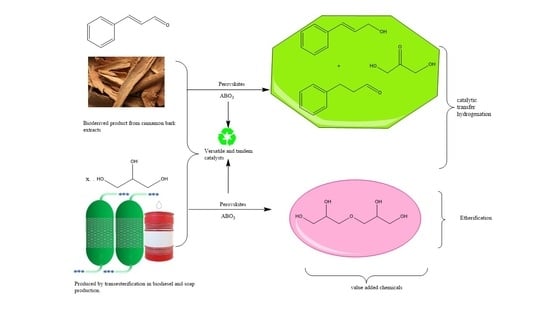
The Inorganic Perovskite-Catalyzed Transfer Hydrogenation of Cinnamaldehyde Using Glycerol as a Hydrogen Donor
Abstract
:1. Introduction
2. Results and Discussions
2.1. Catalyst’s Characterization
2.2. Catalytic Performance
2.2.1. Reaction Optimization
2.2.2. Recyclability Tests
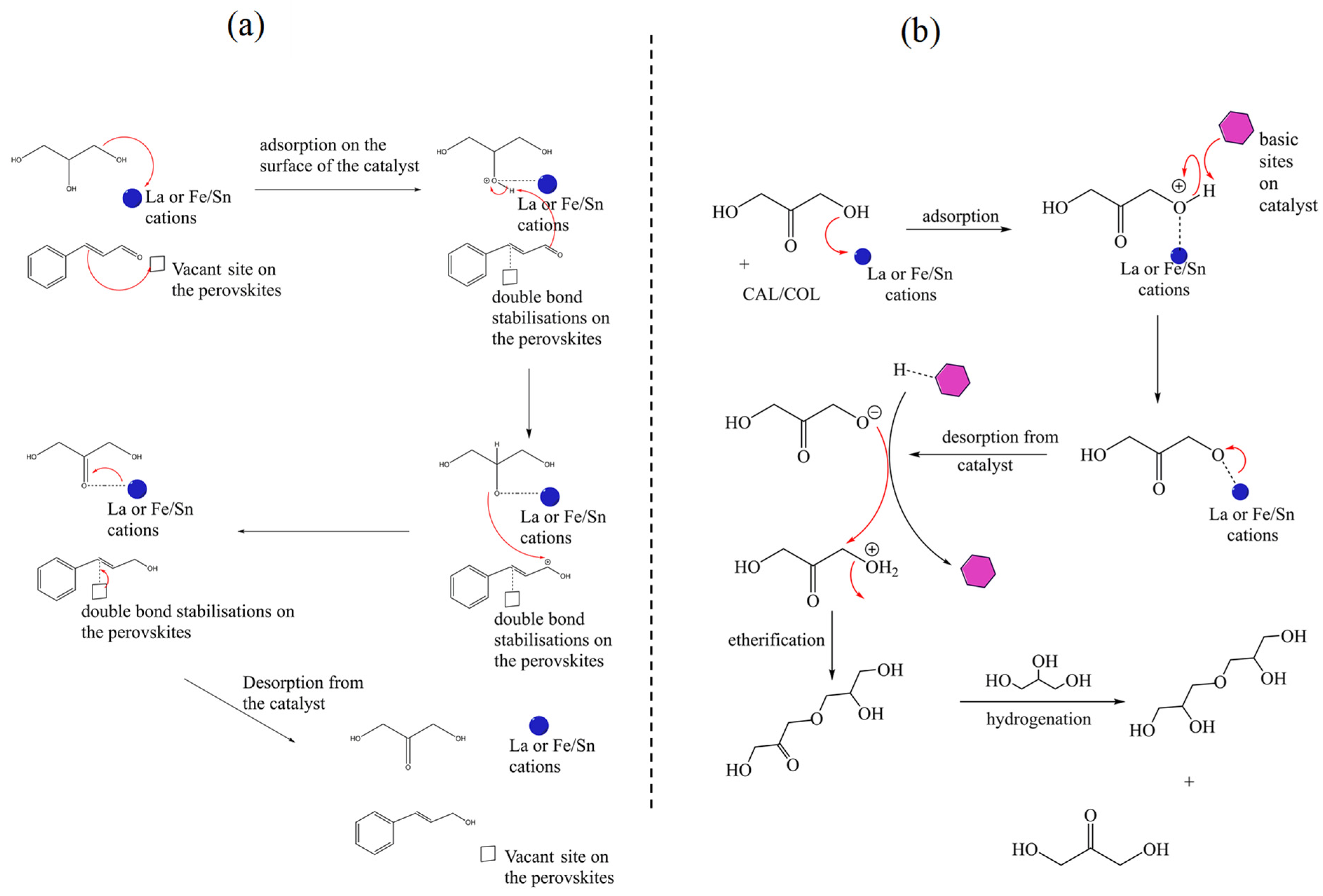
2.3. Proposed Reaction Mechanism
3. Experimental
3.1. Utilized Reagents
3.2. Catalyst Preparation
3.2.1. Synthesis of the Hard-Template and Perovskites (LaSnO3 and LaFeO3)
3.2.2. Synthesis of SnO2
3.3. Catalyst Characterization
3.4. Catalytic Studies
3.5. Recyclability Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Y.; Zhang, Z.; Wang, H.; Wang, Y. Toward efficient single-atom catalysts for renewable fuels and chemicals production from biomass and CO2. Appl. Catal. B Environ. 2021, 292, 120162. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Kalinke, C.; Rocha, R.G.; Dos Santos, L.; Rocha, D.P.; Oliveira, P.R.; Janegitz, B.C.; Bonacin, J.A.; Richter, E.M.; Munoz, R.A.A. Additive-manufactured (3D-printed) electrochemical sensors: A critical review. Anal. Chim. Acta 2020, 1118, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Su, J.; Liu, Y.; Yang, L. Application of Hydrothermal Reactions to Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 2014; pp. 109–138. [Google Scholar] [CrossRef]
- Jin, F.; Wang, Y.; Zeng, X.; Shen, Z.; Yao, G. Water under High Temperature and Pressure Conditions and Its Applications to Develop Green Technologies for Biomass Conversion. In Application of Hydrothermal Reactions to Biomass Conversion; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–28. [Google Scholar] [CrossRef]
- Asomaning, J.; Haupt, S.; Chae, M.; Bressler, D.C. Recent developments in microwave-assisted thermal conversion of biomass for fuels and chemicals. Renew. Sustain. Energy Rev. 2018, 92, 642–657. [Google Scholar] [CrossRef]
- Wang, G.H.; Deng, X.; Gu, D.; Chen, K.; Tüysüz, H.; Spliethoff, B.; Bongard, H.J.; Weidenthaler, C.; Schmidt, W.; Schüth, F. Co3O4 Nanoparticles Supported on Mesoporous Carbon for Selective Transfer Hydrogenation of α,β-Unsaturated Aldehydes. Angew. Chem. 2016, 55, 11101–11105. [Google Scholar] [CrossRef] [PubMed]
- Gilkey, M.J.; Panagiotopoulou, P.; Mironenko, A.V.; Jenness, G.R.; Vlachos, D.G.; Xu, B. Mechanistic Insights into Metal Lewis Acid-Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran. ACS Catal. 2015, 5, 3988–3994. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Rinaldi, R. Exploiting H-transfer reactions with RANEY® Ni for upgrade of phenolic and aromatic biorefinery feeds under unusual, low-severity conditions. Energy Environ. Sci. 2012, 5, 8244–8260. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zhou, H.; Fu, Y. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol over Nitrogen-Doped Carbon-Supported Iron Catalysts. ChemSusChem 2016, 9, 1339–1347. [Google Scholar] [CrossRef]
- Xiao, P.; Zhu, J.; Zhao, D.; Zhao, Z.; Zaera, F.; Zhu, Y. Porous LaFeO3 Prepared by an in Situ Carbon Templating Method for Catalytic Transfer Hydrogenation Reactions. ACS Appl. Mater. Interfaces 2019, 11, 15517–15527. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z. Catalytic Transfer Hydrogenation of Furfural into Furfuryl Alcohol over Magnetic γ-Fe2O3@HAP Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 942–947. [Google Scholar] [CrossRef]
- Wolfson, A.; Dlugy, C.; Shotland, Y.; Tavor, D. Glycerol as solvent and hydrogen donor in transfer hydrogenation–dehydrogenation reactions. Tetrahedron Lett. 2009, 50, 5951–5953. [Google Scholar] [CrossRef]
- Tavor, D.; Gefen, I.; Dlugy, C.; Wolfson, A. Transfer hydrogenations of nitrobenzene using glycerol as solvent and hydrogen donor. Synth. Commun. 2011, 41, 3409–3416. [Google Scholar] [CrossRef]
- Bewana, S.; Ndolomingo, M.J.; Carleschi, E.; Doyle, B.P.; Meijboom, R.; Bingwa, N. Inorganic Perovskite-Induced Synergy on Highly Selective Pd-Catalyzed Hydrogenation of Cinnamaldehyde. ACS Appl. Mater. Interfaces 2019, 11, 32994–33005. [Google Scholar] [CrossRef] [PubMed]
- Breen, J.P.; Burch, R.; Gomez-Lopez, J.; Griffin, K.; Hayes, M. Steric effects in the selective hydrogenation of cinnamaldehyde to cinnamyl alcohol using an Ir/C catalyst. Appl. Catal. A Gen. 2004, 268, 267–274. [Google Scholar] [CrossRef]
- Tuley, W.F.; Adams, R. The reduction of cinnamic aldehyde to cinnamyl alcohol in the presence of platinum-oxide platinum black and promoters. XI. J. Am. Chem. Soc. 1925, 47, 3061–3068. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Surkus, A.E.; Junge, H.; Pohl, M.M.; Radnik, J.; Rabeah, J.; Huan, H.; Schünemann, V.; Brückner, A.; Beller, M. Nanoscale Fe2O3-based catalysts for selective hydrogenation of nitroarenes to anilines. Science 2013, 342, 1073–1076. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Natte, K.; Junge, H.; Beller, M. Nitrogen-Doped Graphene-Activated Iron-Oxide-Based Nanocatalysts for Selective Transfer Hydrogenation of Nitroarenes. ACS Catal. 2015, 5, 1526–1529. [Google Scholar] [CrossRef]
- Jagadeesh, R.V.; Wienhöfer, G.; Westerhaus, F.A.; Surkus, A.E.; Pohl, M.M.; Junge, H.; Junge, K.; Beller, M. Efficient and highly selective iron-catalyzed reduction of nitroarenes. Chem. Commun. 2011, 47, 10972–10974. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, X.; Lu, C.; Ma, L.; Zhang, Q. Water-improved heterogeneous transfer hydrogenation using methanol as hydrogen donor over Pd-based catalyst. Appl. Catal. A Gen. 2010, 375, 289–294. [Google Scholar] [CrossRef]
- Nzuzo, Y.; Adeyinka, A.; Carleschi, E.; Doyle, B.P.; Bingwa, N. Effect of d z2 orbital electron-distribution of La-based inorganic perovskites on surface kinetics of a model reaction. Inorg. Chem. Front. 2021, 8, 3037–3048. [Google Scholar] [CrossRef]
- Mhlwatika, Z.; Meijboom, R.; Bingwa, N. Nanocasted perovskites as potential catalysts for acetalization of glycerol. Inorg. Chem. Commun. 2021, 133, 108962. [Google Scholar] [CrossRef]
- Petunchi, J.O.O.; Ulla, M.A.A.; Marcos, J.A.A.; Lombardo, E.A.A. Characterization of hydrogenation active sites on LaCoO3 perovskite. J. Catal. 1981, 70, 356–363. [Google Scholar] [CrossRef]
- Wu, Y.; Cordier, C.; Berrier, E.; Nuns, N.; Dujardin, C.; Granger, P. Surface reconstructions of LaCo1-xFexO3 at high temperature during N2O decomposition in realistic exhaust gas composition: Impact on the catalytic properties. Appl. Catal. B Environ. 2013, 140–141, 151–163. [Google Scholar] [CrossRef]
- Basile, F.; Brenna, G.; Fornasari, G.; Del Gallo, P.; Gary, D.; Vaccari, A. Perovskite-Type Catalysts for the Water-Gas-Shift Reaction. In Studies in Surface Science and Catalysis, Scientific Bases for the Preparation of Heterogeneous Catalysts, Proceedings of the 10th International Symposium, Louvain-la-Neuve, Belgium, 11–15 July 2010; Studies in Surface Science and Catalysis Series; Elsevier Inc.: Amsterdam, The Netherlands, 2010; Volume 175, pp. 471–474. [Google Scholar]
- Guangchuan, L.; Shuguang, L.; Changlong, L.; Xiuqin, O. Preparation of Nanometer La1-x SrxMnO3 and Application in Air-Cathode. J. Rare Earths 2007, 25, 264–267. [Google Scholar] [CrossRef]
- Chen, W.; Chen, X.; Yang, Y.; Yuan, J.; Shangguan, W. Synthesis and performance of layered perovskite-type H-ABi 2Ta2O9 (A = Ca, Sr, Ba, K0.5La 0.5) for photocatalytic water splitting. Int. J. Hydrog. Energy 2014, 39, 13468–13473. [Google Scholar] [CrossRef]
- He, F.; Li, X.; Zhao, K.; Huang, Z.; Wei, G.; Li, H. The use of La1-xSrxFeO3 perovskite-type oxides as oxygen carriers in chemical-looping reforming of methane. Fuel 2013, 108, 465–473. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Trawczynski, J. Potassium-copper perovskite catalysts for mild temperature diesel soot combustion. Appl. Catal. A Gen. 2014, 485, 214–221. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]
- Xiao, P.; Xu, X.; Zhu, J.; Zhu, Y. In situ generation of perovskite oxides and carbon composites: A facile, effective and generalized route to prepare catalysts with improved performance. J. Catal. 2020, 383, 88–96. [Google Scholar] [CrossRef]
- Han, S.; Shi, Y.; Wang, C.; Liu, C.; Zhang, B. Hollow cobalt sulfide nanocapsules for electrocatalytic selective transfer hydrogenation of cinnamaldehyde with water. Cell Rep. Phys. Sci. 2021, 2, 100337. [Google Scholar] [CrossRef]
- Zhang, M.; Su, X.; Ma, L.; Khan, A.; Wang, L.; Wang, J.; Maloletnev, A.S.; Yang, C. Promotion effects of halloysite nanotubes on catalytic activity of Co3O4 nanoparticles toward reduction of 4-nitrophenol and organic dyes. J. Hazard. Mater. 2021, 403, 123870. [Google Scholar] [CrossRef] [PubMed]
- Santi, N.; Morrill, L.C.; Świderek, K.; Moliner, V.; Luk, L.Y.P. Transfer hydrogenations catalyzed by streptavidin-hosted secondary amine organocatalysts. Chem. Commun. 2021, 57, 1919–1922. [Google Scholar] [CrossRef] [PubMed]
- Xantini, Z.; Erasmus, E. Platinum supported on nanosilica and fibrous nanosilica for hydrogenation reactions. Polyhedron 2021, 193, 114769. [Google Scholar] [CrossRef]
- Xin, H.; Zhang, W.; Xiao, X.; Chen, L.; Wu, P.; Li, X. Selective hydrogenation of cinnamaldehyde with NixFe1-xAl2O4+δ composite oxides supported Pt catalysts: CO versus CC selectivity switch by varying the Ni/Fe molar ratios. J. Catal. 2021, 393, 126–139. [Google Scholar] [CrossRef]
- Bingwa, N.; Ndolomingo, M.J.; Mabate, T.; Dube, S.; Meijboom, R. Surface Property-Activity Relations of Co/Sn Oxide Nanocatalysts Evaluated Using a Model Reaction: Surface Characterization Study. Catal. Lett. 2019, 149, 2940–2949. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Wang, L.; Jin, Y. Preparation, characterization and catalytic application of hierarchically porous LaFeO3 from a pomelo peel template. Inorg. Chem. Front. 2017, 4, 994–1002. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Q.; Wan, F.; Gao, T. Gas sensing properties and mechanism of Nano-SnO2-based sensor for hydrogen and carbon monoxide. J. Nanomater. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Schüth, F. The Evolution of Ordered Mesoporous Materials. In Mesoporous Crystals and Related Nano-Structured Materials, Proceedings of the Meeting on Mesoporous Crystals and Related Nano-Structured Materials, Stockholm, Sweden, 1–5 June 2004; Studies in Surface Science and Catalysis Series; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Volume 148, pp. 1–13. [Google Scholar]
- Afzal, S.; Quan, X.; Zhang, J. High surface area mesoporous nanocast LaMO3 (M = Mn, Fe) perovskites for efficient catalytic ozonation and an insight into probable catalytic mechanism. Appl. Catal. B Environ. 2017, 206, 692–703. [Google Scholar] [CrossRef]
- Manjunathan, P.; Marakatti, V.S.; Chandra, P.; Kulal, A.B.; Umbarkar, S.B.; Ravishankar, R.; Shanbhag, G.V. Mesoporous tin oxide: An efficient catalyst with versatile applications in acid and oxidation catalysis. Catal. Today 2018, 309, 61–76. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Li, C.; Tan, L.; Wei, Z. High Selective Electrochemical Hydrogenation of Cinnamaldehyde to Cinnamyl Alcohol on RuO2-SnO2-TiO2/Ti Electrode. ACS Catal. 2019, 9, 11307–11316. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic transfer hydrogenation/hydrogenolysis for reductive upgrading of furfural and 5-(hydroxymethyl)furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Khayoon, M.S.; Abdullah, A.Z. Synthesis of oxygenated fuel additives via the solventless etherification of glycerol. Bioresour. Technol. 2012, 112, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Jie Wei, W.; Ahmad, M.; Mathialagan, R.; Farrukh, S.; Danish, M.; Ullah, S.; Raza Naqvi, S. Glycerol Conversion to Diglycerol via Etherification under Microwave Irradiation. In Apolipoproteins, Triglycerides and Cholesterol; IntechOpen: London, UK, 2020; pp. 2–4. [Google Scholar]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erné, B.H.; Weckhuysen, B.M. Glycerol etherification over highly active CaO-based materials: New mechanistic aspects and related colloidal particle formation. Chem. Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]










| Entry | Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Reference |
|---|---|---|---|---|---|
| 1 | KIT-6 (SiO2) | 625 | 0.67 | 4.3 | This work |
| 2 | LaFeO3 | 135 | 0.37 | 11.0 | |
| 3 | LaSnO3 | 687 | 1.91 | 9.9 | |
| 4 | SnO2 | 32 | 0.11 | 13.1 | |
| 5 | KIT-6 (SiO2) | 772 | 0.74 | 5.2 | [15] |
| 7 | LaFeO3 | 92 | 0.33 | 7.7 | [42] |
| 8 | SnO2 | 50 | 0.06 | 5.3 | [43] |
| Entry | Catalyst | Reaction Time (h) | Basicity (a.u) | Temperature °C | % Conv. (a) | HCAL % Selec. (b) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | LaFeO3 | 6 | 0.369 | 180 | 99.0 | 82.1 | This work |
| 2 | LaSnO3 | 6 | 0.636 | 180 | 99.3 | 4.9 | |
| 3 | SnO2 | 6 | 0.324 | 180 | 98.7 | 22.1 | |
| 4 | LaFeO3 | 3 | - | 80 | 21.8 | 100 | [15] |
| 5 | Ru0.05Sn0.25Ti0.7O2/Ti | - | - | 30 | 86.2 | 19.3 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabate, T.P.; Meijboom, R.; Bingwa, N. The Inorganic Perovskite-Catalyzed Transfer Hydrogenation of Cinnamaldehyde Using Glycerol as a Hydrogen Donor. Catalysts 2022, 12, 241. https://doi.org/10.3390/catal12020241
Mabate TP, Meijboom R, Bingwa N. The Inorganic Perovskite-Catalyzed Transfer Hydrogenation of Cinnamaldehyde Using Glycerol as a Hydrogen Donor. Catalysts. 2022; 12(2):241. https://doi.org/10.3390/catal12020241
Chicago/Turabian StyleMabate, Tafadzwa Precious, Reinout Meijboom, and Ndzondelelo Bingwa. 2022. "The Inorganic Perovskite-Catalyzed Transfer Hydrogenation of Cinnamaldehyde Using Glycerol as a Hydrogen Donor" Catalysts 12, no. 2: 241. https://doi.org/10.3390/catal12020241
APA StyleMabate, T. P., Meijboom, R., & Bingwa, N. (2022). The Inorganic Perovskite-Catalyzed Transfer Hydrogenation of Cinnamaldehyde Using Glycerol as a Hydrogen Donor. Catalysts, 12(2), 241. https://doi.org/10.3390/catal12020241