Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2
Abstract
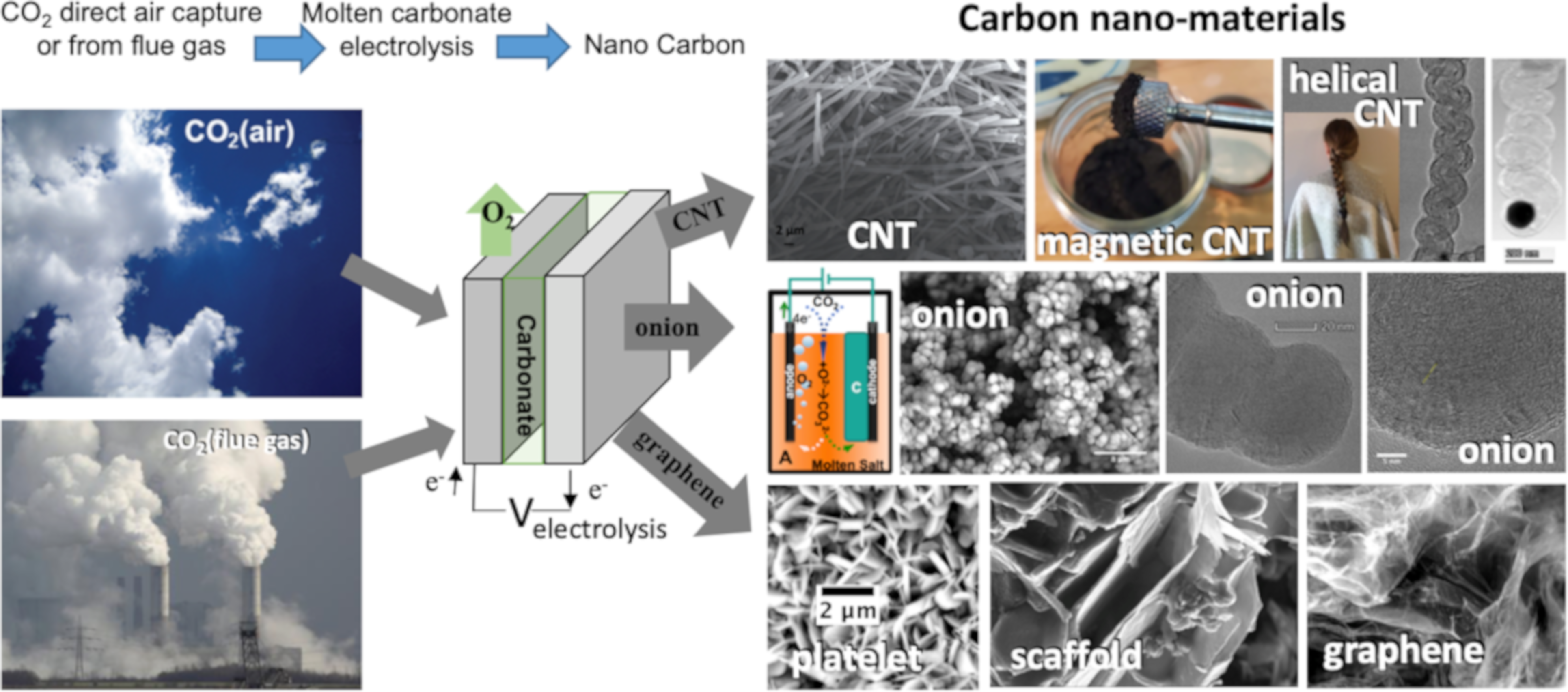
1. Introduction
2. Results and Discussion
2.1. Electrolytic Conditions Varied to Synthesize New Nanocarbon Allotropes from CO2
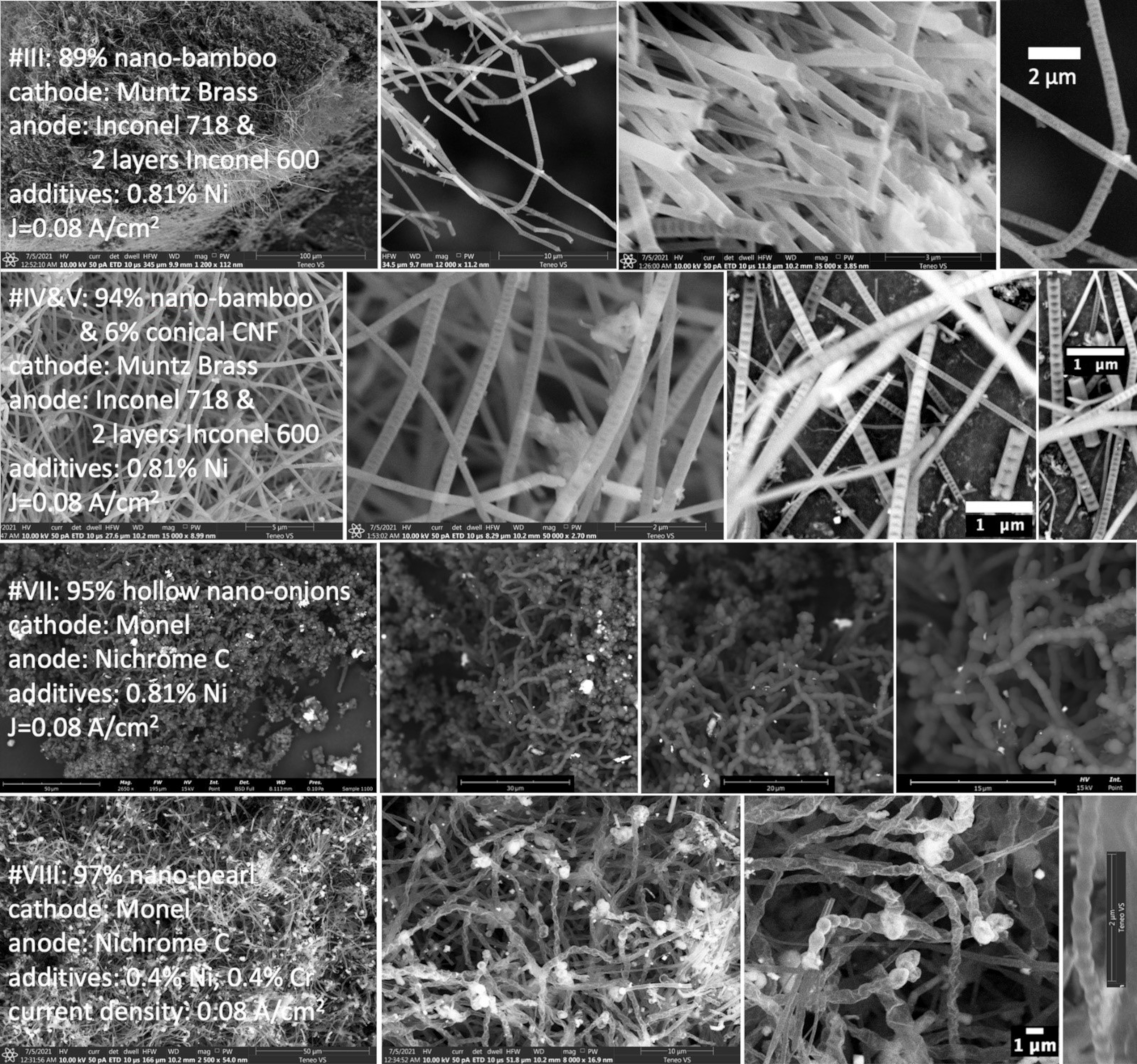
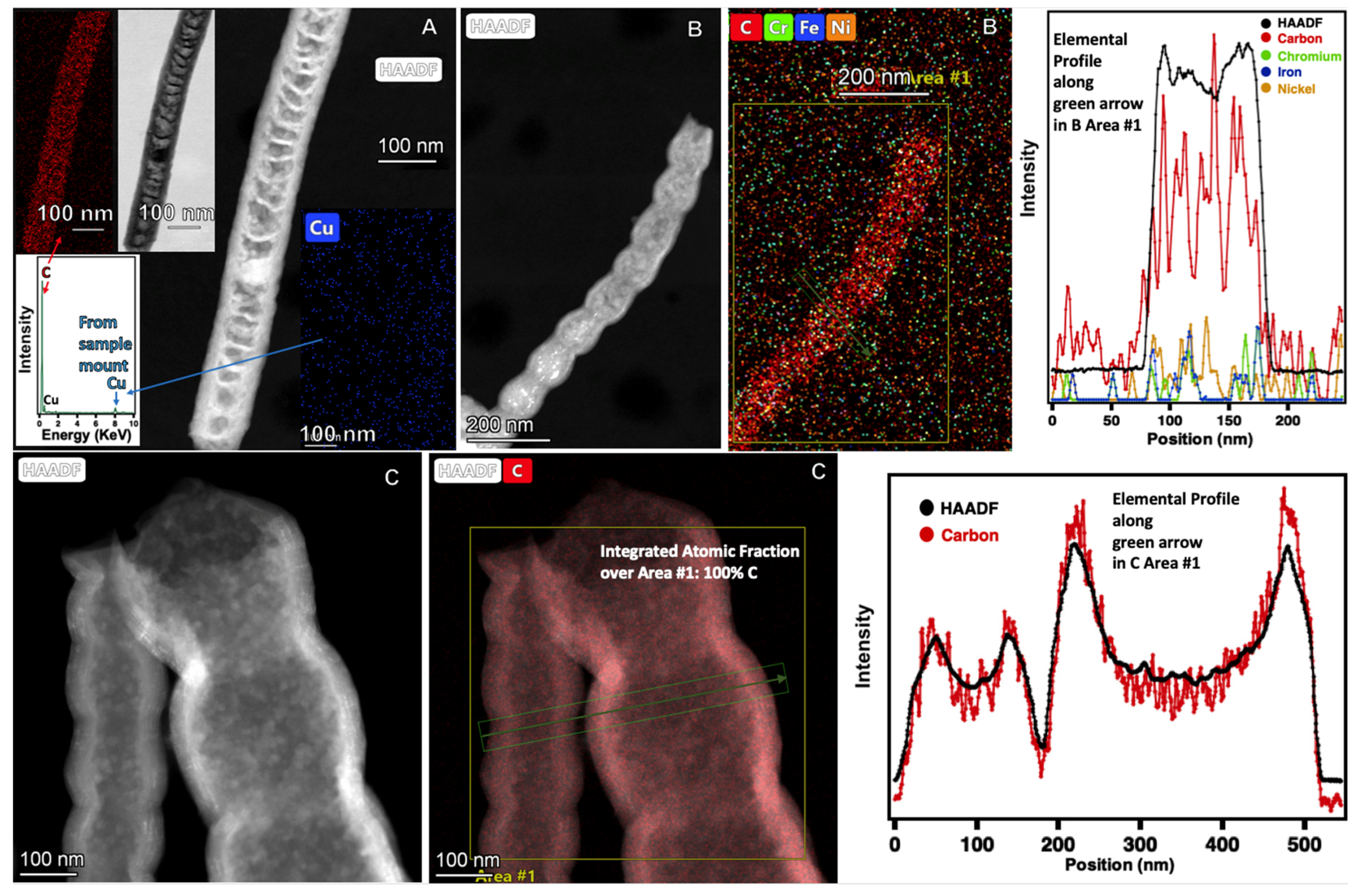
2.2. Electrochemical Conditions to Synthesize Bamboo and Pearl Nanocarbon Allotropes from CO2
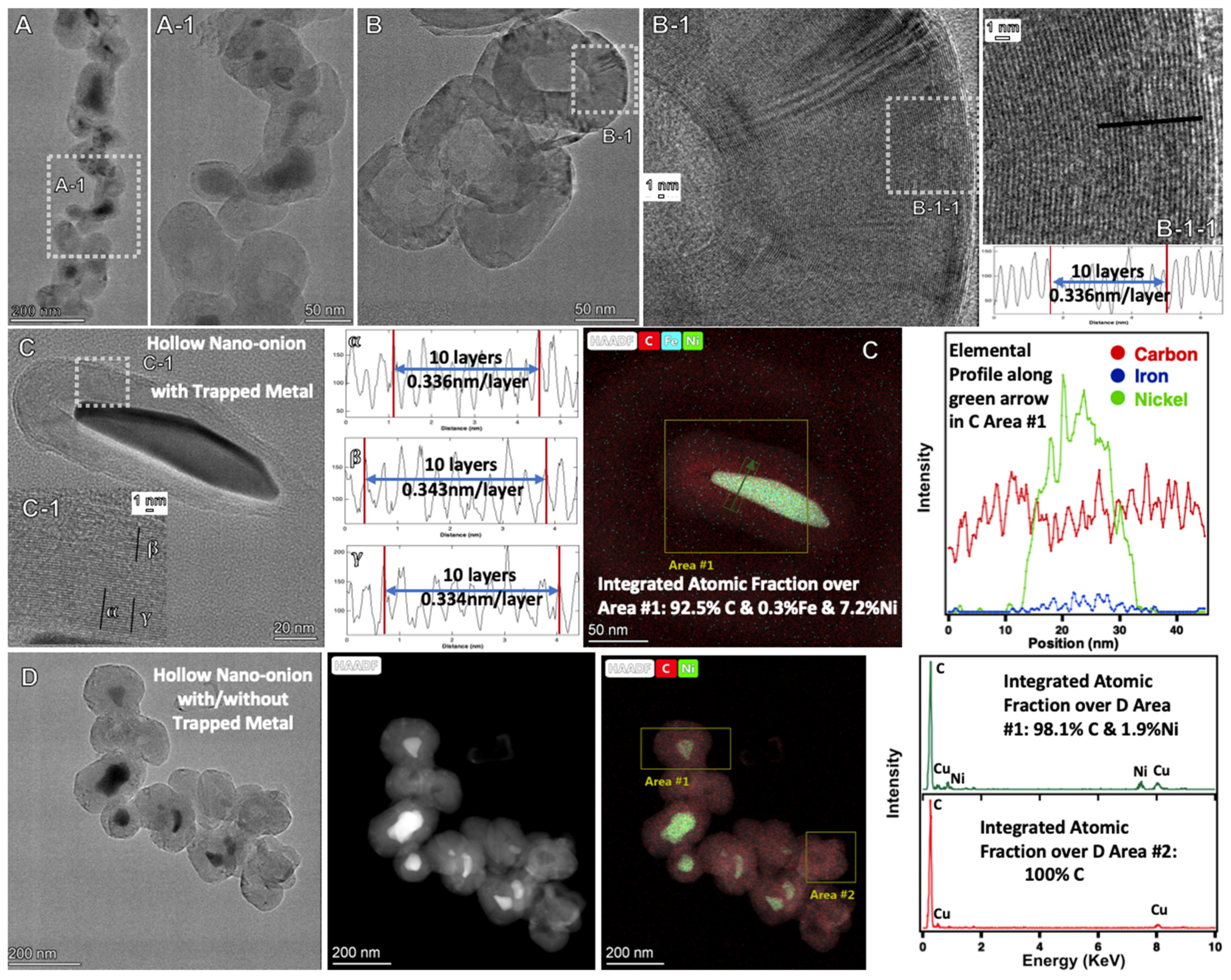
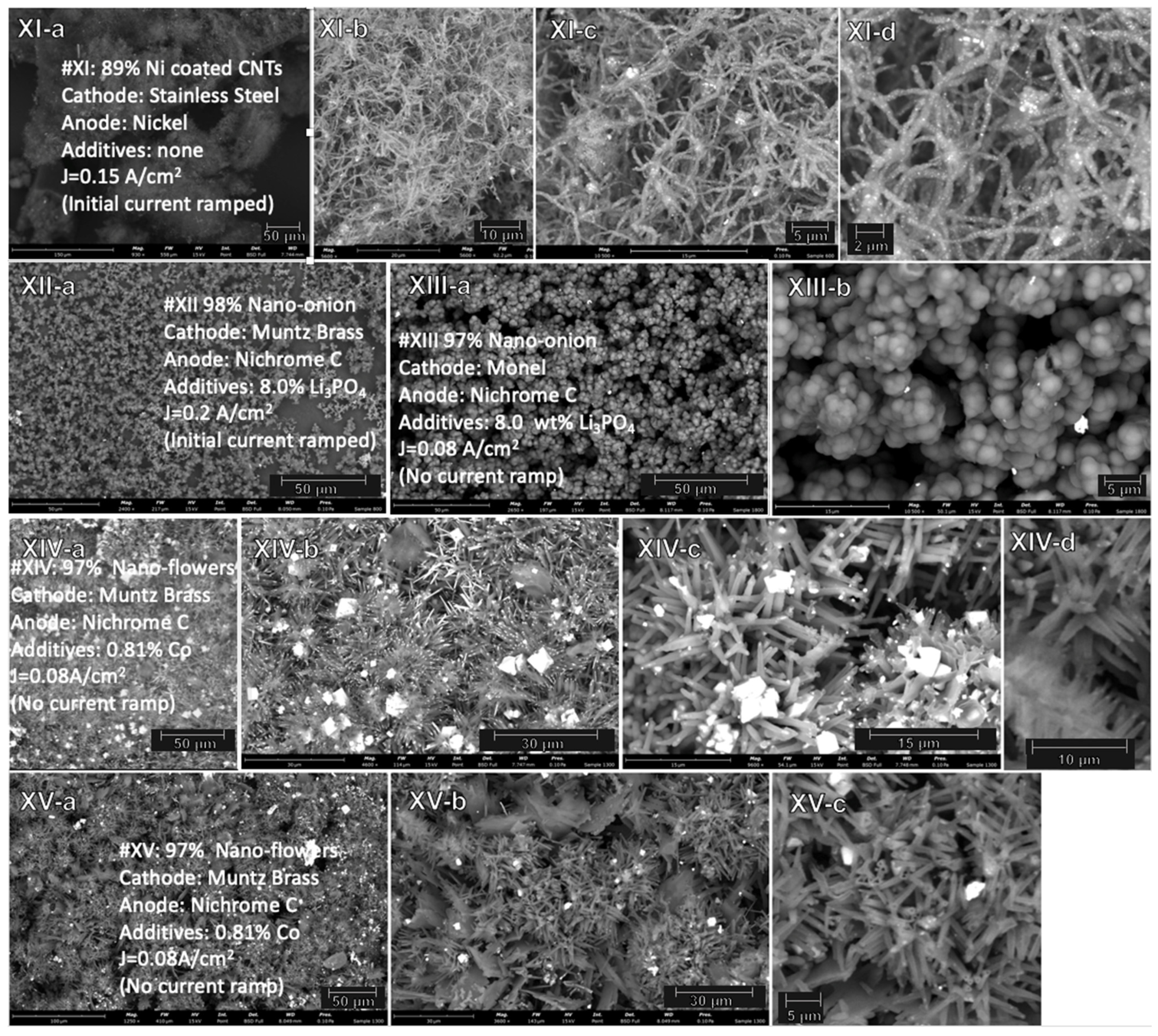
2.3. Electrochemical Conditions to Synthesize Nickel-Coated CNTs, and Onion and Flower Nanocarbon Allotropes from CO2
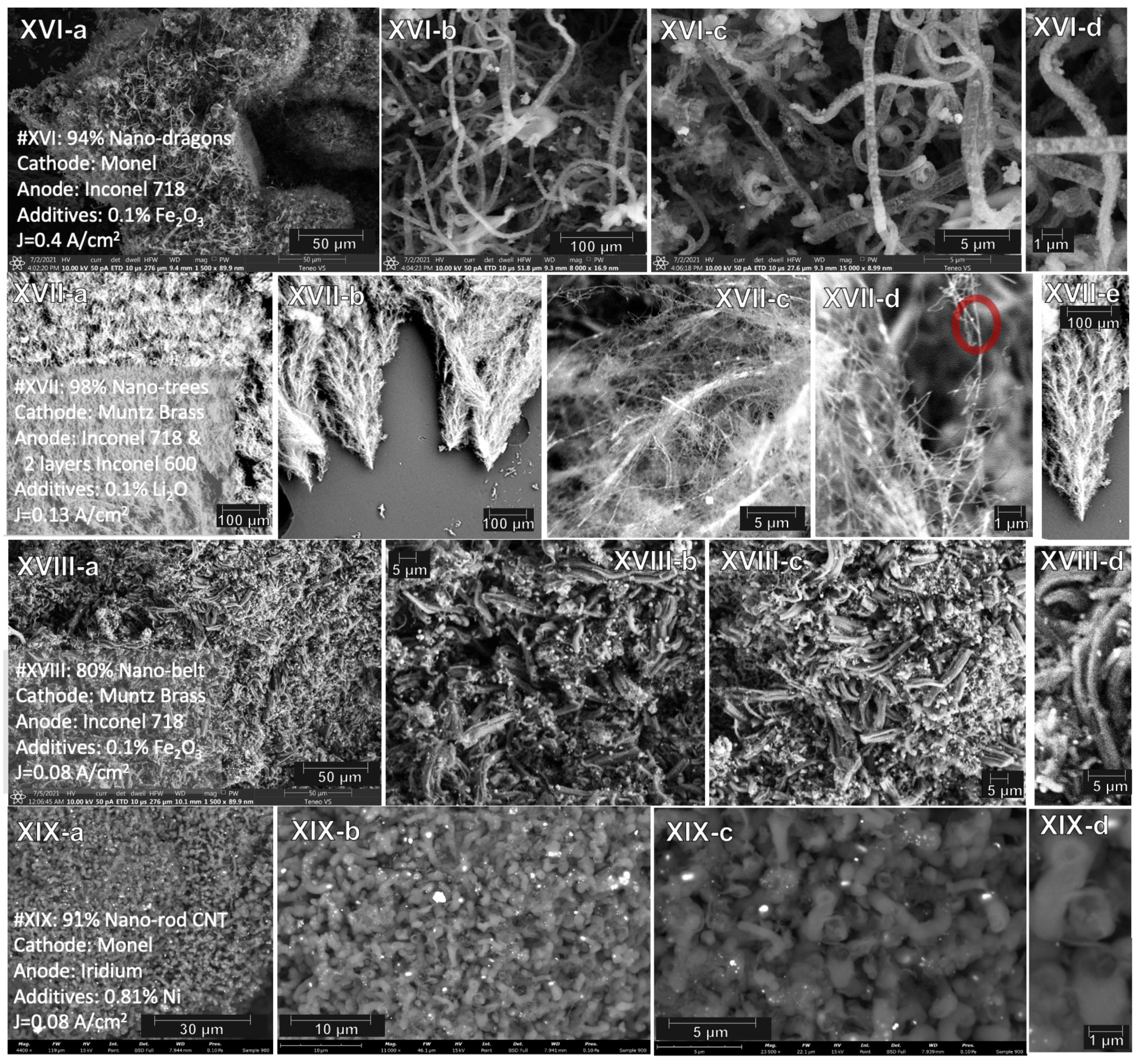
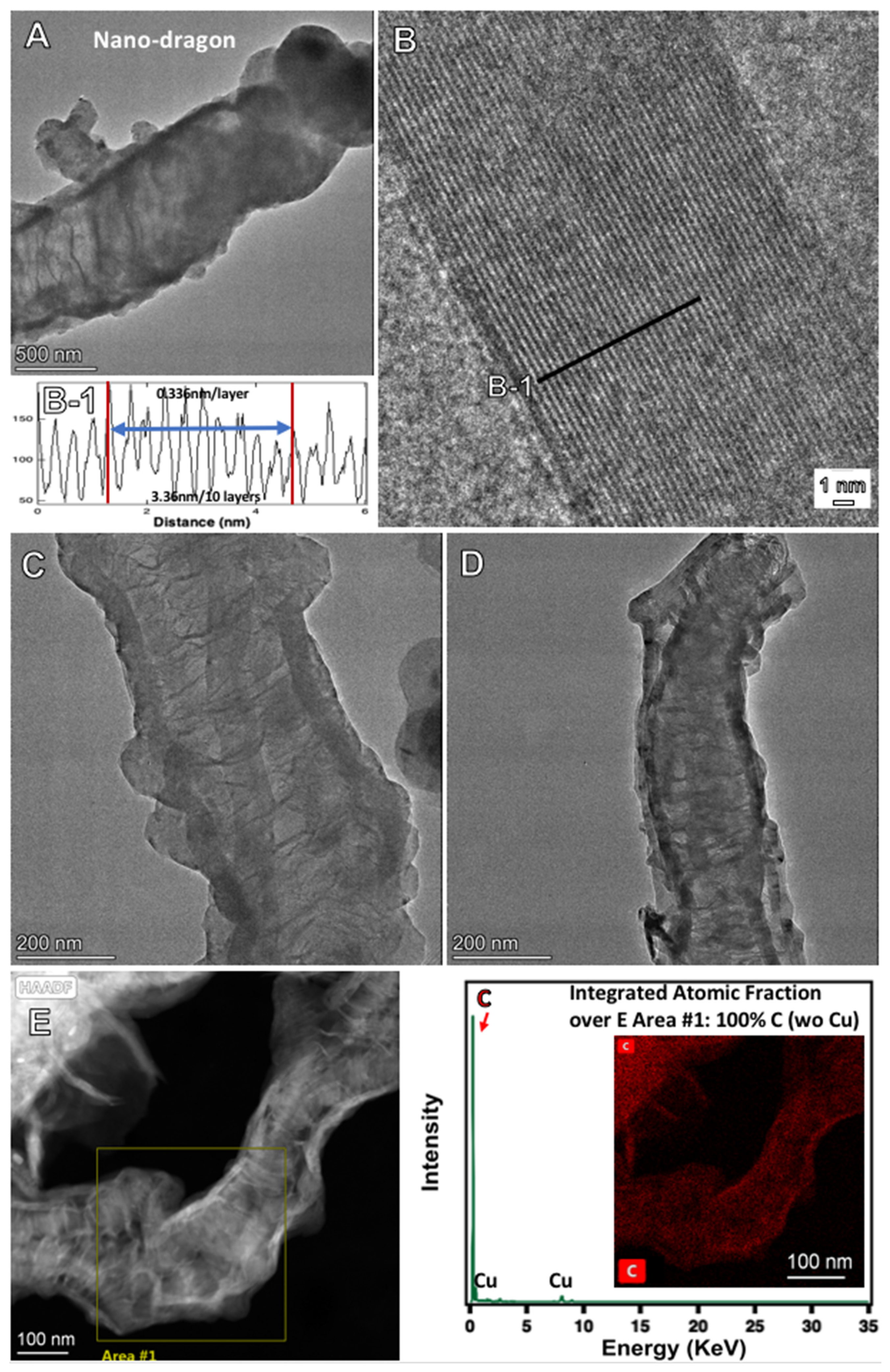
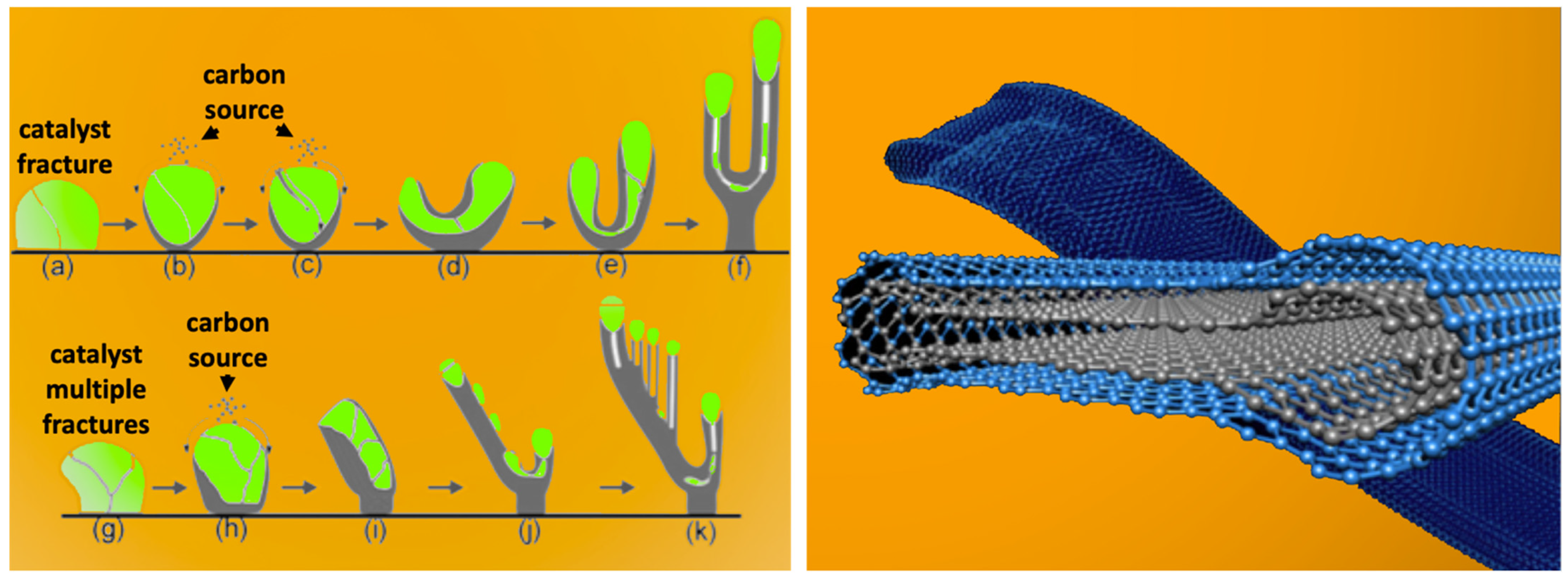
2.4. Electrochemical Conditions to Synthesize Nanocarbon Dragon, Tree, Belt and Rod Allotropes from CO2
2.5. The Diverse Range of Carbon Allotropes Formed by Molten Electrolysis
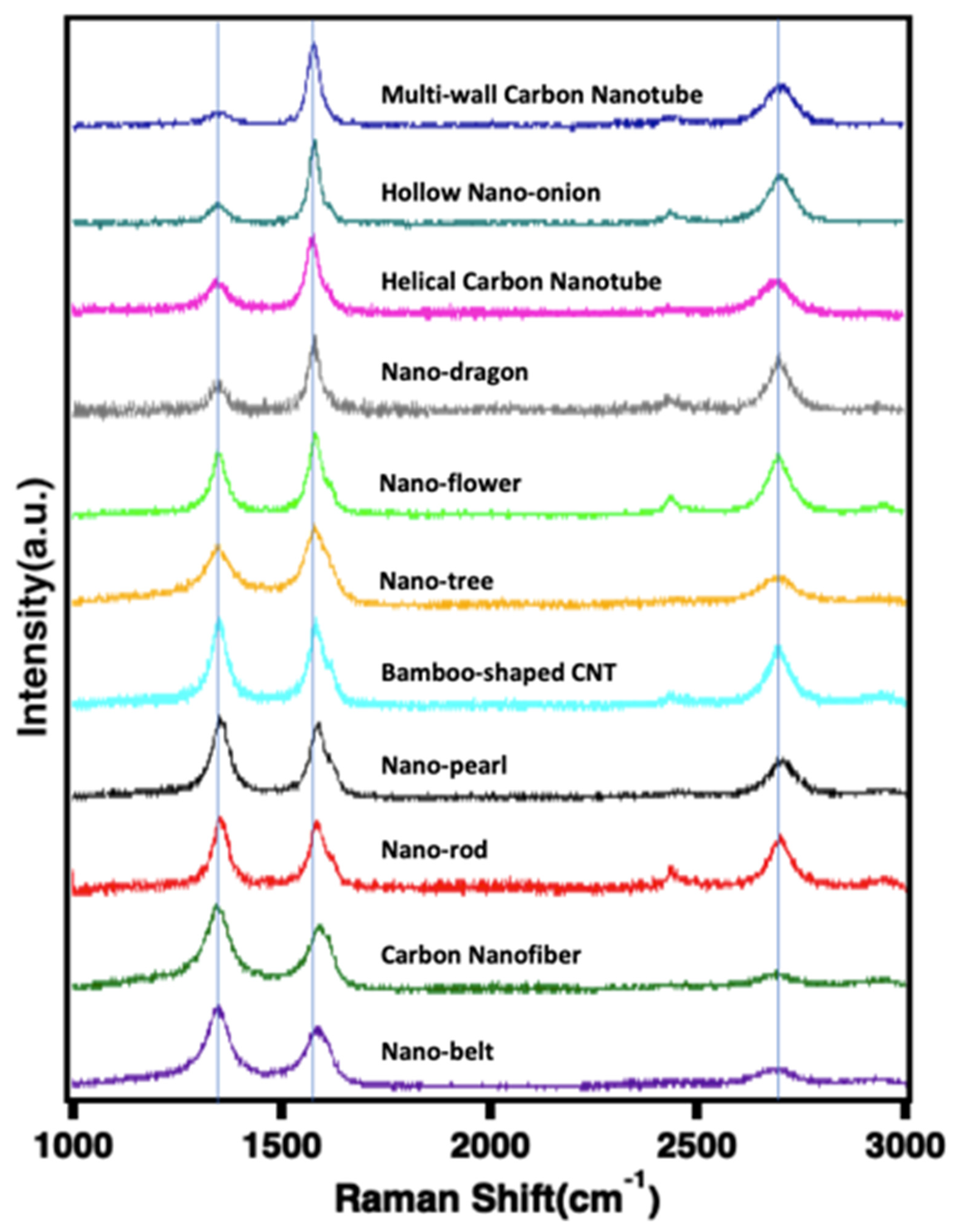
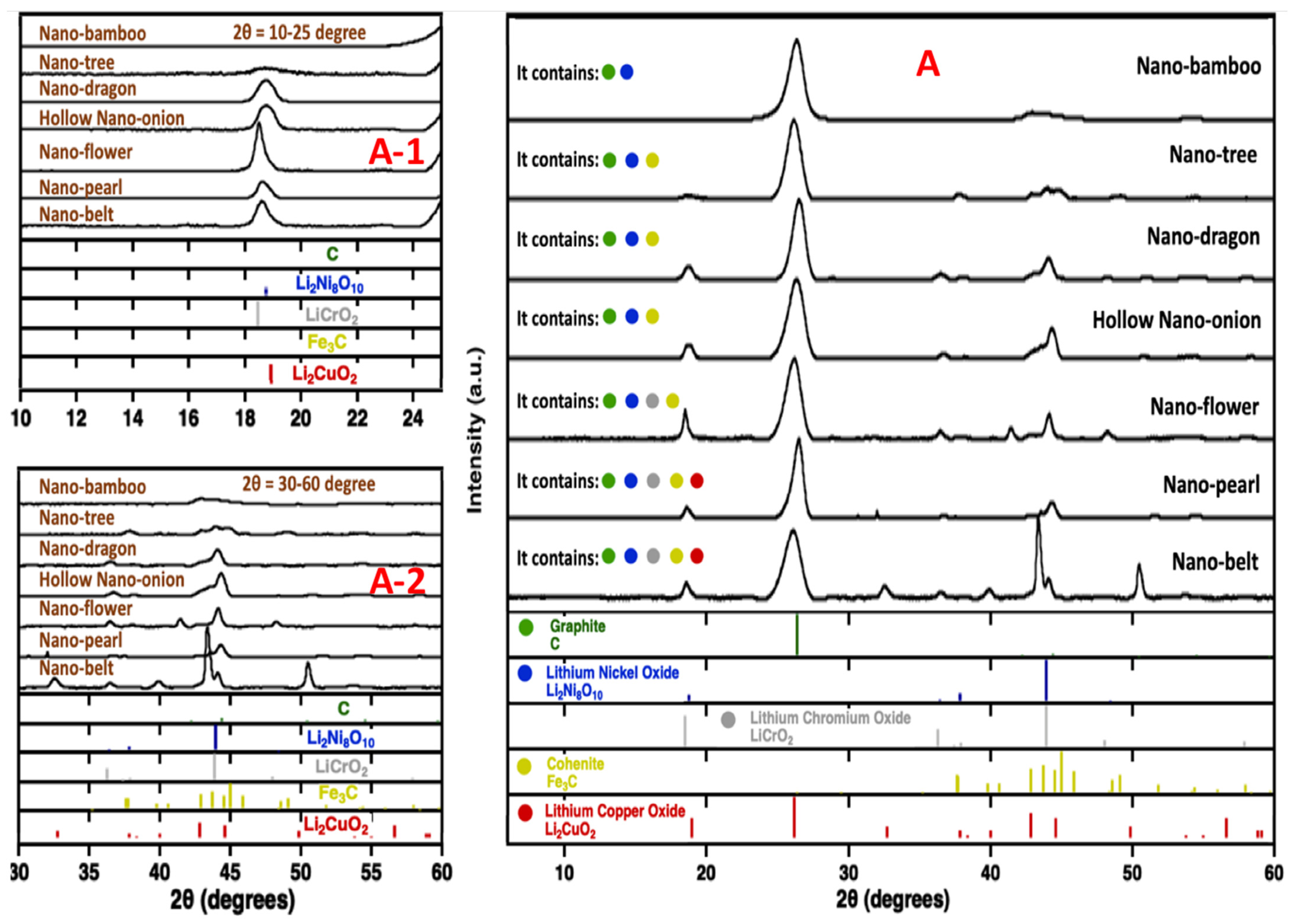
2.6. Raman and XRD of the New Structures Formed by Molten Electrolysis
3. Materials and Methods
3.1. Materials
3.2. Electrolysis and Purification
3.3. Product Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- CO2-Earth: Daily CO2 Values. Available online: https://www.CO2.earth/daily-CO2 (accessed on 7 December 2021).
- NASA: Global Climate Change: The Relentless Rise of Carbon Dioxide. Available online: https://climate.nasa.gov/climate_resources/24/ (accessed on 7 December 2021).
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Pimm, S.L. Climate disruption and biodiversity. Curr. Biol. 2009, 19, R595–R601. [Google Scholar] [CrossRef]
- Praksh, G.K.; Olah, G.A.; Licht, S.; Jackson, N.B. Reversing Global Warming: Chemical Recycling and Utilization of CO2; Report of 2008 NSF Workshop; University of Southern California: Los Angeles, CA, USA, 2008; Available online: https://loker.usc.edu/ReversingGlobalWarming.pdf (accessed on 7 December 2021).
- Khanna, V.; Bakshi, B.R.; Lee, L.J. Carbon Nanofiber Production: Life cycle energy consumption and environmental impact. J. Ind. Ecol. 2008, 12, 394–410. [Google Scholar] [CrossRef]
- Licht, S.; Wang, B.; Ghosh, S.; Ayub, H.; Jiang, D.; Ganley, J. New solar carbon capture process: STEP carbon capture. J. Phys. Chem. Lett. 2010, 1, 2363–2368. [Google Scholar] [CrossRef]
- Ren, J.; Li, F.; Lau, J.; Gonzalez-Urbina, L.; Licht, S. One-pot synthesis of carbon nanofibers from CO2. Nano Lett. 2010, 15, 6142–6148. [Google Scholar] [CrossRef]
- Ren, J.; Licht, S. Tracking airborne CO2 mitigation and low cost transformation into valuable carbon nanotubes. Sci. Rep. 2016, 6, 27760. [Google Scholar] [CrossRef]
- Ren, J.; Lau, J.; Lefler, M.; Licht, S. The minimum electrolytic energy needed to convert carbon dioxide to carbon by electrolysis in carbonate melts. J. Phys. Chem. C 2015, 119, 23342–23349. [Google Scholar] [CrossRef]
- Licht, S.; Liu, X.; Licht, G.; Wang, X.; Swesi, A.; Chan, Y. Amplified CO2 reduction of greenhouse gas emissions with C2CNT carbon nanotube composites. Mater. Today Sustain. 2019, 6, 100023. [Google Scholar] [CrossRef]
- Dey, G.; Ren, J.; El-Ghazawi, O.; Licht, S. How does an amalgamated Ni cathode affect carbon nanotube growth? RSC Adv. 2016, 122, 400–410. [Google Scholar]
- Ren, J.; Johnson, M.; Singhal, R.; Licht, S. Transformation of the greenhouse gas CO2 by molten electrolysis into a wide controlled selection of carbon nanotubes. J. CO2 Util. 2017, 18, 335–344. [Google Scholar] [CrossRef]
- Johnson, M.; Ren, J.; Lefler, M.; Licht, G.; Vicini, J.; Licht, S. Data on SEM, TEM and Raman spectra of doped, and wool carbon nanotubes made directly from CO2 by molten electrolysis. Data Br. 2017, 14, 592–606. [Google Scholar] [CrossRef]
- Johnson, M.; Ren, J.; Lefler, M.; Licht, G.; Vicini, J.; Liu, X.; Licht, S. Carbon nanotube wools made directly from CO2 by molten electrolysis: Value driven pathways to carbon dioxide greenhouse gas mitigation. Mater. Today Energy 2017, 5, 230–236. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Licht, G.; Wang, B.; Licht, S. Exploration of alkali cation variation on the synthesis of carbon nanotubes by electrolysis of CO2 in molten carbonates. J. CO2 Util. 2019, 18, 303–312. [Google Scholar] [CrossRef]
- Wang, X.; Licht, G.; Licht, S. Green and scalable separation and purification of carbon materials in molten salt by efficient high-temperature press filtration. Sep. Purif. Technol. 2021, 244, 117719. [Google Scholar] [CrossRef]
- Wang, X.; Sharif, F.; Liu, X.; Licht, G.; Lefer, M.; Licht, S. Magnetic carbon nanotubes: Carbide nucleated electrochemical growth of ferromagnetic CNTs. J. CO2 Util. 2020, 40, 101218. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Licht, G.; Licht, S. Calcium metaborate induced thin walled carbon nanotube syntheses from CO2 by molten carbonate electrolysis. Sci. Rep. 2020, 10, 15146. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Licht, S. The green synthesis of exceptional braided, helical carbon nanotubes and nanospiral platelets made directly from CO2. Mat. Today Chem. 2021, 22, 100529. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Licht, S. Data for the green synthesis of exceptional braided, helical carbon nanotubes and nano spiral platelets made directly from CO2. arXiv 2021, arXiv:2110.05398. [Google Scholar]
- Licht, S.; Douglas, A.; Carter, R.; Lefler, M.; Pint, C. Carbon Nanotubes Produced from Ambient Carbon Dioxide for Environmentally Sustainable Lithium-Ion and Sodium-Ion Battery Anodes. ACS Cent. Sci. 2016, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Dey, G.; Licht, S. Thermodynamic assessment of CO2 to carbon nanofiber transformation for carbon sequestration in a combined cycle gas or a coal power plant. Energy Conser. Manag. 2016, 122, 400–410. [Google Scholar] [CrossRef]
- Licht, S. Co-production of cement and carbon nanotubes with a carbon negative footprint. J. CO2 Util. 2017, 18, 378–389. [Google Scholar] [CrossRef]
- Liu, X.; Ren, J.; Licht, G.; Wang, X.; Licht, S. Carbon nano-onions made directly from CO2 by molten electrolysis for greenhouse gas mitigation. Adv. Sustain. Syst. 2019, 3, 1900056. [Google Scholar] [CrossRef]
- Ren, J.; Yu, A.; Peng, P.; Lefler, M.; Li, F.F.; Licht, S. Recent advances in solar thermal electrochemical process (STEP) for carbon neutral products and high value nanocarbons. Acc. Chem. Res. 2019, 52, 3177–3187. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Licht, G.; Licht, S. Transformation of the greenhouse gas carbon dioxide to graphene. J. CO2 Util. 2020, 36, 288–294. [Google Scholar] [CrossRef]
- Wang, X.; Licht, G.; Liu, X.; Licht, S. One pot facile transformation of CO2 to an unusual 3-D nan-scaffold morphology of carbon. Sci. Rep. 2020, 10, 21518. [Google Scholar] [CrossRef]
- Liu, X.; Licht, G.; Licht, S. Controlled Transition Metal Nucleated Growth of Carbon Nanotubes by Molten Electrolysis of CO2. Catalysts 2022. submitted. [Google Scholar]
- Yu, M.-F.; Lourie, O.; Dyer, M.; Moloni, K.; Kelly, T.; Ruoff, R. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- XPrize Foundation. Turning CO2 into Products; XPrize Foundation: Culver City, CA, USA, 2021; Available online: http://CarbonXPrize.org (accessed on 7 December 2021).
- XPrize Announces Winners with Each Team Creating Valuable Products from CO2. Available online: https://www.nrg.com/about/newsroom/2021/xprize-announces-the-two-winners-of--20m-nrg-cosia-carbon-xprize.html (accessed on 7 December 2021).
- Chang, C.-C.; Hsu, H.-K.; Aykol, M.; Hung, W.; Chen, C.; Cronin, S. Strain-induced D band observed in carbon nanotubes. ACS Nano 2012, 5, 854–862. [Google Scholar] [CrossRef]
- Tenne, R. Recent advances in the research of inorganic nanotubes and fullerene-like nanoparticles. Front. Phys. 2014, 9, 370–377. [Google Scholar] [CrossRef]
- Pech, D.; Brunet, M.; Durou, H.; Huang, P.; Mochalin, V.; Gogotsi, Y.; Taberna, P.L.; Simon, P. Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat. Nanotechnol. 2010, 5, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Yao, B.; Bai, Y. Preparation of Carbon Nano-Onions and Their Application as Anode Materials for Rechargeable Lithium-Ion Batteries. J. Phys. Chem. C 2011, 115, 8923–8927. [Google Scholar] [CrossRef]
- Zeiger, M.; Jackel, N.; Mochalin, V.N.; Presser, V. Review: Carbon onions for electrochemical energy storage. J. Mater. Chem. A 2016, 4, 3172. [Google Scholar] [CrossRef]
- Sano, N.; Wang, H.; Alexandrou, I.; Chhowalla, M.; Teo, K.B.K.; Amaratunga, G.A.J.; Iimura, K. Properties of carbon onions produced by an arc discharge in water. J. Appl. Phys. 2002, 92, 2783. [Google Scholar] [CrossRef]
- Keller, N.; Maksimova, N.I.; Roddatis, V.V.; Schur, M.; Mestl, G.; Butenko, Y.V.; Kuznetsov, V.L.; Schlogl, R. The Catalytic Use of Onion-Like Carbon Materials for Styrene Synthesis by Oxidative Dehydrogenation of Ethylbenzene. Chem. Int. 2002, 41, 1885. [Google Scholar] [CrossRef]
- Bidsorkhi, H.B.; D’Aloia, A.G.; Tamburrano, A.; Bellis, G.; Delfini, A.; Ballirano, P.; Sarto, M.S. 3D Porous Graphene Based Aerogel for Electromagnetic Applications. Sci. Rep. 2019, 9, 15719. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y.S. Analysis of Thermoelectric Energy Harvesting with Graphene Aerogel-Supported Form-Stable Phase Change Materials. Nanomaterials 2021, 11, 2192. [Google Scholar] [CrossRef]
- Reece, R.; Lekakou, L.; Smith, P.A.; Grilli, R.; Trapalis, C. Sulphur-linked graphitic and graphene oxide platelet-based electrodes for electrochemical double layer capacitors. J. Alloys Compd. 2019, 792, 582–593. [Google Scholar] [CrossRef]
- Tang, N.; Wen, J.; Zhang, Y.; Liu, F.; Lin, K.; Du, Y. Helical Carbon Nanotubes: Catalytic Particle Size-Dependent Growth and Magnetic Properties. ACS Nano 2010, 4, 241. [Google Scholar] [CrossRef]
- Gao, R.; Wang, Z.L.; Fan, S. Kinetically Controlled Growth of Helical and Zigzag Shapes of Carbon Nanotubes. J. Phys. Chem. B 2000, 104, 1227. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Z.; Cui, Z. Helical carbon nanofibers with a symmetric growth mode. Carbon 2004, 42, 1917. [Google Scholar] [CrossRef]
- Suda, Y. Chemical Vapor Deposition of Helical Carbon Nanofibers, Chemical Vapor Deposition for Nanotechnology; IntechOpen: London, UK, 2018. [Google Scholar]
- Bajpai, V.; Dai, L.; Ohashi, T. Large-Scale Synthesis of Perpendicularly Aligned Helical Carbon Nanotubes. J. Am. Chem. Soc. 2004, 126, 5070. [Google Scholar] [CrossRef]
- Tak, K.; Lu, M.; Hui, D. Coiled carbon nanotubes: Synthesis and their potential applications in advanced composite structures. Compos. Part B Eng. 2006, 37, 437. [Google Scholar]
- Zhang, M.; Li, J. Carbon nanotube in different shapes. Mater. Today 2009, 12, 12. [Google Scholar] [CrossRef]
- Wang, W.; Yang, K.; Galliard, J.; Bandaru, P.R.; Rao, Q.M. Rational Synthesis of Helically Coiled Carbon Nanowires and Nanotubes through the Use of Tin and Indium Catalysts. Adv. Mater. 2008, 20, 179. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, M.; Tang, D.; Li, F.; Huang, J.; Liu, B.; Zhu, W.; Zhang, Y.; Wei, F. Carbon-Nanotube-Array Double Helices. Angew. Chem. 2010, 49, 3642. [Google Scholar] [CrossRef] [PubMed]
- Walling, B.E.; Kuang, Z.; Hao, Y.; Estrada, D.; Wood, J.D.; Lian, F.; Miller, L.A.; Shah, A.B.; Jeffries, J.L.; Haasch, R.T. Helical Carbon Nanotubes Enhance the Early Immune Response and Inhibit Macrophage-Mediated Phagocytosis of Pseudomonas aeruginosa. PLoS ONE 2013, 8, e80283. [Google Scholar] [CrossRef]
- Jia, K.; Kou, K.; Qin, M.; Wu, H.; Puleo, F.; Liotta, L.F. Controllable and Large-Scale Synthesis of Carbon Nanostructures: A review of Bamboo-Like Nanotubes. Catalysts 2017, 7, 256. [Google Scholar] [CrossRef]
- Lobo, L.S.; Carabineiro, S.A.C. Explaining Bamboo-Like Carbon Fiber Growth Mechanism: Catalyst Shape Adjustments above Tammann Temperature. J. Carbon Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Gonzalez, I.; De Jesus, J.; Canizales, E. Bamboo-shaped carbon nanotubes generated by methane thermal decomposition using Ni nanoparticles synthesized in water–oil emulsions. Micron 2011, 42, 819–925. [Google Scholar] [CrossRef] [PubMed]
- Maurice, J.-L.; Pribat, D.; He, Z.; Patriarche, G.; Cojocaru, C.S. Catalyst faceting during graphene layer crystallization in the course of carbon nanofiber growth. Carbon 2014, 79, 93–102. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, N.; Sha, J.; Liu, E.; Shi, C.; Li, J.; He, C. Synthesis of novel carbon nano-chains and their application as supercapacitors. J. Mat. Chem. A 2014, 2, 16268. [Google Scholar] [CrossRef]
- Zhang, M.; He, C.; Liu, E.; Zhiu, S.; Shi, C.; Li, J.; Li, Q.; Zhao, N. Activated Carbon Nano-Chains with Tailored Micro-Meso Pore Structures and Their Application for Supercapacitors. J. Phys. Chem. C 2015, 119, 21810–21817. [Google Scholar] [CrossRef]
- Wang, P.; Xiao, P.; Zhong, S.; Chen, J.; Lin, H.; Wu, X.-L. Bamboo-like carbon nanotubes derived from colloidal polymer nanoplates for efficient removal of Bisphenol A. J. Mat. Chem. A 2016, 4, 15450–15456. [Google Scholar] [CrossRef]
- Primo, E.N.; Gutierrz, F.A.; Rubianes, M.D. Bamboo-like multiwall carbon nanotubes dispersed in double stranded calf-thymus DNA as a new analytical platform for building layer-by-layer based biosensors. Electrochim. Acta 2015, 182, 391–397. [Google Scholar] [CrossRef]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963. [Google Scholar] [CrossRef]
- Mohamed, A. Synthesis, Characterization, and Applications Carbon Nanofibers. In Carbon-Based Nanofillers and their Rubber Nanocomposites; Yaragaila, S., Mishra, R., Thomas, S., Kalarikkal, N., Maria, H.J., Eds.; Elselvier: Amsterdam, The Netherlands, 2019; Chapter 9. [Google Scholar]
- Shende, P.; Kasture, P.; Gaud, R.S. Nanoflowers: The future trend of nanotechnology for multi-applications. Artif. Cells Nanomed. Biotechnol. 2019, 46, 413–422. [Google Scholar] [CrossRef]
- Kharisov, B.I. A Review for Synthesis of Nanoflowers. Recent Pat. Nanotechology 2008, 2, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Thongtem, S.; Singjai, P.; Thongtem, T.; Preyachoti, S. Growth of carbon nanoflowers on glass slides using sparked iron as a catalyst. Mat. Sci. Eng. A 2006, 423, 209–213. [Google Scholar] [CrossRef]
- Takai, A.; Ataee-Esfahani, H.; Doi, Y.; Fuziwara, M.; Yamauchi, Y.; Kuroda, K. Pt nanoworms: Creation of a bumpy surface on one-dimensional (1D) Pt nanowires with the assistance of surfactants embedded in mesochannels. Chem. Comm. 2011, 47, 7701–7703. [Google Scholar] [CrossRef]
- He, Z.; Maurice, J.-L.; Lee, C.S.; Cojocaru, C.S.; Pribat, D. Growth mechanisms of carbon nanostructures with branched carbon nanofibers synthesized by plasma-enhanced chemical vapour deposition. Cryst. Eng. Comm. 2014, 16, 2990–29995. [Google Scholar] [CrossRef][Green Version]
- Lin, C.-T.; Chen, R.-H.; Chin, T.-S.; Lee, C.-Y.; Chiu, H.-T. Quasi two-dimensional carbon nanobelts synthesized using a template method. Carbon 2008, 46, 741–746. [Google Scholar] [CrossRef]
- Licht, S. Efficient Solar-Driven Synthesis, Carbon Capture, and Desalinization, STEP: Solar Thermal Electrochemical Production of Fuels, Metals, Bleach. Adv. Mat. 2011, 23, 5592. [Google Scholar] [CrossRef] [PubMed]
- Basheer, B.V.; George, J.; Siengchin, S.; Parameswaranpillai, J. Polymer grafted carbon nanotubes—Synthesis, properties, and applications: A review. Nano-Struct. Nano-Objects 2020, 22, 100429. [Google Scholar] [CrossRef]
- Tian, W.; Yu, W.; Liu, X. A Review on Lattice Defects in Graphene: Types, Generation, Effects and Regulation. Micromachines 2017, 8, 163. [Google Scholar] [CrossRef]




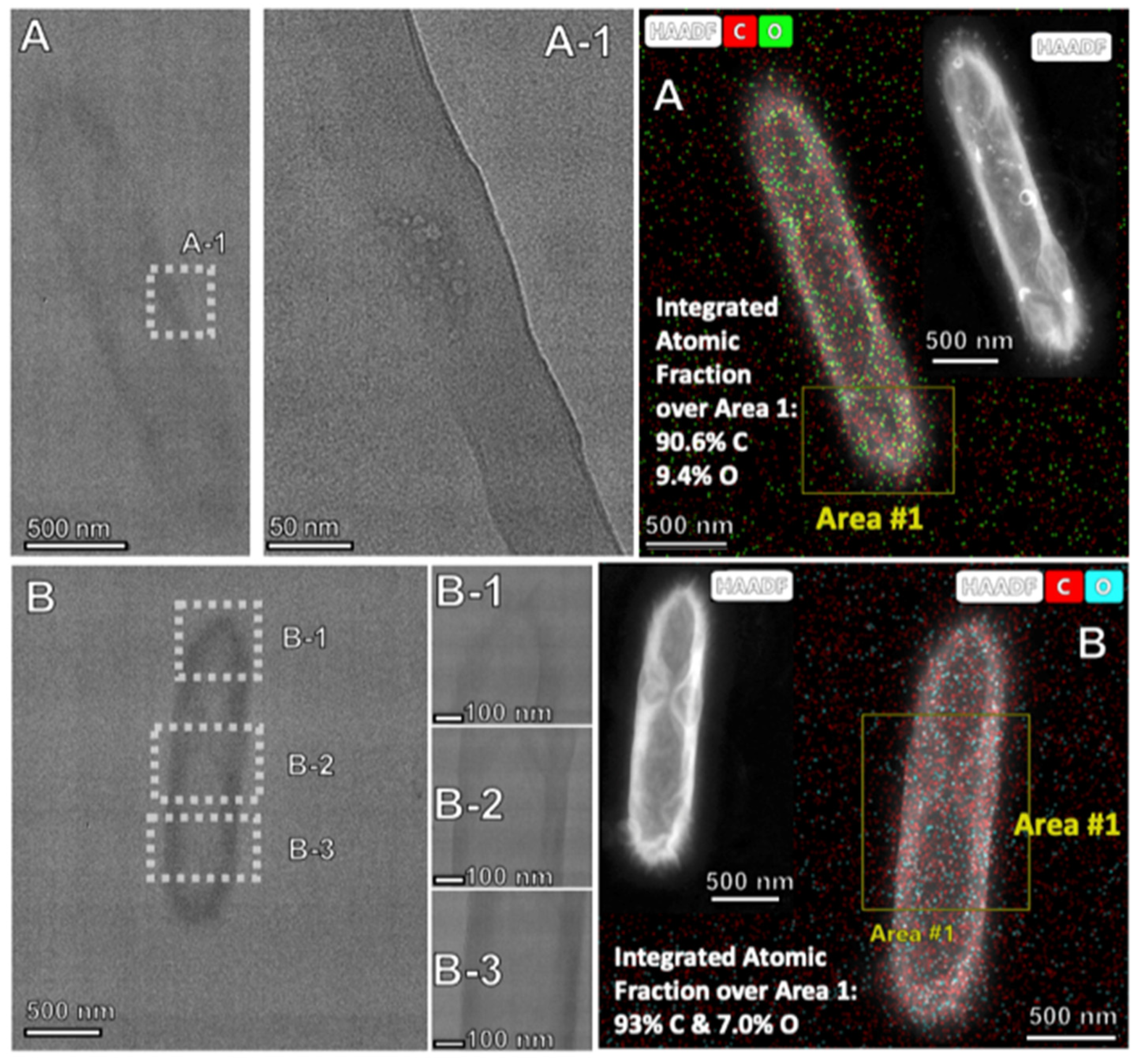
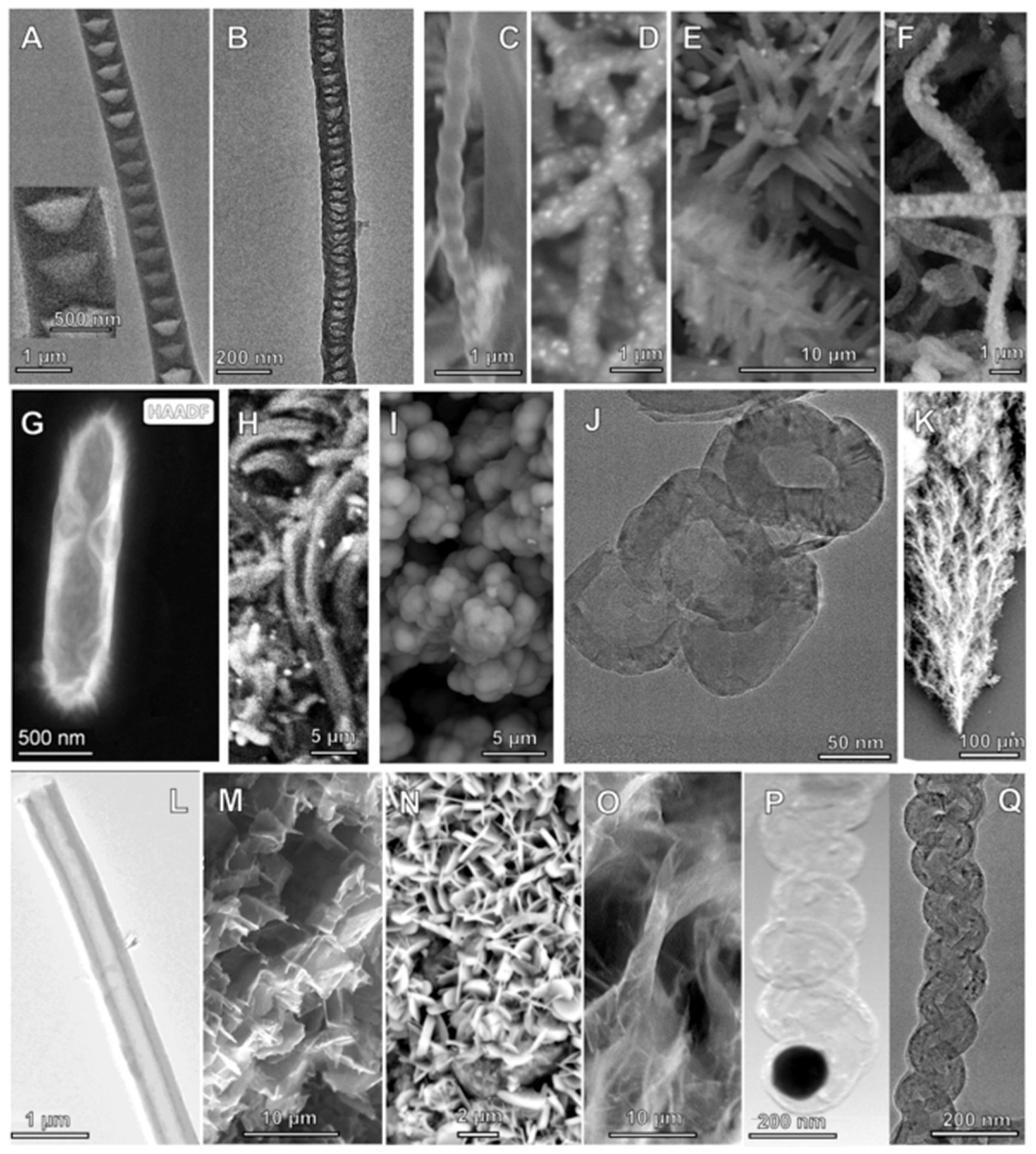
| Alloy | Ni% | Fe% | Cu% | Zn% | Cr% | Mo% | Nb & Ta% |
|---|---|---|---|---|---|---|---|
| Nichrome C | 60 | 24 | 16 | ||||
| Nichrome A | 80 | 20 | |||||
| Inconel 600 | 52.5 | 18.5 | 19.0 | 3.0 | 3.6 | ||
| Inconel 718 | 72% min | 6–10 | 14–17 | ||||
| Inconel 625 | 58 | 5 max | 20–23 | 8–10 | 4.15–3.15 | ||
| Monel | 67 | 31.5 | |||||
| Muntz Brass | 60 | 40 |
| Electrolysis | Cathode | Anode | Additives (wt% Powder) | Electr Time | Current Density A/cm2 | Product Description |
|---|---|---|---|---|---|---|
| I | Nickel | Nickel | - | 4 h | 0.2 | 30% nano-bamboo carbon 40% regular CNT rest: graphitic carbon |
| II | Muntz brass | Iridium | 0.4% Ni 0.4% Cr | 18 h | 0.08 | 60% nano-bamboo carbon 10% regular CNT rest: graphitic carbon |
| III | Muntz brass | Inconel 718 2 layers Inconel 600 | 0.81% Ni powder | 18 h | 0.08 | 89% 30–120 µm nano-bamboo carbon |
| IV | Muntz brass | Inconel 718 2 layers Inconel 600 | 0.81% Ni powder | 18 h | 0.08 | 94% 30–80 µm carbon nano-bamboo, 6% conical carbon nanofiber |
| V | Muntz brass | Inconel 718 2 layers Inconel 600 | 0.81% Ni powder | 18 h | 0.08 | 94% 30–80 µm carbon nano-bamboo, 6% conical carbon nanofiber |
| VI | Nichrome C | Nichrome C | 0.4% Ni 0.4% Cr | 3 h | 0.4 | 95% nano-bamboo carbon |
| VII | Monel | Nichrome C | 0.81% Ni | 18 h | 0.08 | 95% hollow nano-onions |
| VIII | Monel | Nichrome C | 0.4% Ni 0.4% Cr | 18 h | 0.08 | 97% nano-pearl carbon |
| IX | Monel | Nichrome C | 0.4% Ni 0.4% Cr | 18 h | 0.08 | 97% nano-pearl carbon |
| Electrolysis | Cathode | Anode | Additives (wt% Powder) | Electr Time | Current Density A/cm2 | Product Description |
|---|---|---|---|---|---|---|
| X | SST | Nichrome C | 0.81% Ni | 3 h | 0.2 | 60% Ni particle coated CNT 40% 5–10 µm CNT |
| XI | SST | Nickel | - | 4 h | 0.15 | 89% 50–150 µm straight CNT & Ni particle coated CNT |
| XII | Muntz brass | Nichrome C | 8% Li3 PO4 | 4 h | 0.2 | 98% nano-onions |
| XIII | Monel | Nichrome C | 8% Li3 PO4 | 18 h | 0.08 | 97% nano-onions |
| XIV | Muntz brass | Nichrome C | 0.81% Co | 18 h | 0.08 | 97% nano-flowers |
| XV | Muntz brass | Nichrome C | 0.81% Co | 18 h | 0.08 | 97% nano-flowers |
| XVI | Monel | Inconel 718 | 0.1% Fe2O3 | 2 h | 0.4 | 94% 50–100 µm nano-dragon |
| XVII | Muntz brass | Inconnel 718 2 layers Inconel 600 | 0.1% Li2O | 4 h | 0.13 | nano-trees: 98% 80–200 µm CNT with branches and trunk |
| XVIII | Muntz brass | Inconel 718 | 0.1% Fe2O3 | 18 h | 0.08 | 80% nano-belt |
| XIX | Monel | Iridium | 0.81% Ni | 18 h | 0.08 | 91% nano-rod CNT |
| CO2 Molten Electrolysis Product Description | νD (cm−1) | νG (cm−1) | ν2D (cm−1) | ID/IG | I2D/IG |
|---|---|---|---|---|---|
| Multi-wall carbon nanotube | 1342.4 | 1576.5 | 2688.7 | 0.30 | 0.60 |
| Hollow nano-onion | 1346.3 | 1577 | 2694.6 | 0.33 | 0.61 |
| Helical carbon nanotube | 1346.1 | 1578.2 | 2692.8 | 0.45 | 0.40 |
| Nano-dragon | 1346.7 | 1580.3 | 2695.0 | 0.67 | 0.62 |
| Nano-flower | 1347.9 | 1582.7 | 2692.2 | 0.78 | 0.50 |
| Nano-tree | 1343.7 | 1583.7 | 2696.4 | 0.82 | 0.47 |
| Nano-bamboo | 1352.0 | 1586.2 | 2696.9 | 1.04 | 0.72 |
| Nano-pearl | 1352.9 | 1588.5 | 2689.3 | 1.05 | 0.52 |
| Nano-rod | 1351.6 | 1586.0 | 2695.9 | 0.78 | 0.81 |
| Carbon nanofiber | 1349.3 | 1594.9 | 2696.0 | 1.27 | 0.37 |
| Nano-belt | 1348.5 | 1590.5 | 2705.1 | 1.30 | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Licht, G.; Wang, X.; Licht, S. Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Catalysts 2022, 12, 125. https://doi.org/10.3390/catal12020125
Liu X, Licht G, Wang X, Licht S. Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Catalysts. 2022; 12(2):125. https://doi.org/10.3390/catal12020125
Chicago/Turabian StyleLiu, Xinye, Gad Licht, Xirui Wang, and Stuart Licht. 2022. "Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2" Catalysts 12, no. 2: 125. https://doi.org/10.3390/catal12020125
APA StyleLiu, X., Licht, G., Wang, X., & Licht, S. (2022). Controlled Growth of Unusual Nanocarbon Allotropes by Molten Electrolysis of CO2. Catalysts, 12(2), 125. https://doi.org/10.3390/catal12020125






