Theoretical Studies of Acetyl-CoA Synthase Catalytic Mechanism
Abstract
:1. Introduction
2. Computational Details
2.1. Methods of Computation
2.2. Redox Potential
2.3. pK Calculation
2.4. pH Dependent Redox Potentials
3. Results
3.1. Structural Properties
3.2. Electronic Structure
3.3. Energetics of Methylation and Carbonylation Reaction
3.4. pK
3.5. Reduction Potentials
3.6. pH-Dependent Reduction Potentials
4. Discussion
5. Conclusions
- Unligated or water ligated oxidized A-clusters have high reduction potential; the HCOO or OH ligands lower the reduction potential making A less susceptible for reduction.
- Protonation takes place at Ni.
- Protonation stabilizes the two-electron reduced A-cluster.
- The pH-dependent reduction potential for the large model with water molecules agrees with the experimental ones.
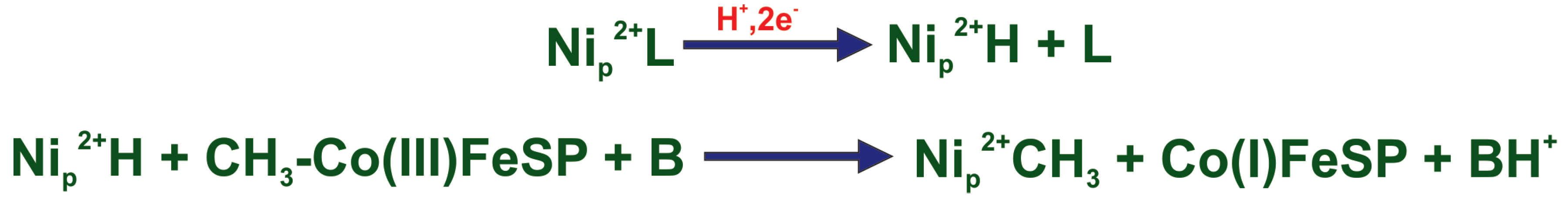
- We propose a mechanism in which two-electron reduction of A–L is coupled to Ni protonation and ligand loss. During methylation reaction Ni is deprotonated by an external base. In the last step, CO binds to the methylated A-cluster and the acetyl group is formed. The role of the external base can be played by a tyrosine residue.
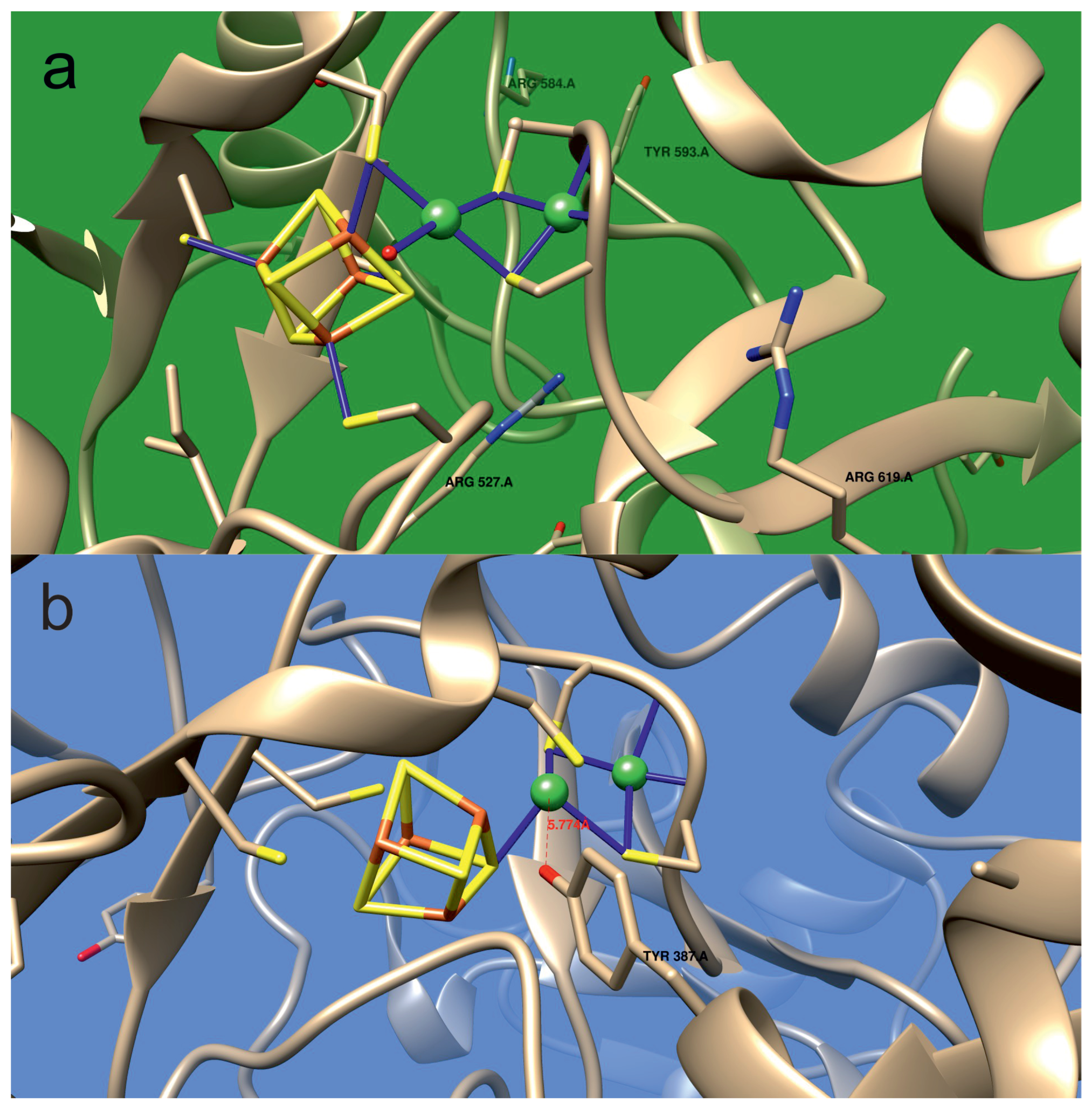
- The arginine residue present in the vicinity of the A-cluster acts as negative ion sink, which facilitates protonation of the A-cluster.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acetyl coenzyme A synthase |
| CoFeSP | Corrinoid Iron-Sulfur Protein |
| Acetyl-CoA | acetyl coenzyme A |
References
- Appel, A.; Bercaw, J.; Bocarsly, A.; Dobbek, H.; Dubois, D.; Dupuis, M.; Ferry, J.; Fujita, E.; Hille, R.; Kenis, P.; et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, G.; Pierce, E.; Hill, J.; Darty, J.; Ragsdale, S. Metal centers in the anaerobic microbial metabolism of CO and CO2. Metallomics 2011, 3, 797–815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragsdale, S. Enzymology of the Wood-Ljungdahl pathway of acetogenesis. Ann. N. Y. Acad. Sci. 2008, 1125, 129–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragsdale, S. Nickel containing CO dehydrogenases and hydrogenases. Sub-Cell. Biochem. 2000, 35, 487–518. [Google Scholar] [CrossRef]
- Ragsdale, S.; Wood, H. Enzymology of the acetyl-coa pathway of CO2 fixation. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 261–300. [Google Scholar] [CrossRef] [PubMed]
- Burton, R.; Can, M.; Esckilsen, D.; Wiley, S.; Ragsdale, S. Production and properties of enzymes that activate and produce carbon monoxide. Methods Enzymol. 2018, 613, 297–324. [Google Scholar] [CrossRef]
- Can, M.; Armstrong, F.; Ragsdale, S. Structure, function, and mechanism of the nickel metalloenzymes, CO dehydrogenase, and acetyl-CoA synthase. Chem. Rev. 2014, 114, 4149–4174. [Google Scholar] [CrossRef]
- Darnault, C.; Volbeda, A.; Kim, E.; Legrand, P.; Vernède, X.; Lindahl, P.; Fontecilla-Camps, J. Ni-Zn-Fe4-S4 and Ni-Ni-Fe4-S4 clusters in closed and open α subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase. Nat. Struct. Biol. 2003, 10, 271–279. [Google Scholar] [CrossRef]
- Lindahl, P.; Graham, D. Acetyl-Coenzyme A Synthases and Nickel-Containing Carbon Monoxide Dehydrogenases; John Wiley & Sons: Chichester, UK, 2007; Volume 2, pp. 357–415. [Google Scholar] [CrossRef]
- Ragsdale, S. Life with carbon monoxide. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 165–195. [Google Scholar] [CrossRef]
- Lindahl, P. The Ni-containing carbon monoxide dehydrogenase family: Light at the end of the tunnel? Biochemistry 2002, 41, 2097–2105. [Google Scholar] [CrossRef] [Green Version]
- Gencic, S.; Grahame, D. Nickel in subunit β of the acetyl-CoA decarbonylase/synthase multienzyme complex in methanogens: Catalytic properties and evidence for a binuclear Ni-Ni site. J. Biol. Chem. 2003, 278, 6101–6110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeoung, J.H.; Fesseler, J.; Goetzl, S.; Dobbek, H. Carbon monoxide. Toxic gas and fuel for anaerobes and aerobes: Carbon monoxide dehydrogenases. Met. Ions Life Sci. 2014, 14-113, 37–69. [Google Scholar] [CrossRef]
- Ragsdale, S. Nickel and the carbon cycle. J. Inorg. Biochem. 2007, 101, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.; Drennan, C. One-Carbon Chemistry of Nickel-Containing Carbon Monoxide Dehydrogenase and Acetyl-CoA Synthase. RSC Metall. 2017, 2017, 121–148. [Google Scholar] [CrossRef]
- Kumar, M.; Lu, W.P.; Liu, L.; Ragsdale, S. Kinetic Evidence that Carbon Monoxide Dehydrogenase Catalyzes the Oxidation of Carbon Monoxide and the Synthesis of Acetyl-CoA at Separate Metal Centers. J. Am. Chem. Soc. 1993, 115, 11646–11647. [Google Scholar] [CrossRef]
- Maynard, E.; Lindahl, P. Catalytic coupling of the active sites in acetyl-CoA synthase, a bifunctional CO-channeling enzyme. Biochemistry 2001, 40, 13262–13267. [Google Scholar] [CrossRef]
- Ragsdale, S.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [Green Version]
- Ragsdale, S.; Riordan, C. The role of nickel in acetyl-CoA synthesis by the bifunctional enzyme CO dehydrogenase/acetyl-CoA synthase: Enzymology and model chemistry. J. Biol. Inorg. Chem. 1996, 1, 489–493. [Google Scholar] [CrossRef]
- Zhu, X.; Tan, X. Metalloproteins/metalloenzymes for the synthesis of acetyl-CoA in the Wood-Ljungdahl pathway. Sci. China Ser. B Chem. 2009, 52, 2071–2082. [Google Scholar] [CrossRef]
- Tan, X.; Volbeda, A.; Fontecilla-Camps, J.; Lindahl, P. Function of the tunnel in acetylcoenzyme A synthase/carbon monoxide dehydrogenase. J. Biol. Inorg. Chem. 2006, 11, 371–378. [Google Scholar] [CrossRef]
- Jeoung, J.H.; Martins, B.; Dobbek, H. X-ray crystallography of carbon monoxide dehydrogenases. Methods Mol. Biol. 2019, 1876, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Harder, S.; Lu, W.P.; Feinberg, B.; Ragsdale, S. Spectroelectrochemical Studies of the Corrinoid/Iron-Sulfur Protein Involved in Acetyl Coenzyme A Synthesis by Clostridium thermoaceticum. Biochemistry 1989, 28, 9080–9087. [Google Scholar] [CrossRef] [PubMed]
- Wirt, M.; Chance, M.; Kumar, M.; Ragsdale, S. X-ray Absorption Spectroscopy of the Corrinoid/Iron-Sulfur Protein Involved in Acetyl Coenzyme A Synthesis by Clostridium thermoaceticum. J. Am. Chem. Soc. 1993, 115, 2146–2150. [Google Scholar] [CrossRef]
- Zhao, S.; Ragsdale, S. A conformational change in the methyltransferase from Clostridium thermoaceticum facilitates the methyl transfer from (6S)-methyltetrahydrofolate to the corrinoid/iron-sulfur protein in the acetyl-CoA pathway. Biochemistry 1996, 35, 2476–2481. [Google Scholar] [CrossRef]
- Svetlitchnaia, T.; Svetlitchnyi, V.; Meyer, O.; Dobbek, H. Structural insights into methyltransfer reactions of a corrinoid iron-sulfur protein involved in acetyl-CoA synthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14331–14336. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, S.; Teutloff, C.; Werther, T.; Hennig, S.; Jeoung, J.H.; Bittl, R.; Dobbek, H. Protein Dynamics in the Reductive Activation of a B12-Containing Enzyme. Biochemistry 2017, 56, 5496–5502. [Google Scholar] [CrossRef] [Green Version]
- Meister, W.; Hennig, S.; Jeoung, J.H.; Lendzian, F.; Dobbek, H.; Hildebrandt, P. Complex formation with the activator RACo affects the corrinoid structure of CoFeSP. Biochemistry 2012, 51, 7040–7042. [Google Scholar] [CrossRef]
- Schrapers, P.; Mebs, S.; Goetzl, S.; Hennig, S.; Dau, H.; Dobbek, H.; Haumann, M. Axial ligation and redox changes at the cobalt ion in cobalamin bound to Corrinoid Iron-Sulfur Protein (CoFeSP) or in solution characterized by XAS and DFT. PLoS ONE 2016, 11, e0158681. [Google Scholar] [CrossRef]
- Matthews, R.; Banerjee, R.; Ragsdale, S. Cobamide-dependent methyl transferases. BioFactors 1990, 2, 147–152. [Google Scholar]
- Seravalli, J.; Brown, K.; Ragsdale, S. Acetyl coenzyme a synthesis from unnatural methylated corrinoids: Requirement for “base-off” coordination at cobalt [14]. J. Am. Chem. Soc. 2001, 123, 1786–1787. [Google Scholar] [CrossRef]
- Fan, C.; Gorst, C.; Ragsdale, S.; Hoffman, B. Characterization of the Ni-Fe-C Complex Formed by Reaction of Carbon Monoxide with the Carbon Monoxide Dehydrogenase from Clostridium thermoaceticum by Q-Band ENDOR. Biochemistry 1991, 30, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Volbeda, A.; Darnault, C.; Tan, X.; Lindahl, P.; Fontecilla-Camps, J. Correction to novel domain arrangement in the crystal structure of a truncated acetyl-CoA synthase from Moorella thermoacetica. Biochemistry 2009, 48, 12058. [Google Scholar] [CrossRef] [Green Version]
- Volbeda, A.; Fontecilla-Camps, J. Crystallographic evidence for a CO/CO2 tunnel gating mechanism in the bifunctional carbon monoxide dehydrogenase/acetyl coenzyme A synthase from Moorella thermoacetica. J. Biol. Inorg. Chem. 2004, 9, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.H.; Bruschi, M.; De Gioia, L.; Blumberger, J. Uncovering a dynamically formed substrate access tunnel in carbon monoxide dehydrogenase/acetyl-CoA synthase. J. Am. Chem. Soc. 2013, 135, 9493–9502. [Google Scholar] [CrossRef]
- Cohen, S.; Can, M.; Wittenborn, E.; Hendrickson, R.; Ragsdale, S.; Drennan, C. Crystallographic Characterization of the Carbonylated A-Cluster in Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. ACS Catal. 2020, 10, 9741–9746. [Google Scholar] [CrossRef]
- Can, M.; Giles, L.; Ragsdale, S.; Sarangi, R. X-ray Absorption Spectroscopy Reveals an Organometallic Ni-C Bond in the CO-Treated Form of Acetyl-CoA Synthase. Biochemistry 2017, 56, 1248–1260. [Google Scholar] [CrossRef] [Green Version]
- Grahame, D. Methods for analysis of acetyl-CoA synthase: Applications to bacterial and archaeal systems. Methods Enzymol. 2011, 494, 189–217. [Google Scholar] [CrossRef]
- Grahame, D. Acetate C-C bond formation and decomposition in the anaerobic world: The structure of a central enzyme and its key active-site metal cluster. Trends Biochem. Sci. 2003, 28, 221–224. [Google Scholar] [CrossRef]
- Gorst, C.; Ragsdale, S. Characterization of the NiFeCO complex of carbon monoxide dehydrogenase as a catalytically competent intermediate in the pathway of acetyl-coenzyme A synthesis. J. Biol. Chem. 1991, 266, 20687–20693. [Google Scholar] [CrossRef]
- Gencic, S.; Duin, E.; Grahame, D. Tight coupling of partial reactions in the Acetyl-CoA Decarbonylase/ Synthase (ACDS) multienzyme complex from Methanosarcina thermophila: Acetyl C-C bond fragmentation at the A cluster promoted by protein conformational changes. J. Biol. Chem. 2010, 285, 15450–15463. [Google Scholar] [CrossRef] [Green Version]
- Gencic, S.; Kelly, K.; Ghebreamlak, S.; Duin, E.; Grahame, D. Different modes of carbon monoxide binding to acetyl-CoA synthase and the role of a conserved phenylalanine in the coordination environment of nickel. Biochemistry 2013, 52, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Bender, G.; Stich, T.; Yan, L.; Britt, R.; Cramer, S.; Ragsdale, S. Infrared and EPR spectroscopic characterization of a Ni(I) species formed by photolysis of a catalytically competent Ni(I)-CO intermediate in the acetyl-CoA synthase reaction. Biochemistry 2010, 49, 7516–7523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bramlett, M.; Stubna, A.; Tan, X.; Surovtsev, I.; Münck, E.; Lindahl, P. Mössbauer and EPR study of recombinant acetyl-CoA synthase from Moorella thermoacetica. Biochemistry 2006, 45, 8674–8685. [Google Scholar] [CrossRef] [PubMed]
- Bramlett, M.; Tan, X.; Lindahl, P. Inactivation of acetyl-CoA synthase/carbon monoxide dehydrogenase by copper. J. Am. Chem. Soc. 2003, 125, 9316–9317. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, O.; Wagner, T. Gas channel rerouting in a primordial enzyme: Structural insights of the carbon-monoxide dehydrogenase/acetyl-CoA synthase complex from the acetogen Clostridium autoethanogenum. Biochim. Biophys. Acta Bioenerg. 2021, 1862. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.; Darensbourg, M.; Lindahl, P.; Hall, M. Structures and Energetics of Models for the Active Site of Acetyl-Coenzyme A Synthase: Role of Distal and Proximal Metals in Catalysis. J. Am. Chem. Soc. 2004, 126, 3410–3411. [Google Scholar] [CrossRef]
- Amara, P.; Volbeda, A.; Fontecilla-Camps, J.; Field, M. A quantum chemical study of the reaction mechanism of acetyl-coenzyme A synthase. J. Am. Chem. Soc. 2005, 127, 2776–2784. [Google Scholar] [CrossRef]
- Chmielowska, A.; Lodowski, P.; Jaworska, M. Redox potentials and protonation of the A-cluster from acetyl-CoA synthase. A density functional theory study. J. Phys. Chem. A 2013, 117, 12484–12496. [Google Scholar] [CrossRef]
- Brunold, T. Spectroscopic and computational insights into the geometric and electronic properties of the A-cluster of acetyl-coenzyme A synthase. J. Biol. Inorg. Chem. 2004, 9, 533–541. [Google Scholar] [CrossRef]
- Elghobashi-Meinhardt, N.; Tombolelli, D.; Mroginski, M.A. QM/MM computations reveal details of the acetyl-CoA synthase catalytic center. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129579. [Google Scholar] [CrossRef]
- Kisgeropoulos, E.; Manesis, A.; Shafaat, H. Ligand Field Inversion as a Mechanism to Gate Bioorganometallic Reactivity: Investigating a Biochemical Model of Acetyl CoA Synthase Using Spectroscopy and Computation. J. Am. Chem. Soc. 2021, 143, 849–867. [Google Scholar] [CrossRef] [PubMed]
- Mathrubootham, V.; Thomas, J.; Staples, R.; McCraken, J.; Shearer, J.; Hegg, E. Bisamidate and mixed amine/amidate NiN2S2 complexes as models for nickel-containing acetyl coenzyme A synthase and superoxide dismutase: An experimental and computational study. Inorg. Chem. 2010, 49, 5393–5406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.L.; Siegbahn, P. Insights into the Chemical Reactivity in Acetyl-CoA Synthase. Inorg. Chem. 2020, 59, 15167–15179. [Google Scholar] [CrossRef] [PubMed]
- Schenker, R.; Brunold, T. Computational Studies on the A Cluster of Acetyl-Coenzyme A Synthase: Geometric and Electronic Properties of the NiFeC Species and Mechanistic Implications. J. Am. Chem. Soc. 2003, 125, 13962–13963. [Google Scholar] [CrossRef]
- Schrapers, P.; Ilina, J.; Gregg, C.; Mebs, S.; Jeoung, J.H.; Dau, H.; Dobbek, H.; Haumann, M. Ligand binding at the A-cluster in full-length or truncated acetyl-CoA synthase studied by X-ray absorption spectroscopy. PLoS ONE 2017, 12, e0171039. [Google Scholar] [CrossRef]
- Cohen, S.; Brignole, E.; Wittenborn, E.; Can, M.; Thompson, S.; Ragsdale, S.; Drennan, C. Negative-Stain Electron Microscopy Reveals Dramatic Structural Rearrangements in Ni-Fe-S-Dependent Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase. Structure 2021, 29, 43–49. [Google Scholar] [CrossRef]
- Chen, J.; Huang, S.; Seravalli, J.; Gutzman, H., Jr.; Swartz, D.; Ragsdale, S.; Bagley, K. Infrared Studies of Carbon Monoxide Binding to Carbon Monoxide Dehydrogenase/Acetyl-CoA Synthase from Moorella thermoacetica. Biochemistry 2003, 42, 14822–14830. [Google Scholar] [CrossRef]
- Kumar, M.; Qiu, D.; Spiro, T.; Ragsdale, S. A methyl-nickel intermediate in a bimetallic mechanism of acetyl-coenzyme A synthesis by anaerobic bacteria. Science 1995, 270, 628–630. [Google Scholar] [CrossRef]
- Maynard, E.; Sewell, C.; Lindahl, P. Kinetic mechanism of acetyl-CoA synthase: Steady-state synthesis at variable CO/CO2 pressures. J. Am. Chem. Soc. 2001, 123, 4697–4703. [Google Scholar] [CrossRef]
- Tan, X.; Bramlett, M.; Lindahl, P. Effect of Zn on Acetyl Coenzyme A Synthase: Evidence for a Conformational Change in the α Subunit during Catalysis. J. Am. Chem. Soc. 2004, 126, 5954–5955. [Google Scholar] [CrossRef]
- Qiu, D.; Kumar, M.; Ragsdale, S.; Spiro, T. Raman and infrared spectroscopy of cyanide-inhibited CO dehydrogenase/acetyl-CoA synthase from Clostridium thermoaceticum: Evidence for bimetallic enzymatic CO oxidation. J. Am. Chem. Soc. 1996, 118, 10429–10435. [Google Scholar] [CrossRef]
- Russell, W.; Støalhandske, C.; Xia, J.; Scott, R.; Lindahl, P. Spectroscopic, redox, and structural characterization of the Ni-labile and nonlabile forms of the acetyl-CoA synthase active site of carbon monoxide dehydrogenase. J. Am. Chem. Soc. 1998, 120, 7502–7510. [Google Scholar] [CrossRef]
- Tan, X.; Sewell, C.; Yang, Q.; Lindahl, P. Reduction and methyl transfer kinetics of the α subunit from acetyl coenzyme A synthase. J. Am. Chem. Soc. 2003, 125, 318–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, X.; Martinho, M.; Stubna, A.; Lindahl, P.; Münck, E. Mössbauer evidence for an exchange-coupled [Fe4S4]1+ A-cluster in isolated α subunits of acetyl-coenzyme A synthase/carbon monoxide dehydrogenase. J. Am. Chem. Soc. 2008, 130, 6712–6713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindahl, P. Acetyl-coenzyme A synthase: The case for a Nip0-based mechanism of catalysis. J. Biol. Inorg. Chem. 2004, 9, 516–524. [Google Scholar] [CrossRef]
- Seravalli, J.; Kumar, M.; Ragsdale, S. Rapid kinetic studies of acetyl-CoA synthesis: Evidence supporting the catalytic intermediacy of a paramagnetic NiFeC species in the autotrophic Wood-Ljungdahl pathway. Biochemistry 2002, 41, 1807–1819. [Google Scholar] [CrossRef]
- George, S.; Seravalli, J.; Ragsdale, S. EPR and infrared spectroscopic evidence that a kinetically competent paramagnetic intermediate is formed when acetyl-coenzyme A synthase reacts with CO. J. Am. Chem. Soc. 2005, 127, 13500–13501. [Google Scholar] [CrossRef]
- Ragsdale, S.; Wood, H.; Antholine, W. Evidence that an iron-nickel-carbon complex is formed by reaction of CO with the CO dehydrogenase from Clostridium thermoaceticum. Proc. Natl. Acad. Sci. USA 1985, 82, 6811–6814. [Google Scholar] [CrossRef] [Green Version]
- Ragsdale, S.; Ljungdahl, L.; DerVartanian, D. 13C and 61Ni isotope substitutions confirm the presence of a nickel(III)-carbon species in acetogenic CO dehydrogenases. Biochem. Biophys. Res. Commun. 1983, 115, 658–665. [Google Scholar] [CrossRef]
- Lindahl, P.; Ragsdale, S.; Munck, E. Mossbauer study of CO dehydrogenase from Clostridium thermoaceticum. J. Biol. Chem. 1990, 265, 3880–3888. [Google Scholar] [CrossRef]
- Menon, S.; Ragsdale, S. Evidence that carbon monoxide is an obligatory intermediate in anaerobic acetyl-CoA synthesis. Biochemistry 1996, 35, 12119–12125. [Google Scholar] [CrossRef] [PubMed]
- Seravalli, J.; Ragsdale, S. Pulse-chase studies of the synthesis of acetyl-CoA by carbon monoxide dehydrogenase/acetyl-CoA synthase: Evidence for a random mechanism of methyl and carbonyl addition. J. Biol. Chem. 2008, 283, 8384–8394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragsdale, S.W. 8.24—Biological Carbon Fixation by an Organometallic Pathway: Evidence Supporting the Paramagnetic Mechanism of the Nickel-Iron-Sulfur Acetyl-CoA Synthase. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Oxford, UK, 2021; pp. 611–633. [Google Scholar] [CrossRef]
- Barondeau, D.; Lindahl, P. Methylation of carbon monoxide dehydrogenase from Clostridium thermoaceticum and mechanism of acetyl coenzyme A synthesis. J. Am. Chem. Soc. 1997, 119, 3959–3970. [Google Scholar] [CrossRef]
- Fraser, D.; Lindahl, P. Evidence for a proposed intermediate redox state in the CO/CO2 active site of acetyl-CoA synthase (carbon monoxide dehydrogenase) from Clostridium thermoaceticum. Biochemistry 1999, 38, 15706–15711. [Google Scholar] [CrossRef]
- Fraser, D.; Lindahl, P. Stoichiometric CO reductive titrations of acetyl-CoA synthase (carbon monoxide dehydrogenase) from Clostridium thermoaceticum. Biochemistry 1999, 38, 15697–15705. [Google Scholar] [CrossRef]
- Lindahl, P. Metal-metal bonds in biology. J. Inorg. Biochem. 2012, 106, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Gencic, S.; Grahame, D. Two separate one-electron steps in the reductive activation of the A cluster in subunit β of the ACDS complex in Methanosarcina thermophila. Biochemistry 2008, 47, 5544–5555. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian˜16 Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Noodleman, L.; Norman, J., Jr. The Xα valence bond theory of weak electronic coupling. Application to the low-lying states of Mo2. J. Chem. Phys. 1979, 70, 4903–4906. [Google Scholar] [CrossRef]
- Noodleman, L. Valence bond description of antiferromagnetic coupling in transition metal dimers. J. Chem. Phys. 1981, 74, 5737–5743. [Google Scholar] [CrossRef]
- Becke, A. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Perdew, J. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Jensen, K.; Ryde, U. Theoretical Prediction of the Co–C Bond Strength in Cobalamins. J. Phys. Chem. A 2003, 107, 7539–7545. [Google Scholar] [CrossRef]
- Kozlowski, P.; Kumar, M.; Piecuch, P.; Li, W.; Bauman, N.; Hansen, J.; Lodowski, P.; Jaworska, M. The cobalt-methyl bond dissociation in methylcobalamin: New benchmark analysis based on density functional theory and completely renormalized coupled-cluster calculations. J. Chem. Theory Comput. 2012, 8, 1870–1894. [Google Scholar] [CrossRef] [PubMed]
- Emelyanova, N.; Sanina, N.; Krivenko, A.; Manzhos, R.; Bozhenko, K.; Aldoshin, S. Comparison of pure and hybrid DFT functionals for geometry optimization and calculation of redox potentials for iron nitrosyl complexes with “μ–SCN” bridging ligands. Theor. Chem. Accounts 2013, 132, 1316. [Google Scholar] [CrossRef]
- Castro, L.; Bühl, M. Calculations of one-electron redox potentials of oxoiron(IV) porphyrin complexes. J. Chem. Theory Comput. 2014, 10, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Fitch, C.; Karp, D.; Lee, K.; Stites, W.; Lattman, E.; Bertrand García-Moreno, E. Experimental pKa values of buried residues: Analysis with continuum methods and role of water penetration. Biophys. J. 2002, 82, 3289–3304. [Google Scholar] [CrossRef] [Green Version]
- Bashford, D.; Karplus, M. pKa’s of Ionizable Groups in Proteins: Atomic Detail from a Continuum Electrostatic Model. Biochemistry 1990, 29, 10219–10225. [Google Scholar] [CrossRef]
- Karp, D.; Gittis, A.; Stahley, M.; Fitch, C.; Stites, W.; García-Moreno E., B. High apparent dielectric constant inside a protein reflects structural reorganization coupled to the ionization of an internal Asp. Biophys. J. 2007, 92, 2041–2053. [Google Scholar] [CrossRef] [Green Version]
- Harms, M.; Schlessman, J.; Chimenti, M.; Sue, G.; Damjanović, A.; García-Moreno, E.B. A buried lysine that titrates with a normal pKa: Role of conformational flexibility at the protein-water interface as a determinant of pKa values. Protein Sci. 2008, 17, 833–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svetlitchnyi, V.; Dobbek, H.; Meyer-Klaucke, W.; Meins, T.; Thiele, B.; Römer, P.; Huber, R.; Meyer, O. A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc. Natl. Acad. Sci. USA 2004, 101, 446–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasatti, S. Components of the absolute electrode potential. conceptions and misinterpretations. Mater. Chem. Phys. 1986, 15, 427–438. [Google Scholar] [CrossRef]
- Lewis, A.; Bumpus, J.; Truhlar, D.; Cramer, C. Molecular modeling of environmentally important processes: Reduction potentials. J. Chem. Educ. 2004, 81, 596–603, Erratum in J. Chem. Educ. 2007, 84, 934. [Google Scholar]
- Noodleman, L.; Han Du, W.G.; McRee, D.; Chen, Y.; Goh, T.; Götz, A. Coupled transport of electrons and protons in a bacterial cytochrome: C oxidase-DFT calculated properties compared to structures and spectroscopies. Phys. Chem. Chem. Phys. 2020, 22, 26652–26668. [Google Scholar] [CrossRef]
- Han, W.G.; Giammona, D.; Bashford, D.; Noodleman, L. Density functional theory analysis of structure, energetics, and spectroscopy for the Mn-Fe active site of chlamydia trachomatis ribonucleotide reductase in four oxidation states. Inorg. Chem. 2010, 49, 7266–7281. [Google Scholar] [CrossRef] [Green Version]
- Han, W.G.; Liu, T.; Lovell, T.; Noodleman, L. Active site structure of class I ribonucleotide reductase intermediate X: A density functional theory analysis of structure, energetics, and spectroscopy. J. Am. Chem. Soc. 2005, 127, 15778–15790. [Google Scholar] [CrossRef]
- Tissandier, M.; Cowen, K.; Feng, W.; Gundlach, E.; Cohen, M.; Earhart, A.; Coe, J.; Tuttle, T., Jr. The proton’s absolute aqueous enthalpy and Gibbs free energy of solvation from cluster-ion solvation data. J. Phys. Chem. A 1998, 102, 7787–7794. [Google Scholar] [CrossRef]
- Tawa, G.; Topol, I.; Burt, S.; Caldwell, R.; Rashin, A. Calculation of the aqueous solvation free energy of the proton. J. Chem. Phys. 1998, 109, 4852–4863. [Google Scholar] [CrossRef]
- Doukov, T.; Iverson, T.; Seravalli, J.; Ragsdale, S.; Drennan, C. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science 2002, 298, 567–572. [Google Scholar] [CrossRef] [Green Version]
- Seravalli, J.; Gu, W.; Tam, A.; Strauss, E.; Begley, T.; Cramer, S.; Ragsdale, S. Functional copper at the acetyl-CoA synthase active site. Proc. Natl. Acad. Sci. USA 2003, 100, 3689–3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seravalli, J.; Xiao, Y.; Gu, W.; Cramer, S.; Antholine, W.; Krymov, V.; Gerfen, G.; Ragsdale, S. Evidence that NiNi Acetyl-CoA Synthase Is Active and that the CuNi Enzyme Is Not. Biochemistry 2004, 43, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- James, C.; Wiley, S.; Ragsdale, S.; Hoffman, B. 13C Electron Nuclear Double Resonance Spectroscopy Shows Acetyl-CoA Synthase Binds Two Substrate CO in Multiple Binding Modes and Reveals the Importance of a CO-Binding “alcove”. J. Am. Chem. Soc. 2020, 142, 15362–15370. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.; Lu, W.P.; Ragsdale, S. Acetyl-coenzyme A synthesis from methyltetrahydrofolate, CO, and coenzyme A by enzymes purified from Clostridium thermoaceticum: Attainment of in vivo rates and identification of rate-limiting steps. J. Bacteriol. 1992, 174, 4667–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhaskar, B.; DeMoll, E.; Grahame, D. Redox-dependent acetyl transfer partial reaction of the acetyl-coa decarbonylase/synthase complex: Kinetics and mechanism. Biochemistry 1998, 37, 14491–14499. [Google Scholar] [CrossRef]
- Fitch, C.; Platzer, G.; Okon, M.; Garcia-Moreno, B.; McIntosh, L. Arginine: Its pKa value revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef] [Green Version]
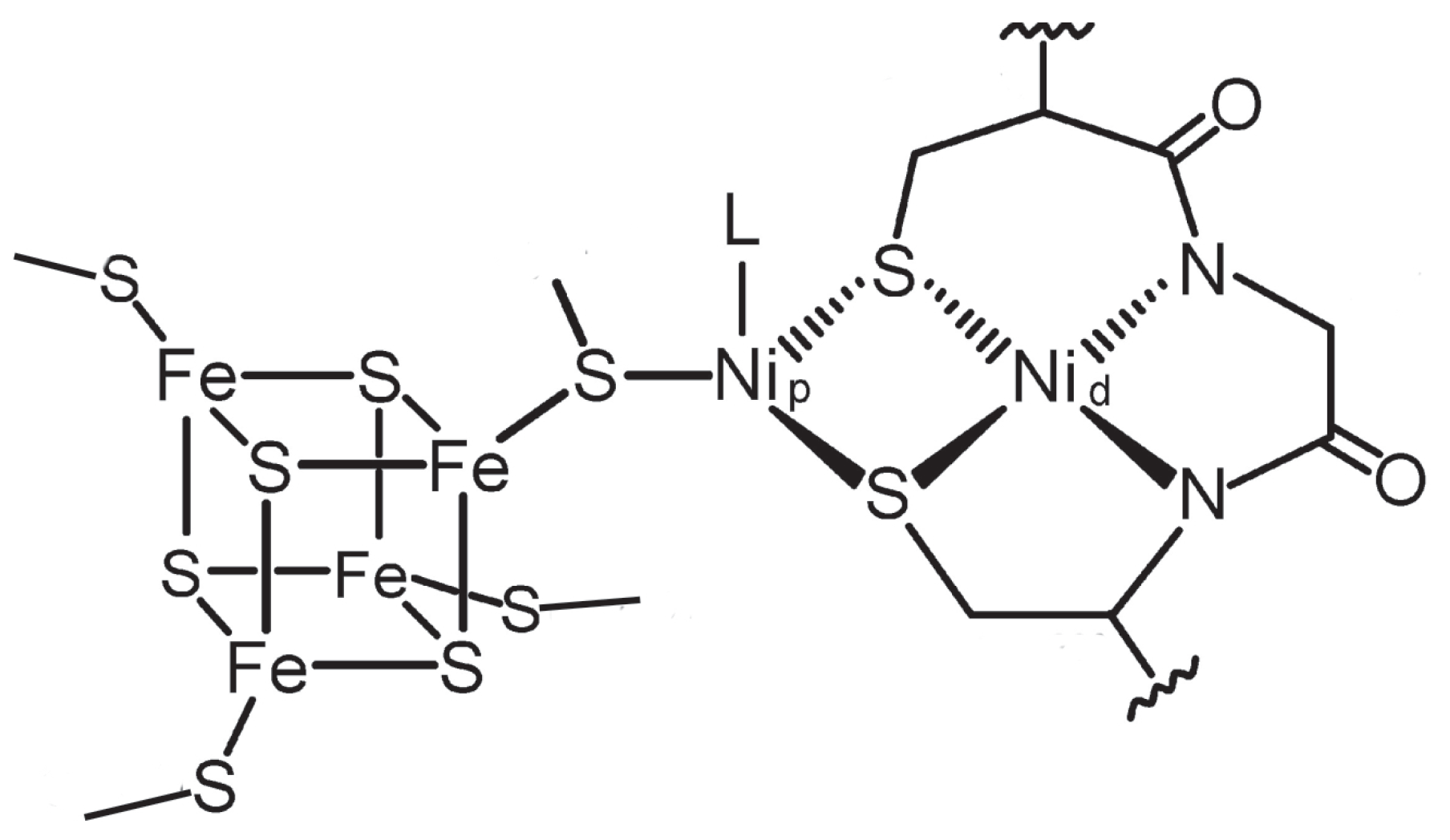
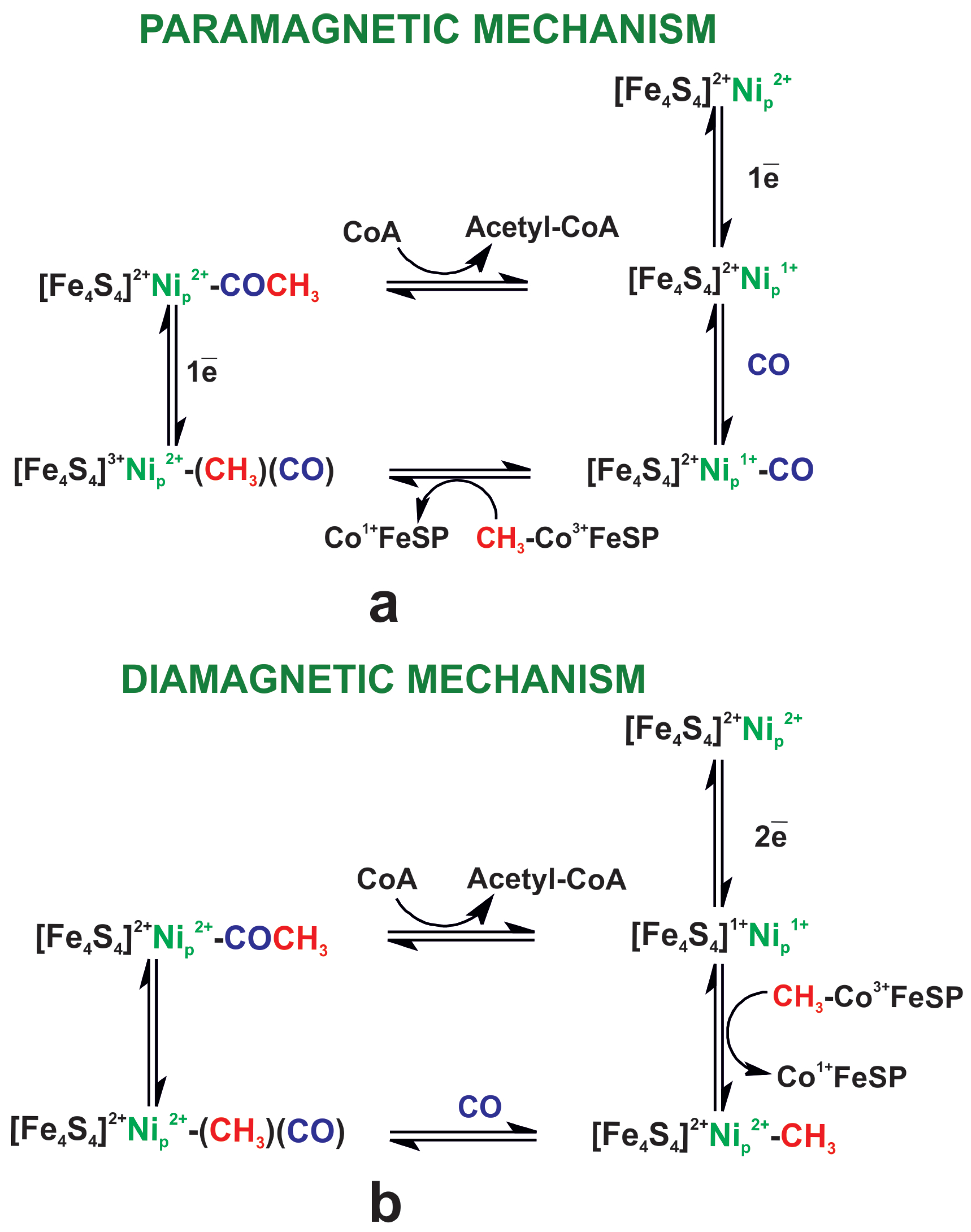

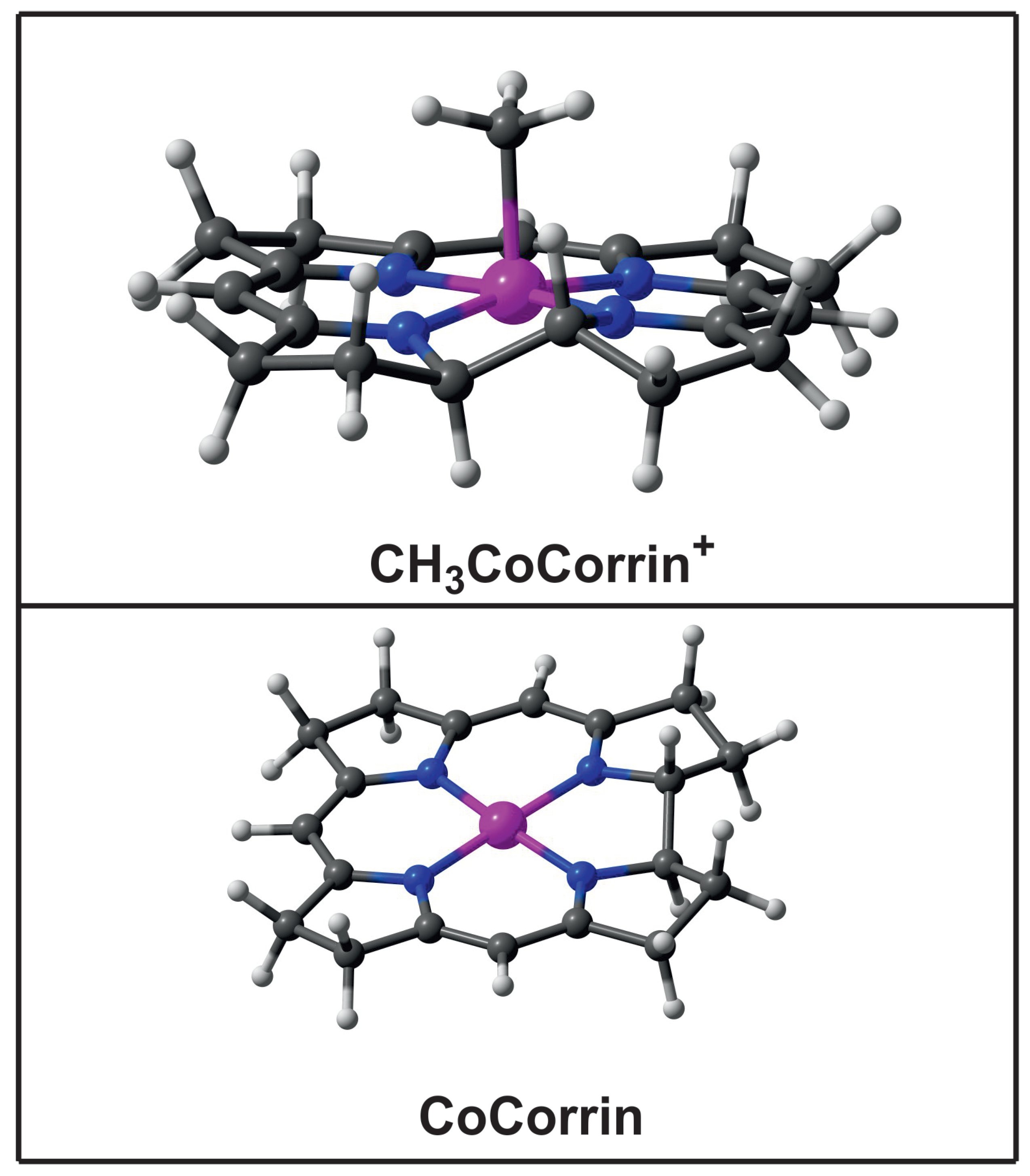
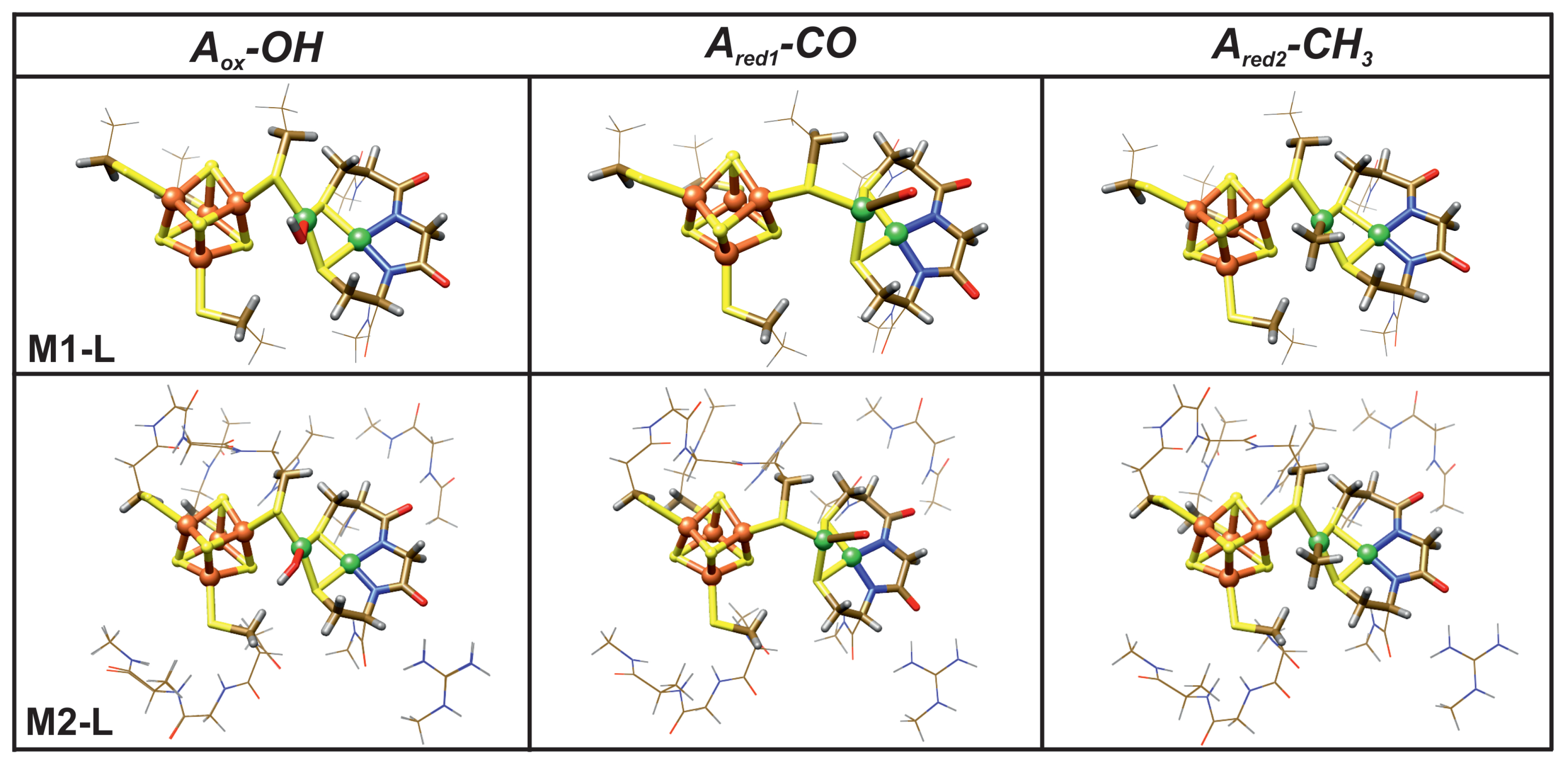
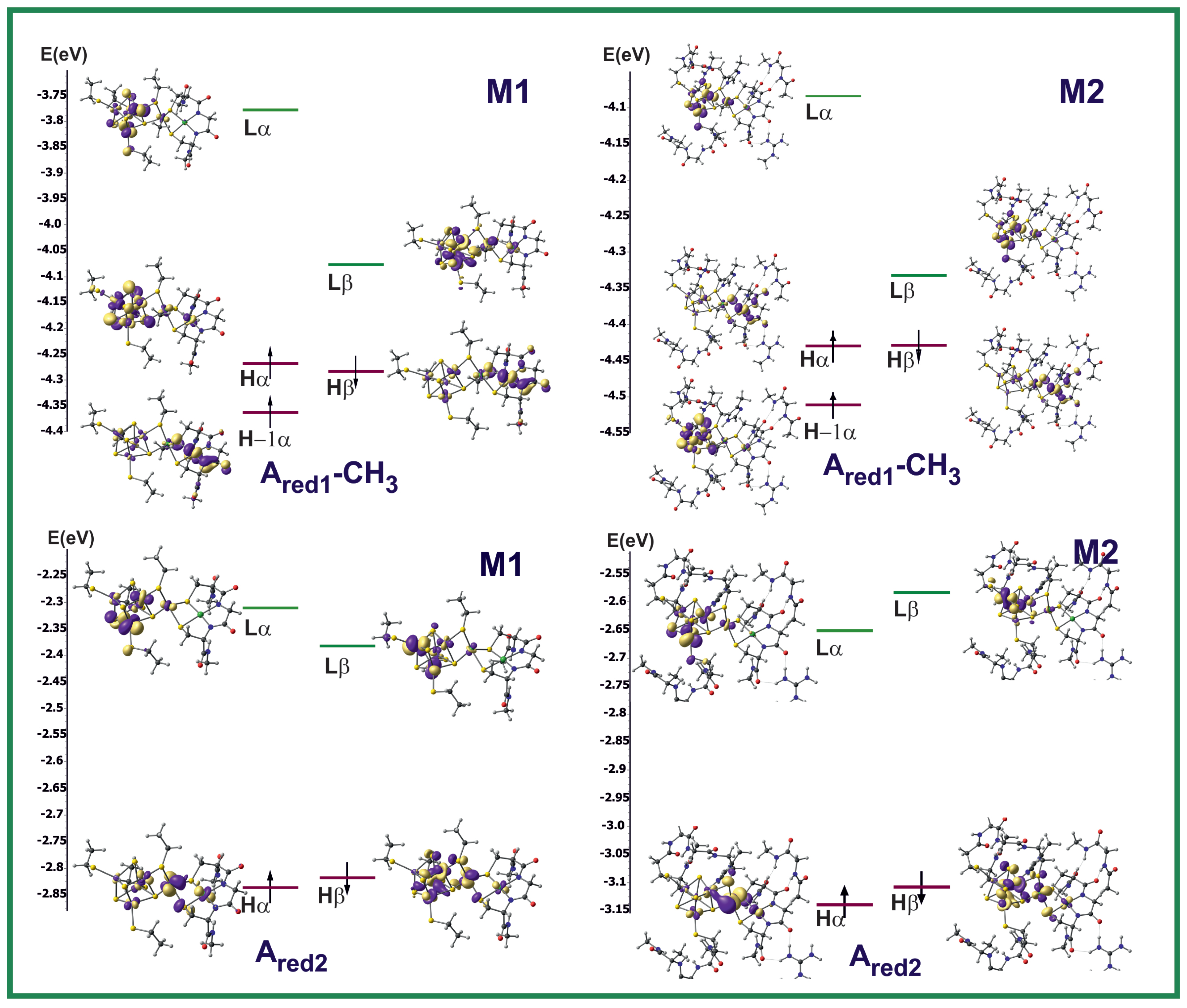
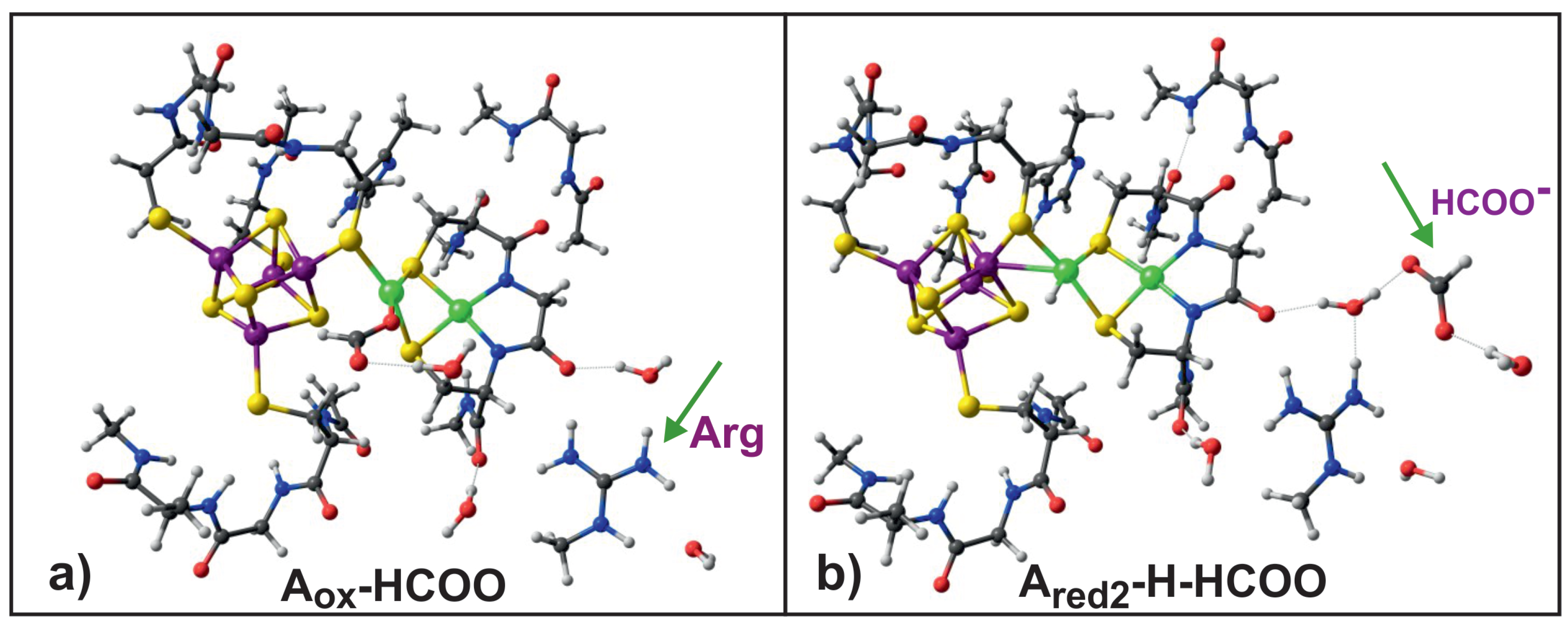
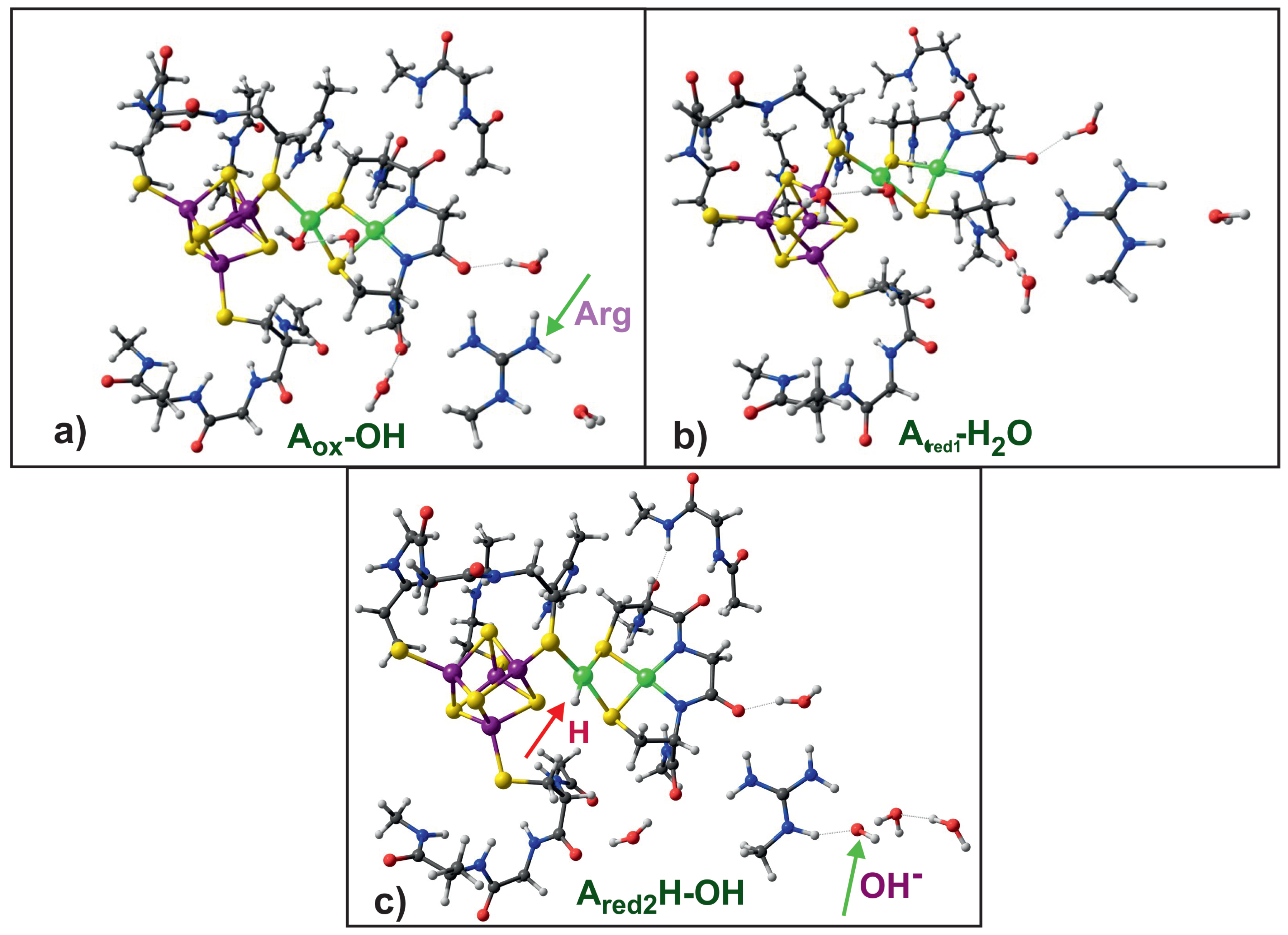
| Name | Electronic Configuration | Spin State | Description | References |
|---|---|---|---|---|
| A | [FeS]NiNi | S = 0 | resting state, oxidized form | [44] |
| stable at potential >−0.5 V | ||||
| A | [FeS]NiNi | S = 1/2 | one–electron reduced | [43] |
| A | [FeS]NiNi | S = 0 | two–electron reduced, | [66] |
| postulated as catalytic intermediate | ||||
| A–CH | [FeS]Ni(CH)Ni | S=0 | methylated form | [44] |
| A–CO | [FeS]Ni(CO)Ni | S = 1/2 | one–electron reduced, | |
| carbonylated form called NiFeC | [32,37,44,67,68,69,70,71] | |||
| A–OH | [FeS]Ni(OH)Ni | S = 0 | [56] | |
| A–HO | [FeS]Ni(HO)Ni | S = 1/2 | [56] |
| X–ray | ||||
|---|---|---|---|---|
| PDB ID: 1RU3 [95] | PDB ID: 1OAO [8] | PDB ID: 6YTT [46] | PDB ID: 6X5K [36] | |
| Ni–Ni | 2.980 | 3.041 | 2.882 | 2.744 |
| Ni–Fe1 | 2.680 | 2.662 | 2.243 | 3.879 |
| Ni–HO | 2.7 | |||
| Ni–CO | 1.630 | |||
| CO–Ni–S1 | 123.9 | |||
| CO–Ni–S5 | 124.3 | |||
| CO–Ni–S6 | 105.3 | |||
| EXAFS [95] | ||||
| Ni–Ni | 2.89 | 2.97/2.96 | ||
| Ni–Fe1 | 2.71 | 2.80 | ||
| Ni–HO | 2.32 | |||
| EXAFS [56] | ||||
| Ni–OH | NiHO | NiCO | NiCH | |
| Ni–Ni | 2.9 | 2.9 | 2.97/2.96 | |
| Ni–Fe1 | 2.7 | 2.7 | 2.80 | |
| Ni–L | 2.0 | 2.1 | 1.7 | 1.95 |
| Model | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 | M1 | M2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distance (Å) | Ni–Ni | Ni–Fe1 | Ni–L | L–Ni–S1 | L–Ni–S5 | L–Ni–S6 | ||||||
| A | 2.964 | 2.937 | 2.833 | 2.880 | – | – | ||||||
| A | 3.121 | 3.091 | 2.600 | 2.583 | – | – | ||||||
| A | 3.132 | 3.129 | 2.490 | 2.510 | – | – | ||||||
| A–OH | 2.938 | 2.907 | 2.900 | 2.892 | 1.876 | 1.882 | 92.1 | 169.9 | 82.7 | |||
| A–HCOO | 2.957 | 2.917 | 2.987 | 2.941 | 1.949 | 1.941 | 90.6 | 170.2 | 85.2 | |||
| A–HO | 2.959 | 2.788 | 2.209 | |||||||||
| A–CO | 2.592 | 2.700 | 4.097 | 3.382 | 1.766 | 1.763 | 108.8 | 134.7 | 98.8 | |||
| A–CH | 2.890 | 2.992 | 2.739 | 2.738 | 1.976 | 1.981 | 89.3 | 165.4 | 85.2 | |||
| A–CH | 3.019 | 2.970 | 2.813 | 2.758 | 1.971 | 1.971 | 89.9 | 166.4 | 85.6 | |||
| A–H | 2.951 | 2.680 | 1.470 | 85.2 | 166.7 | 82.9 | ||||||
| Spin Density | ||||||
|---|---|---|---|---|---|---|
| Ni | FeS | Ni | ||||
| M1 | M2 | M1 | M2 | M1 | M2 | |
| A | −0.103 | 0.124 | −0.140 | 0.002 | 0.090 | −0.111 |
| A | 0.591 | −0.574 | −0.040 | 0.002 | 0.062 | 0.054 |
| A | −0.578 | −0.421 | 0.680 | 0.435 | −0.004 | −0.002 |
| A–OH | −0.132 | −0.138 | 0.004 | −0.002 | −0.005 | 0.000 |
| A–HCOO | −0.121 | 0.109 | −0.020 | −0.002 | −0.006 | 0.005 |
| A–HO | 0.575 | 0.030 | 0.081 | |||
| A–CO | 0.535 | 0.478 | 0.066 | 0.104 | 0.237 | 0.126 |
| A–CH | 0.063 | −0.159 | 0.450 | 0.830 | 0.059 | −0.013 |
| A–CH | −0.158 | −0.178 | 0.011 | 0.063 | −0.006 | −0.009 |
| No. | Reagents | Products | E(kcal) | |||
|---|---|---|---|---|---|---|
| One-elctron reduced | ||||||
| 1 | FeSNi | CO | FeSNiCO | −24.0 | −30.9 | |
| 2 | FeSNi | CHCoCorrin | FeSNiCH | CoCorrin | 1.1 | −1.7 |
| 2a | FeSNiCH | CoCorrin | 1.5 | −1.4 | ||
| 3 | FeSNiCO | CHCoCorrin | FeSNiCH(CO) | CoCorrin | 8.5 | 11.5 |
| 3a | FeSNiCH(CO) | CoCorrin | 5.4 | 12.1 | ||
| 4 | FeSNiCH | CO | FeSNiCH(CO) | −17.5 | −17.7 | |
| 5 | FeSNiCH(CO) | FeSNiacetyl | −10.2 | −13.4 | ||
| 6 | FeSNiacetyl | CoCorrin | FeSNiacetyl | CoCorrin | −3.1 | −7.4 |
| Two-electron reduced | ||||||
| 7 | FeSNi | CHCoCorrin | FeSNiCH | CoCorrin | −12.0 | −15.9 |
| 8 | FeSNiCH | CO | FeSNiCHCO | −18.4 | −17.4 | |
| 9 | FeSNiCH(CO) | FeSNiacetyl | −9.7 | −20.4 | ||
| 20 | 80 | |
|---|---|---|
| A | −1.2 | −2.6 |
| A | 15.6 | 12.6 |
| M2–L | ||
| A–HCOO(HO) | 11.5 | 7.3 |
| A–HCOO(4HO) | 6.1 | 3.0 |
| A–HO(4HO) | 0.6 | 2.2 |
| A–HCOO | 18.8 | 11.9 |
| A–HCOO(HO) | 33.6 | 26.2 |
| A–HCOO(4HO) | 29.0 | 23.3 |
| A–OH(4HO) | 32.6 | 25.5 |
| A–HO(4HO) | 16.0 | 16.8 |
| M2–OH/M2–HO | ||
| A–OH | 9.1 | 7.2 |
| A–OH(4HO) | 8.9 | 7.1 |
| A–OH(4HO) | 17.5 | 14.0 |
| 20 | 80 | 20 | 80 | |
|---|---|---|---|---|
| E | (A/A) | (A/A) | ||
| −0.324 | −0.232 | −1.052 | −0.849 | |
| L=HCOO | ||||
| M2–L | −1.065 | −0.839 | −1.384 | −1.045 |
| M2–L(HO) | −0.960 | −0.755 | −1.479 | −1.152 |
| M2–L(4HO) | −0.894 | −0.701 | −1.532 | −1.231 |
| L=OH | ||||
| M2–L(4HO) | −1.104 | −0.892 | −1.669 | −1.351 |
| L=HO | ||||
| M2–L(4HO) | −0.591 | −0.482 | −1.279 (−0.879 ) | −1.060 (−0.766 ) |
| L=CH | ||||
| M2–CH | −0.071 | |||
| 20 | 80 | |||
|---|---|---|---|---|
| E | (A–L/A–NiH–L) | Expt. | ||
| [79,108] | [44] | |||
| M2-HCOO | ||||
| pH = 6.5 | −0.861 | −0.782 | −0.463 | −0.479 |
| pH = 7.2 | −0.881 | −0.803 | −0.490 | −0.495 |
| pH = 7.9 | −0.902 | −0.824 | −0.547 | −0.524 |
| M2-HCOO(HO) | ||||
| pH = 6.5 | −0.418 | −0.371 | ||
| pH = 7.2 | −0.439 | −0.392 | ||
| pH = 7.9 | −0.460 | −0.413 | ||
| M2-HCOO(4HO) | ||||
| pH = 6.5 | −0.547 | −0.469 | ||
| pH = 7.2 | −0.568 | −0.490 | ||
| pH = 7.9 | −0.588 | −0.510 | ||
| M2-OH(4HO) | ||||
| pH = 6.5 | −0.615 | −0.560 | ||
| pH = 7.2 | −0.637 | −0.580 | ||
| pH = 7.9 | −0.657 | −0.601 | ||
| AOH/AHO | ||||
| pH = 6.5 | −0.445 | |||
| pH = 7.2 | −0.490 | |||
| pH = 7.9 | −0.531 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworska, M.; Lodowski, P. Theoretical Studies of Acetyl-CoA Synthase Catalytic Mechanism. Catalysts 2022, 12, 195. https://doi.org/10.3390/catal12020195
Jaworska M, Lodowski P. Theoretical Studies of Acetyl-CoA Synthase Catalytic Mechanism. Catalysts. 2022; 12(2):195. https://doi.org/10.3390/catal12020195
Chicago/Turabian StyleJaworska, Maria, and Piotr Lodowski. 2022. "Theoretical Studies of Acetyl-CoA Synthase Catalytic Mechanism" Catalysts 12, no. 2: 195. https://doi.org/10.3390/catal12020195
APA StyleJaworska, M., & Lodowski, P. (2022). Theoretical Studies of Acetyl-CoA Synthase Catalytic Mechanism. Catalysts, 12(2), 195. https://doi.org/10.3390/catal12020195







