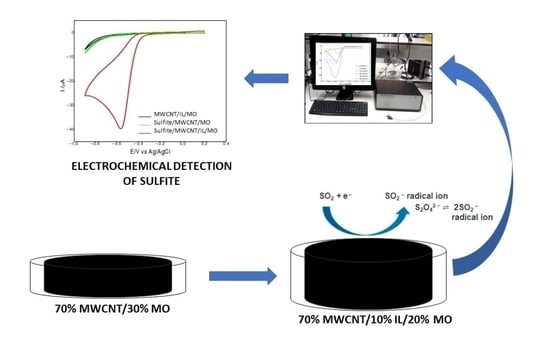
Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid
Abstract
:1. Introduction
2. Results and Discussion
2.1. Obtaining and Characterization of MWCNT/IL/MO Carbon Paste Electrodes
2.2. Electrochemical Characterization of MWCNT/IL/MO Carbon Paste Electrodes
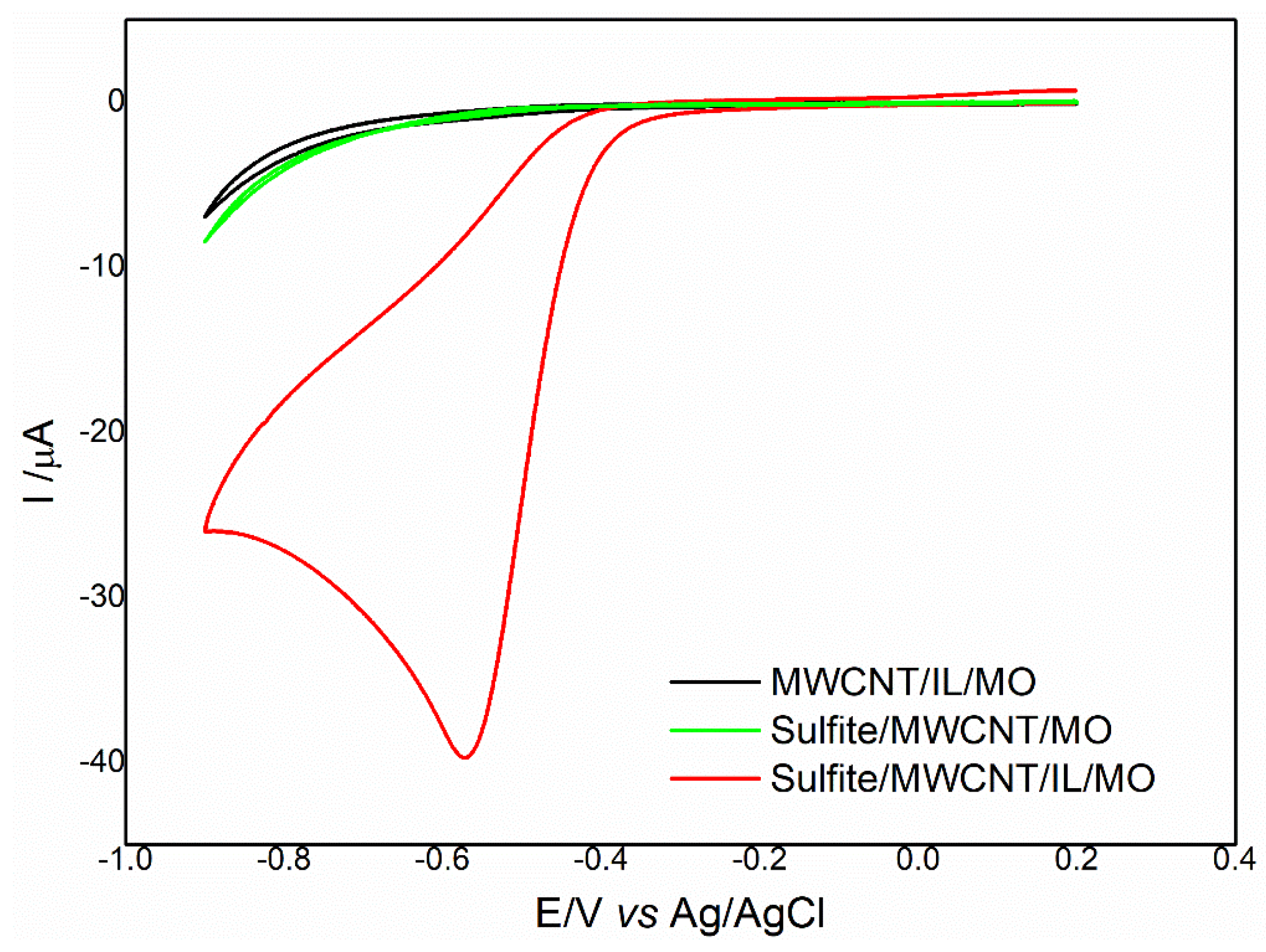
2.3. Electrochemical Behavior of Sulfite on MWCNT/IL/MO
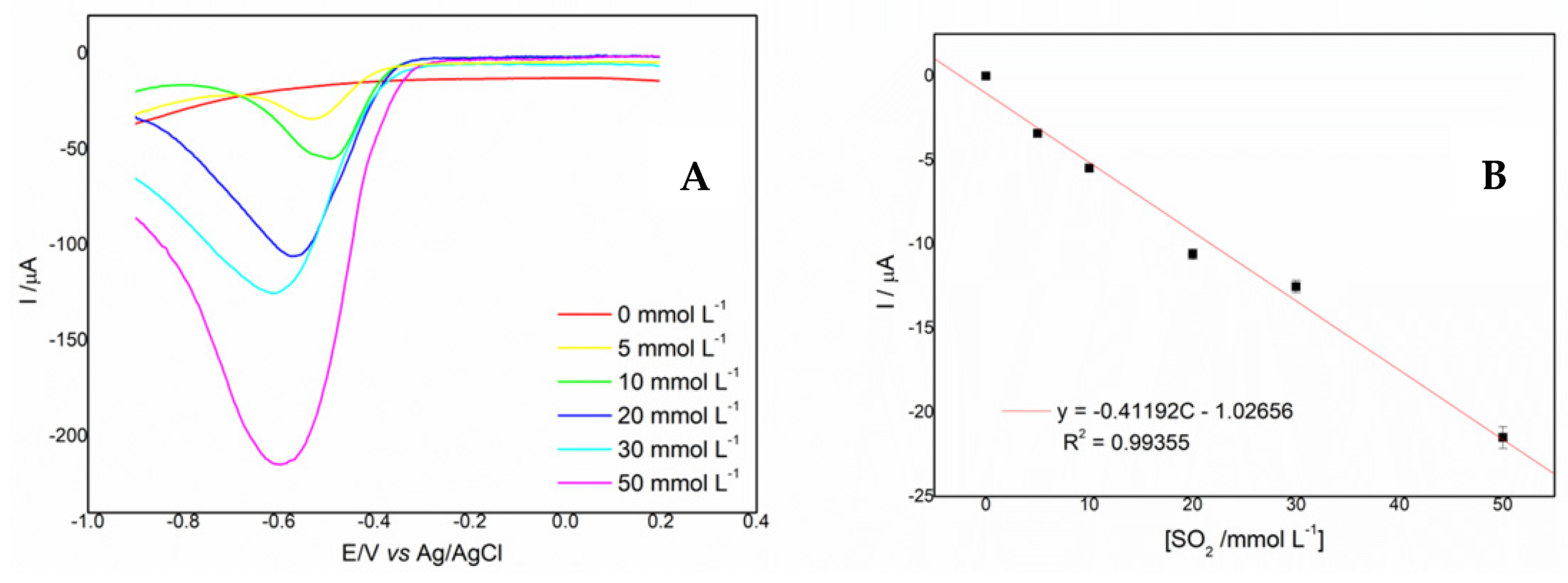
2.4. Voltammetric Determination of Sulfite
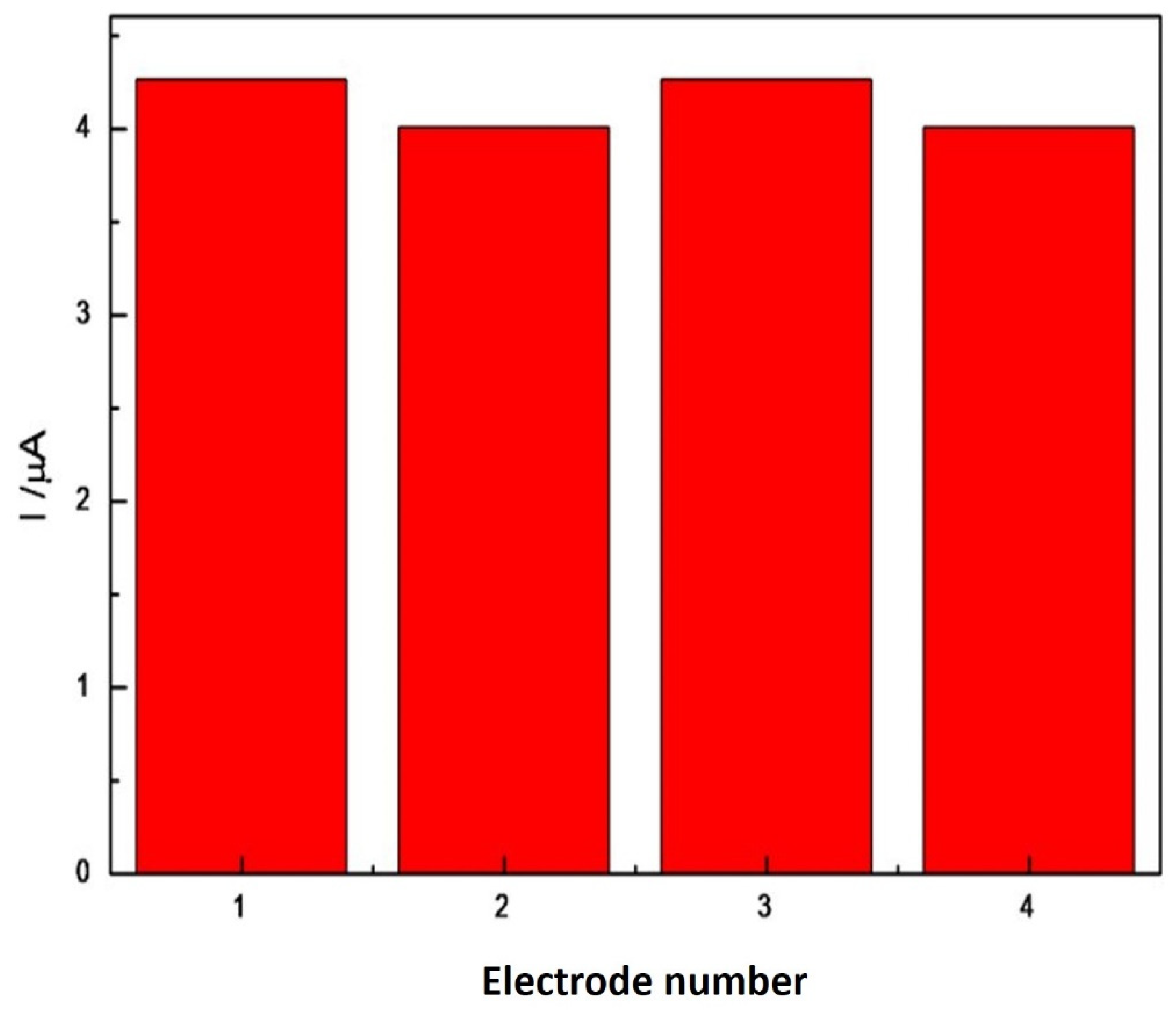
2.5. Analytical Parameters
3. Experimental
3.1. Materials, Reagents, and Equipment
3.2. Paste Electrode Preparation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inetianbor, J.; Yakubu, J.; Ezeonu, S. Effects of food additives and preservatives on man-a review. Asian J. Sci. Technol. 2015, 6, 1118–1135. [Google Scholar]
- Ruiz-Capillas, C.; Jiménez-Colmenero, F. Application of flow injection analysis for determining sulphites in food and beverages: A review. Food Chem. 2009, 112, 487–493. [Google Scholar] [CrossRef]
- Isaac, A.; Davis, J.; Livingstone, C.; Wain, A.J.; Compton, R.G. Electroanalytical methods for the determination of sulfite in food and beverages. TrAC Trends Anal. Chem. 2006, 25, 589–598. [Google Scholar] [CrossRef]
- Gupta, M.K.; Basavaraj, G. Sulphites in food & drinks in asthmatic adults & children: What we need to know. Indian J. Allergy Asthma Immunol. 2021, 35, 43. [Google Scholar]
- Vally, H.; Misso, N.L. Adverse reactions to the sulphite additives. Gastroenterol. Hepatol. Bed Bench 2012, 5, 16. [Google Scholar]
- Lacava, A. Fadugba, Sulfite hypersensitivity: A case of asthma triggered by sulfites. Ann. Allergy Asthma Immunol. 2020, 125, S98. [Google Scholar] [CrossRef]
- Galil, A.; Kumar, Y.; Sathish, M.A.; Nagendrappa, G. Simple spectrophotometric method for the determination of sulfur dioxide by its decolorizing effect on the peroxovanadate complex. Anal. Chem. 2008, 63, 239–243. [Google Scholar]
- Rankine, B.C.; Pocock, K.F. Alkalimetric determination of sulphur dioxide in wine. Aust. Wine Brew. Spirit Rev. 1970, 29, 40–44. [Google Scholar]
- Zamora, F. Elaboración y Crianza del Vino Tinto: Aspectos Científicos y Prácticos; AMV Ediciones, Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Monier-Williams, G.W. Determination of Sulphur Dioxide in Foods. In Public Health and Medical Subjects Report No. 43; Ministry of Health: London, UK, 1927; pp. 415–416. [Google Scholar]
- Wang, M.; Guo, L.; Cao, D. Amino-Functionalized Luminescent Metal–Organic Framework Test Paper for Rapid and Selective Sensing of SO2 Gas and Its Derivatives by Luminescence Turn-On Effect. Anal. Chem. 2018, 90, 3608–3614. [Google Scholar] [CrossRef]
- Al-Hosney, H.A.; Grassian, V.H. Water, sulfur dioxide and nitric acid adsorption on calcium carbonate: A transmission and ATR-FTIR study. Phys. Chem. Chem. Phys. 2005, 7, 1266–1276. [Google Scholar] [CrossRef]
- Baltrusaitis, J.; Cwiertny, D.M.; Grassian, V.H. Adsorption of sulfur dioxide on hematite and goethite particle surfaces. Phys. Chem. Chem. Phys. 2007, 9, 5542–5554. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tan, F.; Hartman, R. Simple spectrophotometry method for the determination of sulfur dioxide in an alcohol-thionyl chloride reaction. Anal. Chim. Acta 2015, 891, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Guo, J.; Wang, C. Dispersible and discrete metalloporphyrin-based CMP nanoparticles enabling colorimetric detection and quantitation of gaseous SO2. Chem. Commun. 2014, 50, 695–697. [Google Scholar] [CrossRef] [PubMed]
- Carrascon, V.; Ontañón, I.; Bueno, M.; Ferreira, V. Gas chromatography-mass spectrometry strategies for the accurate and sensitive speciation of sulfur dioxide in wine. J. Chromatogr. A 2017, 1504, 27–34. [Google Scholar] [CrossRef]
- Koch, M.; Köppen, R.; Siegel, D.; Witt, A.; Nehls, I. Determination of total sulfite in wine by ion chromatography after in-sample oxidation. J. Agric. Food Chem. 2010, 58, 9463–9467. [Google Scholar] [CrossRef]
- Zenga, R.-F.; Lan, J.-S.; Wu, T.; Liu, L.; Lui, Y.; Ho, R.; Ding, Y.; Zhang, T. A novel mitochondria-targetted near-infrared fluorescent probe for selective and colorimetric detection of sulfite and its application in vitro and vivo. Food Chem. 2020, 318, 126358–126365. [Google Scholar] [CrossRef]
- Sung, Q.; Zhang, W.; Qian, J. A ratiometric fluorescence probe for selective detection of sulfite and its application in realistic samples. Talanta 2017, 162, 107–113. [Google Scholar]
- Chandra, S.; Rachna, R. Determination of sulfite, with emphasis on methods of biological detection: A review. Department of Biochemistry, University of Maryland. Bioanal. Chem. 2013, 405, 2599–2608. [Google Scholar]
- Dinçkaya, E.; Sezgintürk, M.K.; Akyılmaz, E.; Ertaş, F.N. Sulfite determination using sulfite oxidase biosensor based glassy carbon electrode coated with thin mercury film. Food Chem. 2007, 101, 1540–1544. [Google Scholar] [CrossRef]
- Silva, E.M.; Takeuchi, R.M.; Santos, A.L. Carbon nanotubes for voltammetric determination of sulphite in some beverages. Food Chem. 2015, 173, 763–769. [Google Scholar] [CrossRef]
- Ahmed, M.I.; Aziz, M.A.; Helal, A.; Shaikh, M.N. Direct electrodeposition of nanogold on gallium-doped zinc oxide by cyclic voltammetry and constant-potential techniques: Application to electro-oxidation of sulfite. J. Electrochem. Soc. 2016, 163, D277. [Google Scholar] [CrossRef]
- Moghaddam, H.M.; Malakootian, M.; Beitollah, H.; Biparva, P. Nanostructured base electrochemical sensor for determination of sulfite. Int. J. Electrochem. Sci. 2014, 9, 327–341. [Google Scholar]
- Kovaleva, S.; Aksinenko, O.; Korshunov, A. Electrooxidation of sulfite ions on a composite carbon-containing electrode modified with submicron gold particles. J. Anal. Chem. 2020, 75, 1348–1357. [Google Scholar] [CrossRef]
- Dadamos, T.R.; Teixeira, M.F. Electrochemical sensor for sulfite determination based on a nanostructured copper-salen film modified electrode. Electrochim. Acta 2009, 54, 4552–4558. [Google Scholar] [CrossRef]
- Isaac, A.; Wain, A.J.; Compton, R.G.; Livingstone, C.; Davis, J. A novel electroreduction strategy for the determination of sulfite. Analyst 2005, 130, 1343–1344. [Google Scholar] [CrossRef]
- Ordeig, O.; Banks, C.E.; Del Campo, F.J.; Muñoz, F.X.; Davis, J.; Compton, R.G. Sulfite determination at in situ plated copper modified gold ultramicroelectrode arrays. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 247–252. [Google Scholar] [CrossRef]
- Montes, R.H.; Richter, E.M.; Munoz, R.A. Low-potential reduction of sulfite at a ruthenium-oxide hexacyanoferrate modified electrode. Electrochem. Commun. 2012, 21, 26–29. [Google Scholar] [CrossRef]
- Yu, H.; Feng, X.; Chen, X.X.; Wang, S.S.; Jin, J. A highly sensitive determination of sulfite using a glassy carbon electrode modified with gold nanoparticles-reduced graphene oxide nanocomposites. J. Electroanal. Chem. 2017, 801, 488–495. [Google Scholar] [CrossRef]
- Wildgoose, G.G.; Banks, C.E.; Leventis, H.C.; Compton, R.G. Chemically modified carbon nanotubes for use in electroanalysis. Microchim. Acta 2006, 152, 187–214. [Google Scholar] [CrossRef]
- Roldan Luna, S.M. Supercondensadores Basados en Electrolitos Redox Activos. Ph.D. Thesis, Departamento de Ciencia de los Materiales e Ingeniería Metalúrgica de la, Universidad de Oviedo, Asturias, Spain, 2013. [Google Scholar]
- Streeter, I.; Wain, A.J.; Davis, J.; Compton, R.G. Cathodic reduction of bisulfite and sulfur dioxide in aqueous solutions on copper electrodes: An electrochemical ESR study. J. Phys. Chem. A 2005, 109, 18500–18506. [Google Scholar] [CrossRef]
- Winiarski, J.P.; Reginato de Barros, M.; Magosso, H.A.; Jost, C.L. Electrochemical reduction of sulfite based on gold nanoparticles/silsesquioxane-modified electrode. Electrochim. Acta 2017, 251, 522–531. [Google Scholar] [CrossRef]
- Gidi, L.; Honores, J.; Arce, R.; Arévalo, M.C.; Aguirre, M.J.; Ramírez, G. Enhanced Electrocatalysis of the Oxygen Reduction Reaction Using Cobalt and Iron Porphyrin/Ionic Liquid Systems. Energy Technol. 2019, 7, 1900698. [Google Scholar] [CrossRef]
- Domańska, U.; Skiba, K.; Zawadzkia, M.; Paduszyńskia, K.; Królikowskia, M. Synthesis, physical, and thermodynamic properties of 1-alkyl-cyanopyridinium bis{(trifluoromethyl)sulfonyl}imide ionic liquids. J. Chem. Thermodyn. 2013, 56, 153–161. [Google Scholar] [CrossRef]
- Figueiredo-Filho, L.C.; Brownson, D.A.; Gómez-Mingot, M.; Iniesta, J.; Fatibello-Filho, O.; Banks, C.E. Exploring the electrochemical performance of graphitic paste electrodes: Graphene vs. graphite. Analyst 2013, 138, 6354–6364. [Google Scholar] [CrossRef]






| Electrode | %C | %O | %F | %P |
|---|---|---|---|---|
| MWCNT/MO | 96.81 ± 0.21 | 3.19 ± 0.26 | --- | --- |
| MWCNT/IL/MO | 93.55 ± 2.81 | 4.02 ± 0.12 | 1.53 ± 0.05 | 0.90 ± 0.03 |
| Paste Electrode | Rs (Ω) | Rct (Ω) | CPE (F cm−2) |
|---|---|---|---|
| MWCNT/MO | 2926 | 776,610 | 0.89 |
| MWCNT/IL/MO | 2850 | 99,275 | 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, M.B.; Ibarra, J.; Gidi, L.; Cavieres, V.; Aguirre, M.J.; Ramírez, G.; Arce, R. Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid. Catalysts 2022, 12, 1675. https://doi.org/10.3390/catal12121675
Villalobos MB, Ibarra J, Gidi L, Cavieres V, Aguirre MJ, Ramírez G, Arce R. Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid. Catalysts. 2022; 12(12):1675. https://doi.org/10.3390/catal12121675
Chicago/Turabian StyleVillalobos, Maicol Bustos, José Ibarra, Leyla Gidi, Valentina Cavieres, María Jesús Aguirre, Galo Ramírez, and Roxana Arce. 2022. "Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid" Catalysts 12, no. 12: 1675. https://doi.org/10.3390/catal12121675
APA StyleVillalobos, M. B., Ibarra, J., Gidi, L., Cavieres, V., Aguirre, M. J., Ramírez, G., & Arce, R. (2022). Electrochemical Detection of Sulfite by Electroreduction Using a Carbon Paste Electrode Binder with N-octylpyridinium Hexafluorophosphate Ionic Liquid. Catalysts, 12(12), 1675. https://doi.org/10.3390/catal12121675