Purification and Structural Characterization of the Auxiliary Activity 9 Native Lytic Polysaccharide Monooxygenase from Thermoascus aurantiacus and Identification of Its C1- and C4-Oxidized Reaction Products
Abstract
:1. Introduction
2. Results and Discussion
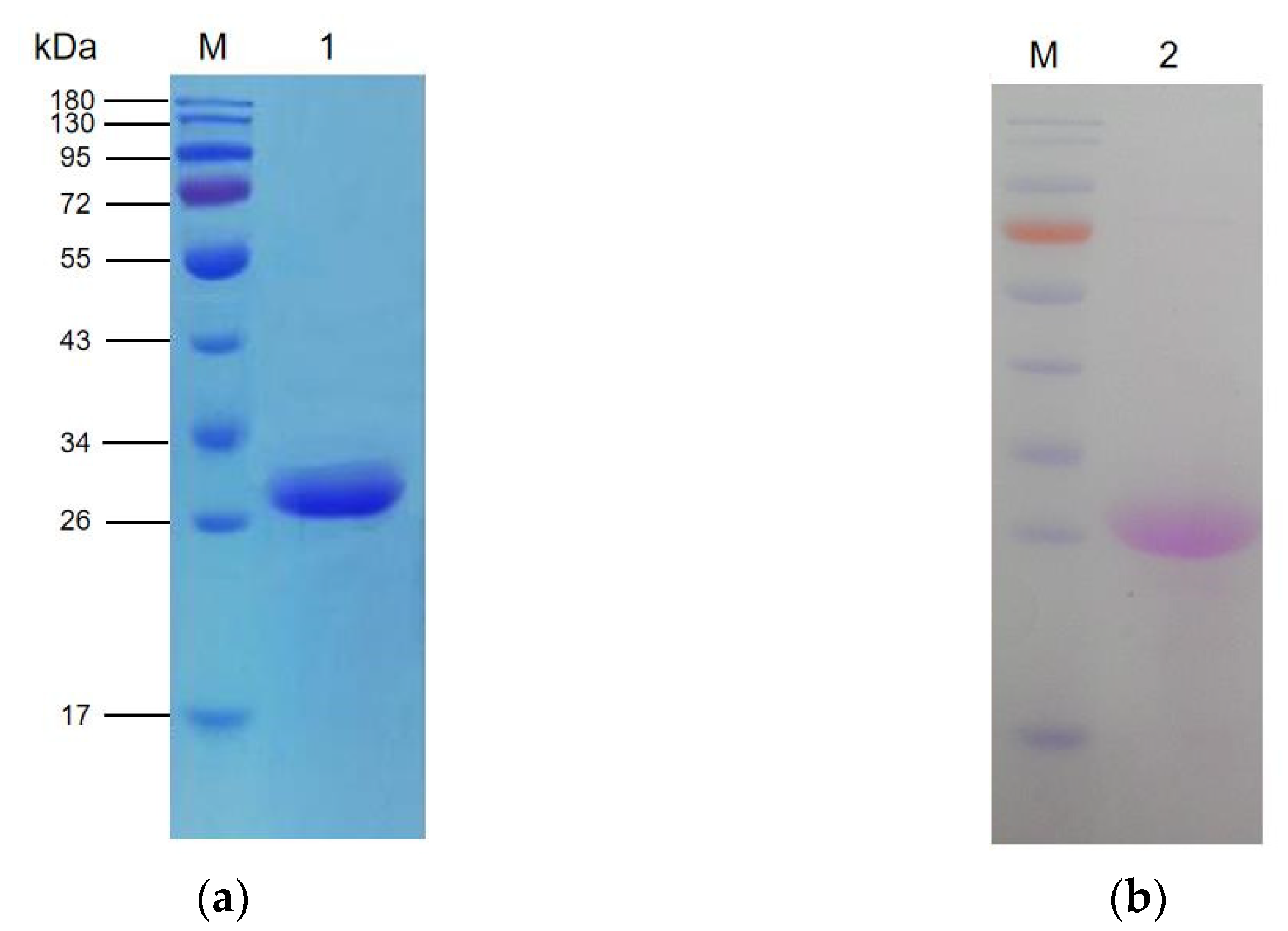
2.1. Purification
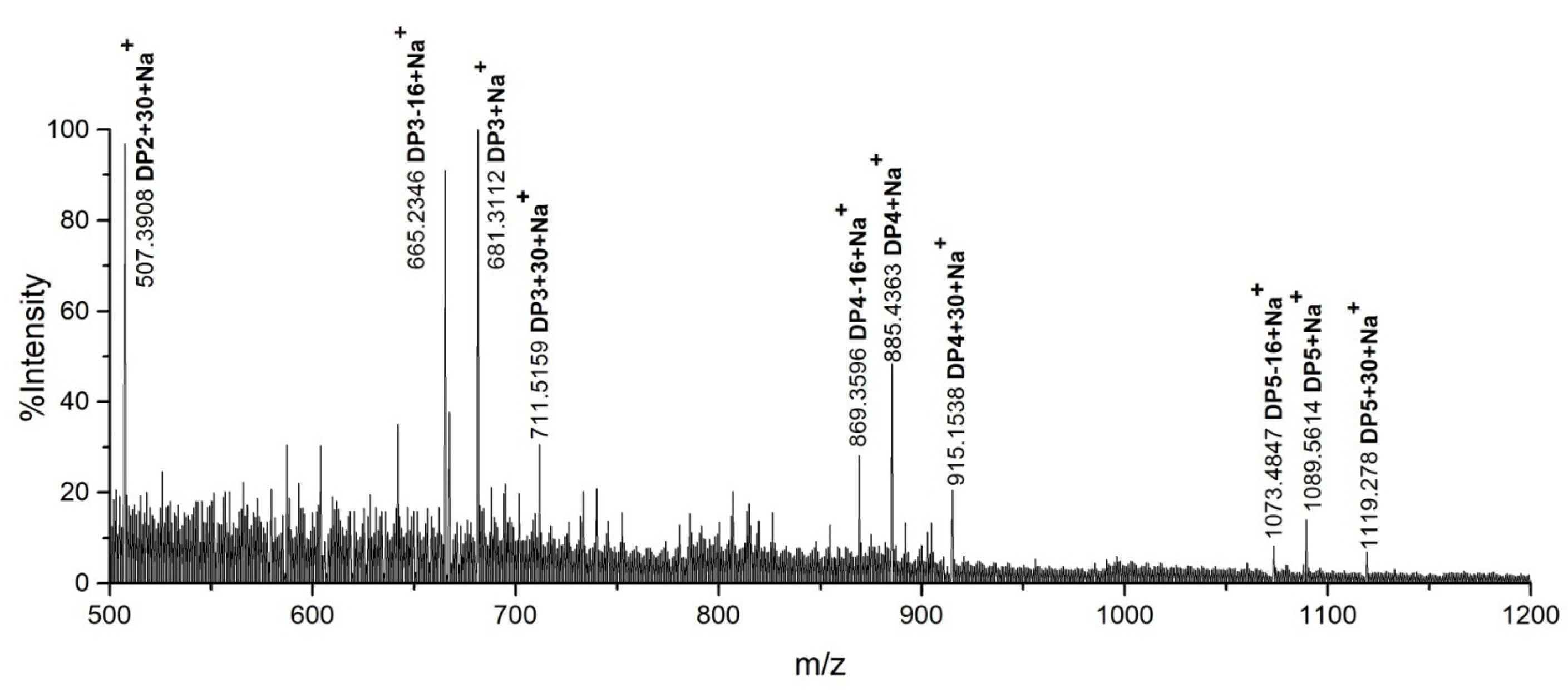
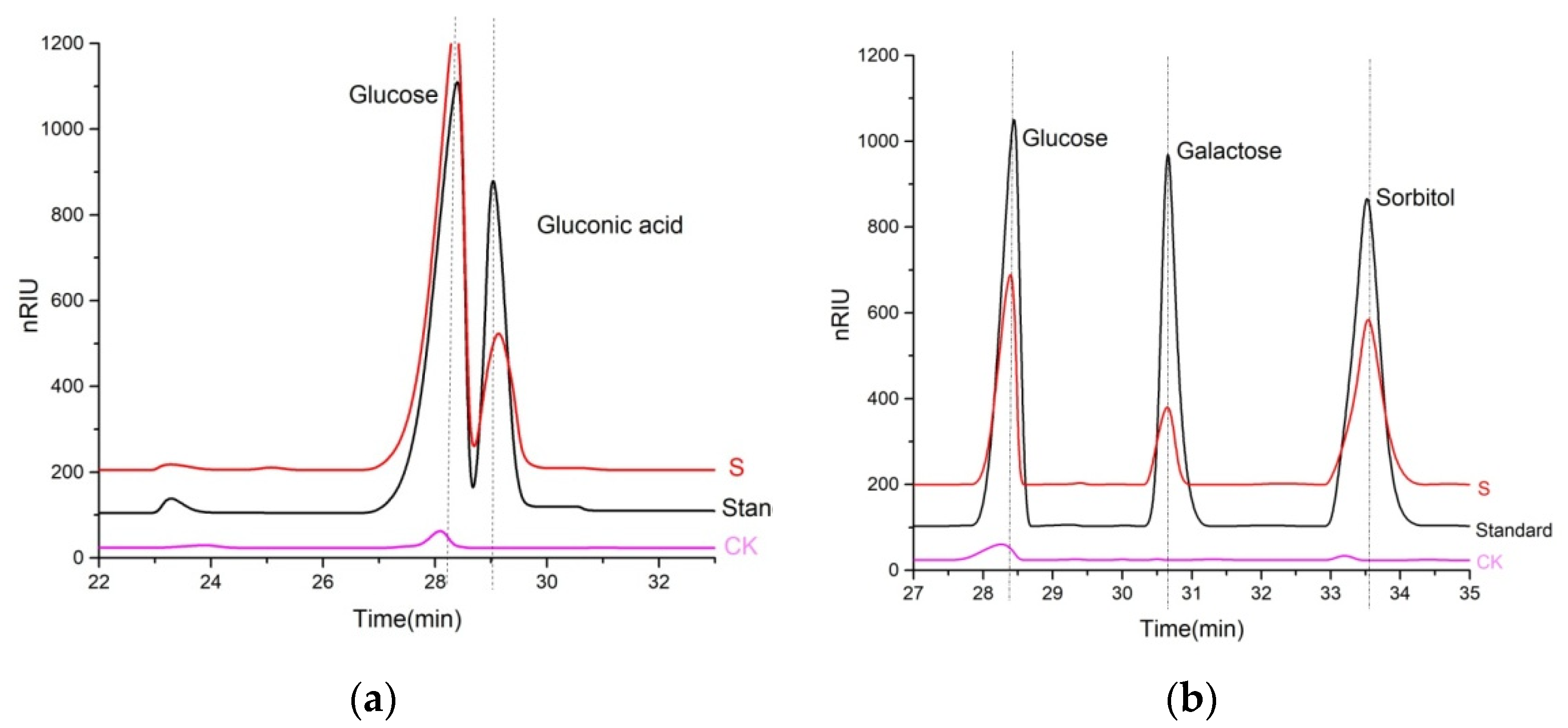
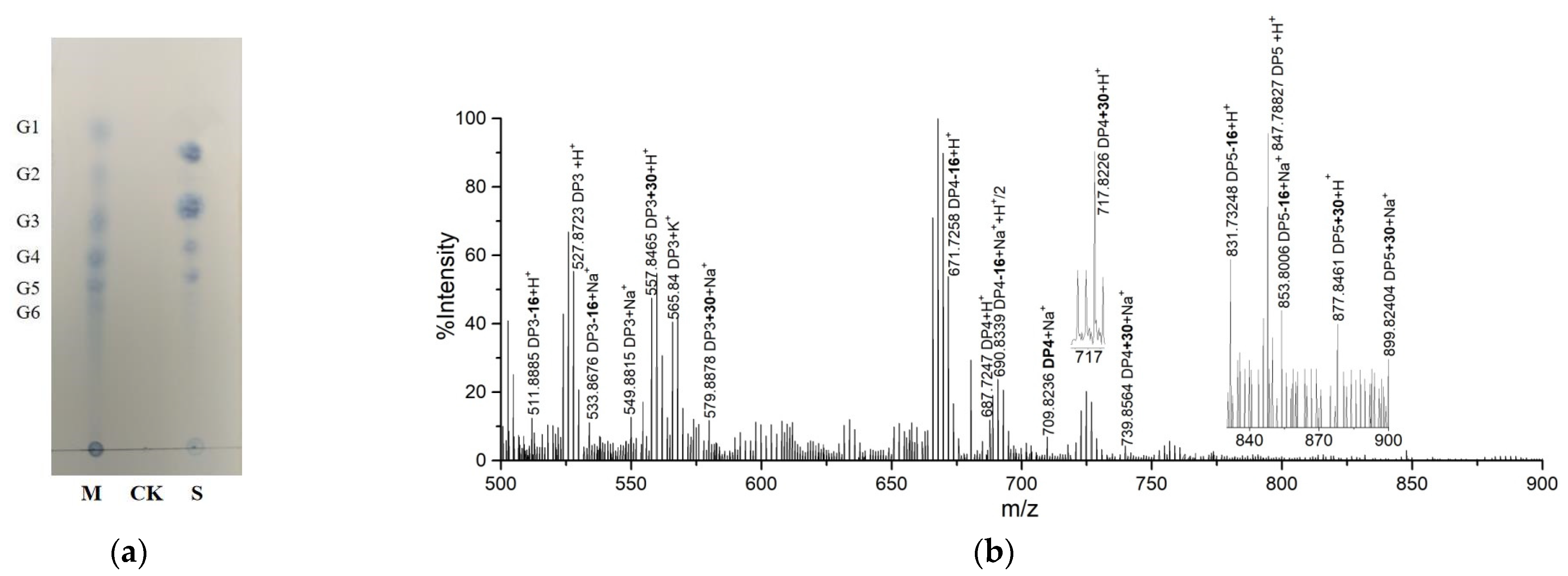
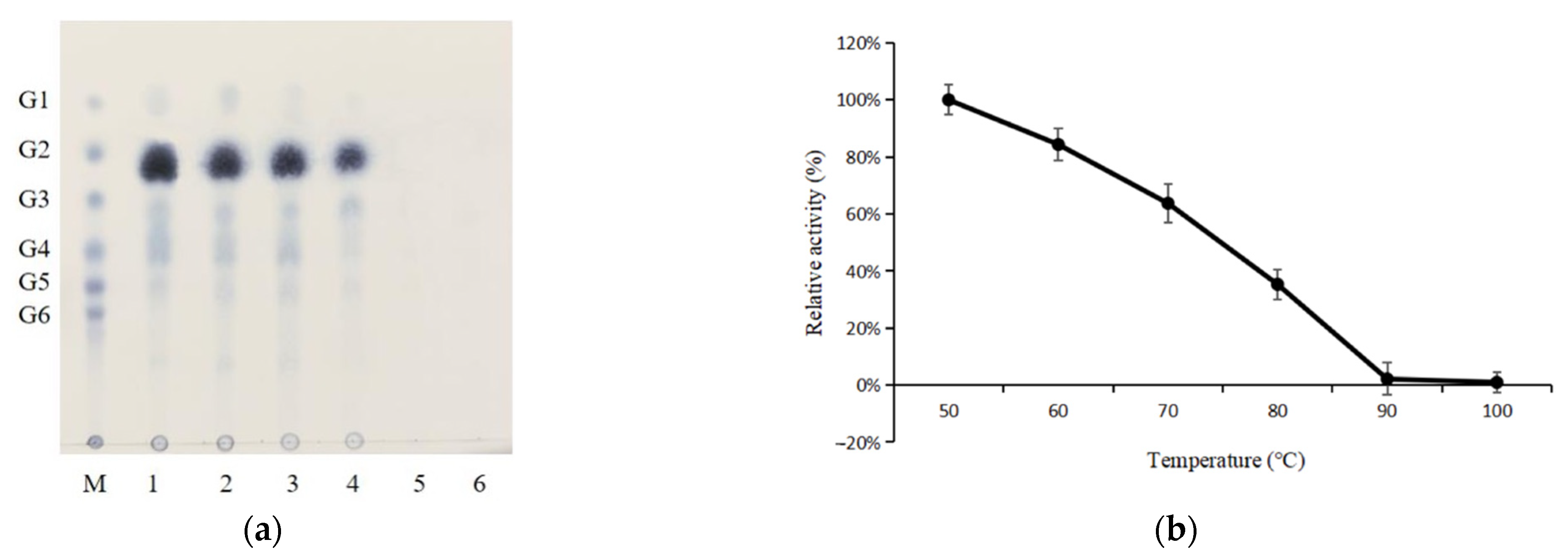
2.2. Product Identification
2.3. Structure Quality and Description
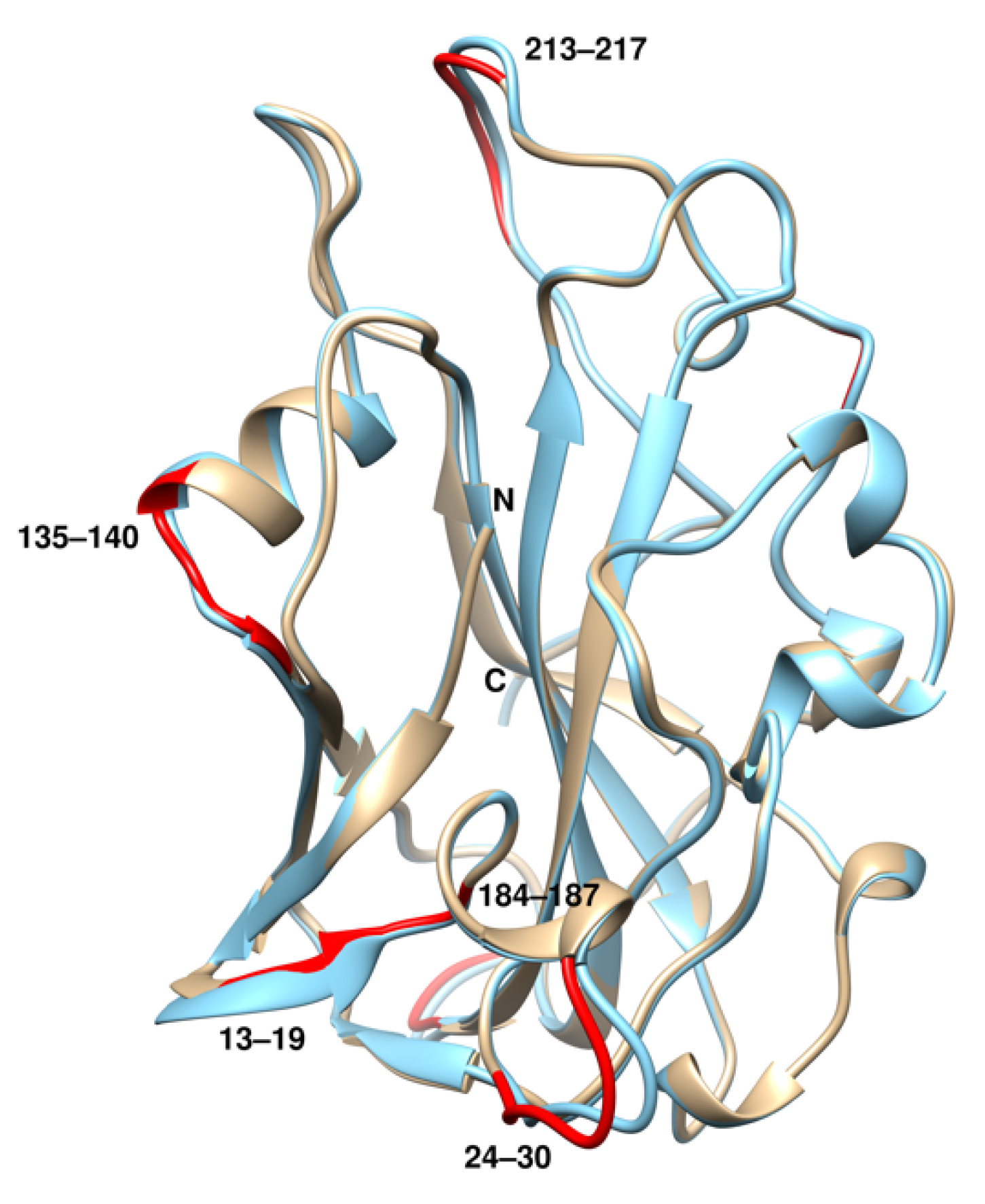
2.4. Structural Comparison with rTaAA9A
2.5. Oligosaccharide Binding
2.6. Thermostability Properties
3. Materials and Methods
3.1. Strains and Chemicals
3.2. Purification and Identification of nTaAA9A from Thermoascus aurantiacus
3.3. Protein Determination, SDS-PAGE, and Carbohydrate Staining
3.4. nTaAA9A Activity Assay
3.5. TLC and MALDI–TOF MS
3.6. Permethylation and Reduction of nTaAA9A Reaction Products
3.7. HPLC–RID
3.8. Protein Crystallization
3.9. Structure Determination and Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Service, R.F. Is there a road ahead for cellulosic ethanol? Science 2010, 329, 784–785. [Google Scholar] [CrossRef] [Green Version]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Vaaje-Kolstad, G.; Forsberg, Z.; Loose, J.S.; Bissaro, B.; Eijsink, V.G. Structural diversity of lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2017, 44, 67–76. [Google Scholar] [CrossRef]
- Bissaro, B.; Várnai, A.; Røhr, Å.K.; Eijsink, V.G.H. Oxidoreductases and Reactive Oxygen Species in Conversion of Lignocellulosic Biomass. Microbiol. Mol. Biol. Rev. 2018, 82, e00029-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levasseur, A.; Drula, E.; Lombard, V.; Coutinho, P.M.; Henrissat, B. Expansion of the enzymatic repertoire of the CAZy database to integrate auxiliary redox enzymes. Biotechnol. Biofuels 2013, 6, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, Z.; Sørlie, M.; Petrović, D.; Courtade, G.; Aachmann, F.L.; Vaaje-Kolstad, G.; Bissaro, B.; Røhr, Å.K.; Eijsink, V.G. Polysaccharide degradation by lytic polysaccharide monooxygenases. Curr. Opin. Struct. Biol. 2019, 59, 54–64. [Google Scholar] [CrossRef]
- Tamburrini, K.C.; Terrapon, N.; Lombard, V.; Bissaro, B.; Longhi, S.; Berrin, J.-G. Bioinformatic Analysis of Lytic Polysaccharide Monooxygenases Reveals the Pan-Families Occurrence of Intrinsically Disordered C-Terminal Extensions. Biomolecules 2021, 11, 1632. [Google Scholar] [CrossRef]
- Chylenski, P.; Bissaro, B.; Sørlie, M.; Røhr, Å.K.; Várnai, A.; Horn, S.J.; Eijsink, V.G.H. Lytic Polysaccharide Monooxygenases in Enzymatic Processing of Lignocellulosic Biomass. ACS Catal. 2019, 9, 4970–4991. [Google Scholar] [CrossRef]
- Tandrup, T.; Frandsen, K.E.H.; Johansen, K.S.; Berrin, J.-G.; Leggio, L.L. Recent insights into lytic polysaccharide monooxygenases (LPMOs). Biochem. Soc. Trans. 2018, 46, 1431–1447. [Google Scholar] [CrossRef]
- Askarian, F.; Uchiyama, S.; Masson, H.; Sørensen, H.V.; Golten, O.; Bunæs, A.C.; Mekasha, S.; Røhr, Å.K.; Kommedal, E.; Ludviksen, J.A.; et al. The lytic polysaccharide monooxygenase CbpD promotes Pseudomonas aeruginosa virulence in systemic infection. Nat. Commun. 2021, 12, 1230. [Google Scholar] [CrossRef]
- Chiu, E.; Hijnen, M.; Bunker, R.D.; Boudes, M.; Rajendran, C.; Aizel, K.; Olieric, V.; Schulze-Briese, C.; Mitsuhashi, W.; Young, V.; et al. Structural basis for the enhancement of virulence by viral spindles and their in vivo crystallization. Proc. Natl. Acad. Sci. USA 2015, 112, 3973–3978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, T.J.; Frandsen, K.E.H.; Ciano, L.; Tryfona, T.; Lenfant, N.; Poulsen, J.C.; Wilson, L.F.L.; Tandrup, T.; Tovborg, M.; Schnorr, K.; et al. Structural and electronic determinants of lytic polysaccharide monooxygenase reactivity on polysaccharide substrates. Nat. Commun. 2017, 8, 1064. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Johnston, E.M.; Li, P.; Shaik, S.; Davies, G.J.; Walton, P.H.; Rovira, C. QM/MM Studies into the H2O2-Dependent Activity of Lytic Polysaccharide Monooxygenases: Evidence for the Formation of a Caged Hydroxyl Radical Intermediate. ACS Catal. 2018, 8, 1346–1351. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Walton, P.H.; Rovira, C. Molecular Mechanisms of Oxygen Activation and Hydrogen Peroxide Formation in Lytic Polysaccharide Monooxygenases. ACS Catal. 2019, 9, 4958–4969. [Google Scholar] [CrossRef] [Green Version]
- Paradisi, A.; Johnston, E.M.; Tovborg, M.; Nicoll, C.R.; Ciano, L.; Dowle, A.; McMaster, J.; Hancock, Y.; Davies, G.J.; Walton, P.H. Formation of a Copper(II)–Tyrosyl Complex at the Active Site of Lytic Polysaccharide Monooxygenases Following Oxidation by H2O2. J. Am. Chem. Soc. 2019, 141, 18585–18599. [Google Scholar] [CrossRef] [Green Version]
- Frandsen, K.E.H.; Leggio, L.L. Lytic polysaccharide monooxygenases: A crystallographer’s view on a new class of biomass-degrading enzymes. IUCrJ 2016, 3, 448–467. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, R.J.; Sweeney, M.D.; Leggio, L.L.; Otten, H.; Poulsen, J.-C.N.; Johansen, K.S.; Krogh, K.B.R.M.; Jørgensen, C.I.; Tovborg, M.; Anthonsen, A.; et al. Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components. Proc. Natl. Acad. Sci. USA 2011, 108, 15079–15084. [Google Scholar] [CrossRef] [Green Version]
- Leggio, L.L.; Weihe, C.D.; Poulsen, J.-C.N.; Sweeney, M.; Rasmussen, F.; Lin, J.; De Maria, L.; Wogulis, M. Structure of a lytic polysaccharide monooxygenase from Aspergillus fumigatus and an engineered thermostable variant. Carbohydr. Res. 2018, 469, 55–59. [Google Scholar] [CrossRef]
- Bissaro, B.; Isaksen, I.; Vaaje-Kolstad, G.; Eijsink, V.G.H.; Røhr, Å.K. How a Lytic Polysaccharide Monooxygenase Binds Crystalline Chitin. Biochemistry 2018, 57, 1893–1906. [Google Scholar] [CrossRef]
- Leggio, L.L.; Simmons, T.J.; Poulsen, J.-C.N.; Frandsen, K.E.H.; Hemsworth, G.R.; Stringer, M.A.; von Freiesleben, P.; Tovborg, M.; Johansen, K.S.; De Maria, L.; et al. Structure and boosting activity of a starch-degrading lytic polysaccharide monooxygenase. Nat. Commun. 2015, 6, 5961. [Google Scholar] [CrossRef] [Green Version]
- Mazurkewich, S.; Seveso, A.; Hüttner, S.; Brändén, G.; Larsbrink, J. Structure of a C1/C4-oxidizing AA9 lytic polysaccharide monooxygenase from the thermophilic fungus Malbranchea cinnamomea. Acta Crystallogr. Sect. D Struct. Biol. 2021, D77, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Beeson, W.T.; Phillips, C.M.; Cate, J.H.D.; Marletta, M.A. Oxidative Cleavage of Cellulose by Fungal Copper-Dependent Polysaccharide Monooxygenases. J. Am. Chem. Soc. 2012, 134, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Bey, M.; Zhou, S.; Poidevin, L.; Henrissat, B.; Coutinho, P.M.; Berrin, J.-G.; Sigoillot, J.-C. Cello-Oligosaccharide Oxidation Reveals Differences between Two Lytic Polysaccharide Monooxygenases (Family GH61) from Podospora anserina. Appl. Environ. Microbiol. 2013, 79, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frommhagen, M.; Sforza, S.; Westphal, A.H.; Visser, J.; Hinz, S.W.A.; Koetsier, M.J.; Van Berkel, W.J.H.; Gruppen, H.; Kabel, M.A. Discovery of the combined oxidative cleavage of plant xylan and cellulose by a new fungal polysaccharide monooxygenase. Biotechnol. Biofuels 2015, 8, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerva, A.; Pentari, C.; Grisel, S.; Berrin, J.-G.; Topakas, E. A new synergistic relationship between xylan-active LPMO and xylobiohydrolase to tackle recalcitrant xylan. Biotechnol. Biofuels 2020, 13, 142. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [Green Version]
- Vu, V.V.; Beeson, W.T.; Phillips, C.M.; Cate, J.H.D.; Marletta, M.A. Determinants of Regioselective Hydroxylation in the Fungal Polysaccharide Monooxygenases. J. Am. Chem. Soc. 2014, 136, 562–565. [Google Scholar] [CrossRef]
- Berka, R.M.; Grigoriev, I.V.; Otillar, R.; Salamov, A.; Grimwood, J.; Reid, I.; Ishmael, N.; John, T.; Darmond, C.; Moisan, M.-C.; et al. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 2011, 29, 922–927. [Google Scholar] [CrossRef]
- Petrović, D.M.; Várnai, A.; Dimarogona, M.; Mathiesen, G.; Sandgren, M.; Westereng, B.; Eijsink, V.G.H. Comparison of three seemingly similar lytic polysaccharide monooxygenases from Neurospora crassa suggests different roles in plant biomass degradation. J. Biol. Chem. 2019, 294, 15068–15081. [Google Scholar] [CrossRef]
- Chen, J.; Guo, X.; Zhu, M.; Chen, C.; Li, D. Polysaccharide monooxygenase-catalyzed oxidation of cellulose to glucuronic acid-containing cello-oligosaccharides. Biotechnol. Biofuels 2019, 12, 42. [Google Scholar] [CrossRef]
- McClendon, S.D.; Batth, T.; Petzold, C.J.; Adams, P.D.; Simmons, B.A.; Singer, S.W. Thermoascus aurantiacus is a promising source of enzymes for biomass deconstruction under thermophilic conditions. Biotechnol. Biofuels 2012, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrović, D.M.; Bissaro, B.; Chylenski, P.; Skaugen, M.; Sørlie, M.; Jensen, M.S.; Aachmann, F.L.; Courtade, G.; Várnai, A.; Eijsink, V.G.H. Methylation of the N-terminal histidine protects a lytic polysaccharide monooxygenase from auto-oxidative inactivation. Protein Sci. 2018, 27, 1636–1650. [Google Scholar] [CrossRef] [PubMed]
- Tandrup, T.; Tryfona, T.; Frandsen, K.E.H.; Johansen, K.S.; DuPree, P.; Leggio, L.L. Oligosaccharide Binding and Thermostability of Two Related AA9 Lytic Polysaccharide Monooxygenases. Biochemistry 2020, 59, 3347–3358. [Google Scholar] [CrossRef] [PubMed]
- Harris, P.V.; Welner, D.; McFarland, K.C.; Re, E.; Poulsen, J.-C.N.; Brown, K.; Salbo, R.; Ding, H.; Vlasenko, E.; Merino, S.; et al. Stimulation of Lignocellulosic Biomass Hydrolysis by Proteins of Glycoside Hydrolase Family 61: Structure and Function of a Large, Enigmatic Family. Biochemistry 2010, 49, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.; Bharadwaj, G.; Bhat, M.K. Thermophilic Fungi: Their Physiology and Enzymes. Microbiol. Mol. Biol. Rev. 2000, 64, 461–488. [Google Scholar] [CrossRef] [Green Version]
- Li, D.-C.; Li, A.-N.; Papageorgiou, A.C. Cellulases from Thermophilic Fungi: Recent Insights and Biotechnological Potential. Enzym. Res. 2011, 2011, 308730. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Liu, Y.; Zhang, Y.; Feng, D.; Hou, S.; Guo, W.; Niu, K.; Jiang, Y.; Han, L.; Sindhu, L.; et al. Identification of a thermostable fungal lytic polysaccharide monooxygenase and evaluation of its effect on lignocellulosic degradation. Appl. Microbiol. Biotechnol. 2019, 103, 5739–5750. [Google Scholar] [CrossRef]
- Panja, A.S.; Bandopadhyay, B.; Maiti, S. Protein Thermostability Is Owing to Their Preferences to Non-Polar Smaller Volume Amino Acids, Variations in Residual Physico-Chemical Properties and More Salt-Bridges. PLoS ONE 2015, 10, e0131495. [Google Scholar] [CrossRef]
- Trivedi, S.; Gehlot, H.S.; Rao, S.R. Protein thermostability in Archaea and Eubacteria. Genet. Mol. Res. 2006, 5, 816–827. [Google Scholar]
- Pucci, F.; Kwasigroch, J.M.; Rooman, M. SCooP: An accurate and fast predictor of protein stability curves as a function of temperature. Bioinformatics 2017, 33, 3415–3422. [Google Scholar] [CrossRef]
- Costantini, S.; Colonna, G.; Facchiano, A.M. ESBRI: A web server for evaluating salt bridges in proteins. Bioinformation 2008, 3, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-C.; Lu, M.; Li, Y.-L.; Lu, J. Purification and characterization of an endocellulase from the thermophilic fungus Chaetomium thermophilum CT2. Enzym. Microb. Technol. 2003, 33, 932–937. [Google Scholar] [CrossRef]
- Chen, C.; Chen, J.; Geng, Z.; Wang, M.; Liu, N.; Li, D. Regioselectivity of oxidation by a polysaccharide monooxygenase from Chaetomium thermophilum. Biotechnol. Biofuels 2018, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.M.; Beeson, W.T.; Cate, J.H.; Marletta, M.A. Cellobiose Dehydrogenase and a Copper-Dependent Polysaccharide Monooxygenase Potentiate Cellulose Degradation by Neurospora crassa. ACS Chem. Biol. 2011, 6, 1399–1406. [Google Scholar] [CrossRef]
- Needs, P.W.; Selvendran, R.R. Avoiding oxidative degradation during sodium hydroxide/methyl iodide-mediated carbohydrate methylation in dimethyl sulfoxide. Carbohydr. Res. 1993, 245, 1–10. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Struct. Biol. 2010, D66, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.R.; Murshudov, G.N. How good are my data and what is the resolution? Acta Crystallogr. Sect. D Struct. Biol. 2013, D69, 1204–1214. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [Green Version]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.-W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Struct. Biol. 2010, D66, 213–221. [Google Scholar] [CrossRef] [Green Version]
- Chen, V.B.; Arendall, W.B., III; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2010, D66, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Struct. Biol. 2004, D60, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Data Collection | |
| Beamline | P13 (PETRA III, DESY) |
| Wavelength (Å) | 0.9762 |
| Resolution (Å) | 44.63−1.36 (1.41−1.36) |
| Space group | P212121 |
| Unit cell a, b, c (Å) | 37.7, 64.2, 88.5 |
| No. of observations | 559,476 (22,595) |
| No. of unique reflections | 43,359 (2631) |
| Completeness (%) | 92.7 (58.5) |
| Multiplicity | 12.9 (8.6) |
| Mosaicity (°) | 0.22 |
| Rmeas | 0.218 (2.993) |
| CC1/2 | 0.998 (0.251) |
| Wilson B factor (Å2) | 22.1 |
| Refinement | |
| No. of reflections used | 43,220 |
| Rcryst/Rfree | 0.151/0.185 |
| RMSD in bonds (Å) | 0.005 |
| RMSD in angles (°) | 0.867 |
| Number of protein atoms | 1762 |
| No. of water molecules | 307 |
| Average B-factor (Å2) | 17.6 |
| Ramachandran favored/outliers (%) | 99.1/0.0 |
| Clashscore | 2.54 |
| PDB id | 7q1k |
| Parameter | nTaAA9A | McAA9F (7ntl) | CvAA9_A (6yde) | AfuAA9A (6h1z) | LsAA9A (5n04) | TcAA9A GenBank (GAM42970.1) |
|---|---|---|---|---|---|---|
| Asp + Glu (−) # | 19 | 16 | 34 | 18 | 21 | 19 |
| Arg + Lys (+) | 7 | 7 | 24 | 10 | 10 | 9 |
| Pro/Gly | 0.84 | 1.04 | 0.91 | 0.57 | 1.11 | 0.76 |
| Val (%) | 5.3 | 4.1 | 8.7 | 4.4 | 9.8 | 10.3 |
| Amino acid residues | 228 | 222 | 252 | 229 | 235 | 246 |
| SAS (Å2) | 9285.0 | 9034.0 | 9649.0 | 9363.0 | 9456 | - |
| Intra-chain salt bridges § | 3 | 2 | 3 | 3 | 11 | - |
| Melting temperature Tm (°C) ‡ | 56.1 | 49.4 | 57.5 | 57.8 | 51.6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.; Mohsin, I.; Papageorgiou, A.C.; Li, D. Purification and Structural Characterization of the Auxiliary Activity 9 Native Lytic Polysaccharide Monooxygenase from Thermoascus aurantiacus and Identification of Its C1- and C4-Oxidized Reaction Products. Catalysts 2022, 12, 139. https://doi.org/10.3390/catal12020139
Yu W, Mohsin I, Papageorgiou AC, Li D. Purification and Structural Characterization of the Auxiliary Activity 9 Native Lytic Polysaccharide Monooxygenase from Thermoascus aurantiacus and Identification of Its C1- and C4-Oxidized Reaction Products. Catalysts. 2022; 12(2):139. https://doi.org/10.3390/catal12020139
Chicago/Turabian StyleYu, Weishuai, Imran Mohsin, Anastassios C. Papageorgiou, and Duochuan Li. 2022. "Purification and Structural Characterization of the Auxiliary Activity 9 Native Lytic Polysaccharide Monooxygenase from Thermoascus aurantiacus and Identification of Its C1- and C4-Oxidized Reaction Products" Catalysts 12, no. 2: 139. https://doi.org/10.3390/catal12020139








