of Preparation Method and Co-Precipitation PH. J. CO2 Util. 2020, 42, 101354. [Google Scholar] [CrossRef]
- Wu, S.L.; Kuo, J.H.; Wey, M.Y. Design of Catalysts Comprising a Nickel Core and Ceria Shell for Hydrogen Production from Plastic Waste Gasification: An Integrated Test for Anti-Coking and Catalytic Performance. Catal. Sci. Technol. 2020, 10, 3975–3984. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Sun, L.; Wang, W.; Chen, Z. Enhanced Electrocatalytic Activity of PtCu Bimetallic Nanoparticles on CeO2/Carbon Nanotubes for Methanol Electro-Oxidation. Int. J. Hydrogen Energy 2020, 45, 8558–8567. [Google Scholar] [CrossRef]
- Mo, L.; Saw, E.T.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Preparation of Highly Dispersed Cu/SiO2 Doped with CeO2 and Its Application for High Temperature Water Gas Shift Reaction. Int. J. Hydrogen Energy 2018, 43, 15891–15897. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Romero-Sarria, F.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Phosphate-Type Supports for the Design of WGS Catalysts. Appl. Catal. B 2019, 244, 853–862. [Google Scholar] [CrossRef]
- Li, F.; Zou, L.; He, J.; Wu, Y.; Yang, L.; Liu, Q.; Wu, Q.; Yang, X. On the Correlation between Structure and Catalytic Activity of Mesoporous Ceria Nanoparticles. J. Catal. 2021, 402, 300–309. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M. Dry Reforming over Mesoporous Nanocrystalline 5% Ni/M-MgAl2O4 (M: CeO2, ZrO2, La2O3) Catalysts. Int. J. Hydrogen Energy 2019, 44, 16516–16525. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Chan, X.; Kim, T.J.; Kim, D.H. How Pt Interacts with CeO2 under the Reducing and Oxidizing Environments at Elevated Temperature: The Origin of Improved Thermal Stability of Pt/CeO2 Compared to CeO2. J. Phys. Chem. C 2016, 120, 25870–25879. [Google Scholar] [CrossRef]
- Lykhach, Y.; Staudt, T.; Vorokhta, M.; Skála, T.; Johánek, V.; Prince, K.C.; Matolín, V.; Libuda, J. Hydrogen Spillover Monitored by Resonant Photoemission Spectroscopy. J. Catal. 2012, 285, 6–9. [Google Scholar] [CrossRef]
- Vecchietti, J.; Bonivardi, A.; Xu, W.; Stacchiola, D.; Delgado, J.J.; Calatayud, M.; Collins, S.E. Understanding the Role of Oxygen Vacancies in the Water Gas Shift Reaction on Ceria-Supported Platinum Catalysts. ACS Catal. 2014, 4, 2088–2096. [Google Scholar] [CrossRef]
- Aranifard, S.; Ammal, S.C.; Heyden, A. On the Importance of Metal-Oxide Interface Sites for the Water-Gas Shift Reaction over Pt/CeO2 Catalysts. J. Catal. 2014, 309, 314–324. [Google Scholar] [CrossRef]
- Kim, K.J.; Jeon, K.W.; Hong, G.R.; Jeon, B.H.; Wook Bae, J.; Jang, W.J.; Lee, Y.L.; Roh, H.S. Elucidating the Effect of Ce/Zr Ratio on High Temperature Shift Activity with Sulfur Poisoning. J. Ind. Eng. Chem. 2022, 115, 537–543. [Google Scholar] [CrossRef]
- Huang, S.; Cheng, B.; Yu, J.; Jiang, C. Hierarchical Pt/MnO2-Ni(OH)2 Hybrid Nanoflakes with Enhanced Room-Temperature Formaldehyde Oxidation Activity. ACS Sustain. Chem. Eng. 2018, 6, 12481–12488. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria Sandwiched Ni Core–Shell Catalyst for Low Temperature Dry Reforming of Biogas: Coke Resistance and Mechanistic Insights. Appl. Catal. B 2018, 230, 220–236. [Google Scholar] [CrossRef]
- Liu, B.; Li, C.; Zhang, G.; Yao, X.; Chuang, S.S.C.; Li, Z. Oxygen Vacancy Promoting Dimethyl Carbonate Synthesis from CO2 and Methanol over Zr-Doped CeO2 Nanorods. ACS Catal. 2018, 8, 10446–10456. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jha, A.; Jang, W.J.; Shim, J.O.; Rode, C.V.; Jeon, B.H.; Bae, J.W.; Roh, H.S. Effect of Alkali and Alkaline Earth Metal on Co/CeO2 Catalyst for the Water-Gas Shift Reaction of Waste Derived Synthesis Gas. Appl. Catal. A Gen. 2018, 551, 63–70. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Na, H.S.; Jeon, K.W.; Lee, Y.L.; Kim, K.J.; Shim, J.O.; Roh, H.S. Effect of Cu/CeO2 Catalyst Preparation Methods on Their Characteristics for Low Temperature Water−gas Shift Reaction: A Detailed Study. Catal. Today 2020, 352, 166–174. [Google Scholar] [CrossRef]
- Artiglia, L.; Orlando, F.; Roy, K.; Kopelent, R.; Safonova, O.; Nachtegaal, M.; Huthwelker, T.; van Bokhoven, J.A. Introducing Time Resolution to Detect Ce3+ Catalytically Active Sites at the Pt/CeO2 Interface through Ambient Pressure X-Ray Photoelectron Spectroscopy. J. Phys. Chem. Lett. 2017, 8, 102–108. [Google Scholar] [CrossRef]
- Kumar, A.; Sinha, A.S.K. Comparative Study of Hydrogen Production from Steam Reforming of Acetic Acid over Synthesized Catalysts via MOF and Wet Impregnation Methods. Int. J. Hydrogen Energy 2020, 45, 11512–11526. [Google Scholar] [CrossRef]
- Na, H.S.; Shim, J.O.; Ahn, S.Y.; Jang, W.J.; Jeon, K.W.; Kim, H.M.; Lee, Y.L.; Kim, K.J.; Roh, H.S. Effect of Precipitation Sequence on Physicochemical Properties of CeO2 Support for Hydrogen Production from Low-Temperature Water-Gas Shift Reaction. Int. J. Hydrogen Energy 2018, 43, 17718–17725. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, B.J.; Park, H.R.; Ahn, S.Y.; Kim, K.J.; Roh, H.S. Improving the Catalytic Activity in Dry Reforming Reaction by Enhancing the Oxygen Storage Capacity of Ce0.8Zr0.2O2 Support through Hydrogen Heat-Treatment. J. CO2 Util. 2022, 57, 101903. [Google Scholar] [CrossRef]
- Jang, W.J.; Shim, J.O.; Kim, H.M.; Yoo, S.Y.; Roh, H.S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal Today 2019, 15–26. [Google Scholar] [CrossRef]
- Tepamatr, P.; Laosiripojana, N.; Sesuk, T.; Charojrochkul, S. Effect of Samarium and Praseodymium Addition on Water Gas Shift Performance of Co/CeO2 Catalysts. J. Rare Earths 2020, 38, 1201–1206. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; la Parola, V.; Liotta, L.F.; Vodyankina, O.V. The Role of Metal–Support Interaction in Ag/CeO2 Catalysts for CO and Soot Oxidation. Appl. Catal. B 2020, 260, 118148. [Google Scholar] [CrossRef]
- Tiwari, S.; Rathore, G.; Patra, N.; Yadav, A.K.; Bhattacharya, D.; Jha, S.N.; Tseng, C.M.; Liu, S.W.; Biring, S.; Sen, S. Oxygen and Cerium Defects Mediated Changes in Structural, Optical and Photoluminescence Properties of Ni Substituted CeO2. J. Alloys Compd. 2019, 782, 689–698. [Google Scholar] [CrossRef]
- Kim, B.J.; Seo, J.C.; Kim, D.H.; Lee, Y.L.; Lee, K.; Roh, H.S. Oxygen Defective Bimodal Porous Ni-CeO2−x-MgO-Al2O3 Catalyst with Multi-Void Spherical Structure for CO2 Reforming of CH4. J. CO2 Util. 2022, 58, 101917. [Google Scholar] [CrossRef]
- Lee, Y.L.; Kim, H.Y.; Kim, K.J.; Hong, G.R.; Shim, J.O.; Ju, Y.W.; Roh, H.S. Nanofiber Structured Oxygen Defective CoFe2O4-x Catalyst for the Water-Gas Shift Reaction in Waste-to-Hydrogen Processes. Int. J. Hydrogen Energy 2022, 47, 30950–30958. [Google Scholar] [CrossRef]
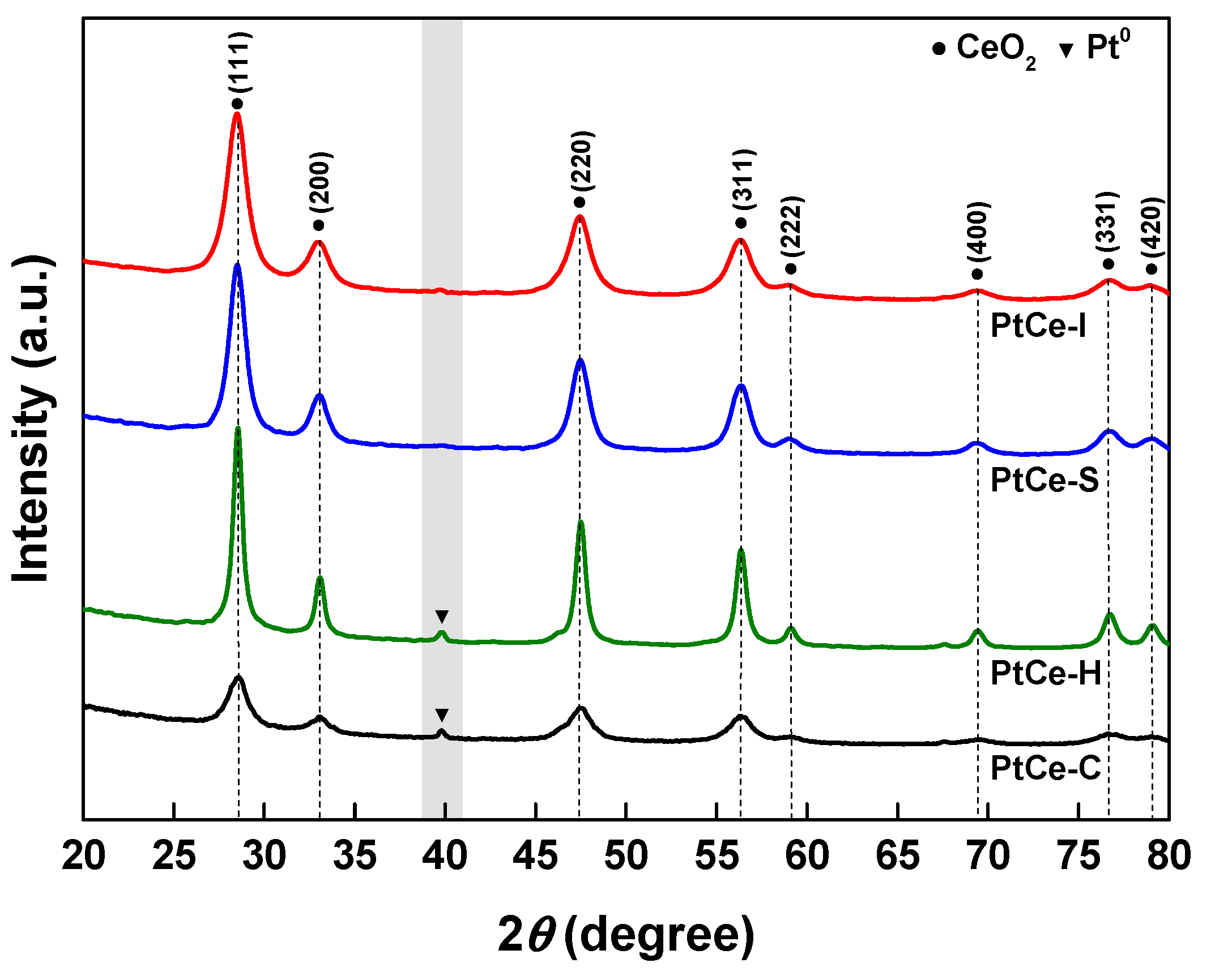
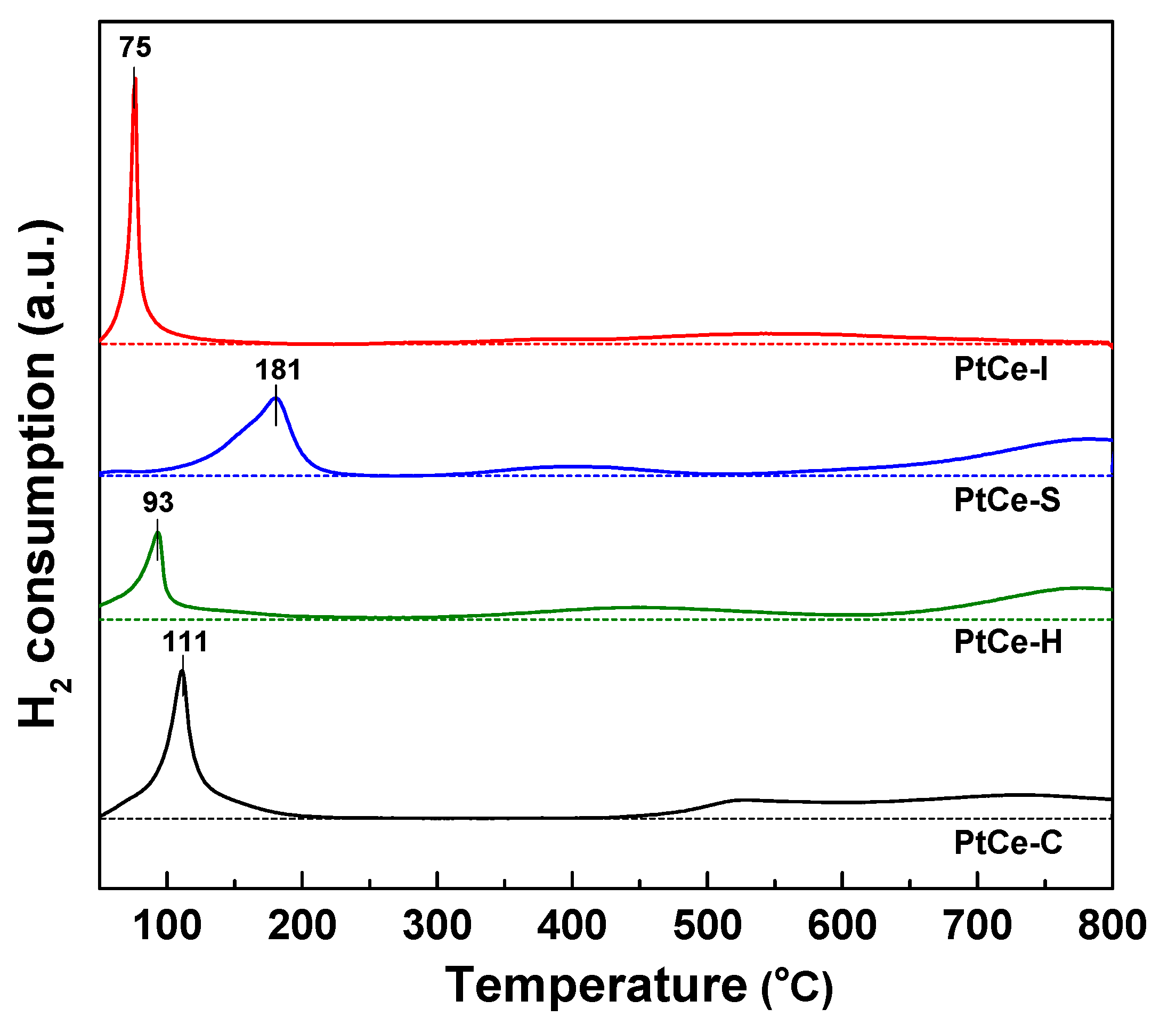
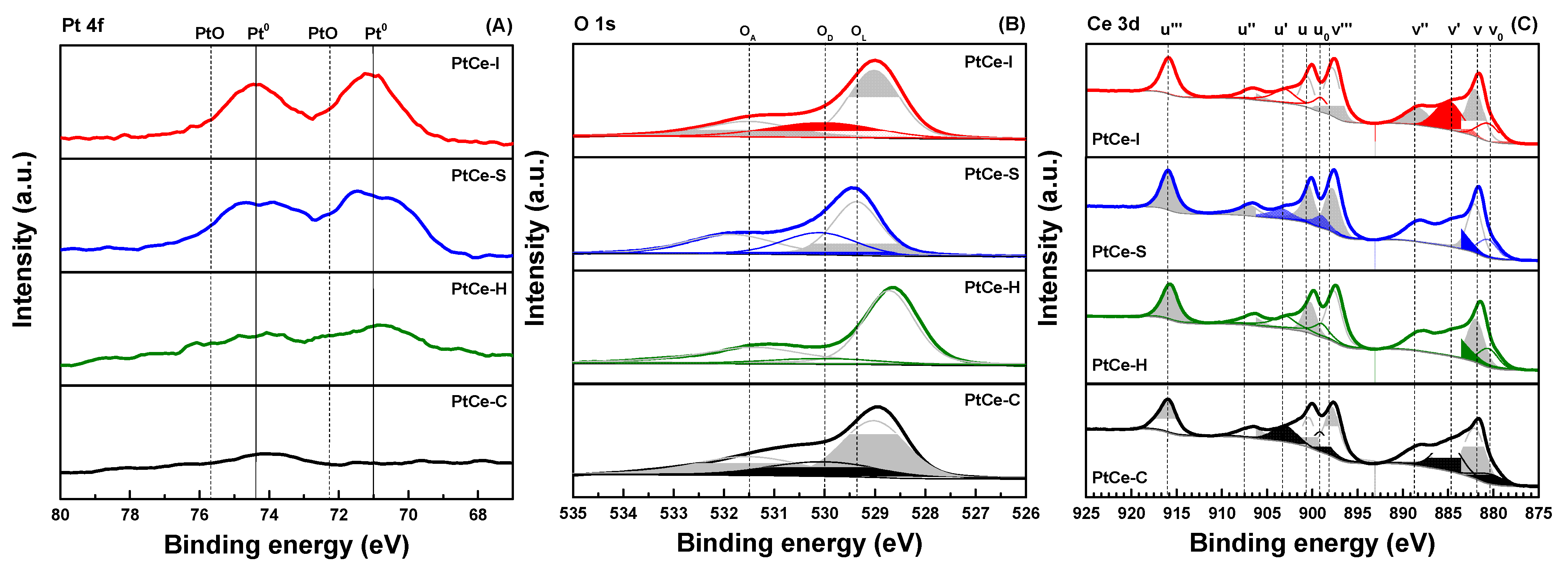

| Catalyst | S.A. (m2/g) a | Pore Volume (cm3/g) a | Pt0 Dispersion (%) b | Pt0 Particle Size (nm) b | H2 Consumption (mmol/g) c |
|---|---|---|---|---|---|
| PtCe-I | 107 | 0.415 | 89.9 | 1.05 | 8.29 |
| PtCe-S | 67 | 0.216 | 53.4 | 1.77 | 5.47 |
| PtCe-H | 78 | 0.357 | 36.2 | 2.60 | 3.52 |
| PtCe-C | 132 | 0.308 | 2.4 | 39.3 | 6.23 |
| Catalyst | OD (%) a | Ce3+ (%) b | OSC (10−4 gmol/gcat) c |
|---|---|---|---|
| PtCe-I | 22.18 | 32.21 | 4.68 |
| PtCe-S | 11.66 | 29.57 | 1.85 |
| PtCe-H | 10.35 | 28.19 | 1.45 |
| PtCe-C | 18.31 | 31.04 | 4.11 |
| Catalyst | Turnover Frequency (s−1) |
|---|---|
| PtCe-I | 0.010 |
| PtCe-S | 0.015 |
| PtCe-H | 0.015 |
| PtCe-C | 0.071 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, G.-R.; Kim, K.-J.; Ahn, S.-Y.; Kim, B.-J.; Park, H.-R.; Lee, Y.-L.; Lee, S.S.; Jeon, Y.; Roh, H.-S. Sulfur-Resistant CeO2-Supported Pt Catalyst for Waste-to-Hydrogen: Effect of Catalyst Synthesis Method. Catalysts 2022, 12, 1670. https://doi.org/10.3390/catal12121670
Hong G-R, Kim K-J, Ahn S-Y, Kim B-J, Park H-R, Lee Y-L, Lee SS, Jeon Y, Roh H-S. Sulfur-Resistant CeO2-Supported Pt Catalyst for Waste-to-Hydrogen: Effect of Catalyst Synthesis Method. Catalysts. 2022; 12(12):1670. https://doi.org/10.3390/catal12121670
Chicago/Turabian StyleHong, Ga-Ram, Kyoung-Jin Kim, Seon-Yong Ahn, Beom-Jun Kim, Ho-Ryong Park, Yeol-Lim Lee, Sang Soo Lee, Yukwon Jeon, and Hyun-Seog Roh. 2022. "Sulfur-Resistant CeO2-Supported Pt Catalyst for Waste-to-Hydrogen: Effect of Catalyst Synthesis Method" Catalysts 12, no. 12: 1670. https://doi.org/10.3390/catal12121670
APA StyleHong, G.-R., Kim, K.-J., Ahn, S.-Y., Kim, B.-J., Park, H.-R., Lee, Y.-L., Lee, S. S., Jeon, Y., & Roh, H.-S. (2022). Sulfur-Resistant CeO2-Supported Pt Catalyst for Waste-to-Hydrogen: Effect of Catalyst Synthesis Method. Catalysts, 12(12), 1670. https://doi.org/10.3390/catal12121670