Electrochemical Degradation of Nitrobenzene Wastewater: From Laboratory Experiments to Pilot-Scale Industrial Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Anode
2.2. Electrochemical Degradation Kinetics of NB
2.2.1. Effect of Applied Current Density
2.2.2. Effect of Electrode Distance and pH
2.2.3. Effect of Initial NB Concentration
2.3. The Mineralization of NB under the Optimum Conditions
2.3.1. Evolution of TOC and Nitrogen-Containing Final Products
2.3.2. Qualitative and Quantitative Determination of •OH
2.3.3. Intermediate Analysis and Degradation Pathway
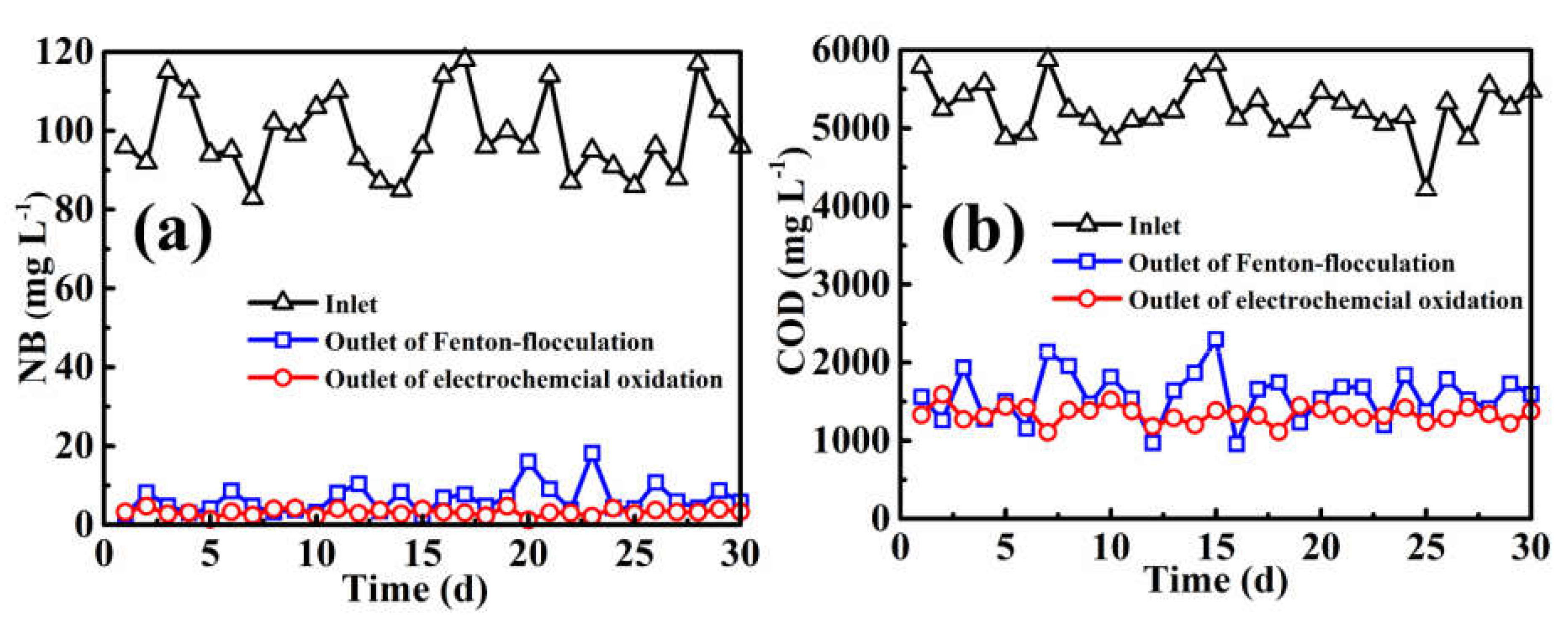
2.4. Pilot-Scale Industrial Application
3. Materials and Methods
3.1. Laboratory Experiments
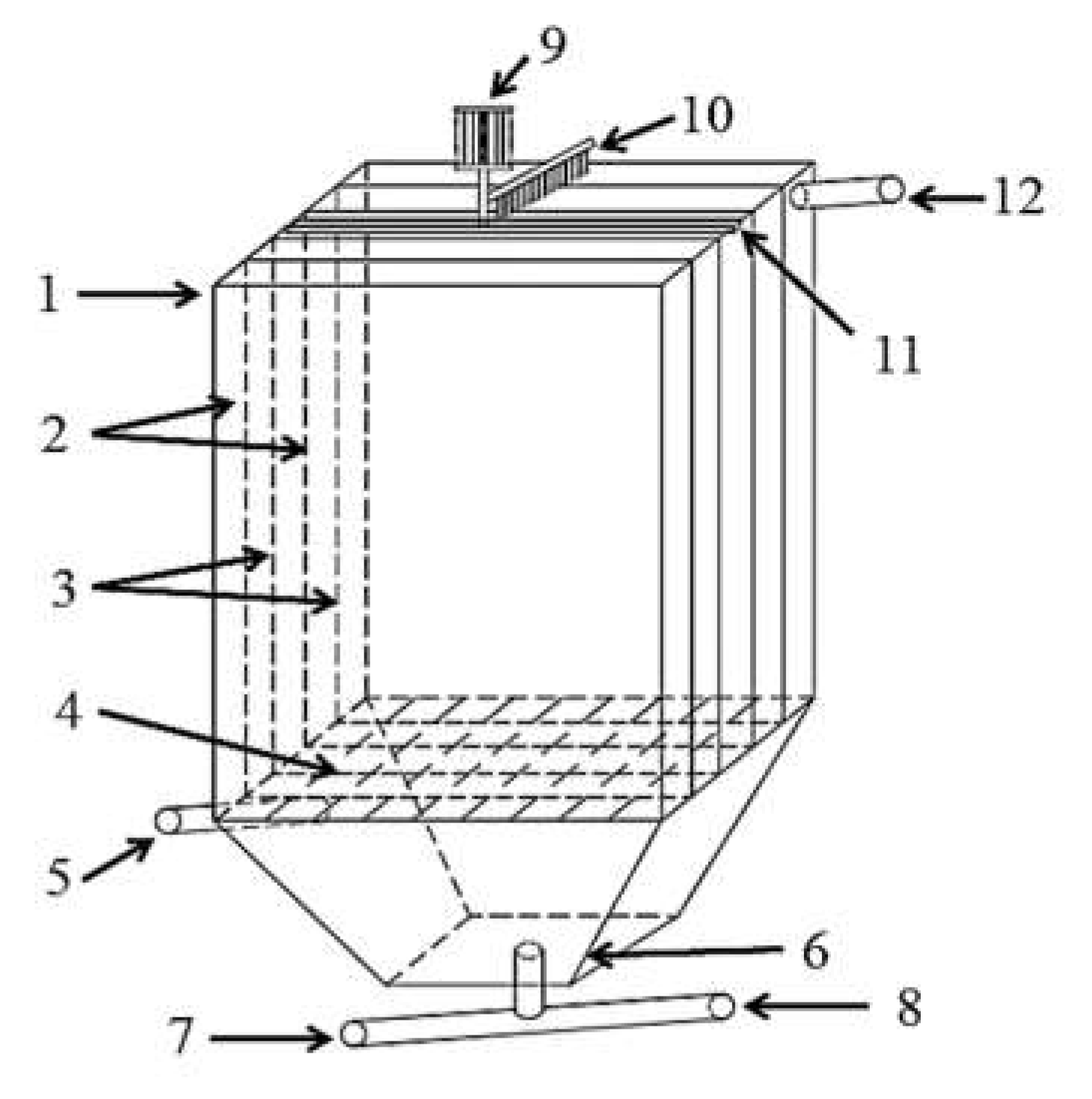
3.2. Pilot-Scale Industrial Application
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, T.; Wu, S.; Hong, H.; Chen, L.; Dong, R. Dynamics of nitrobenzene degradation and interactions with nitrogen transformations in laboratory-scale constructed wetlands. Bioresour. Technol. 2013, 133, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Fu, J.; Du, P.; Zhao, X.; Hao, X.; Liu, W.; Zhao, D. Reduction of nitrobenzene in aqueous and soil phases using carboxymethyl cellulose stabilized zero-valent iron nanoparticles. Chem. Eng. J. 2018, 332, 227–236. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Tian, P.; Sheng, Y.; Xu, J.; Han, Y. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019, 244, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, C.; Lu, J.; Hu, C.; Peng, S.; Chen, T. Electro-catalytic degradation of bisphenol A with modified Co3O4/β-PbO2/Ti electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Hao, X.; Zhou, M.; Xin, Q.; Lei, L. Pulsed discharge plasma induced Fenton-like reactions for the enhancement of the degradation of 4-chlorophenol in water. Chemosphere 2007, 66, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Dong, B.; Zhou, J.; Wang, J.; Jin, R.; Li, J. Enhanced bioreduction of nitrobenzene by reduced graphene oxide materials: Effects of surface modification and coexisting soluble electron shuttles. Environ. Sci. Pollut. Res. 2017, 24, 26874–26880. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Li, J.; Xiong, Z.; Lai, B. Enhanced reactivity of microscale Fe/Cu bimetallic particles (mFe/Cu) with persulfate (PS) for p-nitrophenol (PNP) removal in aqueous solution. Chemosphere 2017, 172, 10–20. [Google Scholar] [CrossRef]
- Gao, J.; Liu, L.; Liu, X.; Zhou, H.; Wang, Z.; Huang, S. Concentration level and geographical distribution of nitrobenzene in Chinese surface waters. J. Environ. Sci. 2008, 20, 803–805. [Google Scholar] [CrossRef]
- Han, Y.; Qi, M.; Zhang, L.; Sang, Y.; Liu, M.; Zhao, T.; Niu, J.; Zhang, S. Degradation of nitrobenzene by synchronistic oxidation and reduction in an internal circulation microelectrolysis reactor. J. Hazard. Mater. 2019, 365, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.; Won, Y.; Hwang, I.; Tayade, R.J. Enhanced photocatalytic degradation of aqueous nitrobenzene using graphitic carbon–TiO2 composites. Ind. Eng. Chem. Res. 2014, 53, 3455–3461. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Liang, C.; Chang, H. Synthesis of graphene-ZnO-Au nanocomposites for efficient photocatalytic reduction of nitrobenzene. Environ. Sci. Technol. 2013, 47, 6688–6695. [Google Scholar] [CrossRef]
- Yen, J.; Lin, K.; Wang, Y. Acute lethal toxicity of environmental pollutants to aquatic organisms. Ecotoxol. Environ. Saf. 2002, 52, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Bonacker, D.; Stoiber, T.; Böhm, K.J.; Unger, E.; Degen, G.H.; Thier, R.; Bolt, H.M. Chromosomal genotoxicity of nitrobenzene and benzonitrile. Arch. Toxicol. 2004, 78, 49–57. [Google Scholar] [PubMed]
- U.S. Environmental Protection Agency. Ambient Water Quality Criteria for Nitrobenzene; U.S. Environmental Protection Agency: Washington, DC, USA, 1980; Volume 10.
- U.S. Environmental Protection Agency. Guidelines for Carcinogen Risk Assessment—Federal Register; U.S. Environmental Protection Agency: Washington, DC, USA, 2005; Volume 804, pp. 636–640.
- State Environmental Protection Administration. Environmental Quality Standard for Surface Water; Regulation GHZB1-1999; State Environmental Protection Administration: Beijing, China, 1999. [Google Scholar]
- Chen, Y.; Li, H.; Liu, W.; Tu, Y.; Zhang, Y.; Han, W.; Wang, L. Electrochemical degradation of nitrobenzene by anodic oxidation on the constructed TiO2-NTs/SnO2-Sb/PbO2 electrode. Chemosphere 2014, 113, 48–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, K.; Dai, C.; Zhou, X.; Si, H. An enhanced Fenton reaction catalyzed by natural heterogeneous pyrite for nitrobenzene degradation in an aqueous solution. Chem. Eng. J. 2014, 244, 438–445. [Google Scholar] [CrossRef]
- Chen, C.; Yan, X.; Yoza, B.A.; Zhou, T.; Li, Y.; Zhan, Y.; Wang, Q.; Li, Q.X. Efficiencies and mechanisms of ZSM5 zeolites loaded with cerium, iron, or manganese oxides for catalytic ozonation of nitrobenzene in water. Sci. Total Environ. 2018, 612, 1424–1432. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sui, M.; Zhang, T.; Guan, C. Effect of pH on MnOx/GAC catalyzed ozonation for degradation of nitrobenzene. Water Res. 2005, 39, 779–786. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, Z.; Ma, J. Novel relationship between hydroxyl radical initiation and surface group of ceramic honeycomb supported metals for the catalytic ozonation of nitrobenzene in aqueous solution. Environ. Sci. Technol. 2009, 43, 4157–4163. [Google Scholar] [CrossRef]
- Nitoi, I.; Oancea, P.; Raileanu, M.; Crisan, M.; Constantin, L.; Cristea, I. UV–VIS photocatalytic degradation of nitrobenzene from water using heavy metal doped titania. J. Ind. Eng. Chem. 2015, 21, 677–682. [Google Scholar] [CrossRef]
- Shen, X.; Zhu, L.; Wang, N.; Zhang, T.; Tang, H. Selective photocatalytic degradation of nitrobenzene facilitated by molecular imprinting with a transition state analog. Catal. Today 2014, 225, 164–170. [Google Scholar] [CrossRef]
- ElShafei, G.M.; Yehia, F.Z.; Dimitry, O.; Badawi, A.M.; Eshaq, G. Degradation of nitrobenzene at near neutral pH using Fe2+–glutamate complex as a homogeneous Fenton catalyst. Appl. Catal. B Environ. 2010, 99, 242–247. [Google Scholar] [CrossRef]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the anode materials on the electrochemical oxidation efficiency. Application to oxidative degradation of the pharmaceutical amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Wang, C.; Yu, Y.; Yin, L.; Niu, J.; Hou, L. Insights of ibuprofen electro-oxidation on metal-oxide-coated Ti anodes: Kinetics, energy consumption and reaction mechanisms. Chemosphere 2016, 163, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, J.; Xu, J.; Huang, H.; Li, D.; Yue, Z.; Feng, C. Highly efficient and mild electrochemical mineralization of long-chain perfluorocarboxylic acids (C9–C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD electrodes. Environ. Sci. Technol. 2013, 47, 13039–13046. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.; Xu, J.; Wu, H.; Li, Y. Electrochemical mineralization of perfluorocarboxylic acids (PFCAs) by Ce-doped modified porous nanocrystalline PbO2 film electrode. Environ. Sci. Technol. 2012, 46, 10191–10198. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, J.; Xu, J.; Li, Y.; Pan, Y. Electrochemical mineralization of sulfamethoxazole by Ti/SnO2-Sb/Ce-PbO2 anode: Kinetics, reaction pathways, and energy cost evolution. Electrochim. Acta. 2013, 97, 167–174. [Google Scholar] [CrossRef]
- Xu, P.; He, X.; Mao, J.; Tang, Y. Fabrication of Ti/SnO2 -Sb/Ce-PbO2 anode from methanesulfonate bath and its electrocatalytic activity. J. Electrochem. Soc. 2019, 166, D638–D644. [Google Scholar] [CrossRef]
- Katsaounis, A.; Souentie, S. Organic pollutants in water using DSA electrodes, in-cell mediated (via active chlorine) electrochemical oxidation. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1407–1416. [Google Scholar]
- Panizza, M.; Cerisola, G. Electrocatalytic materials for the electrochemical oxidation of synthetic dyes. Appl. Catal. B Environ. 2007, 75, 95–101. [Google Scholar] [CrossRef]
- Kitazono, Y.; Ihara, I.; Yoshida, G.; Toyoda, K.; Umetsu, K. Selective degradation of tetracycline antibiotics present in raw milk by electrochemical method. J. Hazard. Mater. 2012, 243, 112–116. [Google Scholar] [CrossRef]
- Xu, L.; Niu, J.; Xie, H.; Ma, X.; Zhu, Y.; Crittenden, J. Effective degradation of aqueous carbamazepine on a novel blue-colored TiO2 nanotube arrays membrane filter anode. J. Hazard. Mater. 2021, 402, 123530. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.; Ding, G.; Wang, H. Electrochemical degradation characteristics of refractory organic pollutants in coking wastewater on multiwall carbon nanotube-modified electrode. J. Nanomater. 2012, 2012, 614032. [Google Scholar] [CrossRef]
- Wang, C.; Niu, J.; Yin, L.; Huang, J.; Hou, L.A. Electrochemical degradation of fluoxetine on nanotube array intercalated anode with enhanced electronic transport and hydroxyl radical production. Chem. Eng. J. 2018, 346, 662–671. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Z.; Chen, X. Effects of experimental parameters on 2,4-dichlorphenol degradation over Er-chitosan-PbO2 electrode. J. Hazard. Mater. 2010, 178, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xia, Y.; Sun, C.; Weng, M.; Chen, J.; Wang, J.; Chen, J. Electrochemical degradation of levodopa with modified PbO2 electrode: Parameter optimization and degradation mechanism. Chem. Eng. J. 2014, 245, 359–366. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Rodrigo, M.A.; Sires, I.; Scialdone, O. Single and coupled electrochemical processes and reactors for the abatement of organic water pollutants: A critical review. Chem. Rev. 2015, 115, 13362–13407. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data. 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Clifton, C.L.; Huie, R.E. Rate constants for hydrogen abstraction reactions of the sulfate radical, SO4−. Alcohols. Int. J. Chem. Kinet. 1989, 21, 677–687. [Google Scholar] [CrossRef]
- Neta, P.; Madhavan, V.; Zemel, H.; Fessenden, R.W. Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds. J. Am. Chem. Soc. 1977, 99, 163–164. [Google Scholar] [CrossRef]
- Kavitha, V.; Palanivelu, K. Degradation of nitrophenols by Fenton and photo-Fenton processes. J. Photochem. Photobiol. A Chem. 2005, 170, 83–95. [Google Scholar] [CrossRef]
- Zhang, L.; Shu, H.; Jia, Y.; Xu, D. Study on solid waste pyrolysis coke catalyst for catalytic cracking of coal tar. Int. J. Hydrog. Energ. 2020, 45, 19280–19290. [Google Scholar]
- Lei, Z.; Gao, H.; Chang, X.; Zhang, L.; Wang, Y. An application of green surfactant synergistically metal supported cordierite catalyst in denitration of selective catalytic oxidation. J. Clean. Prod. 2019, 249, 119307. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Bo, G.; Wu, S.; Luo, L. Degradation of pollutants in polluted river water using Ti/IrO2-Ta2O5 coating electrode and evaluation of electrode characteristics. J. Clean. Prod. 2020, 273, 123019. [Google Scholar] [CrossRef]
- Ramírez, J.I.D.L.; Villegas, V.A.R.; Sicairos, S.P.; Guevara, E.H.; Brito Perea, M.D.C.; Sánchez, B.L. Synthesis and characterization of zinc peroxide nanoparticles for the photodegradation of nitrobenzene assisted by UV-light. Catalysts 2020, 10, 1041. [Google Scholar] [CrossRef]
- Qin, C.; Zhang, J.; Zhang, C.; He, Y.; Tratnyek, P.G. Abiotic transformation of nitrobenzene by zero valent iron under aerobic conditions: Relative contributions of reduction and oxidation in the presence of ethylene diamine tetraacetic acid. Environ. Sci. Technol. 2021, 55, 6828–6837. [Google Scholar] [CrossRef] [PubMed]

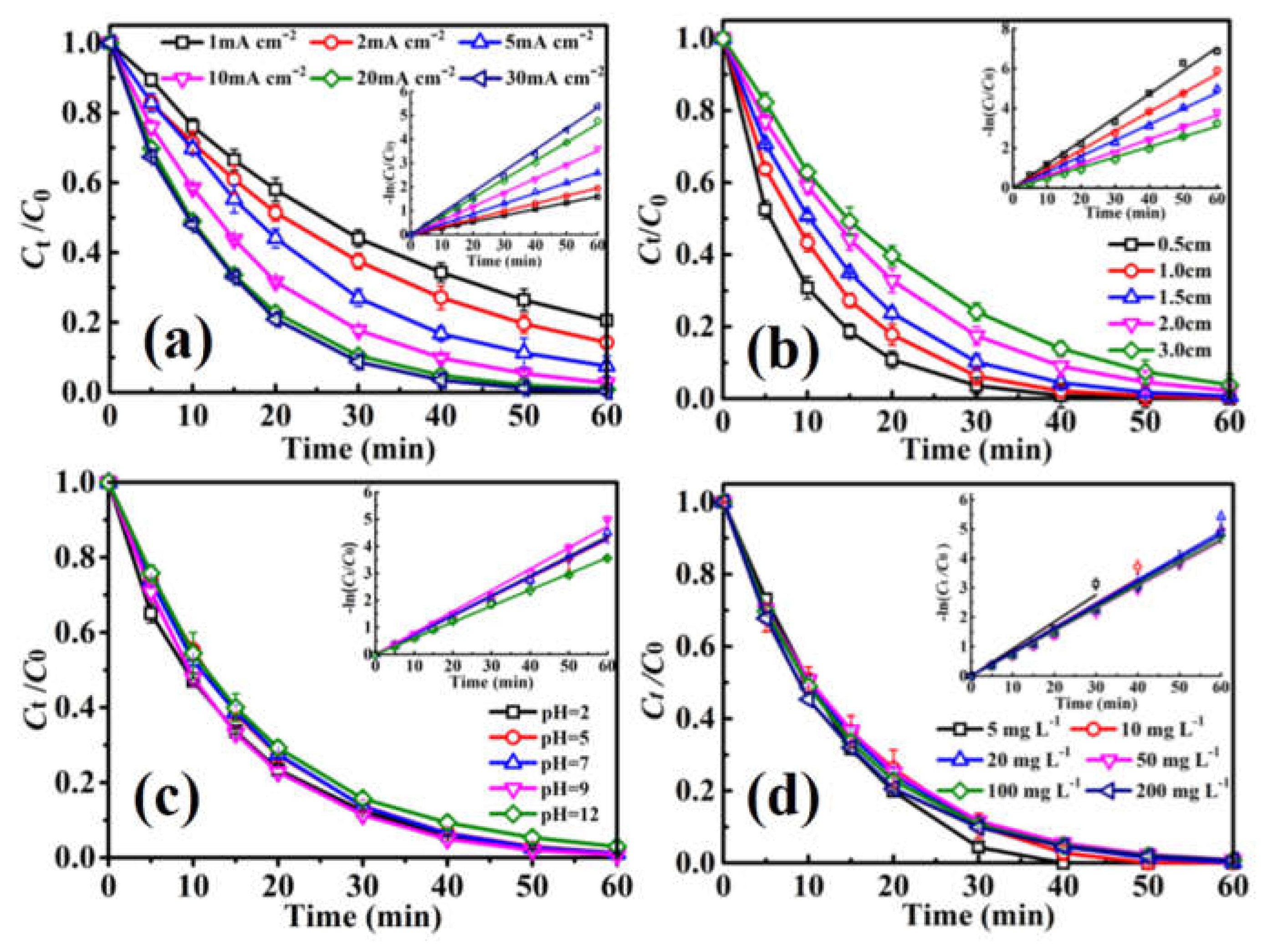

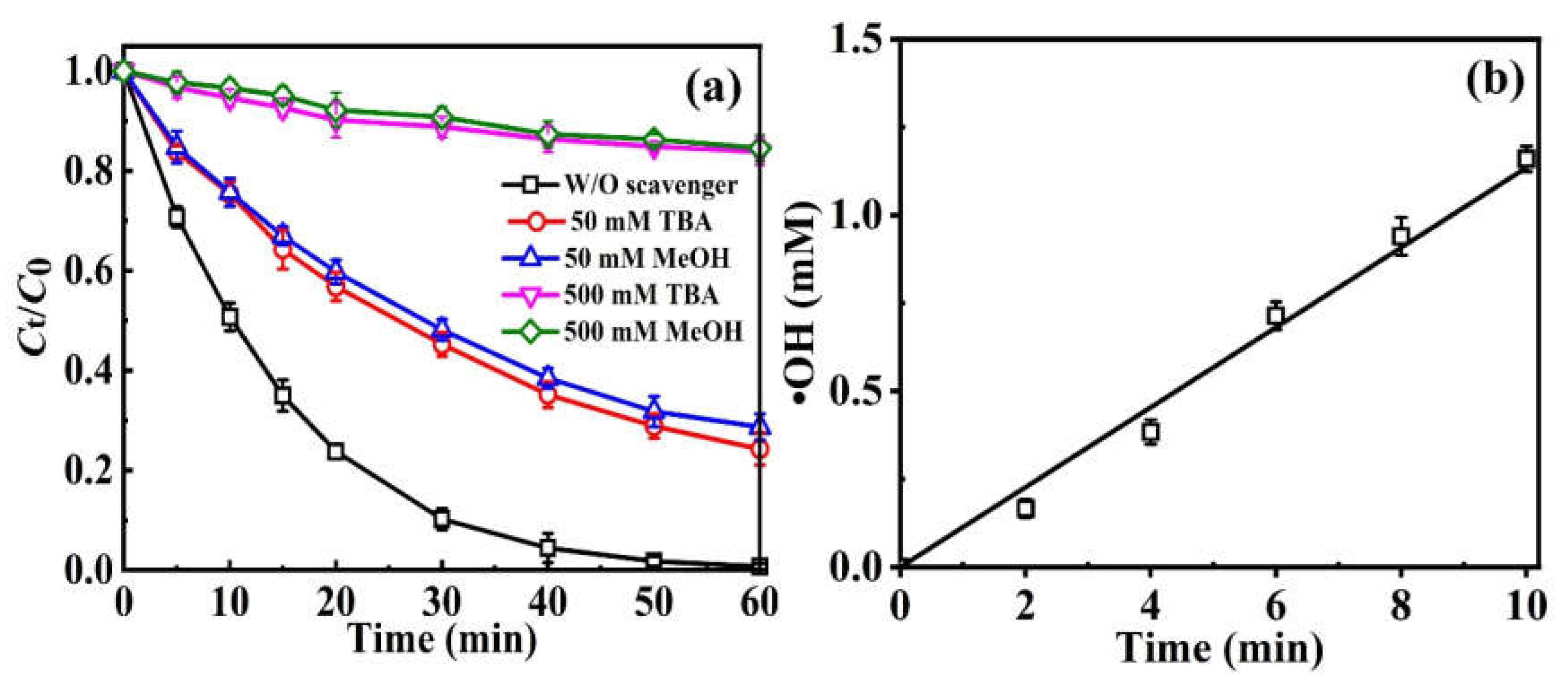
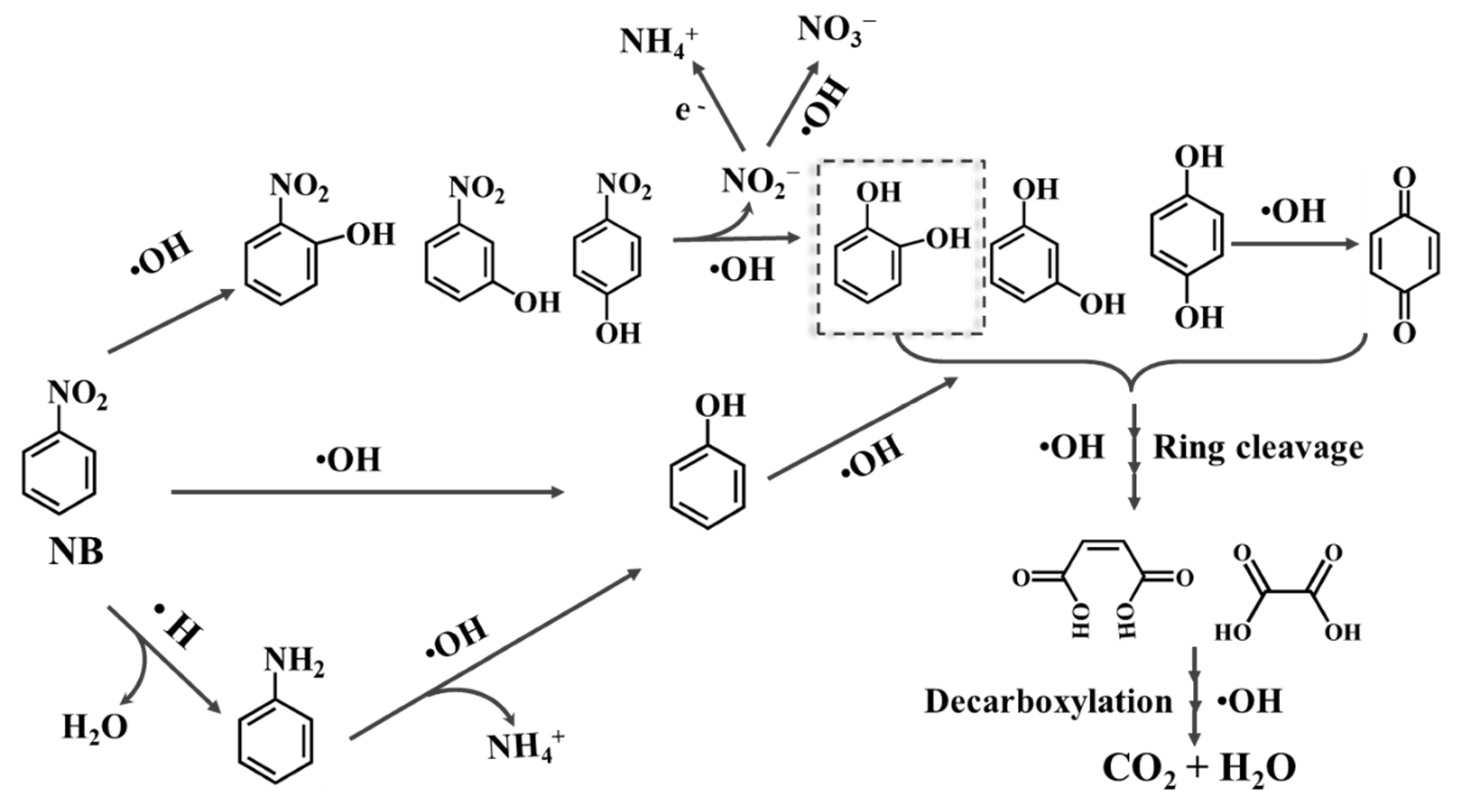
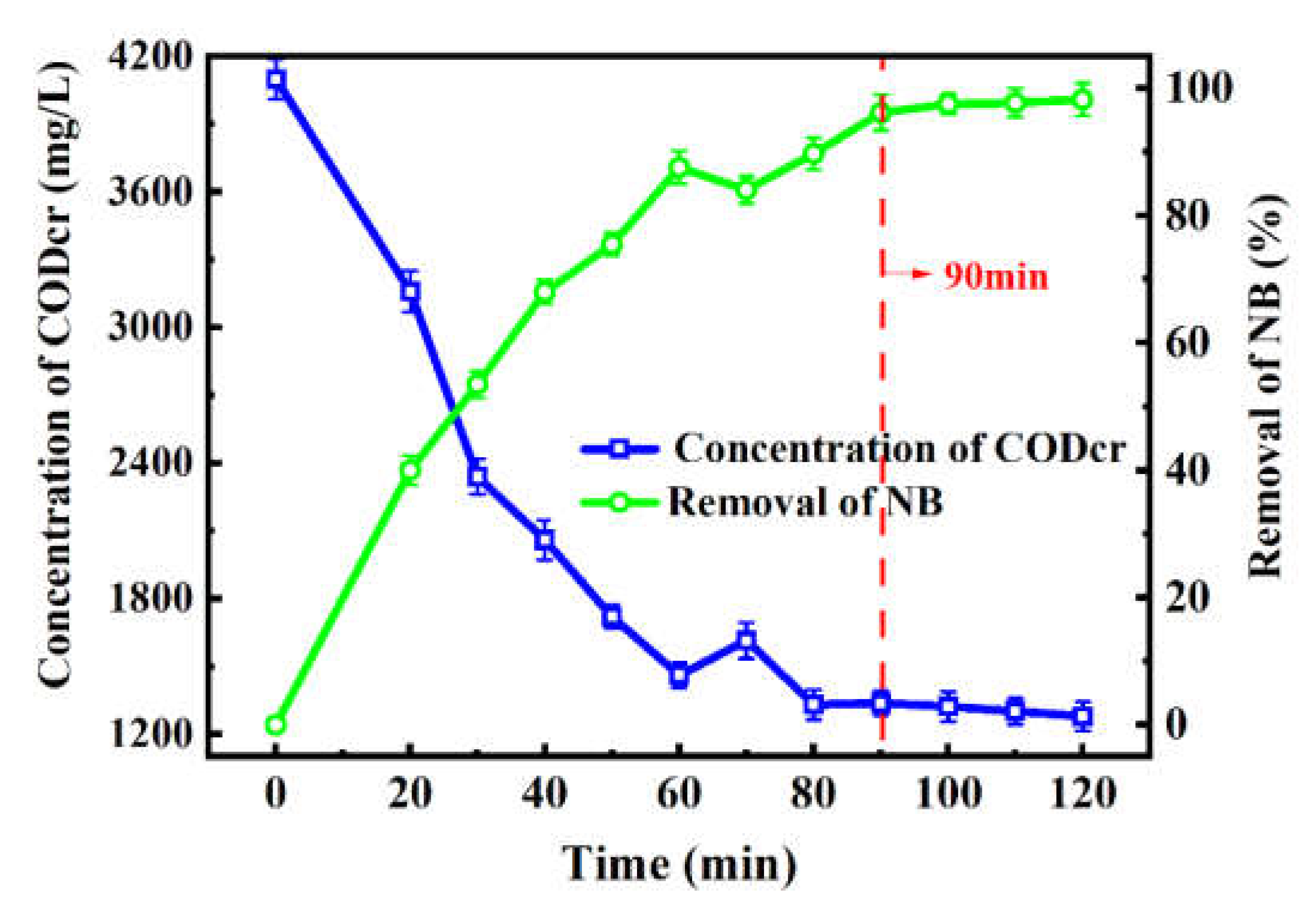
| Parameters | Rate Constant (k, min−1) | Half-Life (min) | Time Range (min) | Average Voltage (V) | R2 | |
|---|---|---|---|---|---|---|
| Current density (mA cm−2) | 1 | 2.7 × 10−2 | 25.7 | 60 | 3.4 | 0.9998 |
| 2 | 3.3 × 10−2 | 21.0 | 60 | 4.2 | 0.9999 | |
| 5 | 4.5 × 10−2 | 15.4 | 60 | 5.2 | 0.9996 | |
| 10 | 5.9 × 10−2 | 11.7 | 60 | 6.6 | 0.9997 | |
| 20 | 7.8 × 10−2 | 8.9 | 60 | 8.5 | 0.9996 | |
| 30 | 8.7 × 10−2 | 8.0 | 60 | 10.5 | 0.9986 | |
| Electrode distance (cm) | 0.5 | 1.2 × 10−1 | 5.8 | 40 | 5.0 | 0.9989 |
| 1.0 | 9.5 × 10−2 | 7.3 | 50 | 5.9 | 0.9993 | |
| 1.5 | 7.8 × 10−2 | 8.8 | 60 | 8.3 | 0.9990 | |
| 2.0 | 6.1 × 10−2 | 11.4 | 60 | 9.2 | 0.9991 | |
| 3. 0 | 5.2 × 10−2 | 13.3 | 60 | 10.6 | 0.9985 | |
| Initial pH | 2 | 6.6 × 10−2 | 9.6 | 60 | 6.8 | 0.9996 |
| 5 | 7.7 × 10−2 | 9.8 | 60 | 7.9 | 0.9988 | |
| 7 | 7.8 × 10−2 | 9.6 | 60 | 7.7 | 0.9986 | |
| 9 | 7.4 × 10−2 | 8.9 | 60 | 7.8 | 0.9987 | |
| 12 | 5.9 × 10−2 | 11.7 | 60 | 7.5 | 0.9998 | |
| Initial concentration (C0, mg L−1) | 5 | 9.2 × 10−2 | 7.5 | 30 | 9.1 | 0.9887 |
| 10 | 8.5 × 10−2 | 8.3 | 40 | 8.8 | 0.9918 | |
| 20 | 8.2 × 10−2 | 8.4 | 60 | 8.7 | 0.9962 | |
| 50 | 7.7 × 10−2 | 9.0 | 60 | 8.4 | 0.9982 | |
| 100 | 7.7 × 10−2 | 9.0 | 60 | 8.2 | 0.9996 | |
| 200 | 8.0 × 10−2 | 8.7 | 60 | 8.0 | 0.9997 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Liao, Z.; Hu, Z.; Shang, E. Electrochemical Degradation of Nitrobenzene Wastewater: From Laboratory Experiments to Pilot-Scale Industrial Application. Catalysts 2022, 12, 190. https://doi.org/10.3390/catal12020190
Liu D, Liao Z, Hu Z, Shang E. Electrochemical Degradation of Nitrobenzene Wastewater: From Laboratory Experiments to Pilot-Scale Industrial Application. Catalysts. 2022; 12(2):190. https://doi.org/10.3390/catal12020190
Chicago/Turabian StyleLiu, Dunyi, Zhangjiu Liao, Ziyi Hu, and Enxiang Shang. 2022. "Electrochemical Degradation of Nitrobenzene Wastewater: From Laboratory Experiments to Pilot-Scale Industrial Application" Catalysts 12, no. 2: 190. https://doi.org/10.3390/catal12020190
APA StyleLiu, D., Liao, Z., Hu, Z., & Shang, E. (2022). Electrochemical Degradation of Nitrobenzene Wastewater: From Laboratory Experiments to Pilot-Scale Industrial Application. Catalysts, 12(2), 190. https://doi.org/10.3390/catal12020190







