Transforming Waste Clamshell into Highly Selective Nanostructured Catalysts for Solvent Free Liquid Phase Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization
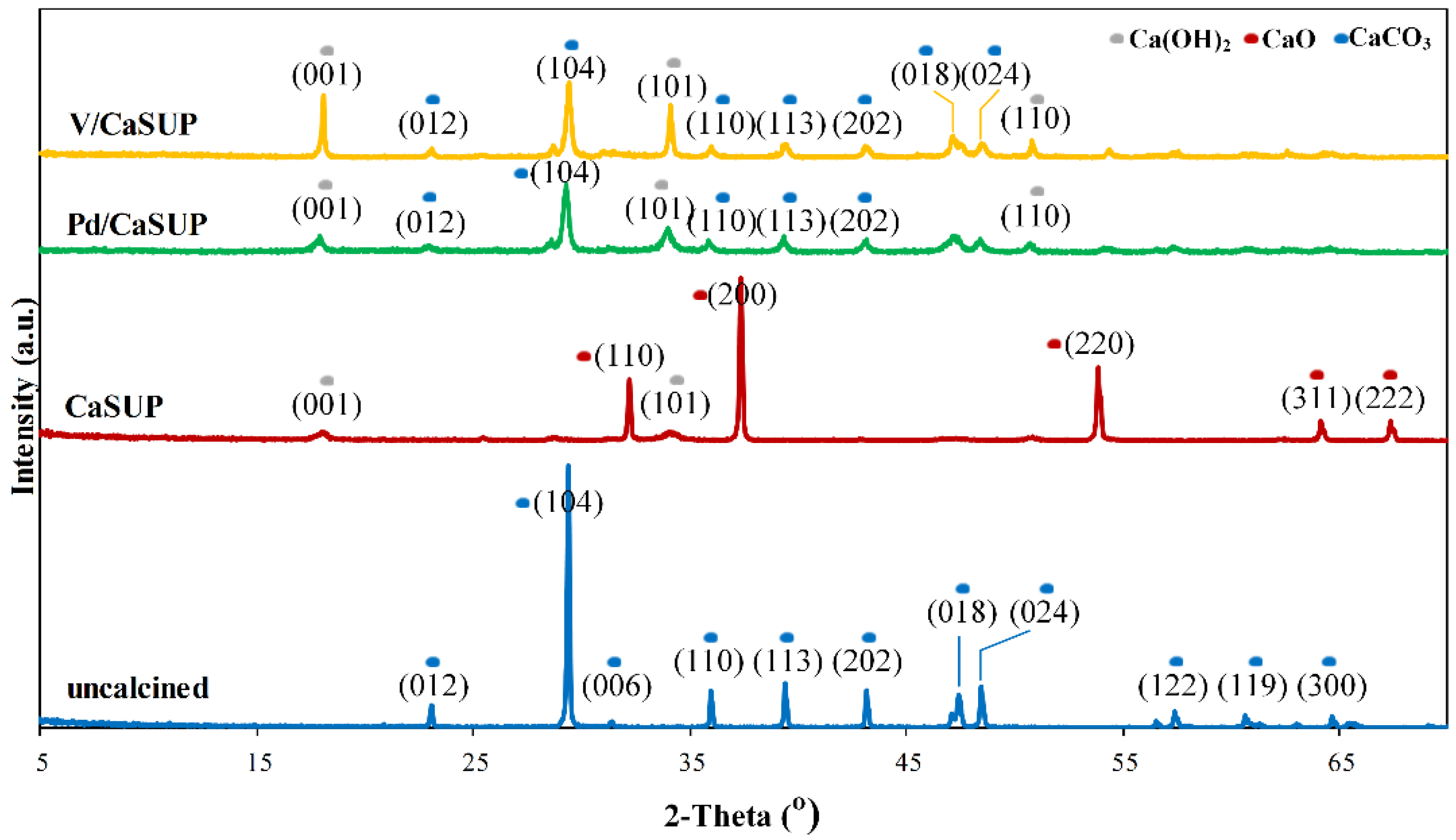
2.1.1. X-ray Diffraction (XRD)
2.1.2. Fourier Transform Infrared Spectroscopy (FTIR)
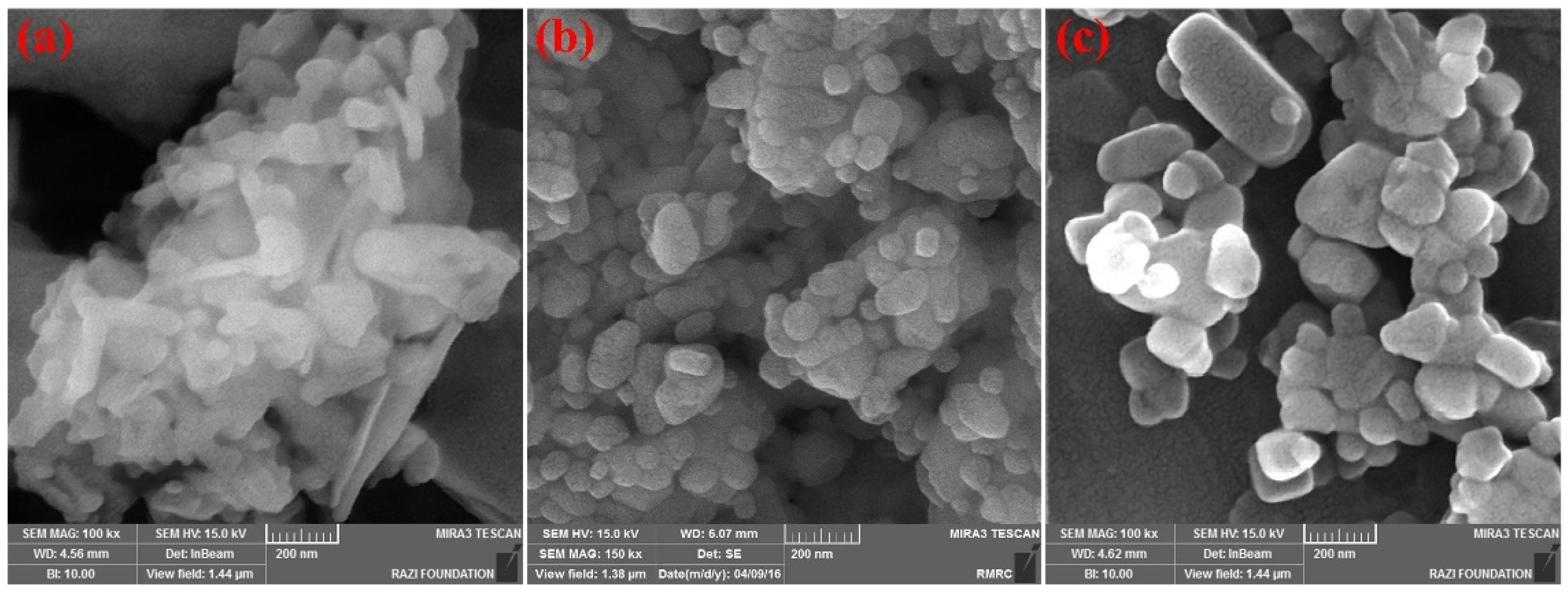
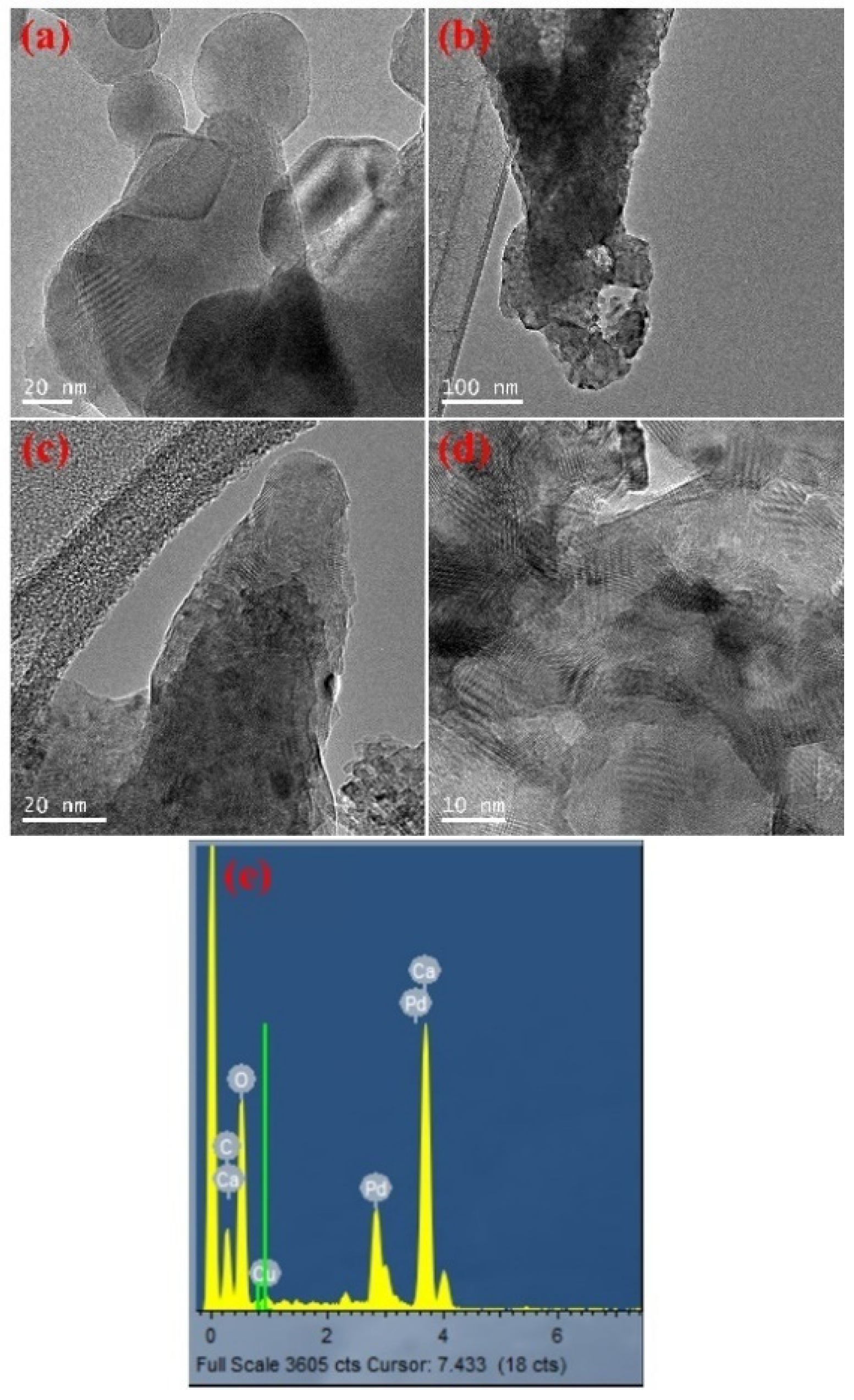
2.1.3. Morphology Characterization and Chemical Composition
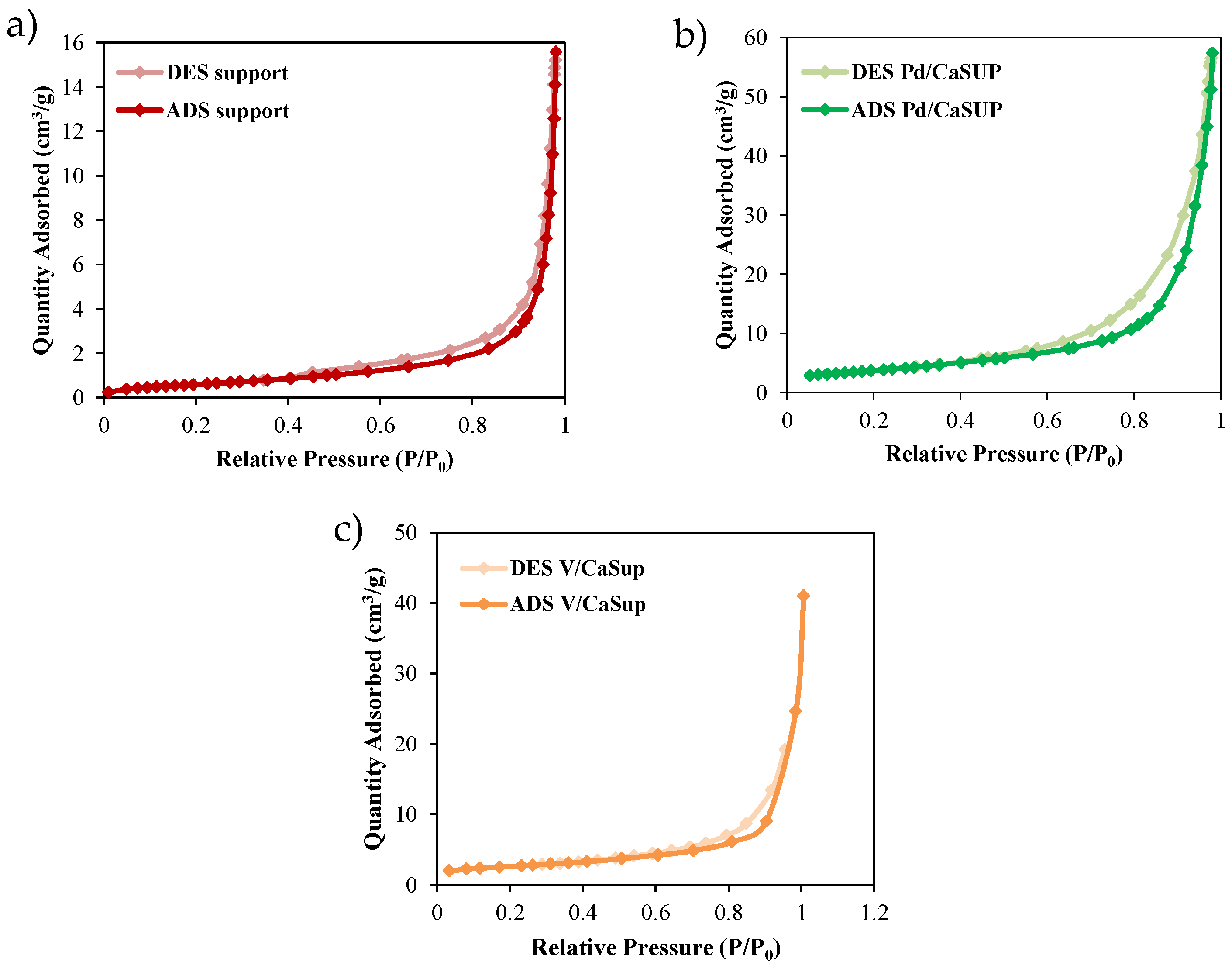
2.1.4. BET Surface Area
2.2. Catalytic Activity
2.2.1. Metal Catalyst Effects
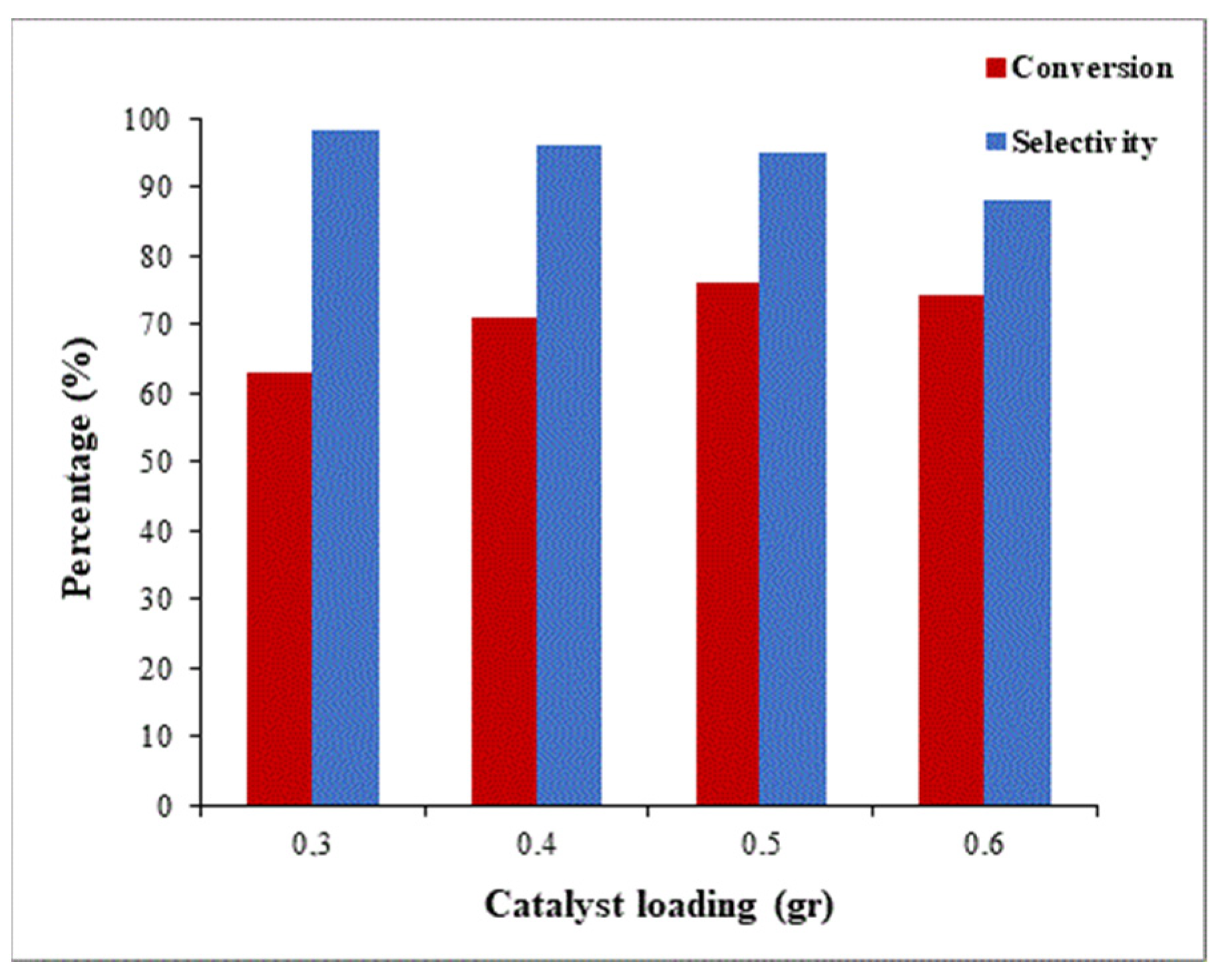
2.2.2. Effect of the Catalyst Loading
2.2.3. Effect of the Molar Ratio of H2O2 to Benzyl Alcohol
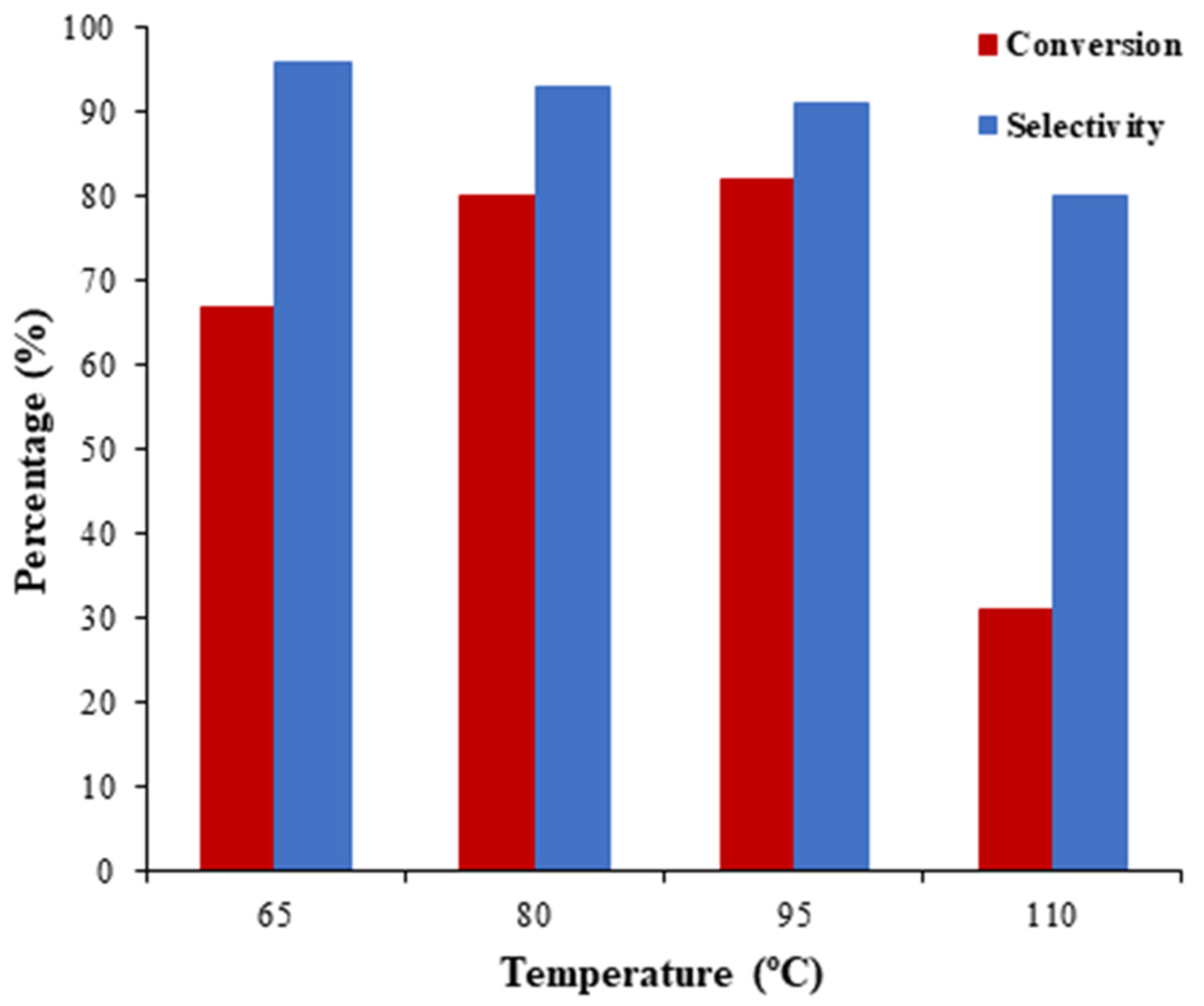
2.2.4. Effect of the Temperature
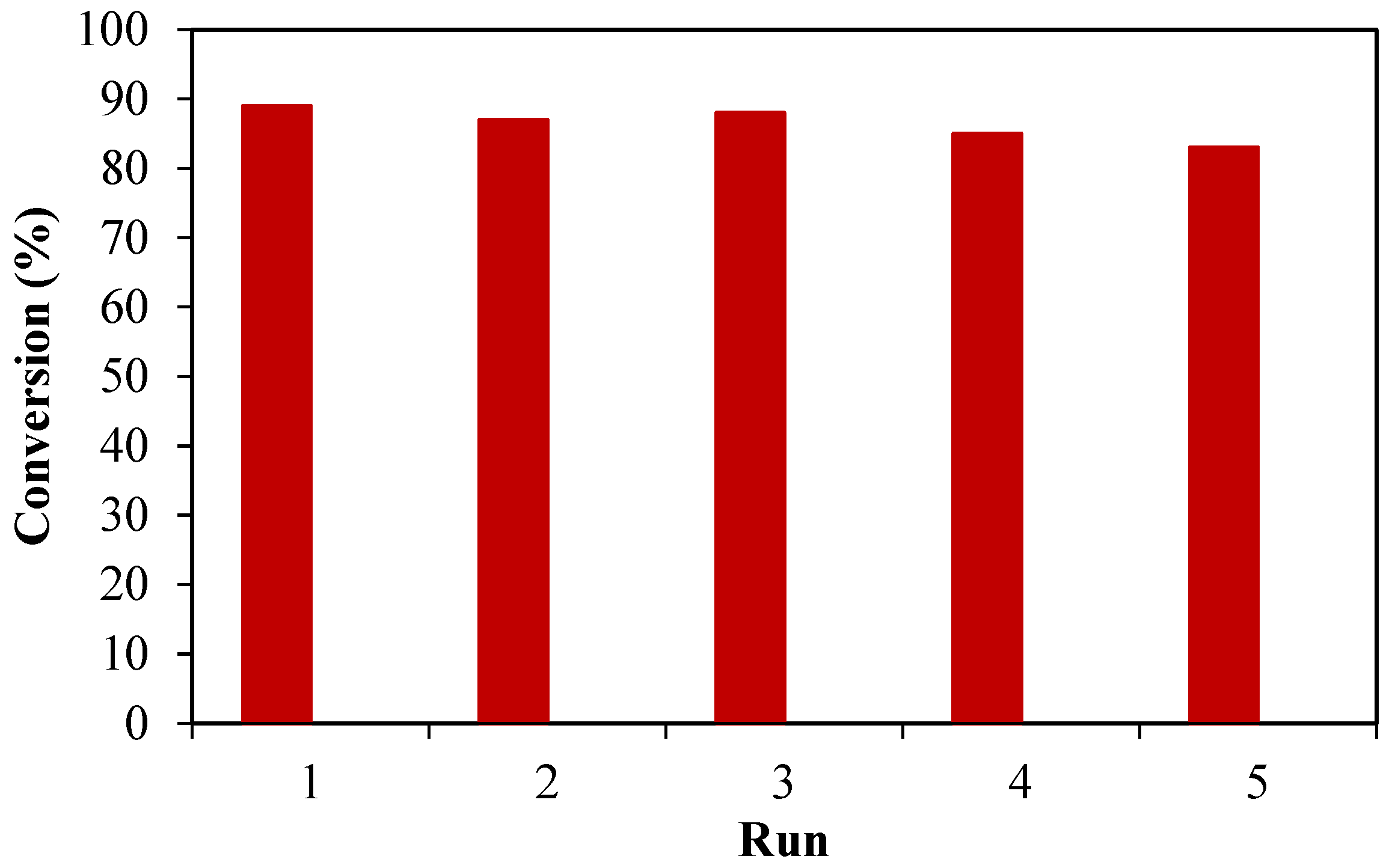
2.2.5. Effect of the Reaction Duration and Stability of the Catalyst
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Characterization of Catalysts
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, G.; Deng, M.; Jiang, Y.; Hu, H.; Huang, J.; Lu, Y. Microstructured Au/Ni-fiber catalyst: Galvanic reaction preparation and catalytic performance for low-temperature gas-phase alcohol oxidation. J. Catal. 2013, 301, 46–53. [Google Scholar] [CrossRef]
- Cang, R.; Lu, B.; Li, X.; Niu, R.; Zhao, J.; Cai, Q. Iron-chloride ionic liquid immobilized on SBA-15 for solvent-free oxidation of benzyl alcohol to benzaldehyde with H2O2. Chem. Eng. Sci. 2015, 137, 268–275. [Google Scholar] [CrossRef]
- Galvanin, F.; Sankar, M.; Cattaneo, S.; Bethell, D.; Dua, V.; Hutchings, G.J.; Gavriilidis, A. On the development of kinetic models for solvent-free benzyl alcohol oxidation over a gold-palladium catalyst. Chem. Eng. J. 2018, 342, 196–210. [Google Scholar] [CrossRef]
- Lukato, S.; Wendt, O.F.; Wallenberg, R.; Kasozi, G.N.; Naziriwo, B.; Persson, A.; Folkers, L.C.; Tebandeke, E. Selective oxidation of benzyl alcohols with molecular oxygen as the oxidant using Ag-Cu catalysts supported on polyoxometalates. Results Chem. 2021, 3, 100150. [Google Scholar] [CrossRef]
- Fei, J.; Sun, L.; Zhou, C.; Ling, H.; Yan, F.; Zhong, X.; Lu, Y.; Shi, J.; Huang, J.; Liu, Z. Tuning the Synthesis of Manganese Oxides Nanoparticles for Efficient Oxidation of Benzyl Alcohol. Nanoscale Res. Lett. 2017, 12, 23. [Google Scholar] [CrossRef] [Green Version]
- Deori, K.; Kalita, C.; Deka, S. (100) surface-exposed CeO2 nanocubes as an efficient heterogeneous catalyst in the tandem oxidation of benzyl alcohol, para-chlorobenzyl alcohol and toluene to the corresponding aldehydes selectively. J. Mater. Chem. A 2015, 3, 6909–6920. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Dumbre, D.K. Supported nano-gold catalysts for epoxidation of styrene and oxidation of benzyl alcohol to benzaldehyde. Top. Catal. 2009, 52, 1677–1687. [Google Scholar] [CrossRef]
- Bijudas, K.; Bashpa, P.; Bibin, V.P.; Nair, L.; Priya, A.P.; Aswathy, M.; Krishnendu, C.; Lisha, P. Selective synthesis of benzaldehydes by hypochlorite oxidation of benzyl alcohols under phase transfer catalysis. Bull. Chem. React. Eng. Catal. 2015, 10, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Göksu, H.; Burhan, H.; Mustafov, S.D.; Şen, F. Oxidation of Benzyl Alcohol Compounds in the Presence of Carbon Hybrid Supported Platinum Nanoparticles (Pt@CHs) in Oxygen Atmosphere. Sci. Rep. 2020, 10, 5439. [Google Scholar] [CrossRef]
- Li, X.; Cao, R.; Lin, Q. Selective oxidation of alcohols with H2O2 catalyzed by long chain multi-SO3H functionalized heteropolyanion-based ionic liquids under solvent-free conditions. Catal. Commun. 2015, 69, 5–10. [Google Scholar] [CrossRef]
- Gaspar, F.; Nunes, C.D. Selective catalytic oxidation of benzyl alcohol by MooO2 nanoparticles. Catalysts 2020, 10, 265. [Google Scholar] [CrossRef] [Green Version]
- Bourbiaux, D.; Mangematin, S.; Djakovitch, L.; Rataboul, F. Selective Aerobic Oxidation of Benzyl Alcohols with Palladium(0) Nanoparticles Suspension in Water. Catal. Lett. 2021, 151, 3239–3249. [Google Scholar] [CrossRef]
- Crombie, C.M.; Lewis, R.J.; Taylor, R.L.; Morgan, D.J.; Davies, T.E.; Folli, A.; Murphy, D.M.; Edwards, J.K.; Qi, J.; Jiang, H.; et al. Enhanced Selective Oxidation of Benzyl Alcohol via in Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 2021, 11, 2701–2714. [Google Scholar] [CrossRef]
- Neves, P.; Valente, A.A.; Lin, Z. Mild Liquid Phase Oxidation of Benzyl Alcohol in the Presence of Microporous Framework Copper Silicates. Eur. J. Inorg. Chem. 2020, 2020, 1172–1176. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective benzyl alcohol oxidation over pd catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Huang, J.; Hu, X.; Lam, F.L.Y.; Wang, W.; Luque, R. Heterogeneous Pd catalyst for mild solvent-free oxidation of benzyl alcohol. J. Mol. Catal. A Chem. 2016, 425, 61–67. [Google Scholar] [CrossRef]
- Hu, Z.; Zhou, G.; Xu, L.; Yang, J.; Zhang, B.; Xiang, X. Preparation of ternary Pd/CeO2-nitroben doped graphene composites as recyclable catalysts for solvent-free aerobic oxidation of benzyl alcohol. Appl. Surf. Sci. 2019, 471, 852–861. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.H.; Yu, Z.X.; Yao, K.F.; Ji, S.F.; Liang, J. Effect of microstructures of Pt catalysts supported on carbon nanotubes (CNTs) and activated carbon (AC) for nitrobenzene hydrogenation. Mater. Chem. Phys. 2007, 103, 225–229. [Google Scholar] [CrossRef]
- Cánepa, A.L.; Elías, V.R.; Vaschetti, V.M.; Sabre, E.V.; Eimer, G.A.; Casuscelli, S.G. Selective oxidation of benzyl alcohol through eco-friendly processes using mesoporous V-MCM-41, Fe-MCM-41 and Co-MCM-41 materials. Appl. Catal. A Gen. 2017, 545, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Ji, W.Y.; Sung, H.J.; Chang, J.S. Vapor-phase oxidation of alkylaromatics over V/TiO2 and VSb/Al2O3 catalysts: Effect of alkali metals. Bull. Korean Chem. Soc. 2007, 28, 2405–2408. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Lin, M.; Ang, T.P.; Chang, J.; Yang, Y.; Borgna, A. Liquid phase aerobic oxidation of benzyl alcohol over Pd and Rh catalysts on N-doped mesoporous carbon: Effect of the surface acido-basicity. Catal. Commun. 2012, 25, 96–101. [Google Scholar] [CrossRef]
- Verziu, M.; Coman, S.M.; Richards, R.; Parvulescu, V.I. Transesterification of vegetable oils over CaO catalysts. Catal. Today 2011, 167, 64–70. [Google Scholar] [CrossRef]
- Aghabarari, B.; Martinez-Huerta, M.V. Biodiesel Production Using Calcined Waste Filter Press Cake from a Sugar Manufacturing Facility as a Highly Economic Catalyst. JAOCS, J. Am. Oil Chem. Soc. 2016, 93, 773–779. [Google Scholar] [CrossRef]
- Asikin-Mijan, N.; Taufiq-Yap, Y.H.; Lee, H.V. Synthesis of clamshell derived Ca(OH)2 nano-particles via simple surfactant-hydration treatment. Chem. Eng. J. 2015, 262, 1043–1051. [Google Scholar] [CrossRef]
- Islam, K.N.; Bakar, M.Z.B.A.; Ali, M.E.; Hussein, M.Z.B.; Noordin, M.M.; Loqman, M.Y.; Miah, G.; Wahid, H.; Hashim, U. A novel method for the synthesis of calcium carbonate (aragonite) nanoparticles from cockle shells. Powder Technol. 2013, 235, 70–75. [Google Scholar] [CrossRef]
- Wan, Z.; Hassan, H. Heterogeneous catalyst acid-activated clamshell for fenton-like oxidation of reactive black 5 solutions. In Proceedings of the 2012 IEEE Symposium on Humanities, Science and Engineering Research, Kuala Lumpur, Malaysia, 24–27 June 2012; pp. 277–281. [Google Scholar]
- Engin, B.; Demirtaş, H.; Eken, M. Temperature effects on egg shells investigated by XRD, IR and ESR techniques. Radiat. Phys. Chem. 2006, 75, 268–277. [Google Scholar] [CrossRef]
- Suprapto, S.; Fauziah, T.R.; Sangi, M.S.; Oetami, T.P.; Qoniah, I.; Prasetyoko, D. Calcium oxide from limestone as solid base catalyst in transesterification of Reutealis trisperma oil. Indones. J. Chem. 2016, 16, 208–213. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, M.; Ge, L.; Chen, T.; Li, H.; Ye, Y.; Zheng, H. Mechanical and thermal properties of polypropylene (PP) composites filled with CaCO3 and shell waste derived bio-fillers. Fibers Polym. 2014, 15, 1278–1287. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Mayeli, M.; Bikas, R.; Ślepokura, K.; Lis, T. Selective catalytic oxidation of benzyl alcohol to benzaldehyde by a mononuclear oxovanadium(V) complex of a bis(phenolate) ligand containing bulky tert-butyl substituents. Transit. Met. Chem. 2014, 39, 33–39. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Dumbre, D.K. Solvent-free selective oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over U3O8-supported nano-gold catalysts. Appl. Catal. A Gen. 2010, 375, 252–257. [Google Scholar] [CrossRef]
- Zhan, G.; Hong, Y.; Mbah, V.T.; Huang, J.; Ibrahim, A.R.; Du, M.; Li, Q. Bimetallic Au-Pd/MgO as efficient catalysts for aerobic oxidation of benzyl alcohol: A green bio-reducing preparation method. Appl. Catal. A Gen. 2012, 439–440, 179–186. [Google Scholar] [CrossRef]
- Adam, F.; Ooi, W.T. Selective oxidation of benzyl alcohol to benzaldehyde over Co-metalloporphyrin supported on silica nanoparticles. Appl. Catal. A Gen. 2012, 445–446, 252–260. [Google Scholar] [CrossRef]
- Wang, X.L.; Wu, G.D.; Zhai, Z.L.; Dai, Y.W. Solvent-Free Oxidation of Benzyl Alcohol by 30% H2O2 over LDH Hosted Chromium Complex. Adv. Mater. Res. 2014, 1015, 497–500. [Google Scholar] [CrossRef]
- Li, Y.; Sabbaghi, A.; Huang, J.; Li, K.C.; Tsui, L.S.; Lam, F.L.Y.; Hu, X. Aerobic oxidation of benzyl alcohol: Influence from catalysts basicity, acidity, and preparation methods. Mol. Catal. 2020, 485, 110789. [Google Scholar] [CrossRef]
- Moeini, S.S.; Battocchio, C.; Casciardi, S.; Luisetto, I.; Lupattelli, P.; Tofani, D.; Tuti, S. Oxidized palladium supported on ceria nanorods for catalytic aerobic oxidation of Benzyl alcohol to benzaldehyde in protic solvents. Catalysts 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Niu, S.; Ji, X.; Yu, W.; Lin, T.W.; Wu, Y.; Li, Y.; Shao, L. Doping carbon networks with phosphorus for supporting Pd in catalyzing selective oxidation of benzyl alcohol. J. Nanoparticle Res. 2018, 20, 180. [Google Scholar] [CrossRef]
- Kong, L.; Wang, C.; Gong, F.; Zhu, W.; Zhong, Y.; Ye, X.; Li, F. Magnetic Core–Shell Nanostructured Palladium Catalysts for Green Oxidation of Benzyl Alcohol. Catal. Lett. 2016, 146, 1321–1330. [Google Scholar] [CrossRef]
- Song, H.; Liu, Z.; Gai, H.; Wang, Y.; Qiao, L.; Zhong, C.; Yin, X.; Xiao, M. Nitrogen-dopped ordered mesoporous carbon anchored Pd nanoparticles for solvent free selective oxidation of benzyl alcohol to benzaldehyde by using O2. Front. Chem. 2019, 7, 458. [Google Scholar] [CrossRef]

| Catalyst | Conversion (%) | Selectivity (%) |
|---|---|---|
| Without catalyst | 2 | 99 |
| CaSUP | 4 | 99 |
| Pd/CaSUP | 71 | 96 |
| V/CaSUP | 37 | 87 |
| H2O2: Benzyl Alcohol Molar Ratio | Conversion (%) | Selectivity (%) |
|---|---|---|
| 5:1 | 69 | 95 |
| 6:1 | 76 | 95 |
| 7:1 | 80 | 93 |
| 8:1 | 83 | 84 |
| Time (h) | Conversion (%) | Selectivity (%) |
|---|---|---|
| 5 | 74 | 92 |
| 6 | 80 | 93 |
| 7 | 88 | 89 |
| 8 | 89 | 85 |
| Catalyst | Reaction Condition | Conversion | Selectivity | Yield | Ref. |
|---|---|---|---|---|---|
| Pdcatalysts supported on magnetic SBA-15 | 95 °C, 3.5 h | 50% | 95% | - | [35] |
| Pd/reduced graphene oxide aerogel | 140 °C, 3 h | ~80% | poor | - | [34] |
| Oxidized Palladium Supported on Ceria Nanorods | 78 °C, 5 h | ~93% | 96% | - | [36] |
| Doping carbon networks with phosphorus for supporting Pd | 70 °C, 10 h | 96.6% | 82.4% | - | [37] |
| Pd/CeO2-nitrogen doped graphene composite | 160 °C, 6 h | 44.5% | 99.6% | - | [17] |
| Pd nanoparticles loaded magnetic SBA-15 | 85 °C, 6 h | 83.2% | 71% | [16] | |
| Magnetic Core–Shell Nanostructured Palladium Catalysts Pd/Fe3O4@CeO2 | 100 °C, 7 h | 80.5% | 94.8% | - | [38] |
| nitrogen-doped ordered mesoporous carbon anchored Pd | 160 °C, 3 h | 24.63% | 85.71% | - | [39] |
| Pd/CaSUP | 80 °C, 7 h | 88 | 89 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saffari, N.S.; Aghabarari, B.; Javaheri, M.; Khanlarkhani, A.; Martinez-Huerta, M.V. Transforming Waste Clamshell into Highly Selective Nanostructured Catalysts for Solvent Free Liquid Phase Oxidation of Benzyl Alcohol. Catalysts 2022, 12, 155. https://doi.org/10.3390/catal12020155
Saffari NS, Aghabarari B, Javaheri M, Khanlarkhani A, Martinez-Huerta MV. Transforming Waste Clamshell into Highly Selective Nanostructured Catalysts for Solvent Free Liquid Phase Oxidation of Benzyl Alcohol. Catalysts. 2022; 12(2):155. https://doi.org/10.3390/catal12020155
Chicago/Turabian StyleSaffari, Nafiseh Sadat, Behzad Aghabarari, Masoumeh Javaheri, Ali Khanlarkhani, and Maria Victoria Martinez-Huerta. 2022. "Transforming Waste Clamshell into Highly Selective Nanostructured Catalysts for Solvent Free Liquid Phase Oxidation of Benzyl Alcohol" Catalysts 12, no. 2: 155. https://doi.org/10.3390/catal12020155








