Recent Advances in Seawater Electrolysis
Abstract
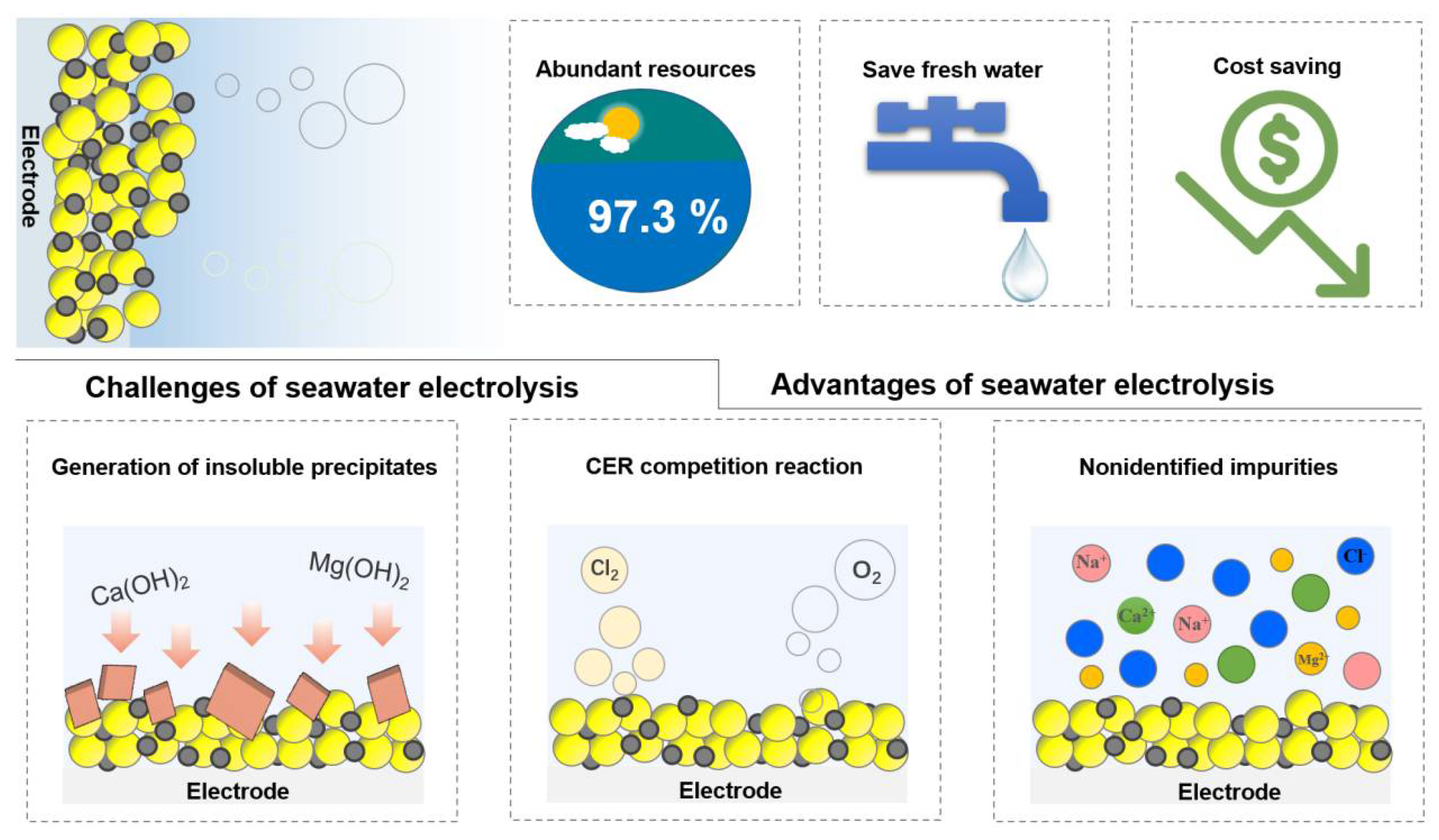
:1. Introduction
2. Reaction Mechanism
2.1. Mechanism of HER and OER
2.2. Characteristics of Seawater Catalytic Reaction
2.2.1. Effects of Complex Cation Components
2.2.2. Effects of Chloride Ions
3. Electrocatalysts for Driving Seawater Electrolysis
3.1. Electrocatalysts for OER
3.1.1. Metal Oxides and Hydroxides for OER
3.1.2. Metal Phosphides for OER
3.1.3. Metal Nitrides for OER
3.1.4. Metal Borides for OER
3.1.5. Hybrid Electrocatalysts for OER
3.2. Electrocatalysts for HER
3.2.1. Noble Metal Alloy Electrocatalysts for HER
3.2.2. Carbon-Supported Noble Metals for HER
3.2.3. MXene-Based Complexes for HER
3.2.4. Metal Phosphides for HER
3.2.5. Metal Oxides and Hydroxides for HER
3.2.6. Metal Nitrides for HER
3.2.7. Hybrid Electrocatalysts for HER
4. Conclusions and Outlook
- (1)
- Combining experimental and theoretical analyses to further confirm the reaction pathways and active sites of catalysts for HER and OER in seawater electrolysis: in addition to monometallic compounds, various polymetallic compounds and heterostructured catalysts have been extensively investigated as catalysts in seawater electrolysis. It is a trend to design catalysts composed of more than a single metal component by taking advantage of the synergistic effect of multimetal components. As catalyst components become more complex, it is more difficult to identify the electrocatalytic reaction pathways as well as the active sites. Therefore, systematic research based on theoretical analyses is necessary, which can provide guidance for designing materials with desirable structures and properties.
- (2)
- Employing in situ characterization methods to unravel the true active sites of catalysts: many current electrocatalysts, such as metal oxides, phosphides, and nitrides, undergo surface oxidation or reconstitution during seawater electrolysis, which means that the true active sites of the catalyst may be altered during the reaction. As each step in the catalytic reaction process changes rapidly, we need in situ characterization techniques to track changes in the intermediates during the catalytic reaction process. It will provide clear principle and guidance to design high-efficiency catalysts if more in situ techniques such as in situ XAS, Raman, Fourier transform infrared spectroscopy, and other novel techniques are involved for the mechanistic studies.
- (3)
- Exploring and developing electrocatalysts with high activity and stability in seawater: Not only multiple cations but also chloride anions in seawater interfere with the water splitting reactions. It is highly desirable to synthesize catalysts with higher selectivity to HER and OER than other competitive reactions. To obtain highly efficient electrocatalysts for seawater electrolysis, modulating the electronic structure of active sites is of great significance and plays a major role in the improvement of catalytic performance. To optimize the electronic structure of catalysts, alloying, vacancy engineering, heteroelement doping, and interface engineering are common methods. Integration of different active materials into a hybrid catalyst is also a good solution for developing high-performance catalysts.
- (4)
- Designing advanced reactors specific for seawater electrolysis: The current research of seawater electrolysis for hydrogen production is mostly focused on the catalysts. To realize the electrocatalytic production of hydrogen, we need to consider the entire reactor rather than the catalysts only. It is necessary to reasonably design reactors which are adaptable to specific seawater electrolysis. For example, the design of asymmetric reactors is considered to be more promising [31,88], which consists of alkaline water in the anode chamber and seawater in the cathode chamber. Such design not only facilitates the diffusion of Cl− to the anode but also protects the anode catalyst, which is of significance in seawater electrolysis.
Author Contributions
Funding
Conflicts of Interest
References
- Chu, S.; Cui, Y.; Liu, N. The path towards sustainable energy. Nat. Mater. 2017, 16, 16–22. [Google Scholar] [CrossRef]
- Jacobson, M.Z.; Colella, W.; Golden, D. Cleaning the air and improving health with hydrogen fuel-cell vehicles. Science 2005, 308, 1901–1905. [Google Scholar] [CrossRef] [Green Version]
- Fukuzumi, S.; Lee, Y.M.; Nam, W. Fuel production from seawater and fuel cells using seawater. ChemSusChem 2017, 10, 4264–4276. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, V.; Abdel-Wahab, A. A short review on hydrogen, biofuel, and electricity production using seawater as a medium. Energy Fuels 2018, 32, 6423–6437. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, W.; Cao, S.; Piao, L. Recent progress for hydrogen production by photocatalytic natural or simulated seawater splitting. Nano Res. 2020, 13, 2313–2322. [Google Scholar] [CrossRef]
- Tian, L.; Zhai, X.; Wang, X.; Pang, X.; Li, J.; Li, Z. Morphology and phase transformation of α-MnO2/MnOOH modulated by N-CDs for efficient electrocatalytic oxygen evolution reaction in alkaline medium. Electrochim. Acta 2020, 337, 135823. [Google Scholar] [CrossRef]
- Li, Z.; Song, M.; Zhu, W.; Zhuang, W.; Du, X.; Tian, L. MOF-derived hollow heterostructures for advanced electrocatalysis. Coord. Chem. Rev. 2021, 439, 213946. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: Recent advances and future prospects. J. Mater. Chem. A 2020, 8, 19729–19745. [Google Scholar] [CrossRef]
- Dresp, S.R.; Dionigi, F.; Klingenhof, M.; Strasser, P. Direct electrolytic splitting of seawater: Opportunities and challenges. ACS Energy Lett. 2019, 4, 933–942. [Google Scholar] [CrossRef]
- Tong, W.; Forster, M.; Dionigi, F.; Dresp, S.; Erami, R.S.; Strasser, P.; Cowan, A.J.; Farràs, P. Electrolysis of low-grade and saline surface water. Nat. Energy 2020, 5, 367–377. [Google Scholar] [CrossRef]
- Cheng, F.; Feng, X.; Chen, X.; Lin, W.; Rong, J.; Yang, W. Synergistic action of Co-Fe layered double hydroxide electrocatalyst and multiple ions of sea salt for efficient seawater oxidation at near-neutral pH. Electrochim. Acta 2017, 251, 336–343. [Google Scholar] [CrossRef]
- Jin, H.; Liu, X.; Vasileff, A.; Jiao, Y.; Zhao, Y.; Zheng, Y.; Qiao, S.-Z. Single-crystal nitrogen-rich two-dimensional Mo5N6 nanosheets for efficient and stable seawater splitting. ACS Nano 2018, 12, 12761–12769. [Google Scholar] [CrossRef] [PubMed]
- Ge, R.; Wang, S.; Su, J.; Dong, Y.; Lin, Y.; Zhang, Q.; Chen, L. Phase-selective synthesis of self-supported RuP films for efficient hydrogen evolution electrocatalysis in alkaline media. Nanoscale 2018, 10, 13930–13935. [Google Scholar] [CrossRef]
- Gao, S.; Li, G.-D.; Liu, Y.; Chen, H.; Feng, L.-L.; Wang, Y.; Yang, M.; Wang, D.; Wang, S.; Zou, X. Electrocatalytic H2 production from seawater over Co, N-codoped nanocarbons. Nanoscale 2015, 7, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Lai, W.-H.; Xiao, F.; Lyu, Y.; Liao, C.; Shao, M. Approaching a high-rate and sustainable production of hydrogen peroxide: Oxygen reduction on Co–N–C single-atom electrocatalysts in simulated seawater. Energy Environ. Sci. 2021, 14, 5444–5456. [Google Scholar] [CrossRef]
- Kuang, Y.; Kenney, M.J.; Meng, Y.; Hung, W.-H.; Liu, Y.; Huang, J.E.; Prasanna, R.; Li, P.; Li, Y.; Wang, L. Solar-driven, highly sustained splitting of seawater into hydrogen and oxygen fuels. Proc. Natl. Acad. Sci. USA 2019, 116, 6624–6629. [Google Scholar] [CrossRef] [Green Version]
- Van de Krol, R.; Grätzel, M. Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2012; Volume 90. [Google Scholar]
- Yu, L.; Zhu, Q.; Song, S.; McElhenny, B.; Wang, D.; Wu, C.; Qin, Z.; Bao, J.; Yu, Y.; Chen, S. Non-noble metal-nitride based electrocatalysts for high-performance alkaline seawater electrolysis. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Wu, L.; McElhenny, B.; Song, S.; Luo, D.; Zhang, F.; Yu, Y.; Chen, S.; Ren, Z. Ultrafast room-temperature synthesis of porous S-doped Ni/Fe (oxy)hydroxide electrodes for oxygen evolution catalysis in seawater splitting. Energy Environ. Sci. 2020, 13, 3439–3446. [Google Scholar] [CrossRef]
- Wu, X.; Yang, Y.; Zhang, T.; Wang, B.; Xu, H.; Yan, X.; Tang, Y. CeOx-decorated hierarchical NiCo2S4 hollow nanotubes arrays for enhanced oxygen evolution reaction electrocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 39841–39847. [Google Scholar] [CrossRef] [PubMed]
- El-Moneim, A.A.; Kumagai, N.; Hashimoto, K. Mn-Mo-W oxide anodes for oxygen evolution in seawater electrolysis for hydrogen production. Mater. Trans. 2009, 50, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T. MnOx/IrOx as selective oxygen evolution electrocatalyst in acidic chloride solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Forster, M.; Yadav, A.; Cowan, A.J.; Patel, N.; Patel, M. Highly efficient and selective metal oxy-boride electrocatalysts for oxygen evolution from alkali and saline solutions. ACS Appl. Energy Mater. 2020, 3, 7619–7628. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@ FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Li, H.; Tang, Q.; He, B.; Yang, P. Robust electrocatalysts from an alloyed Pt–Ru–M (M = Cr, Fe, Co, Ni, Mo)-decorated Ti mesh for hydrogen evolution by seawater splitting. J. Mater. Chem. A 2016, 4, 6513–6520. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, Y.; Xi, H.; Li, C. Seawater splitting for hydrogen evolution by robust electrocatalysts from secondary M (M = Cr, Fe, Co, Ni, Mo) incorporated Pt. RSC Adv. 2018, 8, 9423–9429. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, W.; Yu, K.; Feng, Y.; Zhu, Z. 2D heterogeneous vanadium compound interfacial modulation enhanced synergistic catalytic hydrogen evolution for full pH range seawater splitting. Nanoscale 2020, 12, 6176–6187. [Google Scholar] [CrossRef]
- Xu, W.; Fan, G.; Zhu, S.; Liang, Y.; Cui, Z.; Li, Z.; Jiang, H.; Wu, S.; Cheng, F. Electronic structure modulation of nanoporous cobalt phosphide by carbon doping for alkaline hydrogen evolution reaction. Adv. Funct. Mater. 2021, 46, 2107333. [Google Scholar] [CrossRef]
- Wang, C.; Shang, H.; Jin, L.; Xu, H.; Du, Y. Advances in hydrogen production from electrocatalytic seawater splitting. Nanoscale 2021, 13, 7897–7912. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, X.; Meng, X. Recent advances on electrocatalytic and photocatalytic seawater splitting for hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 9087–9100. [Google Scholar] [CrossRef]
- Khatun, S.; Hirani, H.; Roy, P. Seawater electrocatalysis: Activity and selectivity. J. Mater. Chem. A 2021, 9, 74–86. [Google Scholar] [CrossRef]
- Bolar, S.; Shit, S.; Murmu, N.C.; Kuila, T. Progress in theoretical and experimental investigation on seawater electrolysis: Opportunities and challenges. Sustain. Energy Fuels 2021, 5, 5915–5945. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [Green Version]
- Birss, V.I.; Damjanovic, A.; Hudson, P. Oxygen evolution at platinum electrodes in alkaline solutions: II. Mechanism of the reaction. J. Electrochem. Soc. 1986, 133, 1621. [Google Scholar] [CrossRef]
- Conway, B.E.; Liu, T. Characterization of electrocatalysis in the oxygen evolution reaction at platinum by evaluation of behavior of surface intermediate states at the oxide film. Langmuir 1990, 6, 268–276. [Google Scholar] [CrossRef]
- Niu, X.; Tang, Q.; He, B.; Yang, P. Robust and stable ruthenium alloy electrocatalysts for hydrogen evolution by seawater splitting. Electrochim. Acta 2016, 208, 180–187. [Google Scholar] [CrossRef]
- Millero, F.J.; Feistel, R.; Wright, D.G.; McDougall, T.J. The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 50–72. [Google Scholar] [CrossRef]
- Ayyub, M.M.; Chhetri, M.; Gupta, U.; Roy, A.; Rao, C. Photochemical and photoelectrochemical hydrogen generation by splitting seawater. Chem. Eur. J. 2018, 24, 18455–18462. [Google Scholar] [CrossRef] [PubMed]
- Simamora, A.-J.; Hsiung, T.-L.; Chang, F.-C.; Yang, T.-C.; Liao, C.-Y.; Wang, H.P. Photocatalytic splitting of seawater and degradation of methylene blue on CuO/nano TiO2. Int. J. Hydrogen Energy 2012, 37, 13855–13858. [Google Scholar] [CrossRef]
- Jiang, N.; Meng, H.-M.; Song, L.-J.; Yu, H.-Y. Study on Ni–Fe–C cathode for hydrogen evolution from seawater electrolysis. Int. J. Hydrogen Energy 2010, 35, 8056–8062. [Google Scholar] [CrossRef]
- Song, L.; Meng, H. Effect of carbon content on Ni–Fe–C electrodes for hydrogen evolution reaction in seawater. Int. J. Hydrogen Energy 2010, 35, 10060–10066. [Google Scholar] [CrossRef]
- Li, Y.; Lin, S.; Peng, S.; Lu, G.; Li, S. Modification of ZnS1−x−0.5yOx(OH)y–ZnO photocatalyst with NiS for enhanced visible-light-driven hydrogen generation from seawater. Int. J. Hydrogen Energy 2013, 38, 15976–15984. [Google Scholar] [CrossRef]
- Chang, C.-J.; Huang, K.-L.; Chen, J.-K.; Chu, K.-W.; Hsu, M.-H. Improved photocatalytic hydrogen production of ZnO/ZnS based photocatalysts by Ce doping. J. Taiwan Inst. Chem. Eng. 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.-C.; Chang, F.-C.; Wang, H.P.; Wei, Y.-L.; Jou, C.-J. Photocatalytic splitting of seawater effected by (Ni–ZnO)@ C nanoreactors. Mar. Pollut. Bull. 2014, 85, 696–699. [Google Scholar] [CrossRef]
- Ji, S.M.; Jun, H.; Jang, J.S.; Son, H.C.; Borse, P.H.; Lee, J.S. Photocatalytic hydrogen production from natural seawater. J. Photochem. Photobiol. A Chem. 2007, 189, 141–144. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production–A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Luo, W.; Yang, Z.; Li, Z.; Zhang, J.; Liu, J.; Zhao, Z.; Wang, Z.; Yan, S.; Yu, T.; Zou, Z. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011, 4, 4046–4051. [Google Scholar] [CrossRef]
- Dionigi, F.; Reier, T.; Pawolek, Z.; Gliech, M.; Strasser, P. Design criteria, operating conditions, and nickel-iron hydroxide catalyst materials for selective seawater electrolysis. ChemSusChem 2016, 9, 962–972. [Google Scholar] [CrossRef]
- Okada, T.; Abe, H.; Murakami, A.; Shimizu, T.; Fujii, K.; Wakabayashi, T.; Nakayama, M. A bilayer structure composed of Mg| Co-MnO2 deposited on a Co (OH)2 film to realize selective oxygen evolution from chloride-containing water. Langmuir 2020, 36, 5227–5235. [Google Scholar] [CrossRef]
- Fujimura, K.; Izumiya, K.; Kawashima, A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; Hashimoto, K. Anodically deposited manganese-molybdenum oxide anodes with high selectivity for evolving oxygen in electrolysis of seawater. J. Appl. Electrochem. 1999, 29, 769–775. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G. Electrochemical analysis of seawater electrolysis with molybdenum-oxo catalysts. Int. J. Hydrogen Energy 2013, 38, 2589–2595. [Google Scholar] [CrossRef]
- Venkatkarthick, R.; Elamathi, S.; Sangeetha, D.; Balaji, R.; Kannan, B.S.; Vasudevan, S.; Davidson, D.J.; Sozhan, G.; Ravichandran, S. Studies on polymer modified metal oxide anode for oxygen evolution reaction in saline water. J. Electroanal. Chem. 2013, 697, 1–4. [Google Scholar] [CrossRef]
- Sohrabnejad-Eskan, I.; Goryachev, A.; Exner, K.S.; Kibler, L.A.; Hensen, E.J.; Hofmann, J.P.; Over, H. Temperature-dependent kinetic studies of the chlorine evolution reaction over RuO2 (110) model electrodes. ACS Catal. 2017, 7, 2403–2411. [Google Scholar] [CrossRef]
- Kraft, A.; Stadelmann, M.; Blaschke, M.; Kreysig, D.; Sandt, B.; Schröder, F.; Rennau, J. Electrochemical water disinfection Part I: Hypochlorite production from very dilute chloride solutions. J. Appl. Electrochem. 1999, 29, 859–866. [Google Scholar] [CrossRef]
- Vos, J.; Koper, M. Measurement of competition between oxygen evolution and chlorine evolution using rotating ring-disk electrode voltammetry. J. Electroanal. Chem. 2018, 819, 260–268. [Google Scholar] [CrossRef]
- Juodkazytė, J.; Šebeka, B.; Savickaja, I.; Petrulevičienė, M.; Butkutė, S.; Jasulaitienė, V.; Selskis, A.; Ramanauskas, R. Electrolytic splitting of saline water: Durable nickel oxide anode for selective oxygen evolution. Int. J. Hydrogen Energy 2019, 44, 5929–5939. [Google Scholar] [CrossRef]
- Gayen, P.; Saha, S.; Ramani, V. Selective seawater splitting using pyrochlore electrocatalyst. ACS Appl. Energy Mater. 2020, 3, 3978–3983. [Google Scholar] [CrossRef]
- Tu, Q.; Liu, W.; Jiang, M.; Wang, W.; Kang, Q.; Wang, P.; Zhou, W.; Zhou, F. Preferential adsorption of hydroxide ions onto partially crystalline NiFe-layered double hydroxides leads to efficient and selective OER in alkaline seawater. ACS Appl. Energy Mater. 2021, 4, 4630–4637. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous bimetallic phosphide Ni2P-Fe2P as an efficient bifunctional catalyst for water/seawater splitting. Adv. Funct. Mater. 2021, 31, 2006484. [Google Scholar] [CrossRef]
- Wang, S.; Yang, P.; Sun, X.; Xing, H.; Hu, J.; Chen, P.; Cui, Z.; Zhu, W.; Ma, Z. Synthesis of 3D heterostructure Co-doped Fe2P electrocatalyst for overall seawater electrolysis. Appl. Catal. B Environ. 2021, 297, 120386. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, C.; Fan, J.; Lv, H.; Hao, W. Preparation of Ti@ NiB electrode via electroless plating toward high-efficient alkaline simulated seawater splitting. J. Electroanal. Chem. 2021, 901, 115761. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Chen, H.; Zhang, Z.; Zou, X. Design of a multilayered oxygen-evolution electrode with high catalytic activity and corrosion resistance for saline water splitting. Adv. Funct. Mater. 2021, 2101820. [Google Scholar] [CrossRef]
- Cui, B.; Hu, Z.; Liu, C.; Liu, S.; Chen, F.; Hu, S.; Zhang, J.; Zhou, W.; Deng, Y.; Qin, Z. Heterogeneous lamellar-edged Fe-Ni(OH)2/Ni3S2 nanoarray for efficient and stable seawater oxidation. Nano Res. 2021, 14, 1149–1155. [Google Scholar] [CrossRef]
- Wang, S.; Nai, J.; Yang, S.; Guo, L. Synthesis of amorphous Ni-Zn double hydroxide nanocages with excellent electrocatalytic activity toward oxygen evolution reaction. ChemNanoMat 2015, 1, 324–330. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.; Qin, B.; Fu, L.; Jia, J.; Yi, B.; Shao, Z. Vertically aligned FeOOH/NiFe layered double hydroxides electrode for highly efficient oxygen evolution reaction. ACS Appl. Mater. Interfaces 2017, 9, 464–471. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, W.-J.; Niu, S.; Liu, N.; Luo, H.; Chen, Y.-Y.; Jin, S.-F.; Gao, F.; Wan, L.-J.; Hu, J.-S. Electronic and morphological dual modulation of cobalt carbonate hydroxides by Mn doping toward highly efficient and stable bifunctional electrocatalysts for overall water splitting. J. Am. Chem. Soc. 2017, 139, 8320–8328. [Google Scholar] [CrossRef]
- Izumiya, K.; Akiyama, E.; Habazaki, H.; Kumagai, N.; Kawashima, A.; Hashimoto, K. Anodically deposited manganese oxide and manganese–tungsten oxide electrodes for oxygen evolution from seawater. Electrochim. Acta 1998, 43, 3303–3312. [Google Scholar] [CrossRef]
- Fujimura, K.; Matsui, T.; Izumiya, K.; Kumagai, N.; Akiyama, E.; Habazaki, H.; Kawashima, A.; Asami, K.; Hashimoto, K. Oxygen evolution on manganese–molybdenum oxide anodes in seawater electrolysis. Mater. Sci. Eng. A 1999, 267, 254–259. [Google Scholar] [CrossRef]
- Ghany, N.A.; Kumagai, N.; Meguro, S.; Asami, K.; Hashimoto, K. Oxygen evolution anodes composed of anodically deposited Mn–Mo–Fe oxides for seawater electrolysis. Electrochim. Acta 2002, 48, 21–28. [Google Scholar] [CrossRef]
- Görlin, M.; Ferreira de Araújo, J.; Schmies, H.; Bernsmeier, D.; Dresp, S.R.; Gliech, M.; Jusys, Z.; Chernev, P.; Kraehnert, R.; Dau, H. Tracking catalyst redox states and reaction dynamics in Ni-Fe oxyhydroxide oxygen evolution reaction electrocatalysts: The role of catalyst support and electrolyte pH. J. Am. Chem. Soc. 2017, 139, 2070–2082. [Google Scholar] [CrossRef]
- Speck, F.D.; Dettelbach, K.E.; Sherbo, R.S.; Salvatore, D.A.; Huang, A.; Berlinguette, C.P. On the electrolytic stability of iron-nickel oxides. Chem 2017, 2, 590–597. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Jiang, K.; Hu, Y.; Li, Q.; Deng, Y.; Bao, J.; Lei, Y. Zr-doped CoFe-layered double hydroxides for highly efficient seawater electrolysis. J. Colloid Interface Sci. 2021, 604, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Yu, F.; Zhou, H.; Huang, Y.; Sun, J.; Qin, F.; Bao, J.; Goddard, W.A.; Chen, S.; Ren, Z. High-performance bifunctional porous non-noble metal phosphide catalyst for overall water splitting. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.G. NEXAFS investigations of transition metal oxides, nitrides, carbides, sulfides and other interstitial compounds. Surf. Sci. Rep. 1997, 30, 1–152. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, B.; Xu, J.; Chen, S.; Rawat, R.S.; Fan, H.J. 3D porous hierarchical nickel–molybdenum nitrides synthesized by RF plasma as highly active and stable hydrogen-evolution-reaction electrocatalysts. Adv. Energy Mater. 2016, 6, 1600221. [Google Scholar] [CrossRef]
- Ham, D.J.; Lee, J.S. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells. Energies 2009, 2, 873–899. [Google Scholar] [CrossRef]
- Song, F.; Li, W.; Yang, J.; Han, G.; Liao, P.; Sun, Y. Interfacing nickel nitride and nickel boosts both electrocatalytic hydrogen evolution and oxidation reactions. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Sun, Y.; Yan, F.; Zhu, C.; Gao, P.; Zhang, X.; Chen, Y. Self-supported NiMo-based nanowire arrays as bifunctional electrocatalysts for full water splitting. J. Mater. Chem. A 2018, 6, 8479–8487. [Google Scholar] [CrossRef]
- Zhu, C.; Yin, Z.; Lai, W.; Sun, Y.; Liu, L.; Zhang, X.; Chen, Y.; Chou, S.L. Fe-Ni-Mo nitride porous nanotubes for full water splitting and Zn-air batteries. Adv. Energy Mater. 2018, 8, 1802327. [Google Scholar] [CrossRef]
- Gupta, S.; Patel, M.K.; Miotello, A.; Patel, N. Metal boride-based catalysts for electrochemical water-splitting: A review. Adv. Funct. Mater. 2020, 30, 1906481. [Google Scholar] [CrossRef]
- Chen, Z.; Duan, X.; Wei, W.; Wang, S.; Zhang, Z.; Ni, B.-J. Boride-based electrocatalysts: Emerging candidates for water splitting. Nano Res. 2020, 13, 293–314. [Google Scholar] [CrossRef]
- Masa, J.; Schuhmann, W. The role of non-metallic and metalloid elements on the electrocatalytic activity of cobalt and nickel catalysts for the oxygen evolution reaction. ChemCatChem 2019, 11, 5842–5854. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Zhang, R.; Kong, W.; Qu, F.; Lu, L. Ascorbic acid-loaded apoferritin-assisted carbon dot-MnO2 nanocomposites for the selective and sensitive detection of trypsin. ACS Appl. Bio Mater. 2018, 1, 777–782. [Google Scholar] [CrossRef]
- Xiong, Y.; Dong, J.; Huang, Z.-Q.; Xin, P.; Chen, W.; Wang, Y.; Li, Z.; Jin, Z.; Xing, W.; Zhuang, Z. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 2020, 15, 390–397. [Google Scholar] [CrossRef]
- Dresp, S.; Thanh, T.N.; Klingenhof, M.; Brückner, S.; Hauke, P.; Strasser, P. Efficient direct seawater electrolysers using selective alkaline NiFe-LDH as OER catalyst in asymmetric electrolyte feeds. Energy Environ. Sci. 2020, 13, 1725–1729. [Google Scholar] [CrossRef]
- Lu, X.; Pan, J.; Lovell, E.; Tan, T.H.; Ng, Y.H.; Amal, R. A sea-change: Manganese doped nickel/nickel oxide electrocatalysts for hydrogen generation from seawater. Energy Environ. Sci. 2018, 11, 1898–1910. [Google Scholar] [CrossRef]
- Zheng, J. Seawater splitting for high-efficiency hydrogen evolution by alloyed PtNix electrocatalysts. Appl. Surf. Sci. 2017, 413, 360–365. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Yang, X.; Fa, W.; Ge, S. High-efficiency and stable alloyed nickel based electrodes for hydrogen evolution by seawater splitting. J. Alloys Compd. 2018, 732, 248–256. [Google Scholar] [CrossRef]
- Sarno, M.; Ponticorvo, E.; Scarpa, D. Active and stable graphene supporting trimetallic alloy-based electrocatalyst for hydrogen evolution by seawater splitting. Electrochem. Commun. 2020, 111, 106647. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Huang, B.; Xie, Z. Surface engineering of Rh catalysts with N/S-codoped carbon nanosheets toward high-Performance hydrogen evolution from seawater. ACS Sustain. Chem. Eng. 2019, 7, 18835–18843. [Google Scholar] [CrossRef]
- Xiu, L.; Pei, W.; Zhou, S.; Wang, Z.; Yang, P.; Zhao, J.; Qiu, J. Multilevel Hollow MXene Tailored Low-Pt Catalyst for Efficient Hydrogen Evolution in Full-pH Range and Seawater. Adv. Funct. Mater. 2020, 30, 1910028. [Google Scholar] [CrossRef]
- Miao, J.; Lang, Z.; Zhang, X.; Kong, W.; Peng, O.; Yang, Y.; Wang, S.; Cheng, J.; He, T.; Amini, A. Polyoxometalate-derived hexagonal molybdenum nitrides (MXenes) supported by boron, nitrogen codoped carbon nanotubes for efficient electrochemical hydrogen evolution from seawater. Adv. Funct. Mater. 2019, 29, 1805893. [Google Scholar] [CrossRef] [Green Version]
- Lv, Q.; Han, J.; Tan, X.; Wang, W.; Cao, L.; Dong, B. Featherlike NiCoP holey nanoarrys for efficient and stable seawater splitting. ACS Appl. Energy Mater. 2019, 2, 3910–3917. [Google Scholar] [CrossRef]
- Tian, F.; Geng, S.; He, L.; Huang, Y.; Fauzi, A.; Yang, W.; Liu, Y.; Yu, Y. Interface engineering: PSS-PPy wrapping amorphous Ni-Co-P for enhancing neutral-pH hydrogen evolution reaction performance. Chem. Eng. J. 2021, 417, 129232. [Google Scholar] [CrossRef]
- Wu, D.; Chen, D.; Zhu, J.; Mu, S. Ultralow Ru Incorporated Amorphous Cobalt-Based Oxides for High-Current-Density Overall Water Splitting in Alkaline and Seawater Media. Small 2021, 17, 2102777. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, X.; Tang, C.; Vasileff, A.; Li, L.; Slattery, A.; Qiao, S.Z. Stable and highly efficient hydrogen evolution from seawater enabled by an unsaturated nickel surface nitride. Adv. Mater. 2021, 33, 2007508. [Google Scholar] [CrossRef]
- Yu, L.; Wu, L.; Song, S.; McElhenny, B.; Zhang, F.; Chen, S.; Ren, Z. Hydrogen Generation from Seawater Electrolysis over a Sandwich-like NiCoN|NixP|NiCoN Microsheet Array Catalyst. ACS Energy Lett. 2020, 5, 2681–2689. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, L.; Liu, R.; Hu, Y.; Xiong, T.; Qiu, W.; Balogun, M.-S.J.T.; Pan, A.; Tong, Y. Nitrogen treatment generates tunable nanohybridization of Ni5P4 nanosheets with nickel hydr (oxy) oxides for efficient hydrogen production in alkaline, seawater and acidic media. Appl. Catal. B Environ. 2019, 251, 181–194. [Google Scholar] [CrossRef]
- Grigoriev, S.; Millet, P.; Fateev, V. Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177, 281–285. [Google Scholar] [CrossRef]
- Renjith, A.; Roy, A.; Lakshminarayanan, V. In situ fabrication of electrochemically grown mesoporous metallic thin films by anodic dissolution in deep eutectic solvents. J. Colloid Interface Sci. 2014, 426, 270–279. [Google Scholar] [CrossRef]
- Fang, Y.-H.; Liu, Z.-P. Mechanism and tafel lines of electro-oxidation of water to oxygen on RuO2 (110). J. Am. Chem. Soc. 2010, 132, 18214–18222. [Google Scholar] [CrossRef] [PubMed]
- Trasatti, S. Progress in the understanding of the mechanism of chlorine evolution at oxide electrodes. Electrochim. Acta 1987, 32, 369–382. [Google Scholar] [CrossRef]
- Trasatti, S. Work function, electronegativity, and electrochemical behaviour of metals: III. Electrolytic hydrogen evolution in acid solutions. J. Electroanal. Chem. Interfacial Electrochem. 1972, 39, 163–184. [Google Scholar] [CrossRef]
- Conway, B.; Jerkiewicz, G. Nature of electrosorbed H and its relation to metal dependence of catalysis in cathodic H2 evolution. Solid State Ion. 2002, 150, 93–103. [Google Scholar] [CrossRef]
- Conway, B.; Jerkiewicz, G. Relation of energies and coverages of underpotential and overpotential deposited H at Pt and other metals to the ‘volcano curve’for cathodic H2 evolution kinetics. Electrochim. Acta 2000, 45, 4075–4083. [Google Scholar] [CrossRef]
- Yang, S.-C.; Pang, S.H.; Sulmonetti, T.P.; Su, W.-N.; Lee, J.-F.; Hwang, B.-J.; Jones, C.W. Synergy between ceria oxygen vacancies and Cu nanoparticles facilitates the catalytic conversion of CO2 to CO under mild conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Sarno, M.; Sannino, D.; Leone, C.; Ciambelli, P. Evaluating the effects of operating conditions on the quantity, quality and catalyzed growth mechanisms of CNTs. J. Mol. Catal. A Chem. 2012, 357, 26–38. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, H.B.; Sun, R.; Liu, Y.; Liu, Z.; Zhou, A.; Yu, Z.Z. Hydrophobic, flexible, and lightweight MXene foams for high-performance electromagnetic-interference shielding. Adv. Mater. 2017, 29, 1702367. [Google Scholar] [CrossRef]
- Ma, T.Y.; Cao, J.L.; Jaroniec, M.; Qiao, S.Z. Interacting carbon nitride and titanium carbide nanosheets for high-performance oxygen evolution. Angew. Chem. 2016, 128, 1150–1154. [Google Scholar] [CrossRef]
- Pang, S.-Y.; Wong, Y.-T.; Yuan, S.; Liu, Y.; Tsang, M.-K.; Yang, Z.; Huang, H.; Wong, W.-T.; Hao, J. Universal strategy for HF-free facile and rapid synthesis of two-dimensional MXenes as multifunctional energy materials. J. Am. Chem. Soc. 2019, 141, 9610–9616. [Google Scholar] [CrossRef]
- Ma, Y.-Y.; Wu, C.-X.; Feng, X.-J.; Tan, H.-Q.; Yan, L.-K.; Liu, Y.; Kang, Z.-H.; Wang, E.-B.; Li, Y.-G. Highly efficient hydrogen evolution from seawater by a low-cost and stable CoMoP@ C electrocatalyst superior to Pt/C. Energy Environ. Sci. 2017, 10, 788–798. [Google Scholar] [CrossRef]
- Lovell, E.C.; Lu, X.; Zhang, Q.; Scott, J.; Amal, R. From passivation to activation–tunable nickel/nickel oxide for hydrogen evolution electrocatalysis. Chem. Commun. 2020, 56, 1709–1712. [Google Scholar] [CrossRef] [PubMed]
- Anantharaj, S.; Noda, S. Amorphous catalysts and electrochemical water splitting: An untold story of harmony. Small 2020, 16, 1905779. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, W.; Lai, W.; Wang, M.; Li, Q.; Wang, Z.; Yuan, J.; Deng, Y.; Bao, J.; Ji, H. NiFe layered double hydroxide/FeOOH heterostructure nanosheets as an efficient and durable bifunctional electrocatalyst for overall seawater splitting. Inorg. Chem. 2021, 60, 17371–17378. [Google Scholar] [CrossRef]
- Pi, Y.; Shao, Q.; Wang, P.; Lv, F.; Guo, S.; Guo, J.; Huang, X. Trimetallic oxyhydroxide coralloids for efficient oxygen evolution electrocatalysis. Angew. Chem. Int. Ed. 2017, 56, 4502–4506. [Google Scholar] [CrossRef]
- Xu, H.; Wang, B.; Shan, C.; Xi, P.; Liu, W.; Tang, Y. Ce-doped NiFe-layered double hydroxide ultrathin nanosheets/nanocarbon hierarchical nanocomposite as an efficient oxygen evolution catalyst. ACS Appl. Mater. Interfaces 2018, 10, 6336–6345. [Google Scholar] [CrossRef]
- Huang, L.; Chen, D.; Luo, G.; Lu, Y.R.; Chen, C.; Zou, Y.; Dong, C.L.; Li, Y.; Wang, S. Zirconium-regulation-induced bifunctionality in 3D cobalt–iron oxide nanosheets for overall water splitting. Adv. Mater. 2019, 31, 1901439. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; He, B.; Peng, H.Q.; Zhao, Y.; Cheng, J.; Xia, J.; Shen, J.; Ng, T.W.; Meng, X.; Lee, C.S. Unconventional nickel nitride enriched with nitrogen vacancies as a high-efficiency electrocatalyst for hydrogen evolution. Adv. Sci. 2018, 5, 1800406. [Google Scholar] [CrossRef] [Green Version]
- Jin, H.; Gu, Q.; Chen, B.; Tang, C.; Zheng, Y.; Zhang, H.; Jaroniec, M.; Qiao, S.-Z. Molten salt-directed catalytic synthesis of 2D layered transition-metal nitrides for efficient hydrogen evolution. Chem 2020, 6, 2382–2394. [Google Scholar] [CrossRef]
- Niu, S.; Fang, Y.; Zhou, J.; Cai, J.; Zang, Y.; Wu, Y.; Ye, J.; Xie, Y.; Liu, Y.; Zheng, X. Manipulating the water dissociation kinetics of Ni3N nanosheets via in situ interfacial engineering. J. Mater. Chem. A 2019, 7, 10924–10929. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, Y.; Qian, Q.; Li, Z.; Zhu, Y.; Zhang, G. Manipulating dehydrogenation kinetics through dual-doping Co3N electrode enables highly efficient hydrazine oxidation assisting self-powered H2 production. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, M.; Yang, H.; Xu, M.; Zuo, C.; Feng, K.; Xie, M.; Deng, J.; Zhong, J.; Zhou, W. Weakening hydrogen adsorption on nickel via interstitial nitrogen doping promotes bifunctional hydrogen electrocatalysis in alkaline solution. Energy Environ. Sci. 2019, 12, 3522–3529. [Google Scholar] [CrossRef]
- Salamat, A.; Hector, A.L.; Kroll, P.; McMillan, P.F. Nitrogen-rich transition metal nitrides. Coord. Chem. Rev. 2013, 257, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- You, B.; Liu, X.; Hu, G.; Gul, S.; Yano, J.; Jiang, D.-E.; Sun, Y. Universal surface engineering of transition metals for superior electrocatalytic hydrogen evolution in neutral water. J. Am. Chem. Soc. 2017, 139, 12283–12290. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Yu, X.; Lin, Z.; Zhang, R.; He, D.; Qin, J.; Zhu, J.; Han, J.; Wang, L.; Mao, H.-K. Synthesis, crystal structure, and elastic properties of novel tungsten nitrides. Chem. Mater. 2012, 24, 3023–3028. [Google Scholar] [CrossRef]
- Imbihl, R.; Behm, R.J.; Ertl, G.; Moritz, W. The structure of atomic nitrogen adsorbed on Fe (100). Surf. Sci. 1982, 123, 129–140. [Google Scholar] [CrossRef] [Green Version]









| Catalyst | Electrolytes | Electrodes | Overpotential@10mA cm−2 (mV) | Tafel Slope (mV dec−1) | Mass Loading (mg cm−2) | Ref. |
|---|---|---|---|---|---|---|
| FTO/NiO | 1.0 M KOH + 0.5 M NaCl | FTO | 401 | [57] | ||
| Pb2Ru2O7−x | 0.6 M NaCl | GCE | 500 | 48 | 0.2 | [58] |
| Pb2Ru2O7−x | 0.6 M NaCl + 0.1 M NaOH | GCE | 200 | 45 | 0.2 | [58] |
| Co(OH)2 | 0.25 M Mg(ClO4)2 | FTO | 125 | [50] | ||
| Co(OH)2 | MgCl2 | FTO | 63 | [50] | ||
| Mg|Co-MnO2/Co(OH)2 | 0.25 M Mg(ClO4)2 | FTO | 151 | [50] | ||
| Mg|Co-MnO2/Co(OH)2 | 0.25 M MgCl2 | FTO | 144 | [50] | ||
| NiFe-LDH | 1 M KOH + 0.5 M NaCl | NF | 227 (100 mA/cm2) | 0.32 | [59] | |
| S-(Ni,Fe)OOH | 1 M KOH + Seawater | NF | 300 (100 mA/cm2) | [19] | ||
| S-(Ni,Fe)OOH | 1 M KOH | NF | 281 (100 mA/cm2) | 48.9 | [19] | |
| Ni2P-Fe2P | 1 M KOH | NF | 452 (100 mA/cm2) | 58 | 15.0 | [60] |
| Ni2P-Fe2P | 1 M KOH + Seawater | NF | 581 (100 mA/cm2) | 15.0 | [60] | |
| Co-Fe2P | 1 M KOH | NF | 274 (100 mA/cm2) | 45 | 2.0 | [61] |
| Co-Fe2P | 1 M KOH + 0.5 M NaCl | NF | 460 (100 mA/cm2) | 2.0 | [61] | |
| Ti@NiB | 1 M KOH + 0.5 M NaCl | Ti plate | 397 (50 mA/cm2) | 34.2 | 3.2 | [62] |
| Co-Fe-O-B | 1 M KOH + 0.5 M NaCl | GCE | 294 | 0.1 | [23] | |
| multilayered NiFeBx | 1 M KOH + 0.5 M NaCl | 263 ± 14 | [63] | |||
| NiMoN@NiFeN | 1 M KOH + Seawater | NF | 369 (500 mA/cm2) | [18] | ||
| Fe-Ni(OH)2/Ni3S2 | 1 M KOH + 0.5 M NaCl | NF | 269 | 46 | [64] | |
| CoPx@FeOOH | 1 M KOH Seawater | NF | 283 (100 mA/cm2) | 1.82 | [24] |
| Catalyst | Eectrode | Electrolytes | Onset Potential (mV) | Overpotential @10mA cm−2 (mV) | Tafel Slope (mV dec−1) | Exchange Current Density (mA cm−2) | Mass Loading (mg cm−2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Pt | Ti mesh | seawater | 151.80 | 285 | 45.8 | 7.336 × 10−5 | [25] | |
| Pt-Ru-Cr | Ti mesh | seawater | 129.89 | 256 | 45.7 | 9.280 × 10−5 | [25] | |
| Pt-Ru-Fe | Ti mesh | seawater | 125.92 | 248 | 45.2 | 9.337 × 10−5 | [25] | |
| Pt-Ru-Co | Ti mesh | seawater | 112.79 | 222 | 44.8 | 9.339 × 10−5 | [25] | |
| Pt-Ru-Ni | Ti mesh | seawater | 103.25 | 206 | 44.5 | 1.006 × 10−4 | [25] | |
| Pt-Ru-Mo | Ti mesh | seawater | 96.22 | 196 | 44.0 | 1.080 × 10−4 | [25] | |
| Pt/C | GCE | seawater | 185 | 59 | 1.05 × 10−4 | 0.199 | [90] | |
| PtNi5 | GCE | seawater | 380 | 119 | 8.51 × 10−5 | 0.199 | [90] | |
| PtCr0.1 | Ti mesh | seawater | 166.03 | 283.8 | 3.90 × 10−5 | [26] | ||
| PtFe0.1 | Ti mesh | seawater | 157.69 | 275.2 | 4.55 × 10−5 | [26] | ||
| PtCo0.1 | Ti mesh | seawater | 149.93 | 266.5 | 5.18 × 10−5 | [26] | ||
| PtNi0.1 | Ti mesh | seawater | 147.75 | 263.3 | 5.27 × 10−5 | [26] | ||
| PtMo0.1 | Ti mesh | seawater | 142.50 | 254.6 | 5.35 × 10−5 | [26] | ||
| Ti/NiPt | Ti foil | seawater | 230 | 111 | 9.59 × 10−3 | [91] | ||
| Ti/NiAu | Ti foil | seawater | 410 | 170 | 3.35 × 10−3 | [91] | ||
| RuCo | Ti foil | seawater | 253 | 107 | 4.71 × 10-3 | [36] | ||
| RuCoMo1 | Ti foil | seawater | 354 | 137 | 2.69 × 10−3 | [36] | ||
| NiRuIr_G | seawater | 80 | 48 | [92] | ||||
| 0.5Rh-G1000 | GCE | 1 M PBS | 250 | 1.0 | [93] | |||
| 0.5Rh-G1000 | GCE | seawater | 340 | 1.0 | [93] | |||
| 0.5Rh-GS1000 | GCE | 1 M PBS | 19 | 1.0 | [93] | |||
| 0.5Rh-GS1000 | GCE | seawater | 320 | 1.0 | [93] | |||
| 2.4% Pt@mh-3D MXene | GCE | seawater | 280 | 0.2 | [94] | |||
| VS2@V2C | GCE | seawater (PH = 0) | 148 (20 mA cm−2) | 37 | 27.55 | [27] | ||
| h-MoN@ BNCNT | GCE | seawater | 128 | 0.254 | [95] | |||
| NiCoP/NF | NF | seawater | 287 mV | 2.0 | [96] | |||
| PSS-PPy/Ni-Co-P | CF | artificial seawater | 144 | [97] | ||||
| C-Co2P | GCE | 1 M KOH | 30 | 2.18 | [28] | |||
| C-Co2P | GCE | 1 M KOH, 0.5 M NaCl, 41.2 × 10−3 M MgCl2 and 12.5 × 10−3 M CaCl2 | 192 (1000 mA cm−2) | 2.18 | [28] | |||
| Ru-CoOx | NF | 1 M KOH + seawater | 630 (100 mA cm−2) | [98] | ||||
| Mo5N6 | GCE | 1 M KOH | 94 | 0.41 | [12] | |||
| Ni-SN@C | GCE | 1 M KOH | 28 | 0.255 | [99] | |||
| Ni-SN@C | GCE | 1 M KOH seawater | 23 | 0.255 | [99] | |||
| NiCoN|NixP| NiCoN | NF | seawater | 165 | 1.26 | [100] | |||
| Ni5P4/ Ni2+δOδ(OH)2-δ | CC | seawater | 144 | [101] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Suo, H.; Zhang, T.; Liao, C.; Wang, Y.; Zhao, Q.; Lai, W. Recent Advances in Seawater Electrolysis. Catalysts 2022, 12, 123. https://doi.org/10.3390/catal12020123
Jiang S, Suo H, Zhang T, Liao C, Wang Y, Zhao Q, Lai W. Recent Advances in Seawater Electrolysis. Catalysts. 2022; 12(2):123. https://doi.org/10.3390/catal12020123
Chicago/Turabian StyleJiang, Siqi, Hongli Suo, Teng Zhang, Caizhi Liao, Yunxiao Wang, Qinglan Zhao, and Weihong Lai. 2022. "Recent Advances in Seawater Electrolysis" Catalysts 12, no. 2: 123. https://doi.org/10.3390/catal12020123
APA StyleJiang, S., Suo, H., Zhang, T., Liao, C., Wang, Y., Zhao, Q., & Lai, W. (2022). Recent Advances in Seawater Electrolysis. Catalysts, 12(2), 123. https://doi.org/10.3390/catal12020123






