Production of Amorphous Silicon Dioxide Derived from Aluminum Fluoride Industrial Waste and Consideration of the Possibility of Its Use as Al2O3-SiO2 Catalyst Supports
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Experimental
4. Results and Discussion
4.1. Silica Gel Purification
4.2. Preparation of Catalyst Supports Based on Amorphous Silicon Dioxide
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahn, W.S.; Kang, K.K.; Kim, K.Y. Synthesis of TS-1 by microwave heating of template-impregnated SiO2-TiO2 xerogels. Catal. Lett. 2001, 72, 229–232. [Google Scholar] [CrossRef]
- Potapov, A.I. Estimation of the relation of strength and ultrasound speed in glass-reinforce plastic. J. Min. Inst. 2018, 230, 176–184. [Google Scholar] [CrossRef]
- Kononova, I.E.; Kononov, P.V.; Moshnikov, V.A. Development of a Model for the Formation of Materials with a Hierarchical Pore Structure Produced under Sol–Gel Processing Conditions. Inorg. Mater. 2018, 54, 478–489. [Google Scholar] [CrossRef]
- Smirnova, O.M. Development of classification of rheologically active microfillers for disperse systems with portland cement and superplasticizer Smirnova. Int. J. Civ. Eng. Technol. 2018, 9, 1966–1973. [Google Scholar]
- Ferch, H. Amorphous synthetic silica products in powder form. Part 2. Applications. Prog. Org. Coat. 1982, 10, 91–118. [Google Scholar] [CrossRef]
- Quang, D.V.; Kim, J.K.; Park, J.K.; Park, S.H.; Elineema, G.; Sarawade, P.B.; Kim, H.T. Effect of the gelation on the properties of precipitated silica powder produced by acidizing sodium silicate solution at the pilot scale. Chem. Eng. J. 2012, 209, 531–536. [Google Scholar] [CrossRef]
- Shcherbakova, T.P. A method of obtaining biogenic silica. Theor. Found. Chem. Technol. 2020, 54, 185–191. [Google Scholar] [CrossRef]
- Jesionowski, T. Preparation and characterisation of silicon dioxide obtained via emulsion method. Pigment Resin Technol. 2006, 35, 252–259. [Google Scholar] [CrossRef]
- Filipovic, R. Synthesis of mesoporous silica particles with controlled pore structure. Ceram. Int. 2009, 35, 3347–3353. [Google Scholar] [CrossRef]
- Sierra, L. Synthesis of mesoporous silica with tunable pore size from sodium silicate solutions and a polyethylene oxide surfactant. Micropor. Mesopor. Mater. 1999, 27, 243–253. [Google Scholar] [CrossRef]
- Smirnova, O.M.; Menéndez Pidal de Navascués, I.; Mikhailevskii, V.R.; Kolosov, O.I.; Skolota, N.S. Sound-Absorbing Composites with Rubber Crumb from Used Tires. Appl. Sci. 2021, 11, 7347. [Google Scholar] [CrossRef]
- Tereschenko, I.M. Prospects for the Organization of Production of Silicon Dioxide Based on Raw Materials of the Republic of Belarus. 2020, pp. 263–267. Available online: https://elib.belstu.by/handle/123456789/36873 (accessed on 7 January 2022).
- Tirk, I.G.; Schmidt, G. Precipitated Silica. 1998, p. 18. Available online: https://www.mrs.org/spring1998/symposium-p (accessed on 7 January 2022).
- Gun’ko, V.M.; Zarko, V.I.; Leboda, R.; Chibowski, E. Aqueous suspension of fumed oxides: Particle size distribution and zeta potential. Adv. Colloid Interface Sci. 2001, 91, 1–112. [Google Scholar] [CrossRef]
- Ivanschenko, S.N. Study of the Possibility of Import Substitution of Pyrogenic Amorphous Silicon Dioxide in the Preparation of Manganese Suspension. Perm Natl. Res. Polytech. Univ. 2016, 3, 93–101. [Google Scholar] [CrossRef]
- Kazanskaya, L.; Smirnova, O. Influence of mixture composition on fresh concrete workability for ballastless track slabs. E3S Web Conf. 2020, 157, 06022. [Google Scholar] [CrossRef] [Green Version]
- Ibragimov, M.A. Analysis of the market for organosilicon products on the example of the project «Construction of a separate industrial production of methylchlorosilanes of JSC KZSK-silicone. Innov. Invest. 2019, 10, 343–348. [Google Scholar]
- Rimsza, J.M.; Du, J. Nanoporous silica gel structures and evolution from reactive force field-based molecular dynamics simulations. NPJ Mater. Degrad. 2018, 2, 1–10. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Nakamura, T.; Yamamuro, T. Apatite Formation Induced by Silica Gel in a Simulated Body Fluid. J. Am. Ceram. Soc. 1992, 75, 2094–2097. [Google Scholar] [CrossRef]
- Eränen, S.; Törmä, P.; Gonzalez, P.; Rottenberg, X.; Puurunen, R.L.; Putkonen, M.; Vogl, A.; Tyholdt, F.; Tofteberg, H.; Muralt, P.; et al. Thin Films on Silicon; 2015; ISBN 9780323312233. Available online: https://doi.org/10.1016/B978-0-323-29965-7.00006-3 (accessed on 7 January 2022).
- Albiter, E.; Alfaro, S.; Valenzuela, M.A. Photosensitized oxidation of 9,10-dimethylanthracene on dye-doped silica composites. Int. J. Photoenergy 2012, 2012, 987606. [Google Scholar] [CrossRef]
- Pshchelko, N.S. Use of nano-dimensional hydrophobic coatings for obtaining electrets based on silicon dioxide. J. Min. Inst. 2018, 230, 146–152. [Google Scholar] [CrossRef]
- Gorlanov, E.S.; Brichkin, V.N.; Polyakov, А.А. Electrolytic production of aluminium. Review. Part 1. Conventional areas of development. Tsvetnye Met. 2020, 10, 36–41. [Google Scholar] [CrossRef]
- Kosov, Y.I.; Bazhin, V.Y. Synthesis of an Aluminum–Erbium Master Alloy from Chloride–Fluoride Melts. Russ. Metall. 2018, 2018, 139–148. [Google Scholar] [CrossRef]
- Vaičiukyniene, D.; Kantautas, A.; Vaitkevičius, V.; Sasnauskas, V. Using of modified AlF3 production waste in cement-based materials. Medziagotyra 2009, 15, 255–261. [Google Scholar]
- Gineika, A.; Siauciunas, R.; Baltakys, K. Synthesis of wollastonite from AlF3-rich silica gel and its hardening in the CO2 atmosphere. Sci. Rep. 2019, 9, 18063. [Google Scholar] [CrossRef] [Green Version]
- Vaičiukyniene, D.; Vaitkevičius, V.; Kantautas, A.; Sasnauskas, V. Utilization of by-product waste silica in concrete-based materials. Mater. Res. 2012, 15, 561–567. [Google Scholar] [CrossRef]
- Kogan, V.E.; Shakhparonova, T.S. Chemistry as a basis for solving environmental issues. J. Min. Inst. 2017, 224, 223–228. [Google Scholar] [CrossRef]
- Tereshchenko, I.M. Problems and perspectives of silica gel using in the large-tonnage productions. Belarusian State Technol. Univ. 2018, 2, 126–131. [Google Scholar]
- Mamchenkov, E.A.; Prokof’ev, V.Y. Sodium silicate manufacturing from modified silica gel as by-product of aluminum fluoride. ChemChemTech 2019, 62, 81–93. [Google Scholar] [CrossRef]
- Vaičiukyniene, D.; Kantautas, A.; Vaitkevičius, V.; Jakevičius, L.; Rudžionis, Ž.; Paškevičius, M. Effects of ultrasonic treatment on zeolite NaA synthesized from by-product silica. Ultrason. Sonochem. 2015, 27, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Girskas, G.; Skripkiunas, G.; Šahmenko, G.; Korjakins, A. Durability of concrete containing synthetic zeolite from aluminum fluoride production waste as a supplementary cementitious material. Constr. Build. Mater. 2016, 117, 99–106. [Google Scholar] [CrossRef]
- Palubinskaite, D. Technogenic Materials for the Synthesis of Zeolites and KHS Insulation Materials. Ph.D. Thesis, Chemistry (Easton) University, St. Davids, PA, USA, 1998; p. 103. [Google Scholar]
- Vaičiukyniene, D.; Vaitkevičius, V.; Kantautas, A.; Sasnauskas, V. Effect of AlF3 production waste on the properties of hardened cement paste. Medziagotyra 2012, 18, 187–191. [Google Scholar] [CrossRef]
- Kazanskaya, L.F.; Isakovsky, V.I.; Fadeeva, S. Technological properties of self-compacting concrete mixtures with ground quartz sand. Int. J. Innov. Technol. Explor. Eng. 2019, 8, 799–803. [Google Scholar] [CrossRef]
- Kubiliute, R.; Kaminskas, R. The pozzolanic activity of calcined clay-silica gel composites. Medziagotyra 2013, 19, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Wang, L.; Zhu, J.; Liu, L.; Qian, Y. A novel SiO2–GO/acrylic resin nanocomposite: Fabrication, characterization and properties. Appl. Phys. A 2019, 125, 551. [Google Scholar] [CrossRef]
- Kholodnaya, G.; Sazonov, R.; Ponomarev, D.; Zhirkov, I. Obtaining Silicon Oxide Nanoparticles Doped with Fluorine and Gold Particles by the Pulsed Plasma-Chemical Method. J. Nanotechnol. 2019, 2019, 7062687. [Google Scholar] [CrossRef] [Green Version]


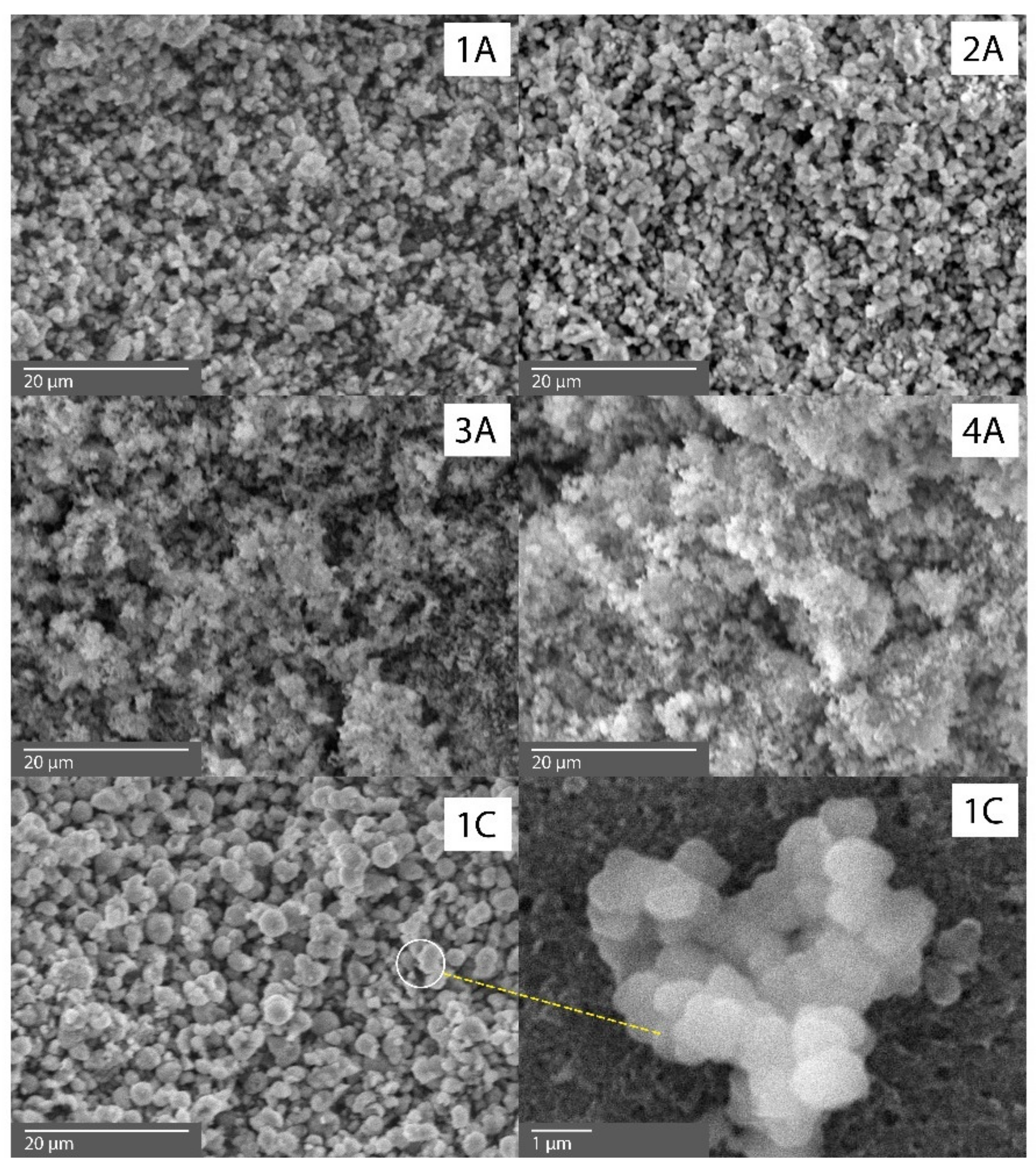
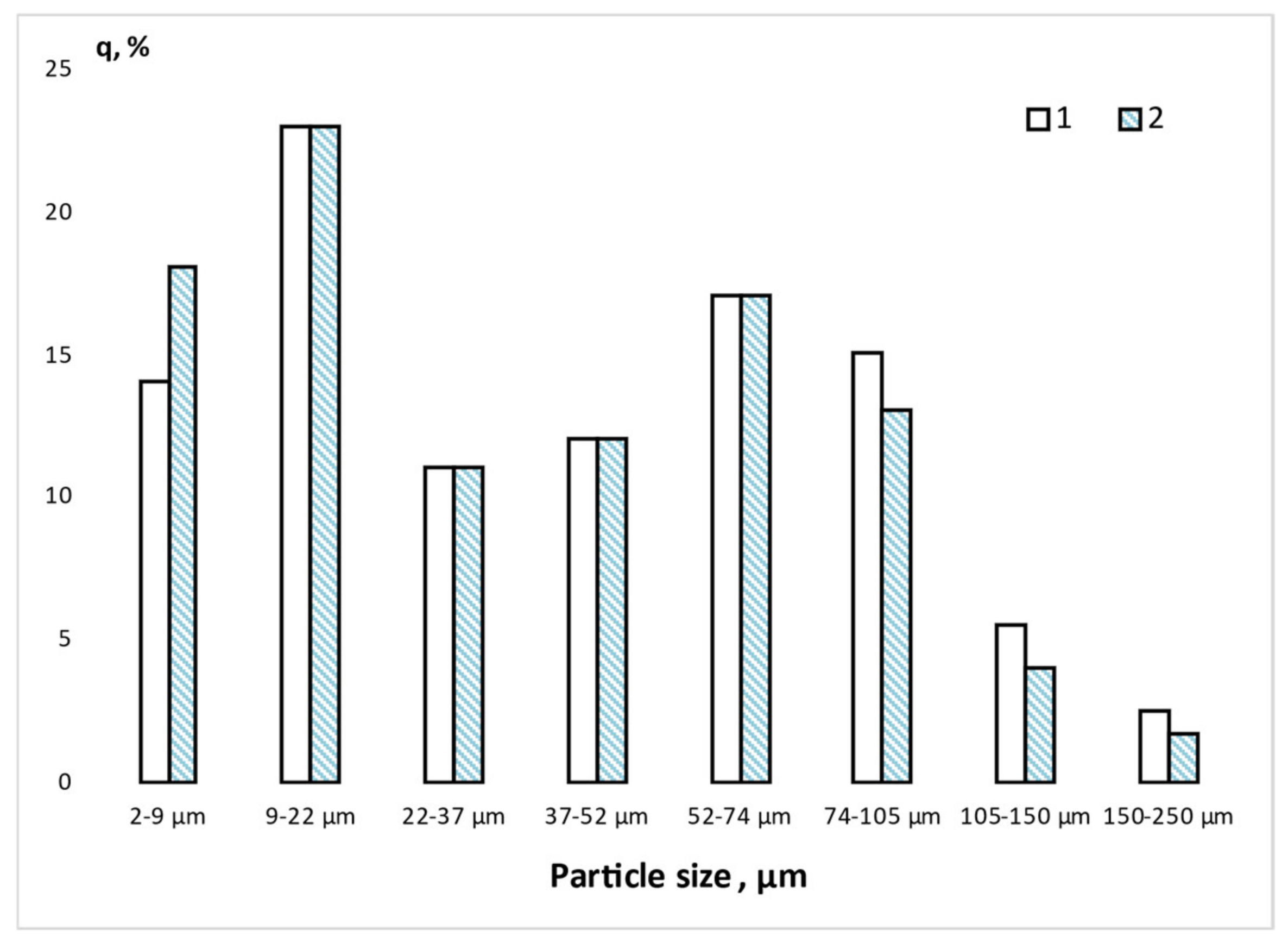
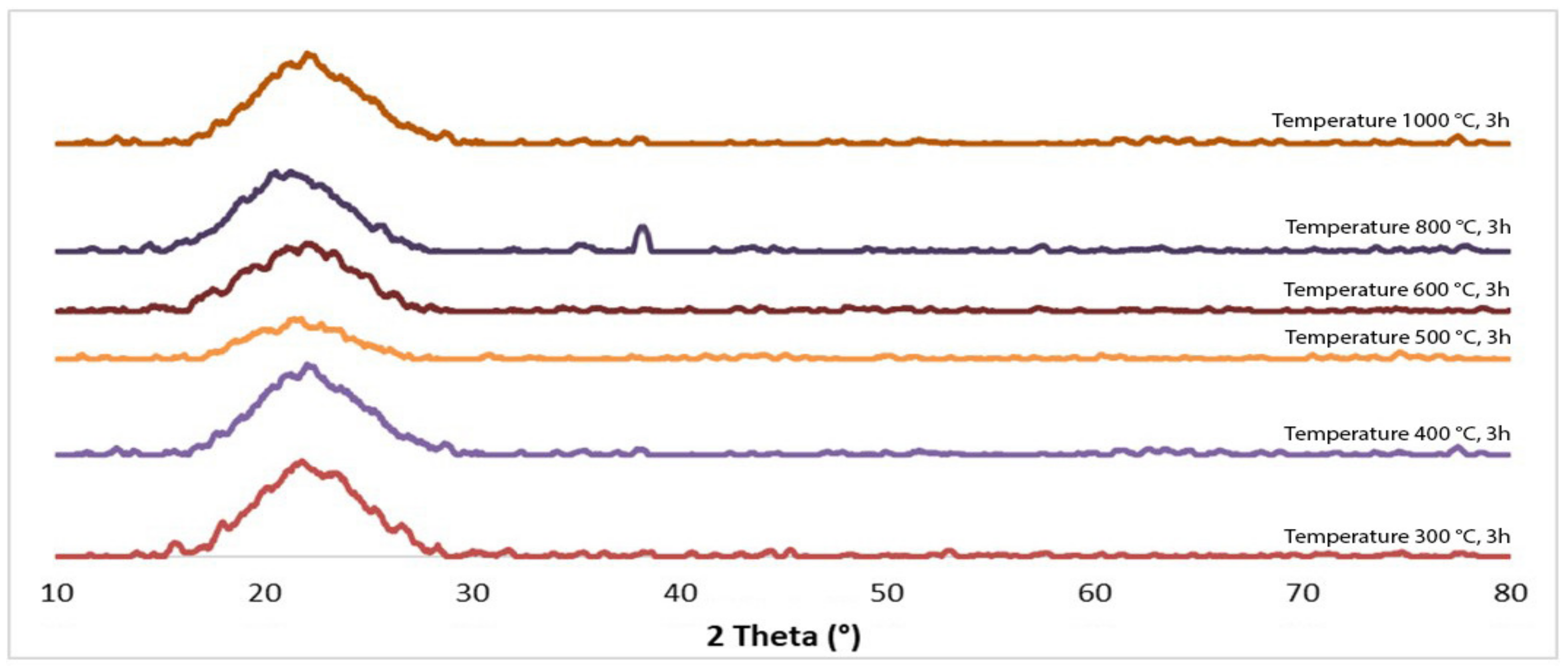

| Component | SiO2 | AlF3 | H2O | SO3 | Cl− | CaO | Fe2O3 | К2О |
|---|---|---|---|---|---|---|---|---|
| Content, wt.% | 76.35 | 20.50 | 3.00 | 0.08 | 0.02 | 0.03 | 0.01 | 0.01 |
| Sample Index | Change in Mass of the Solid Phase after Leaching, % | Reagent Name | Reagent Concentration, % | Liquid-to-Solid Ratio |
|---|---|---|---|---|
| 1А | 9 | NaOH | 0.5 | 10 |
| 2А | 10 | 1.0 | ||
| 3А | 68 | 5.0 | ||
| 4А | 74 | 10.0 | ||
| 5А | 85 | 25.0 | ||
| 1В | 30 | HCl | 0.1 | |
| 2В | 31 | 0.2 | ||
| 3В | 31 | 0.5 | ||
| 4В | 31 | 1.0 | ||
| 1С | 32 | H2SO4 | 0.1 | |
| 2С | 32 | 0.3 | ||
| 3С | 32 | 0.5 | ||
| 4С | 32 | 1.0 |
| Component | Content of Components in Silica Gel after Processing, % | ||
|---|---|---|---|
| 1А (NaOH—0.5 wt.%) | 2А (NaОН—1.0 wt.%) | 3А (NaОН—5.0 wt.%) | |
| SiO2 | 77.81 | 89.30 | 45.50 |
| Al2O3 | 7.25 | 6.99 | 22.42 |
| F | 11.86 | - | - |
| Na2О | 3.08 | 3.71 | 15.00 |
| Component | Content of Components in Silica Gel after Processing, % | |||
|---|---|---|---|---|
| 1В (HCl—0.1 wt.%) | 2С (HCl—0.3 wt.%) | 3С (HCl—0.5 wt.%) | 4С (HCl—1.0 wt.%) | |
| SiO2 | 97.35 | 98.85 | 99.23 | 99.61 |
| Al2O3 | 0.84 | 0.76 | 0.52 | 0.35 |
| F | 1.79 | 0.35 | 0.18 | - |
| CаO | 0.02 | 0.02 | 0.04 | 0.02 |
| Fe2O3 | - | 0.02 | 0.03 | 0.02 |
| Component | Content of Components in Silica Gel after Processing, % | |||
|---|---|---|---|---|
| 1С (H2SO4—0.1%) | 2С (H2SO4—0.3%) | 3С (H2SO4—0.5%) | 4С (H2SO4—1.0%) | |
| SiO2 | 98.35 | 98.73 | 99.78 | 99.88 |
| Al2O3 | 0.54 | 0.27 | 0.15 | 0.06 |
| F | 1.09 | 0.96 | - | - |
| CаO | 0.02 | 0.02 | 0.04 | 0.02 |
| Fe2O3 | - | 0.02 | 0.03 | 0.02 |
| Sample Number | The Composition of the Charge | Peptizer | |||||
|---|---|---|---|---|---|---|---|
| Binder | Filler | ||||||
| AHO Type | Content in the Charge, wt.% | dgrains, μm | Type | Content in the Charge, wt.% | dgrains, μm | ||
| 0 (Al2O3-100) | PAHO | 100 | Up to 40 | SiO2 | 0 | Up to 50 | Nitric acid (HNO3) |
| 1 (Al2O3-80:SiO2-20) | 80 | 20 | |||||
| 2 (Al2O3-60:SiO2-40) | 60 | 40 | |||||
| 3 (Al2O3-35:SiO2-65) | 35 | 65 | |||||
| 4 (Al2O3-20:SiO2-80) | 20 | 80 | |||||
| 5 (Al2O3-15:SiO2-85) | 15 | 85 | |||||
| Sample Number | Strength of Samples | Granule Diameter after Heat, mm | The Total Degree of Shrinkage of Granules in Diameter after Heating, % | Moisture Capacity, сm3/g |
|---|---|---|---|---|
| kg/сm2 | ||||
| Heat treatment at 550 °С | ||||
| 0 (Al2O3-100) | 90 | 1.6 | 20.0 | 0.46 |
| 1 (Al2O3-80:SiO2-20) | 60 | 3.46 | 30.8 | 0.46 |
| 2 (Al2O3-60:SiO2-40) | 88 | 3.50 | 30.0 | 0.47 |
| 3 (Al2O3-35:SiO2-65) | 68 | 3.63 | 27.4 | 0.50 |
| 4 (Al2O3-20:SiO2-80) | 62 | 3.35 | 33.0 | 0.56 |
| 5 (Al2O3-15:SiO2-85) | 50 | 3.66 | 26.8 | 0.58 |
| Heat treatment at 750 °С | ||||
| 0 (Al2O3-100) | 95 | 3.23 | 35.4 | 0.50 |
| 1 (Al2O3-80:SiO2-20) | 85 | 3.40 | 32.0 | 0.50 |
| 2 (Al2O3-60:SiO2-40) | 73 | 3.50 | 30.0 | 0.48 |
| 3 (Al2O3-35:SiO2-65) | 65 | 3.67 | 26.6 | 0.53 |
| 5 (Al2O3-20:SiO2-80) | 65 | 3.33 | 33.4 | 0.55 |
| Heat treatment at 900 °С | ||||
| 0 (Al2O3-100) | 95 | 3.21 | 35.8 | 0.42 |
| 1 (Al2O3-80:SiO2-20) | 89 | 3.35 | 33.0 | 0.43 |
| 2 (Al2O3-60:SiO2-40) | 84 | 3.40 | 32.0 | 0.45 |
| 3 (Al2O3-35:SiO2-65) | 81 | 3.59 | 28.2 | 0.46 |
| 5 (Al2O3-20:SiO2-80) | 57 | 3.38 | 32.4 | 0.49 |
| Heat treatment at 1150 °С | ||||
| 0 (Al2O3-100) | 120 | 2.89 | 42.2 | 0.31 |
| 1 (Al2O3-80:SiO2-20) | 92 | 3.19 | 36.2 | 0.37 |
| 2 (Al2O3-60:SiO2-40) | 78 | 3.26 | 34.8 | 0.37 |
| 3 (Al2O3-35:SiO2-65) | 83 | 3.53 | 29.4 | 0.42 |
| 5 (Al2O3-20:SiO2-80) | 81 | 3.30 | 34.0 | 0.49 |
| Name | Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| 550 °С | 750 °С | 900 °С | 1150 °С | |||||
| Pore Size, Ǻ | Pore vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % |
| 0–40 | 0.014 | 3.00 | 0.001 | 0.27 | 0.000 | 0.00 | 0.0002 | 0.06 |
| 40–50 | 0.059 | 12.68 | 0.015 | 3.13 | 0.004 | 0.83 | 0.0013 | 0.38 |
| 50–60 | 0.031 | 6.75 | 0.017 | 3.65 | 0.005 | 1.21 | 0.0019 | 0.54 |
| 60–70 | 0.109 | 23.27 | 0.062 | 13.09 | 0.025 | 5.61 | 0.0155 | 4.33 |
| 70–100 | 0.084 | 17.95 | 0.169 | 35.47 | 0.114 | 25.31 | 0.0301 | 8.40 |
| 100–240 | 0.014 | 3.09 | 0.052 | 11.03 | 0.155 | 34.36 | 0.1027 | 28.63 |
| 240–380 | 0.004 | 0.90 | 0.005 | 0.97 | 0.010 | 2.11 | 0.0198 | 5.52 |
| 380–1000 | 0.005 | 1.00 | 0.007 | 1.56 | 0.005 | 1.15 | 0.0042 | 1.16 |
| >1000 | 0.147 | 31.36 | 0.147 | 30.83 | 0.132 | 29.42 | 0.1830 | 50.98 |
| Pore volume in H2O, сm3/g | 0.47 | 0.48 | 0.45 | 0.36 | ||||
| Specific surface area (BJH method), m2/g | 190.7 | 148.5 | 107.5 | 50.1 | ||||
| Name | Samples Heat-Treated at 550 °С | |||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3-80:SiO2-20 | Al2O3-60:SiO2-40 | Al2O3-35:SiO2-65 | Al2O3-20:SiO2-80 | |||||
| Pore Size. Ǻ | Pore Vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % | Pore Vol., сm3/g | Vol. Fraction, % |
| 0–40 | 0.022 | 4.85 | 0.014 | 3.00 | 0.016 | 3.14 | 0.014 | 2.42 |
| 40–50 | 0.078 | 16.88 | 0.059 | 12.68 | 0.041 | 8.20 | 0.034 | 6.02 |
| 50–60 | 0.065 | 13.95 | 0.032 | 6.75 | 0.031 | 6.17 | 0.042 | 7.54 |
| 60–70 | 0.139 | 30.13 | 0.109 | 23.27 | 0.055 | 11.08 | 0.014 | 2.48 |
| 70–100 | 0.100 | 21.74 | 0.084 | 17.95 | 0.032 | 6.48 | 0.015 | 2.75 |
| 100–240 | 0.015 | 3.44 | 0.015 | 3.09 | 0.009 | 1.89 | 0.009 | 1.52 |
| 240–380 | 0.005 | 0.94 | 0.004 | 0.90 | 0.005 | 0.91 | 0.005 | 0.83 |
| 380–1000 | 0.003 | 0.64 | 0.005 | 1.00 | 0.003 | 0.68 | 0.005 | 0.88 |
| >1000 | 0.034 | 7.41 | 0.147 | 31.36 | 0.308 | 61.45 | 0.423 | 75.56 |
| Pore volume in H2O, сm3/g | 0.46 | 0.47 | 0.50 | 0.56 | ||||
| Specific surface area (BJH method), m2/g | 260.9 | 190.7 | 119.4 | 86.8 | ||||
| No. | Raw Material Type | g Н2О/g Al2O3 | Assessment of the Molded Paste | Properties of Cylindrical Granules after Heat Treatment at 550 °C | ||
|---|---|---|---|---|---|---|
| Specific Surface, m2/g | Pore Vol., сm3/g | Mechanical Crushing Strength, kg/сm2 | ||||
| 1 | PAHO (up to 87 wt.% pseudoboehmite). | 1.06 | Solid paste, highly plastic, excellent molding | 320 | 0.63 | 84 |
| 2 | Pural SB1 (up to 95 wt.% well-crystallized boehmite). | 1.23 | The paste is very dense, rubbery, translucent, good molding | 230 | 0.48 | 24 |
| 3 | AHO obtained by precipitation of sodium aluminate with nitric acid at 50 °C (up to 85 wt.% poorly crystallized boehmite). | 1.38 | Low-plasticity paste, thixotropic, difficult to mold | 200 | 0.98 | 10 |
| 4 | AHO mixture obtained by precipitation of sodium aluminate with nitric acid at 20 °C and at 100 °C (up to 80 wt.% well-crystallized boehmite). | 0.95 | Low-plasticity paste, soft, difficult to mold | - | 0.70 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyagay, I.N.; Shaidulina, A.A.; Konoplin, R.R.; Artyushevskiy, D.I.; Gorshneva, E.A.; Sutyaginsky, M.A. Production of Amorphous Silicon Dioxide Derived from Aluminum Fluoride Industrial Waste and Consideration of the Possibility of Its Use as Al2O3-SiO2 Catalyst Supports. Catalysts 2022, 12, 162. https://doi.org/10.3390/catal12020162
Pyagay IN, Shaidulina AA, Konoplin RR, Artyushevskiy DI, Gorshneva EA, Sutyaginsky MA. Production of Amorphous Silicon Dioxide Derived from Aluminum Fluoride Industrial Waste and Consideration of the Possibility of Its Use as Al2O3-SiO2 Catalyst Supports. Catalysts. 2022; 12(2):162. https://doi.org/10.3390/catal12020162
Chicago/Turabian StylePyagay, Igor N., Alina A. Shaidulina, Rostislav R. Konoplin, Dmitriy I. Artyushevskiy, Ekaterina A. Gorshneva, and Michail A. Sutyaginsky. 2022. "Production of Amorphous Silicon Dioxide Derived from Aluminum Fluoride Industrial Waste and Consideration of the Possibility of Its Use as Al2O3-SiO2 Catalyst Supports" Catalysts 12, no. 2: 162. https://doi.org/10.3390/catal12020162
APA StylePyagay, I. N., Shaidulina, A. A., Konoplin, R. R., Artyushevskiy, D. I., Gorshneva, E. A., & Sutyaginsky, M. A. (2022). Production of Amorphous Silicon Dioxide Derived from Aluminum Fluoride Industrial Waste and Consideration of the Possibility of Its Use as Al2O3-SiO2 Catalyst Supports. Catalysts, 12(2), 162. https://doi.org/10.3390/catal12020162





