Skeletal Nickel Catalyst for the Methanation Reaction Developed by Laser-Engineered Net-Shaping Technology
Abstract
:1. Introduction
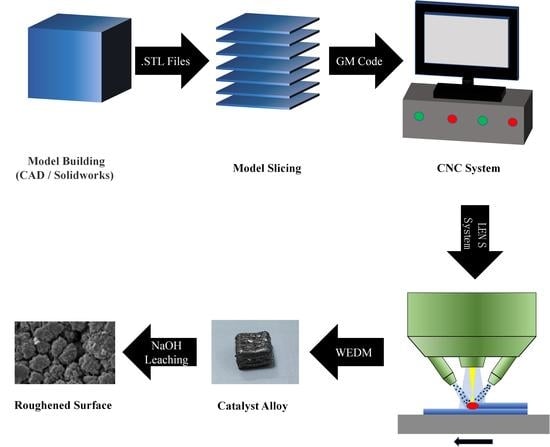
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, W.; Su, M.; Fath, B.D.; Zhang, M.; Hao, Y. A systematic method of evaluation of the Chinese natural gas supply security. Appl. Energy 2016, 165, 858–867. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Production of synthetic natural gas (SNG) from coal and dry biomass—A technology review from 1950 to 2009. Fuel 2010, 89, 1763–1783. [Google Scholar] [CrossRef]
- Lebarbier, V.M.; Dagle, R.A.; Kovarik, L.; Albrecht, K.O.; Li, X.; Li, L.; Taylor, C.E.; Bao, X.; Wang, Y. Sorption-enhanced synthetic natural gas (SNG) production from syngas: A novel process combining CO methanation, water-gas shift, and CO2 capture. Appl. Catal. B: Environ. 2014, 144, 223–232. [Google Scholar] [CrossRef]
- Yi, Q.; Wu, G.-S.; Gong, M.-H.; Huang, Y.; Feng, J.; Hao, Y.-H.; Li, W.-Y. A feasibility study for CO2 recycle assistance with coke oven gas to synthetic natural gas. Appl. Energy 2017, 193, 149–161. [Google Scholar] [CrossRef]
- Raney, M. Catalysts From Alloys. Ind. Eng. Chem. 2002, 32, 1199–1203. [Google Scholar] [CrossRef]
- Covert, L.W.; Adkins, H. Nickel by the Raney Process as a Catalyst of Hydrogenation. J. Am. Chem. Soc. 2002, 54, 4116–4117. [Google Scholar] [CrossRef]
- Lustemberg, P.G.; Ramírez, P.J.; Liu, Z.; Gutiérrez, R.A.; Grinter, D.G.; Carrasco, J.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V. Room-Temperature Activation of Methane and Dry Re-forming with CO2 on Ni-CeO2(111) Surfaces: Effect of Ce3+ Sites and Metal–Support Interactions on C–H Bond Cleavage. ACS Catal. 2016, 6, 8184–8191. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marina, G.B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B Environ. 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, Y.; Li, H.; Li, H. CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability. Green Chem. 2018, 20, 2781–2787. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, Z.L.; Wen, D.S.; Ghadiri, M. Hydrodynamics of gas–solid two-phase mixtures flowing upward through packed beds. Powder Technol. 2005, 153, 13–22. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation–From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Wolf, A.; Turek, T.; Mleczko, L. Structured Raney Nickel Catalysts for Liquid-Phase Hydrogenation. Chem. Eng. Technol. 2016, 39, 1933–1938. [Google Scholar] [CrossRef]
- Rashid, M.U.; Daud, W.M.A.W.; Abbas, H.F. Corrigendum to “Dry reforming of methane: Influence of process parameters—A review” [Renew Sustain Energy Rev 45 (2015) 710–744]. Renew. Sustain. Energy Rev. 2020, 133. [Google Scholar] [CrossRef]
- Mardani, S.; Ojala, L.S.; Uusi-Kyyny, P.; Alopaeus, V. Development of a unique modular distillation column using 3D printing. Chem. Eng. Processing-Process Intensif. 2016, 109, 136–148. [Google Scholar] [CrossRef]
- Duda, T.; Raghavan, L.V. 3D Metal Printing Technology. IFAC-Pap. 2016, 49, 103–110. [Google Scholar] [CrossRef]
- Stuecker, J.N.; Miller, J.E.; Ferrizz, R.E.; Mudd, J.E.; Cesarano, J. Advanced Support Structures for Enhanced Catalytic Activity. Ind. Eng. Chem. Res. 2004, 43, 51–55. [Google Scholar] [CrossRef]
- Lacey, S.D.; Kirsch, D.J.; Li, Y.; Morgenstern, J.T.; Zarket, B.C.; Yao, Y.; Dai, J.; Garcia, L.Q.; Liu, B.; Gao, T.; et al. Extrusion-Based 3D Printing of Hierarchically Porous Advanced Battery Electrodes. Adv. Mater 2018, 30, e1705651. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Moo, J.G.S.; Pumera, M. Helical 3D-Printed Metal Electrodes as Custom-Shaped 3D Platform for Electrochemical Devices. Adv. Funct. Mater. 2016, 26, 698–703. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Thomson, A.R.; Schneider, K.; Cameron, P.J.; Ieropoulos, I. A small-scale air-cathode microbial fuel cell for on-line monitoring of water quality. Biosens Bioelectron 2014, 62, 182–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.D.; Kim, E.Y.; Humes, V.P.; Kizuka, J.; Thompson, L.T. Inkjet printing of carbon supported platinum 3-D catalyst layers for use in fuel cells. J. Power Sources 2007, 171, 101–106. [Google Scholar] [CrossRef]
- Chaparro-Garnica, C.Y.; Jorda-Faus, P.; Bailon-Garcia, E.; Ocampo-Perez, R.; Aguilar-Madera, C.G.; Davo-Quinonero, A.; Lozano-Castello, D.; Bueno-Lopez, A. Customizable Heterogeneous Catalysts: Nonchanneled Advanced Monolithic Supports Manufactured by 3D-Printing for Improved Active Phase Coating Performance. ACS Appl. Mater. Interfaces 2020, 12, 54573–54584. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Xu, G.; Chen, Z.; Liu, C.; Wang, D.; Lao, C. Multiple metals doped polymer-derived SiOC ceramics for 3D printing. Ceram. Int. 2018, 44, 11030–11038. [Google Scholar] [CrossRef]
- Danaci, S.; Protasova, L.; Snijkers, F.; Bouwen, W.; Bengaouer, A.; Marty, P. Innovative 3D-manufacture of structured copper supports post-coated with catalytic material for CO2 methanation. Chem. Eng. Process.-Process Intensif. 2018, 127, 168–177. [Google Scholar] [CrossRef]
- Davo-Quinonero, A.; Sorolla-Rosario, D.; Bailon-Garcia, E.; Lozano-Castello, D.; Bueno-Lopez, A. Improved asymmetrical honeycomb monolith catalyst prepared using a 3D printed template. J. Hazard. Mater. 2019, 368, 638–643. [Google Scholar] [CrossRef]
- Tubío, C.R.; Azuaje, J.; Escalante, L.; Coelho, A.; Guitián, F.; Sotelo, E.; Gil, A. 3D printing of a heterogeneous copper-based catalyst. J. Catal. 2016, 334, 110–115. [Google Scholar] [CrossRef]
- Heßelmann, C.; Wolf, T.; Galgon, F.; Körner, C.; Albert, J.; Wasserscheid, P. Additively manufactured RANEY®-type copper catalyst for methanol synthesis. Catal. Sci. Technol. 2020, 10, 164–168. [Google Scholar] [CrossRef]
- Wolf, T.; Fu, Z.; Ye, J.; Heßelmann, C.; Pistor, J.; Albert, J.; Wasserscheid, P.; Körner, C. Periodic Open Cellular Raney-Copper Catalysts Fabricated via Selective Electron Beam Melting. Adv. Eng. Mater. 2020, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Hu, Y.; Cong, W.; Zhi, L.; Guo, Z. Additive manufacturing of alumina using laser engineered net shaping: Effects of deposition variables. Ceram. Int. 2017, 43, 7768–7775. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.L.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; Wilson-Heid, A.; De, A.; Zhang, W. Additive manufacturing of metallic components–Process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–224. [Google Scholar] [CrossRef]
- Zhi, C.; Yang, W. Improvement of Mo-doping on sulfur-poisoning of Ni catalyst: Activity and selectivity to CO methanation. Comput. Theor. Chem. 2021, 1197. [Google Scholar] [CrossRef]
- Bian, Z.; Meng, X.; Tao, M.; Lv, Y.; Xin, Z. Effect of MoO3 on catalytic performance and stability of the SBA-16 supported Ni-catalyst for CO methanation. Fuel 2016, 179, 193–201. [Google Scholar] [CrossRef]
- Cao, K.; Gong, M.; Yang, J.; Cai, J.; Chu, S.; Chen, Z.; Shan, B.; Chen, R. Nickel catalyst with atomically-thin meshed cobalt coating for improved durability in dry reforming of methane. J. Catal. 2019, 373, 351–360. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Z.; Gu, F.; Wang, X.; Lu, X.; Li, H.; Xu, G.; Su, F. CO methanation on ordered mesoporous Ni–Cr–Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232. [Google Scholar] [CrossRef]
- Krishna, B.V.; Xue, W.; Bose, S.; Bandyopadhyay, A. Functionally graded Co-Cr-Mo coating on Ti-6Al-4V alloy structures. Acta Biomater. 2008, 4, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.D.; Moon, M.J.; Park, J.H.; Park, S.S.; Hong, S.S. Raney Ni Catalysts Derived from Different Alloy Precursors Part II. CO and CO2 Methanation Activity. Korean J. Chem. Eng. 2005, 22, 541–546. [Google Scholar] [CrossRef]









| Batch Number | Powder Feeder Speed (r/min) | |
|---|---|---|
| Ni | Al-CoCrMo | |
| 1 | 2 | 2 |
| 2 | 2 | 3 |
| 3 | 2 | 4 |
| 4 | 3 | 2 |
| 5 | 4 | 2 |
| Temperature (°C) | Leaching Time (h) |
|---|---|
| 40 | 2 |
| 40 | 6 |
| 40 | 12 |
| 40 | 18 |
| 20 | 2 |
| 60 | 2 |
| 80 | 2 |
| 80 | 12 |
| XRF | Nominal | |
|---|---|---|
| Element | Mass% | Mass% |
| Al | 59.13 | 64.00 |
| Ni | 34.06 | 32.00 |
| Co | 1.87 | 2.60 |
| Cr | 1.49 | 1.20 |
| Mo | 0.46 | 0.20 |
| others | 0.30 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, K.; Lin, Z.; Li, X.; Chen, M.; Wang, X.; Wang, Y.; Wang, J.; Jiang, H. Skeletal Nickel Catalyst for the Methanation Reaction Developed by Laser-Engineered Net-Shaping Technology. Catalysts 2022, 12, 208. https://doi.org/10.3390/catal12020208
Yan K, Lin Z, Li X, Chen M, Wang X, Wang Y, Wang J, Jiang H. Skeletal Nickel Catalyst for the Methanation Reaction Developed by Laser-Engineered Net-Shaping Technology. Catalysts. 2022; 12(2):208. https://doi.org/10.3390/catal12020208
Chicago/Turabian StyleYan, Kuo, Zaiwen Lin, Xu Li, Meng Chen, Xiaolong Wang, Yuren Wang, Jun Wang, and Heng Jiang. 2022. "Skeletal Nickel Catalyst for the Methanation Reaction Developed by Laser-Engineered Net-Shaping Technology" Catalysts 12, no. 2: 208. https://doi.org/10.3390/catal12020208