Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials
Abstract
:1. Introduction
2. Endocrine Disrupting Compounds (EDCs)
2.1. Historical Definition and Regulatory Evolution
2.2. Main Natural and Synthetic EDCs
2.3. Main EDC Sources as Risks for Natural Ecosystems
3. Chemical Degradation Approaches
3.1. Photocatalytic Processes
3.2. Other Heterogeneous Catalytic Processes
4. Metal Oxide-Based Nanomaterials for EDCs Removal
4.1. Photocatalytic and Catalytic Degradation of Plastic Components and Additives
4.1.1. Bisphenol A
4.1.2. Phthalates
4.2. Photocatalytic and Catalytic Degradation of Agricultural Chemicals
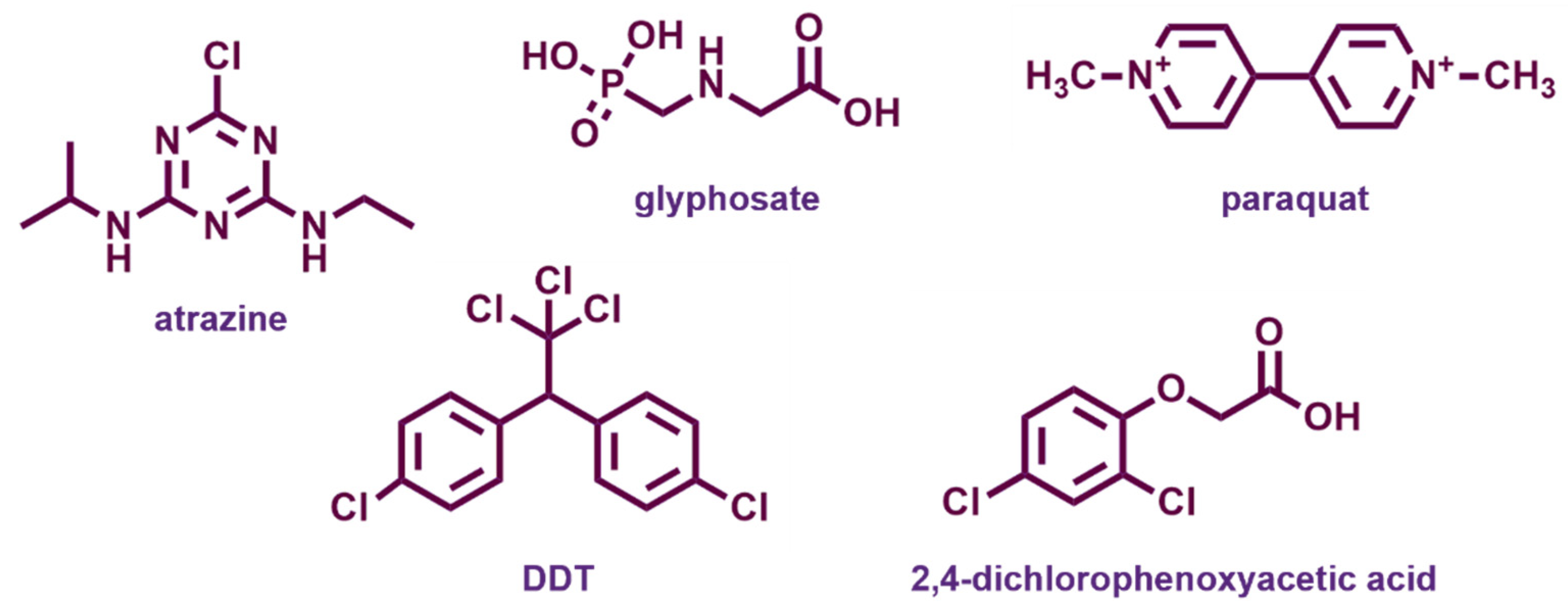
4.2.1. Atrazine
4.2.2. Glyphosate
4.2.3. Paraquat
4.2.4. Chlorinated Phenoxyalkanoic Herbicides
4.2.5. Other Pesticides
4.3. Photocatalytic and Catalytic Degradation of Pharmaceuticals and Personal Care Products
4.3.1. Triclosan
4.3.2. Parabens
4.3.3. Steroid Hormones
4.3.4. Other Pharmaceuticals
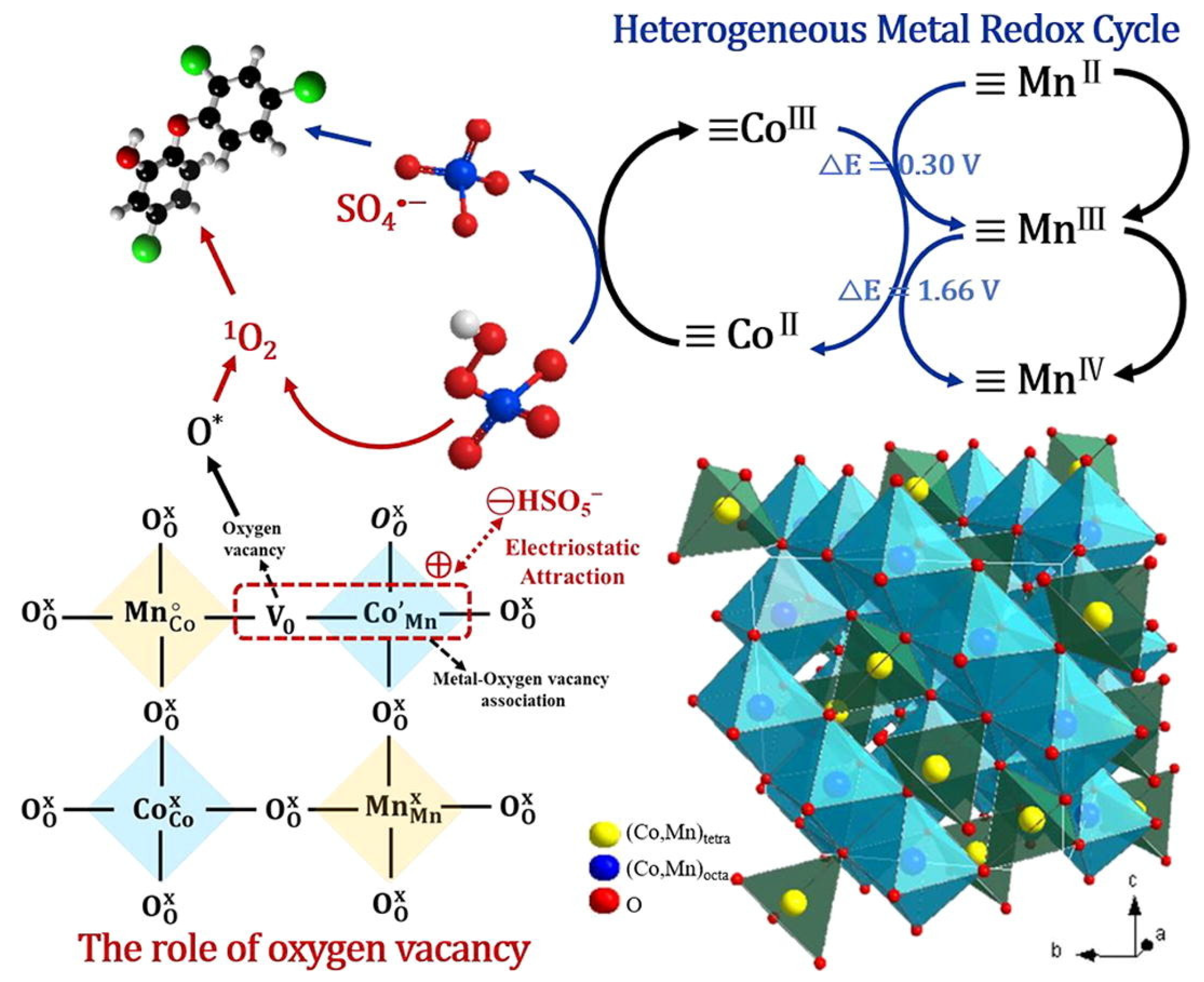
4.4. Degradation of Other EDCs
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Vieira, W.T.; De Farias, M.B.; Spaolonzi, M.P.; Da Silva, M.G.C.; Vieira, M.G.A. Latest Advanced Oxidative Processes Applied for the Removal of Endocrine Disruptors from Aqueous Media—A Critical Report. J. Environ. Chem. Eng. 2021, 9, 105748. [Google Scholar] [CrossRef]
- Vieira, W.T.; De Farias, M.B.; Spaolonzi, M.P.; Da Silva, M.G.C.; Vieira, M.G.A. Endocrine-Disrupting Compounds: Occurrence, Detection Methods, Effects and Promising Treatment Pathways—A Critical Review. J. Environ. Chem. Eng. 2021, 9, 104558. [Google Scholar] [CrossRef]
- Pironti, C.; Ricciardi, M.; Proto, A.; Bianco, P.M.; Montano, L.; Motta, O. Endocrine-Disrupting Compounds: An Overview on Their Occurrence in the Aquatic Environment and Human Exposure. Water 2021, 13, 1347. [Google Scholar] [CrossRef]
- Kabir, E.R.; Rahman, M.S.; Rahman, I. A Review on Endocrine Disruptors and Their Possible Impacts on Human Health. Environ. Toxicol. Pharmacol. 2015, 40, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Schjenken, J.E.; Green, E.S.; Overduin, T.S.; Mah, C.Y.; Russell, D.L.; Robertson, S.A. Endocrine Disruptor Compounds—A Cause of Impaired Immune Tolerance Driving Inflammatory Disorders of Pregnancy? Front. Endocrinol. 2021, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Kang, S.; Xiong, R.; Chen, M. Environment-Friendly Removal Methods for Endocrine Disrupting Chemicals. Sustainability 2020, 12, 7615. [Google Scholar] [CrossRef]
- Medhi, R.; Marquez, M.D.; Lee, T.R. Visible-Light-Active Doped Metal Oxide Nanoparticles: Review of Their Synthesis, Properties, and Applications. ACS Appl. Nano Mater. 2020, 3, 6156–6185. [Google Scholar] [CrossRef]
- Yoon, Y.; Truong, P.L.; Lee, D.; Ko, S.H. Metal-Oxide Nanomaterials Synthesis and Applications in Flexible and Wearable Sensors. ACS Nanosci. Au 2021. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of Bioinspired Metal and Metal Oxide Nanoparticles in Biomedical Sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef]
- Costantini, A.; Venezia, V.; Pota, G.; Bifulco, A.; Califano, V.; Sannino, F. Adsorption of Cellulase on Wrinkled Silica Nanoparticles with Enhanced Inter-Wrinkle Distance. Nanomaterials 2020, 10, 1799. [Google Scholar] [CrossRef]
- Selvaraj, M.; Hai, A.; Banat, F.; Haija, M.A. Application and Prospects of Carbon Nanostructured Materials in Water Treatment: A Review. J. Water Process Eng. 2020, 33, 100996. [Google Scholar] [CrossRef]
- Ojha, A.; Tiwary, D.; Oraon, R.; Singh, P. Degradations of Endocrine-Disrupting Chemicals and Pharmaceutical Compounds in Wastewater with Carbon-Based Nanomaterials: A Critical Review. Environ. Sci. Pollut. Res. 2021, 28, 30573–30594. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Astruc, D. Nanocatalysts and Other Nanomaterials for Water Remediation from Organic Pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Mehmood, S.; Tang, H.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Mitigation of Environmentally-Related Hazardous Pollutants from Water Matrices Using Nanostructured Materials—A Review. Chemosphere 2020, 253, 126770. [Google Scholar] [CrossRef] [PubMed]
- González-González, R.B.; Parra-Arroyo, L.; Parra-Saldívar, R.; Ramirez-Mendoza, R.A.; Iqbal, H.M.N. Nanomaterial-Based Catalysts for the Degradation of Endocrine-Disrupting Chemicals—A Way Forward to Environmental Remediation. Mater. Lett. 2022, 308, 131217. [Google Scholar] [CrossRef]
- Colborn, T.; vom Saal, F.S.; Soto, A.M. Developmental Effects of Endocrine-Disrupting Chemicals in Wildlife and Humans. Environ. Health Perspect. 1993, 101, 378–384. [Google Scholar] [CrossRef]
- Dirac, P.; Kapitza, P.; Zichichi, A. Statement from the Work Session on Environmental Endocrine-Disrupting Chemical: Neural, Endocrine and Behavioral Effects. Toxicol. Ind. Health 1998, 14, 1–8. [Google Scholar]
- Short, P. Statement from the Work Session on Health Effects of Contemporary-Use Pesticides: The Wildlife/Human Connection. Toxicol. Ind. Health 1999, 15, 1–5. [Google Scholar] [CrossRef]
- Colborn, T.; Clement, C. Chemically-Induced Alterations in Sexual and Functional Development; Princeton Scientific Pub. Co.: Princeton, NJ, USA, 1992. [Google Scholar]
- Thomas Zoeller, R.; Brown, T.R.; Doan, L.L.; Gore, A.C.; Skakkebaek, N.E.; Soto, A.M.; Woodruff, T.J.; Vom Saal, F.S. Endocrine-Disrupting Chemicals and Public Health Protection: A Statement of Principles from the Endocrine Society. Endocrinology 2012, 153, 4097–4110. [Google Scholar] [CrossRef]
- Register-Notices. Available online: https://Www.Epa.Gov/Endocrine-Disruption/Endocrine-Disruptor-Screening-Program-Edsp-1998-Federal (accessed on 28 December 2021).
- Chemicals Strategy for Sustainability towards a Toxic-Free Environment; European Commision: Brussels, Belgium, 2020.
- La Merrill, M.A.; Vandenberg, L.N.; Smith, M.T.; Goodson, W.; Browne, P.; Patisaul, H.B.; Guyton, K.Z.; Kortenkamp, A.; Cogliano, V.J.; Woodruff, T.J.; et al. Consensus on the Key Characteristics of Endocrine-Disrupting Chemicals as a Basis for Hazard Identification. Nat. Rev. Endocrinol. 2020, 16, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. A Review of Phytoestrogens: Their Occurrence and Fate in the Environment. Water Res. 2010, 44, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y. Endocrine Disruptor Compounds (EDCs) and Agriculture: The Case of Pesticides. C. R. Biol. 2017, 340, 406–409. [Google Scholar] [CrossRef] [PubMed]
- González-Davis, O.; Chauhan, K.; Zapian-Merino, S.J.; Vazquez-Duhalt, R. Bi-Enzymatic Virus-like Bionanoreactors for the Transformation of Endocrine Disruptor Compounds. Int. J. Biol. Macromol. 2020, 146, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Dichiarante, V.; Cavallo, G.; Metrangolo, P. Endocrine-Disrupting Pollutants Properties Affecting Their Bioactivity, Remediation, and Detection. Curr. Opin. Green Sustain. Chem. 2021, 30, 100485. [Google Scholar] [CrossRef]
- Barra, R.O.; Chiang, G.; Saavedra, M.F.; Orrego, R.; Servos, M.R.; Hewitt, L.M.; McMaster, M.E.; Bahamonde, P.; Tucca, F.; Munkittrick, K.R. Endocrine Disruptor Impacts on Fish from Chile: The Influence of Wastewaters. Front. Endocrinol. 2021, 12, 208. [Google Scholar] [CrossRef]
- Al Sharabati, M.; Abokwiek, R.; Al-Othman, A.; Tawalbeh, M.; Karaman, C.; Orooji, Y.; Karimi, F. Biodegradable Polymers and Their Nano-Composites for the Removal of Endocrine-Disrupting Chemicals (EDCs) from Wastewater: A Review. Environ. Res. 2021, 202, 111694. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-Driven Advanced Oxidation Processes in the Disposal of Emerging Pharmaceutical Contaminants in Aqueous Media: A Brief Review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs New Advanced Treatment Methods for the Removal of Contaminants of Emerging Concern from Urban Wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Canle, M.; Fernández Pérez, M.I.; Santaballa, J.A. Photocatalyzed Degradation/Abatement of Endocrine Disruptors. Curr. Opin. Green Sustain. Chem. 2017, 6, 101–138. [Google Scholar] [CrossRef]
- Ushavipinachandran, V.; Rajendran, S.; Badagoppam Haroon, K.H.; Ashokan, I.; Mondal, A.; Bhunia, S.K. Detoxification of Endocrine Disruptors in Water Using Visible-Light-Active Nanostructures: A Review. ACS Appl. Nano Mater. 2020, 3, 11659–11687. [Google Scholar] [CrossRef]
- Wang, R.; Ma, X.; Liu, T.; Li, Y.; Song, L.; Tjong, S.C.; Cao, L.; Wang, W.; Yu, Q.; Wang, Z. Degradation Aspects of Endocrine Disrupting Chemicals: A Review on Photocatalytic Processes and Photocatalysts. Appl. Catal. A Gen. 2020, 597, 117547. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, V.; Kumar, A.; Krishnan, V. Nanomaterials for Photocatalytic Decomposition of Endocrine Disruptors in Water. In Nanostructured Materials for Environmental Applications; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 299–320. [Google Scholar]
- Velempini, T.; Prabakaran, E.; Pillay, K. Recent Developments in the Use of Metal Oxides for Photocatalytic Degradation of Pharmaceutical Pollutants in Water—A Review. Mater. Today Chem. 2021, 19, 100380. [Google Scholar] [CrossRef]
- Luciani, G.; Imparato, C.; Vitiello, G. Photosensitive Hybrid Nanostructured Materials: The Big Challenges for Sunlight Capture. Catalysts 2020, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.; Li, Y.; Sharma, P.R.; He, H.; Sharma, S.K.; Wang, R.; Hsiao, B.S. A Study of TiO2 Nanocrystal Growth and Environmental Remediation Capability of TiO2/CNC Nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Shen, H.; Shuai, D. Simultaneous Coupling of Photocatalytic and Biological Processes: A Promising Synergistic Alternative for Enhancing Decontamination of Recalcitrant Compounds in Water. Chem. Eng. J. 2021, 403, 126365. [Google Scholar] [CrossRef]
- Yu, M.; Wang, J.; Tang, L.; Feng, C.; Liu, H.; Zhang, H.; Peng, B.; Chen, Z.; Xie, Q. Intimate Coupling of Photocatalysis and Biodegradation for Wastewater Treatment: Mechanisms, Recent Advances and Environmental Applications. Water Res. 2020, 175, 115673. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A Versatile Strategy to Eliminate Emerging Contaminants from the Aqueous Environment: Heterogeneous Fenton Process. J. Clean. Prod. 2021, 278, 124014. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent Advances in Nano-Fenton Catalytic Degradation of Emerging Pharmaceutical Contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-Mediated Activation of Persulfate and Peroxymonosulfate in Both Homogeneous and Heterogeneous Ways: A Review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Duan, X.; Yang, S.; Wacławek, S.; Fang, G.; Xiao, R.; Dionysiou, D.D. Limitations and Prospects of Sulfate-Radical Based Advanced Oxidation Processes. J. Environ. Chem. Eng. 2020, 8, 103849. [Google Scholar] [CrossRef]
- Xia, X.; Zhu, F.; Li, J.; Yang, H.; Wei, L.; Li, Q.; Jiang, J.; Zhang, G.; Zhao, Q. A Review Study on Sulfate-Radical-Based Advanced Oxidation Processes for Domestic/Industrial Wastewater Treatment: Degradation, Efficiency, and Mechanism. Front. Chem. 2020, 8, 592056. [Google Scholar] [CrossRef]
- Sannino, F.; Pirozzi, D.; Vitiello, G.; D’Errico, G.; Aronne, A.; Fanelli, E.; Pernice, P. Oxidative Degradation of Phenanthrene in the Absence of Light Irradiation by Hybrid ZrO2-Acetylacetonate Gel-Derived Catalyst. Appl. Catal. B Environ. 2014, 156–157, 101–107. [Google Scholar] [CrossRef]
- Sannino, F.; Pernice, P.; Imparato, C.; Aronne, A.; D’Errico, G.; Minieri, L.; Perfetti, M.; Pirozzi, D. Hybrid TiO2-Acetylacetonate Amorphous Gel-Derived Material with Stably Adsorbed Superoxide Radical Active in Oxidative Degradation of Organic Pollutants. RSC Adv. 2015, 5, 93831–93839. [Google Scholar] [CrossRef]
- Pirozzi, D.; Imparato, C.; D’Errico, G.; Vitiello, G.; Aronne, A.; Sannino, F. Three-Year Lifetime and Regeneration of Superoxide Radicals on the Surface of Hybrid TiO2 Materials Exposed to Air. J. Hazard. Mater. 2020, 387, 121716. [Google Scholar] [CrossRef]
- Imparato, C.; Passaro, J.; Bifulco, A.; Branda, F.; Pirozzi, D.; Aronne, A. Development of Hybrid Titanium Oxide-Based Systems for the Surface Stabilization of Reactive Oxygen Radicals. Chem. Eng. Trans. 2021, 84, 139–144. [Google Scholar] [CrossRef]
- Ritacco, I.; Imparato, C.; Falivene, L.; Cavallo, L.; Magistrato, A.; Caporaso, L.; Farnesi Camellone, M.; Aronne, A. Spontaneous Production of Ultrastable Reactive Oxygen Species on Titanium Oxide Surfaces Modified with Organic Ligands. Adv. Mater. Interfaces 2021, 8, 2100629. [Google Scholar] [CrossRef]
- Liang, Z.; Yan, C.F.; Rtimi, S.; Bandara, J. Piezoelectric Materials for Catalytic/Photocatalytic Removal of Pollutants: Recent Advances and Outlook. Appl. Catal. B Environ. 2019, 241, 256–269. [Google Scholar] [CrossRef]
- Nie, G.; Yao, Y.; Duan, X.; Xiao, L.; Wang, S. Advances of Piezoelectric Nanomaterials for Applications in Advanced Oxidation Technologies. Curr. Opin. Chem. Eng. 2021, 33, 100693. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V.; Palma, V. Non-Thermal Plasma Coupled with Catalyst for the Degradation of Water Pollutants: A Review. Catalysts 2020, 10, 1438. [Google Scholar] [CrossRef]
- Nam, S.-N.; Choong, C.E.; Hoque, S.; Farouk, T.I.; Cho, J.; Jang, M.; Snyder, S.A.; Meadows, M.E.; Yoon, Y. Catalytic Non-Thermal Plasma Treatment of Endocrine Disrupting Compounds, Pharmaceuticals, and Personal Care Products in Aqueous Solution: A Review. Chemosphere 2022, 290, 133395. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A Review of the Environmental Fate, Effects, and Exposures of Bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Ismail, N.A.H.; Wee, S.Y.; Aris, A.Z. Multi-Class of Endocrine Disrupting Compounds in Aquaculture Ecosystems and Health Impacts in Exposed Biota. Chemosphere 2017, 188, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive Removal of Bisphenol A (BPA) from Aqueous Solution: A Review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Bisphenol A, Nonylphenols, Benzophenones, and Benzotriazoles in Soils, Groundwater, Surface Water, Sediments, and Food: A Review. Environ. Sci. Pollut. Res. 2015, 22, 5711–5741. [Google Scholar] [CrossRef] [Green Version]
- Rastkari, N.; Jeddi, M.Z.; Yunesian, M.; Ahmadkhaniha, R. Effect of Sunlight Exposure on Phthalates Migration from Plastic Containers to Packaged Juices. J. Environ. Health Sci. Eng. 2018, 16, 27–33. [Google Scholar] [CrossRef]
- Russo, G.; Laneri, S.; di Lorenzo, R.; Ferrara, L.; Grumetto, L. The Occurrence of Selected Endocrine-Disrupting Chemicals in Water and Sediments from an Urban Lagoon in Southern Italy. Water Environ. Res. 2021, 93, 1944–1958. [Google Scholar] [CrossRef]
- Jiang, J.; Mu, D.; Ding, M.; Zhang, S.; Zhang, H.; Hu, J. Simultaneous Determination of Primary and Secondary Phthalate Monoesters in the Taihu Lake: Exploration of Sources. Chemosphere 2018, 202, 17–24. [Google Scholar] [CrossRef]
- Salaudeen, T.; Okoh, O.; Agunbiade, F.; Okoh, A. Phthalates Removal Efficiency in Different Wastewater Treatment Technology in the Eastern Cape, South Africa. Environ. Monit. Assess. 2018, 190, 299. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic Activity and Mechanism of Bisphenol a Removal over TiO2−x/RGO Nanocomposite Driven by Visible Light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Wang, G.; Dai, J.; Luo, Q.; Deng, N. Photocatalytic Degradation of Bisphenol A by TiO2@aspartic Acid-β-Cyclodextrin@reduced Graphene Oxide. Sep. Purif. Technol. 2021, 254, 117574. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, N.; Cheng, G.; Guo, H.; Shen, Z.; Yang, L.; Zhao, Y.; Alsaedi, A.; Hayat, T.; Wang, X. Preparing a Photocatalytic Fe Doped TiO2/RGO for Enhanced Bisphenol A and Its Analogues Degradation in Water Sample. Appl. Surf. Sci. 2020, 505, 144640. [Google Scholar] [CrossRef]
- Garg, A.; Singhania, T.; Singh, A.; Sharma, S.; Rani, S.; Neogy, A.; Yadav, S.R.; Sangal, V.K.; Garg, N. Photocatalytic Degradation of Bisphenol-A Using N,Co Codoped TiO2 Catalyst under Solar Light. Sci. Rep. 2019, 9, 765. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, R.; Lu, Z.; Wang, W.; Yan, Y. Dual Sensitization Effect and Conductive Structure of Fe3O4@mTiO2/C Photocatalyst towards Superior Photodegradation Activity for Bisphenol A under Visible Light. J. Photochem. Photobiol. A Chem. 2019, 382, 111902. [Google Scholar] [CrossRef]
- He, J.; Zeng, X.; Lan, S.; Lo, I.M.C. Reusable Magnetic Ag/Fe, N-TiO2/Fe3O4@SiO2 Composite for Simultaneous Photocatalytic Disinfection of E. Coli and Degradation of Bisphenol A in Sewage under Visible Light. Chemosphere 2019, 217, 869–878. [Google Scholar] [CrossRef]
- Ju, L.; Wu, P.; Yang, Q.; Ahmed, Z.; Zhu, N. Synthesis of ZnAlTi-LDO Supported C60@AgCl Nanoparticles and Their Photocatalytic Activity for Photo-Degradation of Bisphenol A. Appl. Catal. B Environ. 2018, 224, 159–174. [Google Scholar] [CrossRef]
- Kumar, A.; Schuerings, C.; Kumar, S.; Kumar, A.; Krishnan, V. Perovskite-Structured CaTiO3 Coupled with g-C3N4 as a Heterojunction Photocatalyst for Organic Pollutant Degradation. Beilstein J. Nanotechnol. 2018, 9, 671–685. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Xu, Y.; Ji, X.; Xie, M.; Jiang, D.; Yan, J.; Song, Z.; Xu, H.; Li, H. Construction of Polythiophene/Bi4O5I2 Nanocomposites to Promote Photocatalytic Degradation of Bisphenol a. J. Alloys Compd. 2020, 823, 153773. [Google Scholar] [CrossRef]
- Wu, R.; Song, H.; Luo, N.; Sheng, Y.; Ji, G. Microwave-Assisted Preparation and Enhanced Photocatalytic Activity of Bi2WO6/BiOI Heterojunction for Organic Pollutants Degradation under Visible-Light Irradiation. Solid State Sci. 2019, 87, 101–109. [Google Scholar] [CrossRef]
- Rao, F.; Zhu, G.; Wang, M.; Zubairu, S.M.; Peng, J.; Gao, J.; Hojamberdiev, M. Constructing the Pd/PdO/β-Bi2O3 Microspheres with Enhanced Photocatalytic Activity for Bisphenol A Degradation and NO Removal. J. Chem. Technol. Biotechnol. 2020, 95, 862–874. [Google Scholar] [CrossRef]
- Li, N.; Zhu, G.; Hojamberdiev, M.; Zhu, R.; Chang, J.; Gao, J.; Guo, Q.; Liu, P. Pd Nanoparticle-Decorated Bi4O5Br2 Nanosheets with Enhanced Visible-Light Photocatalytic Activity for Degradation of Bisphenol A. J. Photochem. Photobiol. A Chem. 2018, 356, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhang, X.; Zhang, Y.; Zhu, J.; Zhu, R. Nano Flake Ag3PO4 Enhanced Photocatalytic Activity of Bisphenol A under Visible Light Irradiation. Colloids Interface Sci. Commun. 2020, 37, 100277. [Google Scholar] [CrossRef]
- Karuppaiah, S.; Annamalai, R.; Muthuraj, A.; Kesavan, S.; Palani, R.; Ponnusamy, S.; Nagarajan, E.R.; Meenakshisundaram, S. Efficient Photocatalytic Degradation of Ciprofloxacin and Bisphenol A under Visible Light Using Gd2WO6 Loaded ZnO/Bentonite Nanocomposite. Appl. Surf. Sci. 2019, 481, 1109–1119. [Google Scholar] [CrossRef]
- Fu, H.; Ma, S.; Zhao, P.; Xu, S.; Zhan, S. Activation of Peroxymonosulfate by Graphitized Hierarchical Porous Biochar and MnFe2O4 Magnetic Nanoarchitecture for Organic Pollutants Degradation: Structure Dependence and Mechanism. Chem. Eng. J. 2019, 360, 157–170. [Google Scholar] [CrossRef]
- Kong, L.; Fang, G.; Kong, Y.; Xie, M.; Natarajan, V.; Zhou, D.; Zhan, J. Cu2O@Β-Cyclodextrin as a Synergistic Catalyst for Hydroxyl Radical Generation and Molecular Recognitive Destruction of Aromatic Pollutants at Neutral PH. J. Hazard. Mater. 2018, 357, 109–118. [Google Scholar] [CrossRef]
- Zhang, C.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. The Ultrasonic-Induced-Piezoelectric Enhanced Photocatalytic Performance of ZnO/CdS Nanofibers for Degradation of Bisphenol A. J. Alloys Compd. 2021, 885, 160987. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, Q.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Environmental Science Nano Rh-Doped SrTiO3 Inverse Opal with Piezoelectric Effect for Enhanced Visible-Light-Driven Photodegradation of Bisphenol A. Environ. Sci. Nano 2020, 7, 2267. [Google Scholar] [CrossRef]
- Huang, R.; Wu, J.; Lin, E.; Kang, Z.; Qin, N.; Bao, D. A New Strategy for Large-Scale Synthesis of Na0.5Bi0.5TiO3 Nanowires and Their Application in Piezocatalytic Degradation. Nanoscale Adv. 2021, 3, 3159–3166. [Google Scholar] [CrossRef]
- Jing, W.W.; Li, D.Q.; Li, J.; Li, X.F.; Wu, Z.H.; Liu, Y.L. Photodegradation of Dimethyl Phthalate (DMP) by UV–TiO2 in Aqueous Solution: Operational Parameters and Kinetic Analysis. Int. J. Environ. Sci. Technol. 2018, 15, 969–976. [Google Scholar] [CrossRef]
- Wang, C.; Zeng, T.; Gu, C.; Zhu, S.; Zhang, Q.; Luo, X. Photodegradation Pathways of Typical Phthalic Acid Esters Under UV, UV/TiO2, and UV-Vis/Bi2WO6 Systems. Front. Chem. 2019, 7, 852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Hu, Y.; Liu, X.; Wei, C. Synergetic Removal of Pb(II) and Dibutyl Phthalate Mixed Pollutants on Bi2O3-TiO2 Composite Photocatalyst under Visible Light. Appl. Catal. B Environ. 2018, 232, 288–298. [Google Scholar] [CrossRef]
- Meenakshi, G.; Sivasamy, A. Nanorod ZnO/SiC Nanocomposite: An Efficient Catalyst for the Degradation of an Endocrine Disruptor under UV and Visible Light Irradiations. J. Environ. Chem. Eng. 2018, 6, 3757–3769. [Google Scholar] [CrossRef]
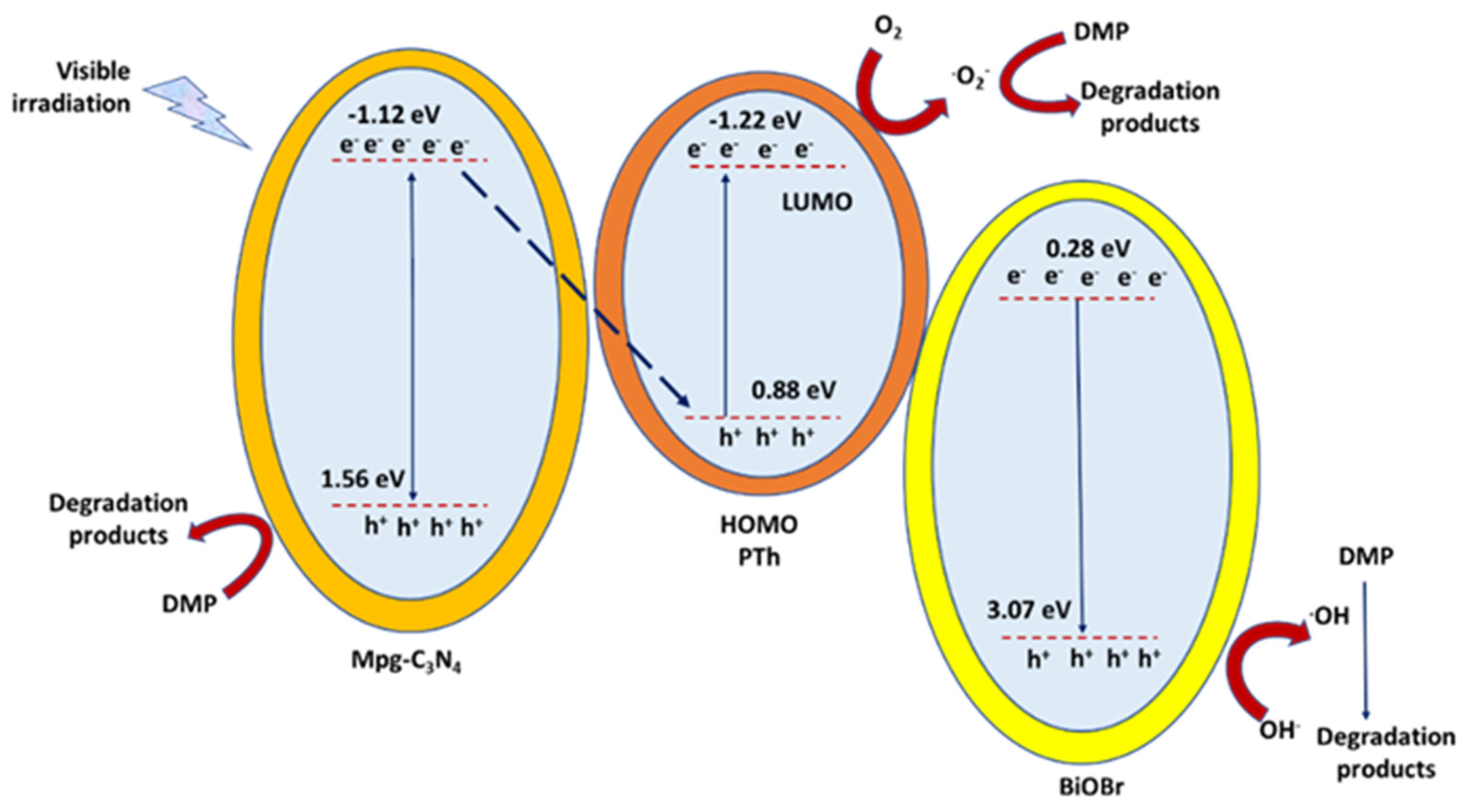
- Li, S.; Lai, C.; Li, C.; Zhong, J.; He, Z.; Peng, Q.; Liu, X.; Ke, B. Enhanced Photocatalytic Degradation of Dimethyl Phthalate by Magnetic Dual Z-Scheme Iron Oxide/Mpg-C3N4/BiOBr/Polythiophene Heterostructure Photocatalyst under Visible Light. J. Mol. Liq. 2021, 342, 116947. [Google Scholar] [CrossRef]
- di Dong, C.; Huang, C.P.; Nguyen, T.B.; Hsiung, C.F.; Wu, C.H.; Lin, Y.L.; Chen, C.W.; Hung, C.M. The Degradation of Phthalate Esters in Marine Sediments by Persulfate over Iron–Cerium Oxide Catalyst. Sci. Total Environ. 2019, 696, 133973. [Google Scholar] [CrossRef]
- Ding, S.; Wan, J.; Ma, Y.; Wang, Y.; Li, X.; Sun, J.; Pu, M. Targeted Degradation of Dimethyl Phthalate by Activating Persulfate Using Molecularly Imprinted Fe-MOF-74. Chemosphere 2021, 270, 128620. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/faostat/ (accessed on 31 January 2022).
- Rostami, S.; Jafari, S.; Moeini, Z.; Jaskulak, M.; Keshtgar, L.; Badeenezhad, A.; Azhdarpoor, A.; Rostami, M.; Zorena, K.; Dehghani, M. Current Methods and Technologies for Degradation of Atrazine in Contaminated Soil and Water: A Review. Environ. Technol. Innov. 2021, 24, 102019. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Moussavi, G.; Mahjoub, A.R.; Satari, M.; Abdolmaleki, P. Synthesis and Visible-Light Photocatalytic Activity of In,S-TiO2@rGO Nanocomposite for Degradation and Detoxification of Pesticide Atrazine in Water. Chem. Eng. J. 2018, 345, 300–311. [Google Scholar] [CrossRef]
- Shawky, A.; Mohamed, R.M.; Mkhalid, I.A.; Youssef, M.A.; Awwad, N.S. Visible Light-Responsive Ag/LaTiO3 Nanowire Photocatalysts for Efficient Elimination of Atrazine Herbicide in Water. J. Mol. Liq. 2020, 299, 112163. [Google Scholar] [CrossRef]
- Shawky, A.; Alhaddad, M.; Mohamed, R.M.; Awwad, N.S.; Ibrahium, H.A. Magnetically Separable and Visible Light-Active Ag/NiCo2O4 Nanorods Prepared by a Simple Route for Superior Photodegradation of Atrazine in Water. Prog. Nat. Sci. Mater. Int. 2020, 30, 160–167. [Google Scholar] [CrossRef]
- Majhi, D.; Das, K.; Mishra, A.; Dhiman, R.; Mishra, B.G. One Pot Synthesis of CdS/BiOBr/Bi2O2CO3: A Novel Ternary Double Z-Scheme Heterostructure Photocatalyst for Efficient Degradation of Atrazine. Appl. Catal. B Environ. 2020, 260, 118222. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Zhu, J.; Wang, Z.; Li, J. Study on the Efficacy and Mechanism of Fe-TiO2 Visible Heterogeneous Fenton Catalytic Degradation of Atrazine. Chemosphere 2020, 252, 126333. [Google Scholar] [CrossRef]
- Huang, Y.; Han, C.; Liu, Y.; Nadagouda, M.N.; Machala, L.; O’Shea, K.E.; Sharma, V.K.; Dionysiou, D.D. Degradation of Atrazine by ZnxCu1−xFe2O4 Nanomaterial-Catalyzed Sulfite under UV–Vis Light Irradiation: Green Strategy to Generate SO4−. Appl. Catal. B Environ. 2018, 221, 380–392. [Google Scholar] [CrossRef]
- Xie, S.; Tang, C.; Shi, H.; Zhao, G. Highly Efficient Photoelectrochemical Removal of Atrazine and the Mechanism Investigation: Bias Potential Effect and Reactive Species. J. Hazard. Mater. 2021, 415, 125681. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Xiao, K.; Shi, J.; Du, X.; Wang, L.; Wu, X. The Intimate Coupling of Photocatalysis and Biodegradation for the Degradation and Mineralization of Atrazine in Water. New J. Chem. 2021, 45, 13029–13039. [Google Scholar] [CrossRef]
- Xu, X.; Chen, W.; Zong, S.; Ren, X.; Liu, D. Atrazine Degradation Using Fe3O4-Sepiolite Catalyzed Persulfate: Reactivity, Mechanism and Stability. J. Hazard. Mater. 2019, 377, 62–69. [Google Scholar] [CrossRef]
- Li, J.; Wan, Y.; Li, Y.; Yao, G.; Lai, B. Surface Fe(III)/Fe(II) Cycle Promoted the Degradation of Atrazine by Peroxymonosulfate Activation in the Presence of Hydroxylamine. Appl. Catal. B Environ. 2019, 256, 117782. [Google Scholar] [CrossRef]
- Wang, G.; Cheng, C.; Zhu, J.; Wang, L.; Gao, S.; Xia, X. Enhanced Degradation of Atrazine by Nanoscale LaFe1−xCuxO3−δ Perovskite Activated Peroxymonosulfate: Performance and Mechanism. Sci. Total Environ. 2019, 673, 565–575. [Google Scholar] [CrossRef]
- Dong, X.; Ren, B.; Zhang, X.; Liu, X.; Sun, Z.; Li, C.; Tan, Y.; Yang, S.; Zheng, S.; Dionysiou, D.D. Diatomite Supported Hierarchical 2D CoNi3O4 Nanoribbons as Highly Efficient Peroxymonosulfate Catalyst for Atrazine Degradation. Appl. Catal. B Environ. 2020, 272, 118971. [Google Scholar] [CrossRef]
- Chen, S.; He, P.; Wang, X.; Xiao, F.; Zhou, P.; He, Q.; Jia, L.; Dong, F.; Zhang, H.; Jia, B.; et al. Co/Sm-Modified Ti/PbO2 Anode for Atrazine Degradation: Effective Electrocatalytic Performance and Degradation Mechanism. Chemosphere 2021, 268, 128799. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, J.; Yao, G.; Zhang, Y.; Li, X.; Lai, B. Improving the Degradation of Atrazine in the Three-Dimensional (3D) Electrochemical Process Using CuFe2O4 as Both Particle Electrode and Catalyst for Persulfate Activation. Chem. Eng. J. 2019, 361, 1317–1332. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, Q.; Zhang, Y. Comparative Study of Low-Index {1 0 1}-TiO2, {0 0 1}-TiO2, {1 0 0}-TiO2 and High-Index {2 0 1}-TiO2 on Glyphosate Adsorption and Photo-Degradation. Chem. Eng. J. 2019, 360, 1247–1254. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Chen, J.; Wang, G.Y.; Wang, D.; Zeng, X.F.; Wang, J.X. Citric Acid-Assisted Ultrasmall CeO2 Nanoparticles for Efficient Photocatalytic Degradation of Glyphosate. Chem. Eng. J. 2021, 425, 130640. [Google Scholar] [CrossRef]
- Russo, M.; Iervolino, G.; Vaiano, V. W-Doped ZnO Photocatalyst for the Degradation of Glyphosate in Aqueous Solution. Catalysts 2021, 11, 234. [Google Scholar] [CrossRef]
- Cao, L.; Ma, D.; Zhou, Z.; Xu, C.; Cao, C.; Zhao, P.; Huang, Q. Efficient Photocatalytic Degradation of Herbicide Glyphosate in Water by Magnetically Separable and Recyclable BiOBr/Fe3O4 Nanocomposites under Visible Light Irradiation. Chem. Eng. J. 2019, 368, 212–222. [Google Scholar] [CrossRef]
- Luo, X.L.; Chen, Z.Y.; Yang, S.Y.; Xu, Y.H. Two-Step Hydrothermal Synthesis of Peanut-Shaped Molybdenum Diselenide/Bismuth Vanadate (MoSe2/BiVO4) with Enhanced Visible-Light Photocatalytic Activity for the Degradation of Glyphosate. J. Colloid Interface Sci. 2018, 532, 456–463. [Google Scholar] [CrossRef]
- Tang, Q.Y.; Chen, W.F.; Lv, Y.R.; Yang, S.Y.; Xu, Y.H. Z-Scheme Hierarchical Cu2S/Bi2WO6 Composites for Improved Photocatalytic Activity of Glyphosate Degradation under Visible Light Irradiation. Sep. Purif. Technol. 2020, 236, 116243. [Google Scholar] [CrossRef]
- Xue, L.; Hao, L.; Ding, H.; Liu, R.; Zhao, D.; Fu, J.; Zhang, M. Complete and Rapid Degradation of Glyphosate with Fe3Ce1Ox Catalyst for Peroxymonosulfate Activation at Room Temperature. Environ. Res. 2021, 201, 111618. [Google Scholar] [CrossRef]
- Marien, C.B.D.; le Pivert, M.; Azaïs, A.; M’Bra, I.C.; Drogui, P.; Dirany, A.; Robert, D. Kinetics and Mechanism of Paraquat’s Degradation: UV-C Photolysis vs UV-C Photocatalysis with TiO2/SiC Foams. J. Hazard. Mater. 2019, 370, 164–171. [Google Scholar] [CrossRef]
- Vanichvattanadecha, C.; Jaroenworaluck, A.; Henpraserttae, P.; Wimuktiwan, P.; Manpetch, P.; Singhapong, W. Ordered Mesoporous Silica (SBA-16) Supporting Titania (TiO2) Nanoparticles for Photodegradation of Paraquat (PQ) Herbicide. J. Porous Mater. 2021, 28, 1137–1153. [Google Scholar] [CrossRef]
- Suwannaruang, T.; Kamonsuangkasem, K.; Kidkhunthod, P.; Chirawatkul, P.; Saiyasombat, C.; Chanlek, N.; Wantala, K. Influence of Nitrogen Content Levels on Structural Properties and Photocatalytic Activities of Nanorice-like N-Doped TiO2 with Various Calcination Temperatures. Mater. Res. Bull. 2018, 105, 265–276. [Google Scholar] [CrossRef]
- Pourzad, A.; Sobhi, H.R.; Behbahani, M.; Esrafili, A.; Kalantary, R.R.; Kermani, M. Efficient Visible Light-Induced Photocatalytic Removal of Paraquat Using N-Doped TiO2@SiO2@Fe3O4 Nanocomposite. J. Mol. Liq. 2020, 299, 112167. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Feyzi, M.; Zinadini, S.; Bahnemann, D.W. Photomineralization of Recalcitrant Wastewaters by a Novel Magnetically Recyclable Boron Doped-TiO2-SiO2 Cobalt Ferrite Nanocomposite as a Visible-Driven Heterogeneous Photocatalyst. J. Environ. Chem. Eng. 2018, 6, 6370–6381. [Google Scholar] [CrossRef]
- Khodkar, A.; Khezri, S.M.; Pendashteh, A.R.; Khoramnejadian, S.; Mamani, L. A Designed Experimental Approach for Photocatalytic Degradation of Paraquat Using α-Fe2O3@MIL-101(Cr)@TiO2 Based on Metal–Organic Framework. Int. J. Environ. Sci. Technol. 2019, 16, 5741–5756. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Guo, C.; Stadler, F.J. Biochar-Templated g-C3N4/Bi2O2CO3/CoFe2O4 Nano-Assembly for Visible and Solar Assisted Photo-Degradation of Paraquat, Nitrophenol Reduction and CO2 Conversion. Chem. Eng. J. 2018, 339, 393–410. [Google Scholar] [CrossRef]
- Mary Nisha, U.; Umapathy, M.J.; Sivasamy, A. Effective Degradation of 2,4-Dichlorophenoxy Acetic Acid Endocrine Disruptor Using CeO2–Bi2O3 Mixed Metal Oxide Photocatalyst under Visible Light Irradiation. J. Mater. Sci. Mater. Electron. 2021, 32, 14791–14800. [Google Scholar] [CrossRef]
- Lima, M.S.; Cruz-Filho, J.F.; Noleto, L.F.G.; Silva, L.J.; Costa, T.M.S.; Luz, G.E. Synthesis, Characterization and Catalytic Activity of Fe3O4@WO3/SBA-15 on Photodegradation of the Acid Dichlorophenoxyacetic (2,4-D) under UV Irradiation. J. Environ. Chem. Eng. 2020, 8, 104145. [Google Scholar] [CrossRef]
- Xu, X.; Cai, J.; Zhou, M.; Du, X.; Zhang, Y. Photoelectrochemical Degradation of 2,4-Dichlorophenoxyacetic Acid Using Electrochemically Self-Doped Blue TiO2 Nanotube Arrays with Formic Acid as Electrolyte. J. Hazard. Mater. 2020, 382, 121096. [Google Scholar] [CrossRef]
- Sharma, R.K.; Arora, B.; Sharma, S.; Dutta, S.; Sharma, A.; Yadav, S.; Solanki, K. In Situ Hydroxyl Radical Generation Using the Synergism of the Co–Ni Bimetallic Centres of a Developed Nanocatalyst with Potent Efficiency for Degrading Toxic Water Pollutants. Mater. Chem. Front. 2020, 4, 605–620. [Google Scholar] [CrossRef]
- Riaz, U.; Zia, J. Microwave-Assisted Rapid Degradation of DDT Using Nanohybrids of PANI with SnO2 Derived from Psidium Guajava Extract. Environ. Pollut. 2020, 259, 113917. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; An, F.; Zhu, C.; Zhou, D. Efficient Transformation of DDT with Peroxymonosulfate Activation by Different Crystallographic MnO2. Sci. Total Environ. 2021, 759, 142864. [Google Scholar] [CrossRef] [PubMed]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Photocatalytic Oxidation of Six Pesticides Listed as Endocrine Disruptor Chemicals from Wastewater Using Two Different TiO2 Samples at Pilot Plant Scale under Sunlight Irradiation. J. Photochem. Photobiol. A Chem. 2018, 353, 271–278. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; el Aatik, A.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Removal of Pesticides with Endocrine Disruptor Activity in Wastewater Effluent by Solar Heterogeneous Photocatalysis Using ZnO/Na2S2O8. Water Air Soil Pollut. 2019, 230, 134. [Google Scholar] [CrossRef]
- Rossetti, M.F.; Stoker, C.; Ramos, J.G. Agrochemicals and Neurogenesis. Mol. Cell. Endocrinol. 2020, 510, 110820. [Google Scholar] [CrossRef]
- Kalofiri, P.; Balias, G.; Tekos, F. The EU Endocrine Disruptors’ Regulation and the Glyphosate Controversy. Toxicol. Rep. 2021, 8, 1193–1199. [Google Scholar] [CrossRef]
- Alulema-Pullupaxi, P.; Fernández, L.; Debut, A.; Santacruz, C.P.; Villacis, W.; Fierro, C.; Espinoza-Montero, P.J. Photoelectrocatalytic Degradation of Glyphosate on Titanium Dioxide Synthesized by Sol-Gel/Spin-Coating on Boron Doped Diamond (TiO2/BDD) as a Photoanode. Chemosphere 2021, 278, 130488. [Google Scholar] [CrossRef]
- Phuinthiang, P.; Kajitvichyanukul, P. Degradation of Paraquat from Contaminated Water Using Green TiO2 Nanoparticles Synthesized from Coffea arabica L. in Photocatalytic Process. Water Sci. Technol. 2018, 79, 905–910. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly Reusable Visible Light Active Hierarchical Porous WO3/SiO2 Monolith in Centimeter Length Scale for Enhanced Photocatalytic Degradation of Toxic Pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Quan, Y.; Yao, J.; Yang, S.; Chen, L.; Liu, Y.; Lang, J.; Zeng, H.; Yang, J.; Gao, M. Detect, Remove and Re-Use: Sensing and Degradation Pesticides via 3D Tilted ZMRs/Ag Arrays. J. Hazard. Mater. 2020, 391, 122222. [Google Scholar] [CrossRef]
- Fidelis, M.Z.; Abreu, E.; Josué, T.G.; de Almeida, L.N.B.; Lenzi, G.G.; Santos, O.A.A. dos Continuous Process Applied to Degradation of Triclosan and 2.8-Dichlorodibenzene-p-Dioxin. Environ. Sci. Pollut. Res. 2021, 28, 23675–23683. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Dahake, R.; Maddigapu, P.R.; Hippargi, G.; Pophali, G.R.; Bansiwal, A. Enhanced Photocatalytic Degradation of Antimicrobial Triclosan Using RGO–TiO2 Composite under Natural Solar Illumination. J. Mater. Sci. Mater. Electron. 2020, 31, 6045–6058. [Google Scholar] [CrossRef]
- Ojha, A.; Singh, P.; Tiwary, D. Photocatalytic Degradation of Triclosan in Visible-Light-Induced via CdS@TiO2-RGO Nanocomposite. Surf. Topogr.: Metrol. Prop. 2021, 9, 035032. [Google Scholar] [CrossRef]
- Chandra Pragada, S.; Thalla, A.K. Polymer-Based Immobilized Fe2O3–TiO2/PVP Catalyst Preparation Method and the Degradation of Triclosan in Treated Greywater Effluent by Solar Photocatalysis. J. Environ. Manag. 2021, 296, 113305. [Google Scholar] [CrossRef]
- Köwitsch, I.; Mehring, M. Coatings of Magnetic Composites of Iron Oxide and Carbon Nitride for Photocatalytic Water Purification. RSC Adv. 2021, 11, 14053–14062. [Google Scholar] [CrossRef]
- Katal, R.; Panah, S.M.; Saeedikhani, M.; Kosari, M.; Sheng, C.C.; Leong, O.S.; Xiao, G.; Jiangyong, H. Pd-Decorated CuO Thin Film for Photodegradation of Acetaminophen and Triclosan under Visible Light Irradiation. Adv. Mater. Interfaces 2018, 5, 1801440. [Google Scholar] [CrossRef]
- Tiwari, A.; Shukla, A.; Lalliansanga; Tiwari, D.; Lee, S.M. Synthesis and Characterization of Ag0(NPs)/TiO2 Nanocomposite: Insight Studies of Triclosan Removal from Aqueous Solutions. Environ. Technol. 2020, 41, 3500–3514. [Google Scholar] [CrossRef]
- Ferreira, O.; Monteiro, O.C.; do Rego, A.M.B.; Ferraria, A.M.; Batista, M.; Santos, R.; Monteiro, S.; Freire, M.; Silva, E.R. Visible Light-Driven Photodegradation of Triclosan and Antimicrobial Activity against Legionella Pneumophila with Cobalt and Nitrogen Co-Doped TiO2 Anatase Nanoparticles. J. Environ. Chem. Eng. 2021, 9, 106735. [Google Scholar] [CrossRef]
- Chen, Z.; Bi, S.; Zhao, G.; Chen, Y.; Hu, Y. Enhanced Degradation of Triclosan by Cobalt Manganese Spinel-Type Oxide Activated Peroxymonosulfate Oxidation Process via Sulfate Radicals and Singlet Oxygen: Mechanisms and Intermediates Identification. Sci. Total Environ. 2020, 711, 134715. [Google Scholar] [CrossRef]
- So, H.L.; Lin, K.Y.; Chu, W. Triclosan Removal by Heterogeneous Fenton-like Process: Studying the Kinetics and Surface Chemistry of Fe3O4 as Catalyst. J. Environ. Chem. Eng. 2019, 7, 103432. [Google Scholar] [CrossRef]
- So, H.L.; Lin, K.Y.; Chu, W.; Gong, H. Degradation of Triclosan by Recyclable MnFe2O4-Activated PMS: Process Modification for Reduced Toxicity and Enhanced Performance. Ind. Eng. Chem. Res. 2020, 59, 4257–4264. [Google Scholar] [CrossRef]
- Huang, Z.; Lin, Q.; Cai, N.; Weng, Q.; Xu, J.; Gan, S.; Chen, C.; Zhong, Q.; Fu, H.; Xia, Y.; et al. Coexistence of Free Radical and Nonradical Mechanisms for Triclosan Degradation by CuO/HNTs. Sep. Purif. Technol. 2021, 276, 119318. [Google Scholar] [CrossRef]
- Song, X.; Ren, C.; Zhao, Q.; Su, B. Simultaneous Removal of Cr(VI) and Triclosan from Aqueous Solutions through Fe3O4 Magnetic Nanoscale-Activated Persulfate Oxidation. Chem. Eng. J. 2020, 381, 122586. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Photocatalytic Removal of Parabens and Halogenated Products in Wastewater: A Review. Environ. Chem. Lett. 2021, 19, 3789–3819. [Google Scholar] [CrossRef]
- de Jesus, R.A.; de Assis, G.C.; de Oliveira, R.J.; Costa, J.A.S.; da Silva, C.M.P.; Bilal, M.; Iqbal, H.M.N.; Ferreira, L.F.R.; Figueiredo, R.T. Environmental Remediation Potentialities of Metal and Metal Oxide Nanoparticles: Mechanistic Biosynthesis, Influencing Factors, and Application Standpoint. Environ. Technol. Innov. 2021, 24, 101851. [Google Scholar] [CrossRef]
- Ngigi, E.M.; Nomngongo, P.N.; Ngila, J.C. Recent Methods Used in Degradation of Parabens in Aqueous Solutions: A Review. Int. J. Environ. Sci. Technol. 2022, 19, 2139–2154. [Google Scholar] [CrossRef]
- Foszpańczyk, M.; Bednarczyk, K.; Drozdek, E.; Martins, R.C.; Ledakowicz, S.; Gmurek, M. Comparison of Photocatalytic and Photosensitized Oxidation of Paraben Aqueous Solutions Under Sunlight. Water Air Soil Pollut. 2018, 229, 362. [Google Scholar] [CrossRef] [Green Version]
- Gomes, J.; Lincho, J.; Domingues, E.; Gmurek, M.; Mazierski, P.; Zaleska-Medynska, A.; Klimczuk, T.; Quinta-Ferreira, R.M.; Martins, R.C. TiO2 Nanotube Arrays-Based Reactor for Photocatalytic Oxidation of Parabens Mixtures in Ultrapure Water: Effects of Photocatalyst Properties, Operational Parameters and Light Source. Sci. Total Environ. 2019, 689, 79–89. [Google Scholar] [CrossRef]
- Lincho, J.; Gomes, J.; Kobylanski, M.; Bajorowicz, B.; Zaleska-Medynska, A.; Martins, R.C. TiO2 Nanotube Catalysts for Parabens Mixture Degradation by Photocatalysis and Ozone-Based Technologies. Process Saf. Environ. Prot. 2021, 152, 601–613. [Google Scholar] [CrossRef]
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar Reclamation of Wastewater Effluent Polluted with Bisphenols, Phthalates and Parabens by Photocatalytic Treatment with TiO2/Na2S2O8 at Pilot Plant Scale. Chemosphere 2018, 212, 95–104. [Google Scholar] [CrossRef]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and Characterization of CoOx/BiVO4 Photocatalysts for the Degradation of Propyl Paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Moschogiannaki, M.; Frontistis, Z.; Kiriakidis, G.; Mantzavinos, D.; Binas, V. Porous CoxNi1−xTiO3 Nanorods for Solar Photocatalytic Degradation of Ethyl Paraben. J. Mater. 2020, 6, 788–799. [Google Scholar] [CrossRef]
- Sheikhmohammadi, A.; Nourmoradi, H.; Manshouri, M.; Asgari, E. Performance Intensification of BzP Photo-Catalytic Degradation through Adding Exogenous Oxidant. Optik 2020, 202, 163571. [Google Scholar] [CrossRef]
- Asgari, E.; Esrafili, A.; Rostami, R.; Farzadkia, M. O3, O3/UV and O3/UV/ZnO for Abatement of Parabens in Aqueous Solutions: Effect of Operational Parameters and Mineralization/Biodegradability Improvement. Process Saf. Environ. Prot. 2019, 125, 238–250. [Google Scholar] [CrossRef]
- Pratush, A.; Ye, X.; Yang, Q.; Kan, J.; Peng, T.; Wang, H.; Huang, T.; Xiong, G.; Hu, Z. Biotransformation Strategies for Steroid Estrogen and Androgen Pollution. Appl. Microbiol. Biotechnol. 2020, 104, 2385–2409. [Google Scholar] [CrossRef]
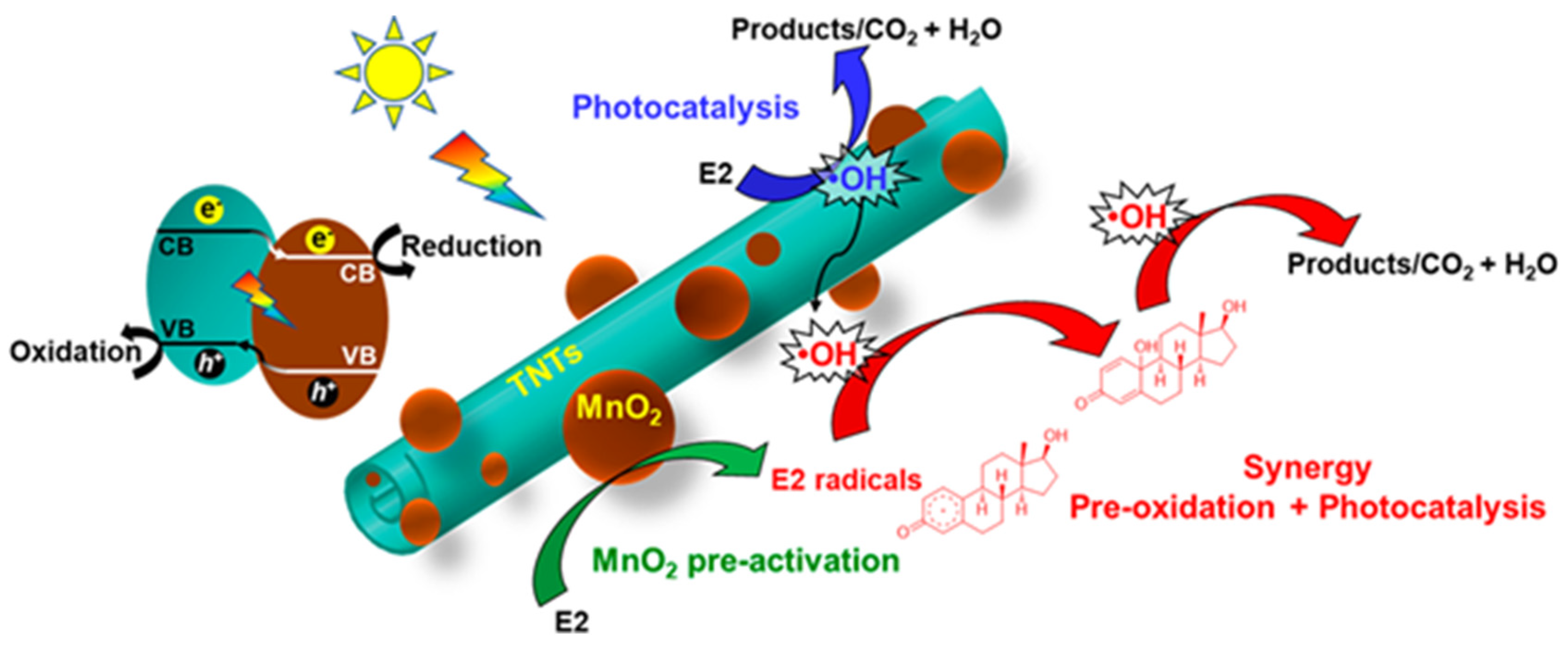
- Du, P.; Chang, J.; Zhao, H.; Liu, W.; Dang, C.; Tong, M.; Ni, J.; Zhang, B. Sea-Buckthorn-Like MnO2 Decorated Titanate Nanotubes with Oxidation Property and Photocatalytic Activity for Enhanced Degradation of 17β-Estradiol under Solar Light. ACS Appl. Energy Mater. 2018, 1, 2123–2133. [Google Scholar] [CrossRef]
- Sun, K.; Liu, Q.; Li, S.; Qi, Y.; Si, Y. MnO2 Nanozyme-Driven Polymerization and Decomposition Mechanisms of 17β-Estradiol: Influence of Humic Acid. J. Hazard. Mater. 2020, 393, 122393. [Google Scholar] [CrossRef]
- Kovacic, M.; Kopcic, N.; Kusic, H.; Bozic, A.L. Solar Driven Degradation of 17β-Estradiol Using Composite Photocatalytic Materials and Artificial Irradiation Source: Influence of Process and Water Matrix Parameters. J. Photochem. Photobiol. A Chem. 2018, 361, 48–61. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, A.K. Enhanced Photocatalytic Degradation of 17β-Estradiol by Polythiophene Modified Al-Doped ZnO: Optimization of Synthesis Parameters Using Multivariate Optimization Techniques. J. Environ. Chem. Eng. 2020, 8, 104463. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Liu, Z.; Yang, X.; Naraginti, S.; Xu, X.; Wang, X. Visible Light Photocatalytic Mineralization of 17α-Ethinyl Estradiol (EE2) and Hydrogen Evolution over Silver and Strontium Modified TiO2 Nanoparticles: Mechanisms and Phytotoxicity Assessment. RSC Adv. 2018, 8, 4329–4339. [Google Scholar] [CrossRef] [Green Version]
- Menon, N.G.; George, L.; Tatiparti, S.S.V.; Mukherji, S. Efficacy and Reusability of Mixed-Phase TiO2–ZnO Nanocomposites for the Removal of Estrogenic Effects of 17β-Estradiol and 17α-Ethinylestradiol from Water. J. Environ. Manag. 2021, 288, 112340. [Google Scholar] [CrossRef] [PubMed]
- Abreu, E.; Fidelis, M.Z.; Fuziki, M.E.; Malikoski, R.M.; Mastsubara, M.C.; Imada, R.E.; Diaz de Tuesta, J.L.; Gomes, H.T.; Anziliero, M.D.; Baldykowski, B.; et al. Degradation of Emerging Contaminants: Effect of Thermal Treatment on nb2o5 as Photocatalyst. J. Photochem. Photobiol. A Chem. 2021, 419, 113484. [Google Scholar] [CrossRef]
- Villa, K.; Parmar, J.; Vilela, D.; Sánchez, S. Core-Shell Microspheres for the Ultrafast Degradation of Estrogen Hormone at Neutral pH. RSC Adv. 2018, 8, 5840–5847. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Li, C.; Bu, L.; Tang, C.; Wang, S.; Duan, P.; Yao, L.; Tang, J.; Dionysiou, D.D.; Wu, Y. Bismuth Impregnated Biochar for Efficient Estrone Degradation: The Synergistic Effect between Biochar and Bi/Bi2O3 for a High Photocatalytic Performance. J. Hazard. Mater. 2020, 384, 121258. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, I.M.; Bandala, E.R. Photocatalytic Degradation of Estriol Using Iron-Doped TiO2 under High and Low UV Irradiation. Catalysts 2018, 8, 625. [Google Scholar] [CrossRef] [Green Version]
- Escudeiro de Oliveira, M.; Barroso, B.L.; de Almeida, J.; Moraes, M.L.L.; de Arruda Rodrigues, C. Photoelectrocatalytic Degradation of 17α-Ethinylestradiol and Estrone under UV and Visible Light Using Nanotubular Oxide Arrays Grown on Ti-0.5wt%W. Environ. Res. 2020, 191, 110044. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Gamallo, M.; Conde, J.J.; Vargas-Osorio, Z.; Vázquez-Vázquez, C.; Piñeiro, Y.; Rivas, J.; Feijoo, G.; Moreira, M.T. Exploiting the Potential of Supported Magnetic Nanomaterials as Fenton-like Catalysts for Environmental Applications. Nanomaterials 2021, 11, 2902. [Google Scholar] [CrossRef]
- Bayode, A.A.; Vieira, E.M.; Moodley, R.; Akpotu, S.; de Camargo, A.S.S.; Fatta-Kassinos, D.; Unuabonah, E.I. Tuning ZnO/GO p-n Heterostructure with Carbon Interlayer Supported on Clay for Visible-Light Catalysis: Removal of Steroid Estrogens from Water. Chem. Eng. J. 2021, 420, 127668. [Google Scholar] [CrossRef]
- Joseita dos Santos Costa, M.; dos Santos Costa, G.; Estefany Brandão Lima, A.; Eduardo da Luz Júnior, G.; Longo, E.; Santos Cavalcante, L.; da Silva Santos, R. Photocurrent Response and Progesterone Degradation by Employing WO3 Films Modified with Platinum and Silver Nanoparticles. ChemPlusChem 2018, 83, 1153–1161. [Google Scholar] [CrossRef]
- Maniakova, G.; Kowalska, K.; Murgolo, S.; Mascolo, G.; Libralato, G.; Lofrano, G.; Sacco, O.; Guida, M.; Rizzo, L. Comparison between Heterogeneous and Homogeneous Solar Driven Advanced Oxidation Processes for Urban Wastewater Treatment: Pharmaceuticals Removal and Toxicity. Sep. Purif. Technol. 2020, 236, 116249. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-Based Adsorbent and Photocatalyst Use for Pharmaceutical Contaminant Removal during Indirect Potable Water Reuse. NPJ Clean Water 2020, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Salimi, M.; Behbahani, M.; Sobhi, H.R.; Gholami, M.; Jonidi Jafari, A.; Rezaei Kalantary, R.; Farzadkia, M.; Esrafili, A. A New Nano-Photocatalyst Based on Pt and Bi Co-Doped TiO2 for Efficient Visible-Light Photo Degradation of Amoxicillin. New J. Chem. 2019, 43, 1562–1568. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, P.; Kaur, D.; Devunuri, N.; Sinha, R.K.; Devi, P. TiO2 Nanofibres Decorated with Green-Synthesized PAu/Ag@CQDs for the Efficient Photocatalytic Degradation of Organic Dyes and Pharmaceutical Drugs. RSC Adv. 2020, 10, 8941–8948. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Wu, X.; Zhang, M.; Liu, C.; Yu, J.; Sun, G.; Si, Y.; Ding, B. Highly Flexible, Core-Shell Heterostructured, and Visible-Light-Driven Titania-Based Nanofibrous Membranes for Antibiotic Removal and E. Coil Inactivation. Chem. Eng. J. 2020, 379, 122269. [Google Scholar] [CrossRef]
- Islam, S.E.; Hang, D.R.; Chen, C.H.; Sharma, K.H. Facile and Cost-Efficient Synthesis of Quasi-0D/2D ZnO/MoS2 Nanocomposites for Highly Enhanced Visible-Light-Driven Photocatalytic Degradation of Organic Pollutants and Antibiotics. Chem. Eur. J. 2018, 24, 9305–9315. [Google Scholar] [CrossRef]
- Lwin, H.M.; Zhan, W.; Song, S.; Jia, F.; Zhou, J. Visible-Light Photocatalytic Degradation Pathway of Tetracycline Hydrochloride with Cubic Structured ZnO/SnO2 Heterojunction Nanocatalyst. Chem. Phys. Lett. 2019, 736, 136806. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Raju, G.S.R.; Pavitra, E.; Kwak, C.H.; Han, Y.K.; Huh, Y.S. Ultrasound-Assisted Heterogeneous Degradation of Tetracycline over Flower-like RGO/CdWO4 Hierarchical Structures as Robust Solar-Light-Responsive Photocatalysts: Optimization, Kinetics, and Mechanism. Appl. Surf. Sci. 2019, 489, 110–122. [Google Scholar] [CrossRef]
- Chen, W.; Chang, L.; Ren, S.B.; He, Z.C.; Huang, G.B.; Liu, X.H. Direct Z-Scheme 1D/2D WO2.72/ZnIn2S4 Hybrid Photocatalysts with Highly-Efficient Visible-Light-Driven Photodegradation towards Tetracycline Hydrochloride Removal. J. Hazard. Mater. 2020, 384, 121308. [Google Scholar] [CrossRef]
- Kandi, D.; Behera, A.; Sahoo, S.; Parida, K. CdS QDs Modified BiOI/Bi2MoO6 Nanocomposite for Degradation of Quinolone and Tetracycline Types of Antibiotics towards Environmental Remediation. Sep. Purif. Technol. 2020, 253, 117523. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Al-Muhtaseb, A.H.; Guo, C.; Iglesias-Juez, A.; Stadler, F.J. High-Performance Photocatalytic Hydrogen Production and Degradation of Levofloxacin by Wide Spectrum-Responsive Ag/Fe3O4 Bridged SrTiO3/g-C3N4 Plasmonic Nanojunctions: Joint Effect of Ag and Fe3O4. ACS Appl. Mater. Interfaces 2018, 10, 40474–40490. [Google Scholar] [CrossRef]
- Szabó-Bárdos, E.; Cafuta, A.; Hegedűs, P.; Fónagy, O.; Kiss, G.; Babić, S.; Škorić, I.; Horváth, O. Photolytic and Photocatalytic Degradation of Nitrofurantoin and Its Photohydrolytic Products. J. Photochem. Photobiol. A Chem. 2020, 386, 112093. [Google Scholar] [CrossRef]
- Devi, M.; Das, B.; Barbhuiya, M.H.; Bhuyan, B.; Dhar, S.S.; Vadivel, S. Fabrication of Nanostructured NiO/WO3 with Graphitic Carbon Nitride for Visible Light Driven Photocatalytic Hydroxylation of Benzene and Metronidazole Degradation. New J. Chem. 2019, 43, 14616–14624. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Z.; He, C.; Faqian, S.; Zhou, Y. Enhanced Photodegradation of Sulfamethoxazole by a Novel WO3-CNT Composite under Visible Light Irradiation. J. Alloys Compd. 2018, 754, 153–162. [Google Scholar] [CrossRef]
- Katal, R.; Davood Abadi Farahani, M.H.; Jiangyong, H. Degradation of Acetaminophen in a Photocatalytic (Batch and Continuous System) and Photoelectrocatalytic Process by Application of Faceted-TiO2. Sep. Purif. Technol. 2020, 230, 115859. [Google Scholar] [CrossRef]
- Namshah, K.S.; Mohamed, R.M. WO3–TiO2 Nanocomposites for Paracetamol Degradation under Visible Light. Appl. Nanosci. 2018, 8, 2021–2030. [Google Scholar] [CrossRef]
- Ranjith Kumar, D.; Ranjith, K.S.; Haldorai, Y.; Kandasami, A.; Rajendra Kumar, R.T. Nitrogen-Implanted ZnO Nanorod Arrays for Visible Light Photocatalytic Degradation of a Pharmaceutical Drug Acetaminophen. ACS Omega 2019, 4, 11973–11979. [Google Scholar] [CrossRef] [Green Version]
- Palas, B.; Ersöz, G.; Atalay, S. Bioinspired Metal Oxide Particles as Efficient Wet Air Oxidation and Photocatalytic Oxidation Catalysts for the Degradation of Acetaminophen in Aqueous Phase. Ecotoxicol. Environ. Saf. 2019, 182, 109367. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt–TiO2–Nb2O5 Heterojunction as Effective Photocatalyst for the Degradation of Diclofenac and Ketoprofen. Mater. Sci. Semicond. Process. 2020, 107, 104839. [Google Scholar] [CrossRef]
- Vitiello, G.; Iervolino, G.; Imparato, C.; Rea, I.; Borbone, F.; de Stefano, L.; Aronne, A.; Vaiano, V. F-Doped ZnO Nano- and Meso-Crystals with Enhanced Photocatalytic Activity in Diclofenac Degradation. Sci. Total Environ. 2021, 762, 143066. [Google Scholar] [CrossRef]
- Yilmaz, E.; Salem, S.; Sarp, G.; Aydin, S.; Sahin, K.; Korkmaz, I.; Yuvali, D. TiO2 Nanoparticles and C-Nanofibers Modified Magnetic Fe3O4 Nanospheres (TiO2@Fe3O4@C–NF): A Multifunctional Hybrid Material for Magnetic Solid-Phase Extraction of Ibuprofen and Photocatalytic Degradation of Drug Molecules and Azo Dye. Talanta 2020, 213, 120813. [Google Scholar] [CrossRef]
- Khammar, S.; Bahramifar, N.; Younesi, H. Preparation and Surface Engineering of CM-β-CD Functionalized Fe3O4@TiO2 Nanoparticles for Photocatalytic Degradation of Polychlorinated Biphenyls (PCBs) from Transformer Oil. J. Hazard. Mater. 2020, 394, 122422. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, T.I.; Kozhevnikova, N.S.; Vorokh, A.S.; Enyashin, A.N.; Pervova, M.G.; Zapevalov, A.Y.; Saloutin, V.I.; Chupakhin, O.N. Photolysis of Polychlorobiphenyls in the Presence of Nanocrystalline TiO2 and CdS/TiO2. React. Kinet. Mech. Catal. 2019, 126, 1115–1134. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, J.; Yuan, C.; Long, Y.; Chen, M.; Tao, J.; Wang, Q.; Cong, Y. CuO@Cu/Ag/MWNTs/Sponge Electrode-Enhanced Pollutant Removal in Dielectric Barrier Discharge (DBD) Reactor. Chemosphere 2019, 229, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Entezari, M.; Godini, H.; Sheikhmohammadi, A.; Esrafili, A. Enhanced Degradation of Polychlorinated Biphenyls with Simultaneous Usage of Reductive and Oxidative Agents over UV/Sulfite/TiO2 Process as a New Approach of Advanced Oxidation/Reduction Processes. J. Water Process Eng. 2019, 32, 100983. [Google Scholar] [CrossRef]
- Lei, M.; Wang, N.; Guo, S.; Zhu, L.; Ding, Y.; Tang, H. A One-Pot Consecutive Photocatalytic Reduction and Oxidation System for Complete Debromination of Tetrabromodiphenyl Ether. Chem. Eng. J. 2018, 345, 586–593. [Google Scholar] [CrossRef]
- Lei, M.; Wang, Z.; Tang, Y.; Wang, H.; Zhu, L.; Tang, H. Peculiar and Full Debromination of Tetrabromodiphenyl Ether on Pd/TiO2: A Competing Route through Hydro-Debromination and Coupling-Debromination. Appl. Catal. B Environ. 2020, 275, 119093. [Google Scholar] [CrossRef]
- Chen, K.; Wang, X.; Xia, P.; Xie, J.; Wang, J.; Li, X.; Tang, Y.; Li, L. Efficient Removal of 2,2′,4,4′-Tetrabromodiphenyl Ether with a Z-Scheme Cu2O-(RGO-TiO2) Photocatalyst under Sunlight Irradiation. Chemosphere 2020, 254, 126806. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Wang, H.; Tao, S.; Kiyama, R. Biological Impact of Environmental Polycyclic Aromatic Hydrocarbons (EPAHs) as Endocrine Disruptors. Environ. Pollut. 2016, 213, 809–824. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Phan Thi, L.-A.; van Le, Q.; Singh, P.; Raizada, P.; Kajitvichyanukul, P. Tailored Photocatalysts and Revealed Reaction Pathways for Photodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) in Water, Soil and Other Sources. Chemosphere 2020, 260, 127529. [Google Scholar] [CrossRef]
- Martínez-Vargas, B.L.; Cruz-Ramírez, M.; Díaz-Real, J.A.; Rodríguez-López, J.L.; Bacame-Valenzuela, F.J.; Ortega-Borges, R.; Reyes-Vidal, Y.; Ortiz-Frade, L. Synthesis and Characterization of N-ZnO/p-MnO Nanocomposites for the Photocatalytic Degradation of Anthracene. J. Photochem. Photobiol. A Chem. 2019, 369, 85–96. [Google Scholar] [CrossRef]
- Mukwevho, N.; Gusain, R.; Fosso-Kankeu, E.; Kumar, N.; Waanders, F.; Ray, S.S. Removal of Naphthalene from Simulated Wastewater through Adsorption-Photodegradation by ZnO/Ag/GO Nanocomposite. J. Ind. Eng. Chem. 2020, 81, 393–404. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Sunlight Mediated Improved Photocatalytic Degradation of Carcinogenic Benz[a]Anthracene and Benzo[a]Pyrene by Zinc Oxide Encapsulated Hexacyanoferrate Nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 381, 111861. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Enhanced Photocatalytic Degradation of Chrysene by Fe2O3@ZnHCF Nanocubes. Chem. Eng. J. 2018, 348, 754–764. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Kao, C.-M.; Chien, C.-C.; Hung, C.-M. Wood-Biochar-Supported Magnetite Nanoparticles for Remediation of PAH-Contaminated Estuary Sediment. Catalysts 2018, 8, 73. [Google Scholar] [CrossRef] [Green Version]
- Qin, Z.; Zhao, Z.; Jiao, W.; Han, Z.; Xia, L.; Fang, Y.; Wang, S.; Ji, L.; Jiang, Y. Coupled Photocatalytic-Bacterial Degradation of Pyrene: Removal Enhancement and Bacterial Community Responses. Environ. Res. 2020, 183, 109135. [Google Scholar] [CrossRef]
- Cai, H.; Sun, L.; Wang, Y.; Song, T.; Bao, M.; Yang, X. Unprecedented Efficient Degradation of Phenanthrene in Water by Intimately Coupling Novel Ternary Composite Mn3O4/MnO2-Ag3PO4 and Functional Bacteria under Visible Light Irradiation. Chem. Eng. J. 2019, 369, 1078–1092. [Google Scholar] [CrossRef]
- Guan, S.H.; Zhao, K.F.; Tong, Q.; Rao, Q.X.; Cheng, L.; Song, W.; Zhang, Q.C.; Wang, X.L.; Song, W.G. A Review of Photocatalytic Materials Application on Nonylphenol Degradation. Environ. Chall. 2021, 4, 100172. [Google Scholar] [CrossRef]
- Tang, C.; Huang, X.; Wang, H.; Shi, H.; Zhao, G. Mechanism Investigation on the Enhanced Photocatalytic Oxidation of Nonylphenol on Hydrophobic TiO2 Nanotubes. J. Hazard. Mater. 2020, 382, 121017. [Google Scholar] [CrossRef]
- Niu, B.; Cai, J.; Song, W.; Zhao, G. Novel Electrochemical Pretreatment for Preferential Removal of Nonylphenol in Industrial Wastewater: Biodegradability Improvement and Toxicity Reduction. Environ. Sci. Technol. 2020, 54, 1258–1266. [Google Scholar] [CrossRef]
- Dong, C.-D.; Chen, C.-W.; Tsai, M.-L.; Chang, J.-H.; Lyu, S.-Y.; Hung, C.-M. Degradation of 4-Nonylphenol in Marine Sediments by Persulfate over Magnetically Modified Biochars. Bioresour. Technol. 2019, 281, 143–148. [Google Scholar] [CrossRef]
- Xu, P.; Chen, M.; Lai, C.; Zeng, G.; Huang, D.; Wang, H.; Gong, X.; Qin, L.; Liu, Y.; Mo, D.; et al. Effects of Typical Engineered Nanomaterials on 4-Nonylphenol Degradation in River Sediment: Based on Bacterial Community and Function Analysis. Environ. Sci. Nano 2019, 6, 2171–2184. [Google Scholar] [CrossRef]

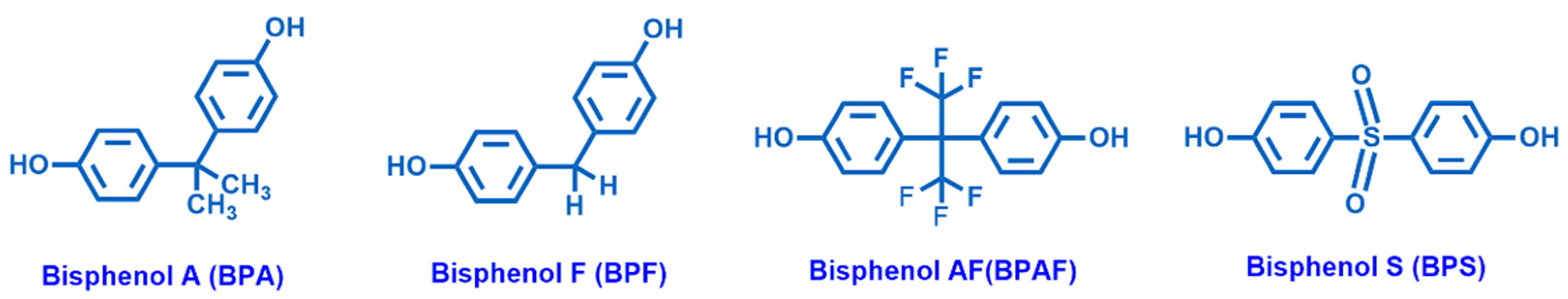




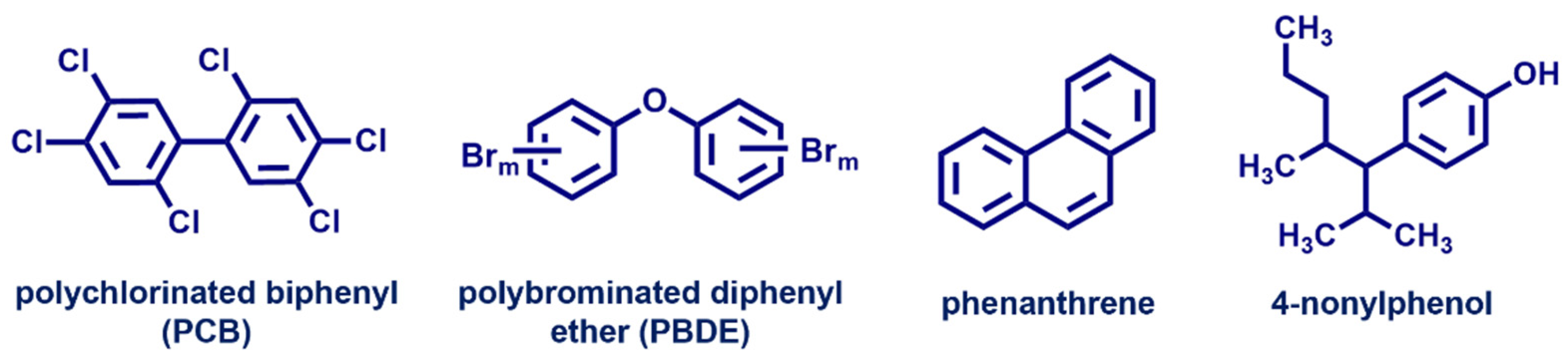

| Endocrine Disrupting Compounds | Main Sources | Description |
|---|---|---|
| Bisphenols (e.g., BPA) | Epoxy composites, polyester resins, polycarbonate plastics, conserved food, dental sealants | Highly pervasive, estrogen mimic |
| Phthalates (e.g., dimethyl phthalate) | Polyvinylchloride (PVC), personal care products, medical devices | Effects on endocrine and reproductive systems |
| Alkylphenols (e.g., nonylphenol, octylphenol) | Non-ionic surfactants, industrial products, cosmetics | Lipophilic properties, estrogenic activity |
| Brominated flame retardants (polybrominated diphenyl ethers) | Household products, industrial products | Carcinogenicity |
| Insecticides (e.g., DDT) Herbicides (e.g., atrazine) Fungicides (e.g., vinclozolin) | Agricultural and household uses | Persistent agents, estrogen mimic |
| Parabens (e.g., methylparaben) | Common preservatives, food, cosmetics, pharmaceuticals | Estrogenic effect |
| Antibacterial agents (e.g., triclosan) | Cosmetics, pharmaceuticals | Effects on reproductive hormons |
| Antibiotics (e.g., cyclines) Anti-inflammatory drugs (e.g., paracetamol, ibuprofen, diclofenac) | Pharmaceutical products | Effects on the level of estrogen hormones |
| Synthetic hormones (e.g., 17β-estradiol) | Oral contraceptive pills, wastewater contamination | Strong estrogenic behavior |
| Material | Preparation | Target | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| (N, Co)-codoped TiO2 | Wet impregnation method | BPA (20 mg/L) | Visible-light irradiation | 97% removal, 0.0195 min−1 | [69] |
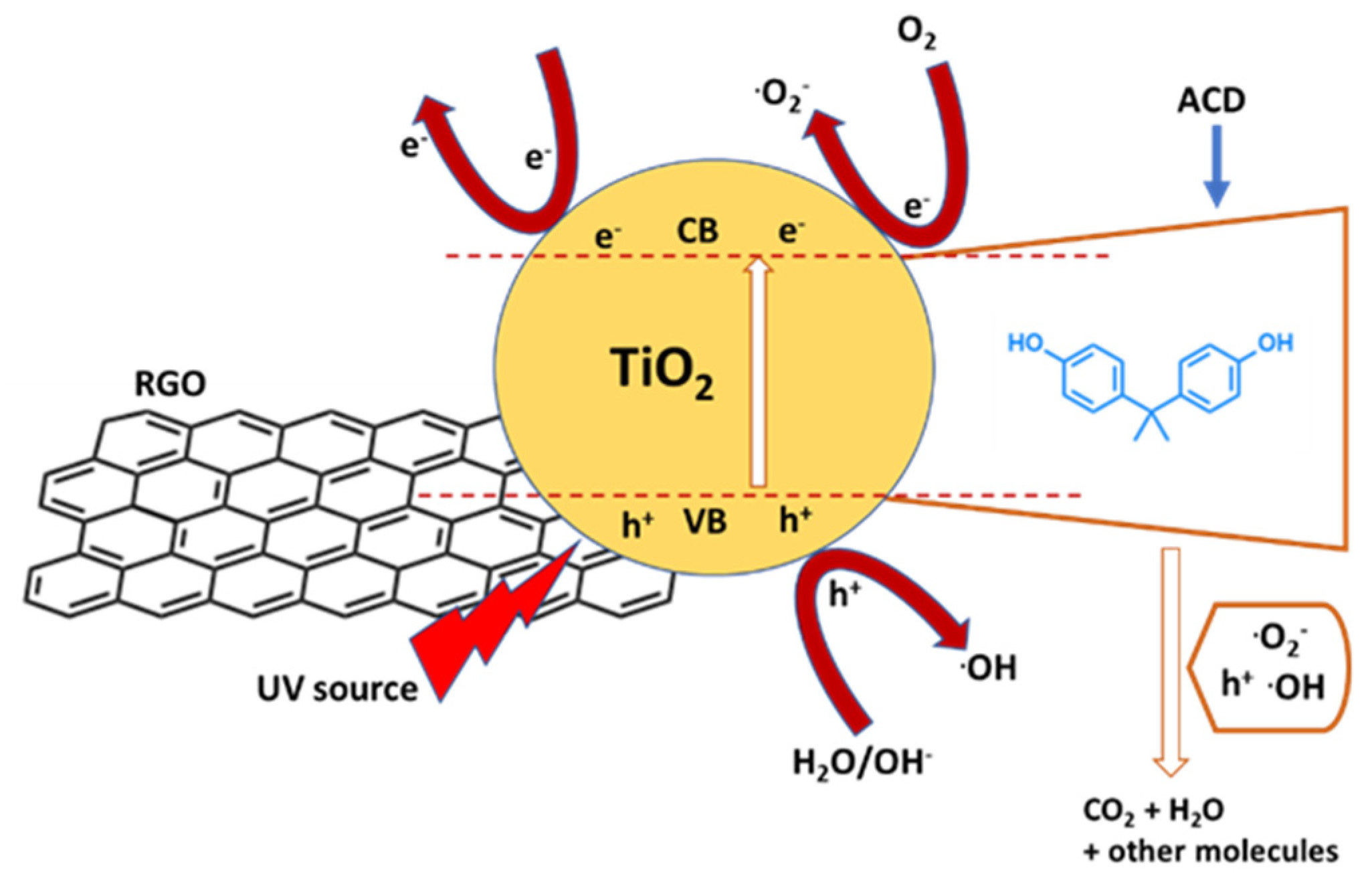
| TiO2−x/rGO nanocomposite | Hydrothermal-calcination method | BPA (2.5 mg/L) | Visible-light irradiation (60 min) | 91% removal, 0.049 min−1 | [66] |
| TiO2@ACD@RGO composite | Photochemical method | BPA (20 mg/L) | UV irradiation (60 min) | 85.6% removal, 0.739 mg/L·min | [67] |
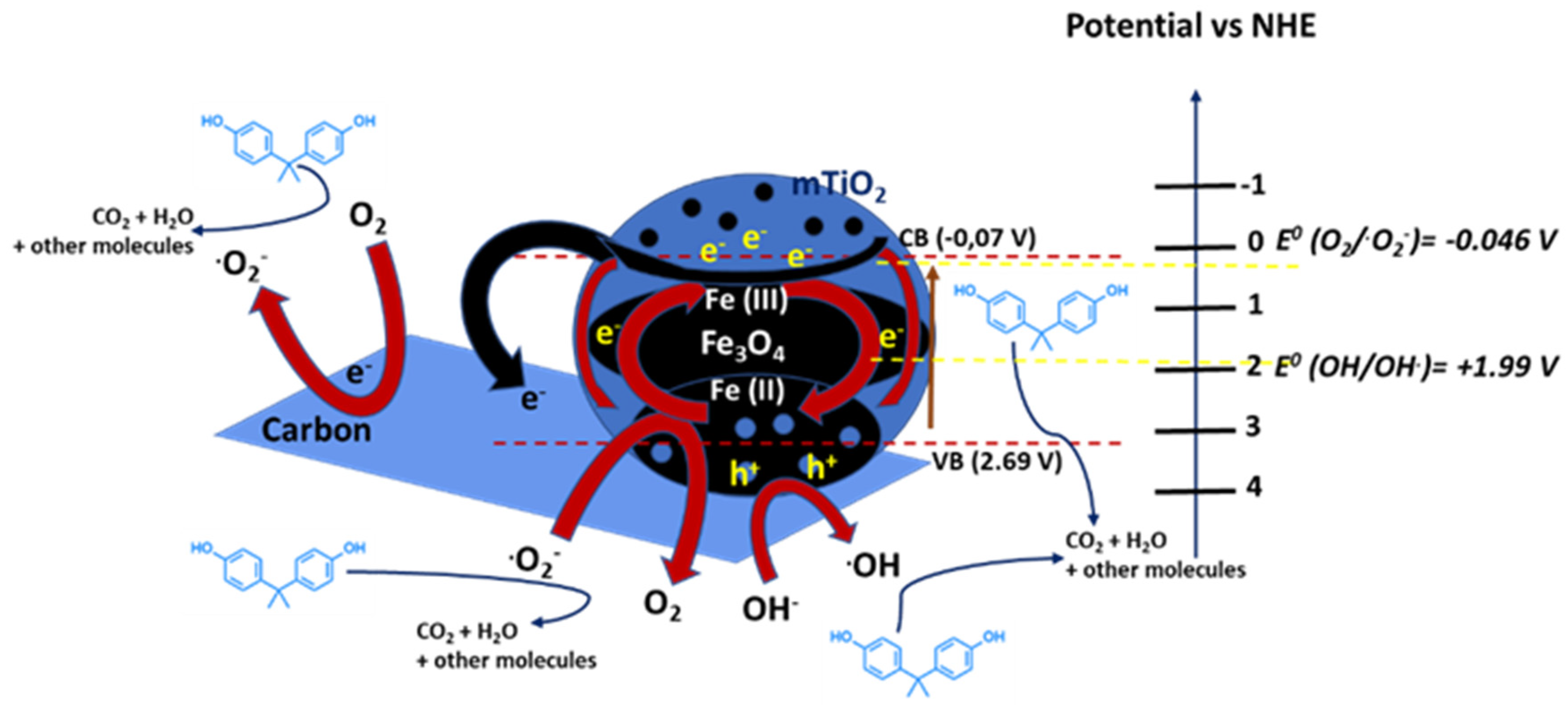
| Fe3O4@mTiO2/C | Sol–gel Methodology | BPA | Visible-light irradiation (120 min) | 100% removal, 0.01045 min−1 | [70] |
| Ag/Fe,N-TiO2/Fe3O4@SiO2 (AgFeNTFS) | Sol–gel Methodology | BPA (2 mg/L), E. coli (106 CFU/mL), | Visible-light irradiation (360 min for BPA, 90 min for E. coli) | 100% removal | [71] |
| (g-C3N4)–CaTiO3 heterojunction | Mixing methodology | BPA | Sunlight irradiation (120 min) | 47% removal | [73] |
| C60@AgCl-ZnAl LDO | Sol–gel Methodology | BPA (0.5 g/L) | Ultraviolet light (5 min) | 100% removal | [72] |
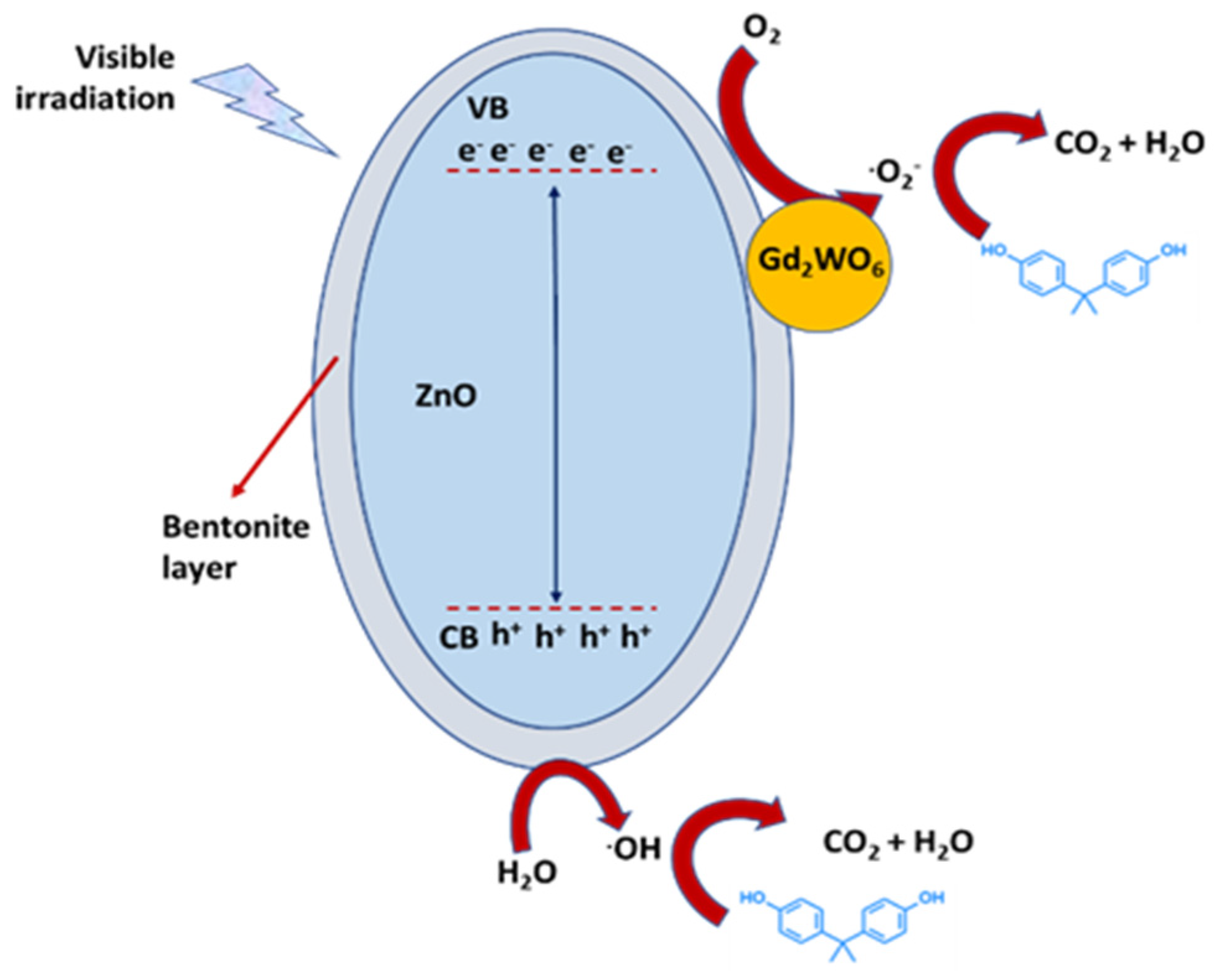
| Gd2WO6/ZnO/bentonite (GWZB) nanocomposite | Hydrothermal process | Ciprofloxacin (CF) (4 × 10−5 M), BPA (4 × 10−5 M) | Visible-light irradiation | Removal 98.3% and 97.9% respectively | [79] |
| MnFe2O4/MX magnetic composites | Sol–gel Methodology | BPA (10 mg/L), sulfadiazine (10 mg/L), ciprofloxacin (10 mg/L) | PMS activation | Removal of 95%, 91% and 85%, respectively | [80] |
| Fe-doped TiO2/rGO | Hydrothermal process | BPA, Bisphenol F (BPF), Bisphenol AF (BPAF), Bisphenol S (BPS) (20 mg/L) | Visible-light irradiation (60 min for BPA, 120 min for BPF, BPAF and BPS) | Removal of 100%, 100%, 100% and 96% respectively | [68] |
| Bi2WO6/BiOI (BWOI-3 morphology) | Microwave-assisted synthetic method | Methyl orange (MO), BPA | Visible-light irradiation (60 min for MO, 100 min for BPA) | Removal 95.0% and 86.8% respectively, MO: 0.04169 min−1, BPA: 0.01778 min−1 | [75] |
| Polythiophene (PTh)/Bi4O5I2 | Sol–gel Methodology | BPA | Visible-light irradiation (30 min) | Removal 99.2%, 0.194 min−1 | [74] |
| Pd/PdO/β-Bi2O3 composite | Simple two-step Sol–gel-based process | BPA | Visible-light irradiation (30 min) | Removal of 97.4%, 0.1129 min−1 | [76] |
| Pd/Bi4O5Br2 | Solvothermal-reduction method | BPA (20 mg/L) | LED visible-light irradiation (70 min) | 95.8% removal, 0.0548 min−1 | [77] |
| Nano flake Ag3PO4 | Sol–gel Methodology | BPA (15 mg/L) | Visible-light irradiation (30 min) | 100% removal efficiency, 0.6324 min−1 | [78] |
| Cu2O@β-CD | Topotaxial conversion | BPA (8 mg/L) | Neutral pH | 83.7% removal, 0.0196 min−1 | [81] |
| TiO2 | Sol–gel Methodology | DMP (5.16 µM) | UV irradiation (75 min) and pH 6–7 | 80.5% removal, 0.0768 min−1 | [85] |
| TiO2 | Sol–gel Methodology | DMP, DEP, DBP (6, 6, and 4.128 mg/L) | UV irradiation (90 min) | Removal 93.0, 92.6, 92.5% respectively, 0.025 min−1 | [86] |
| Bi2O3-TiO2 composite | Hydrothermal process | Pb(II), DBP | Visible-light irradiation (4 h) | 45%-DBP oxidation, 40%-Pb(II) reduction | [87] |
| Nanorod ZnO/SiC nanocomposite | Sol–gel Methodology | DEP (5 ppm) | UV and visible light, neutral pH | 90% removal, UV: 46.86 × 10−3 min−1, Visible: 8.52 × 10−3 min−1. | [88] |
| mpg-C3N4/BiOBr/PTh(Z-scheme) | Sol–gel Methodology | DMP (1 g/L) | Visible-light irradiation | Removal efficiency of 40% and 50% higher than g-C3N4 and BiOBr, 0.193 h−1. | [89] |
| Iron–cerium (Fe-Ce) bimetallic catalysts (FCBCs) | Sol–gel Methodology | PAEs (DMP, DEP, DEHP, DINP, DnOP, DIDP) | PS 1.0 × 10−5 M, FCBC 1.67 g/L, pH 2 | 86% removal, 1.5 × 10−1 h−1. | [90] |
| Material | Preparation | Target | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| In,S-TiO2@rGO | ultrasonic-assisted solvothermal method | ATZ (20 mg/L) | Visible light (20 min) | 100% removal, 95.5% mineralization, k = 0.248 min−1 | [94] |
| Fe-TiO2 | Sol–gel method | ATZ (10 mg/L) | Visible light, 1.6 mM H2O2 (30 min) | 95% degradation at pH 3 k = 0.1021 min−1 | [98] |
| Ag/LaTiO3 nanowires | Hydrothermal method | ATZ (50 mg/L) | Visible light (40 min) | 100% removal k = 0.0434 min−1 | [95] |
| Ag/NiCo2O4 nanorods | Co-precipitation | ATZ (50 mg/L) | Visible light (20 min) | 100% removal, k = 0.049 min−1 | [96] |
| CdS/BiOBr/Bi2O2CO3 | Hydrothermal method | ATZ (50 mg/L) | Visible light (30 min) | 95% removal, k = 0.122 min−1 | [97] |
| ZnxCu1−xFe2O4 | Sol–gel combustion process | ATZ (4.4 μM) | UV-vis light (30 min), Na2SO3 0.5 mM | 95% removal, k = 0.195 min−1 | [99] |
| TiO2 nanotubes | Electrochemical anodization | ATZ (2 mg/L) | UV-vis light, bias 0.2 V vs. SCE (2 h) | 96.8% removal, k = 1.72 h−1 | [100] |
| Bi2WO6/C3N4 | Hydrothermal method | ATZ (20 mg/L) | Visible light, biofilm (8 h) | >50% removal | [101] |
| Fe3O4-sepiolite | Co-precipitation | ATZ (10 mM) | PS 92 mM (1 h) | 71.6% removal, 21% mineralization k = 0.0108 min−1 | [102] |
| Fe3O4 | Commercial | ATZ (23 μM) | PMS 0.4 mM hydroxylamine 0.3 mM (15 min) | 100% degradation, k = 0.152 min−1 | [103] |
| Cu-doped LaFeO3 | Sol–gel method | ATZ (23 μM) | PMS 0.5 mM (1 h) | 100% degradation, 52% mineralization k = 0.1406 min−1 | [104] |
| CoNi3O4 nanoribbons/diatomite | Co-precipitation | ATZ (5 mg/L) | PMS 0.3 mM (30 min) | 93% removal, 56% mineralization k = 0.0842 min−1 | [105] |
| Co,Sm-Ti/PbO2 electrode | Electrochemical deposition | ATZ (20 mg/L) | Current density 20 mA cm−2 (3 h) | 92.6% removal, 84.5% COD decrease | [106] |
| Ti/RuO2-IrO2 anode, CuFe2O4 particles | Sol–gel combustion process | ATZ (46 μM) | Current density 20 mA cm−2, PS 4.0 mM (35 min) | 99% removal, 22.1% mineralization k = 0.0186 min−1 | [107] |
| Faceted TiO2 | Hydrothermal method | PMG (10 mg/L) | UV light | 100% removal in 50 min, k = 3.0 h−1 on {201}-TiO2 | [108] |
| CeO2 NPs | Solution synthesis | PMG (25 mg/L) | UV or visible light | 100% removal in 5 min (UV, k = 0.6601 min−1) or 20 min (visible, 0.3028 min−1) at pH 4 | [109] |
| W/ZnO | Precipitation | PMG (20 mg/L) | Simulated solar light (3 h) | 74% removal, 30% mineralization | [110] |
| BiOBr/Fe3O4 | Solvothermal method | PMG (100 mg/L) | Visible light (1 h) | 97% removal | [111] |
| MoSe2/BiVO4 | Hydrothermal method | PMG (10−4 M) | Visible light (3 h) | 86.1% removal | [112] |
| Cu2S/Bi2WO6 | Hydrothermal method | PMG (10−4 M) | Visible light (3 h) | 73.2% removal | [113] |
| Fe3CeOx | Co-precipitation | PMG (100 mg/L) | PMS 0.5 mM (15 min) | 100% removal, 85.6 TOC decrease 400 mg L−1 h−1 | [114] |
| TiO2 P25 on SiC | Dip coating | Paraquat (5–40 mg/L) | UV-C light | 90% mineralization | [115] |
| TiO2 on SBA-16 SiO2 | Sol–gel method | Paraquat (50 mg/L) | UV light (24 h) | 70% removal, k = 0.0431 min−1 | [116] |
| N-TiO2 | Hydrothermal method | Paraquat (20 mg/L) | UV or visible light (120 min) | Removal 86% (UV, k = 0.0230 min−1), 62% (visible, k = 0.0074 min−1) | [117] |
| N-TiO2@SiO2@Fe3O4 | Sol–gel method | Paraquat (10–40 mg/L) | Visible light (3 h) | 98.7% removal, 84.7% mineralization | [118] |
| B-TiO2-SiO2/CoFe2O4 | Sol–gel, hydrothermal method | Paraquat (300 mg/L COD) | Visible light (3 h) | 82% COD removal k = 0.89 h−1 | [119] |
| TiO2@MIL-101(Cr)@Fe3O4 | Solution synthesis | Paraquat (20 mg/L) | UV light (45 min) | 87% removal, k = 0.0126 min−1 | [120] |
| g-C3N4/Bi2O2CO3/CoFe2O4 on biochar | Solution synthesis | Paraquat (20 mg/L) | Visible light, sunlight, photo-ozonation, PMS | 99% removal in 1.5 h (vis, 0.0596 min−1), 100% mineralization in 30 min (visible-O3-PMS) | [121] |
| CeO2–Bi2O3 | Co-precipitation | 2,4-D | Visible light (13 h) | 90% removal and COD decrease | [122] |
| Fe3O4@WO3/SBA-15 | Co-precipitation hydrothermal method | 2,4-D (10−6 M) | UV light (4 h) | 90.7% removal | [123] |
| TiO2 nanotubes | Anodization | 2,4-D (10 mg/L) | Simulated sunlight, bias 2.4 V (2 h) | 97% removal, k = 0.0295 min−1 | [124] |
| TiO2-acetylacetone | Sol–gel method | 2,4-D, 4-CPA, MCPA, MCPB (≥0.2 mM) | Dark (1 h) | 80–90% removal | [50] |
| Co–Ni@chitosan@Fe3O4 | Co-precipitation, reduction | 2,4-D (100 mg/L) | H2O2 1–2 mL | 95.5% removal, k = 0.07517 min−1 | [125] |
| polyaniline/SnO2 | Polymerization, precipitation | DDT (100–500 mg/L) | Microwave irradiation (12 min) | 80% removal, k = 0.20 min−1 | [126] |
| MnO2 | Oxidation | DDT (0.5 mg/L) | PMS (4 h) | 100% removal | [127] |
| TiO2,ZnO | Commercial | vinclozoline fenarimol, malathion, fenotrothion, quinalphos, dimethoate (0.3 mg/L) | UV light or sunlight, PS (250 mg/L Na2S2O8), 4 h | 70–100% removal, except for fenarimol, 0.0018–0.0292 min−1 (TiO2); 0.0023–0.0872 min−1 (ZnO) | [128,129] |
| Material | Preparation | Target | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| TiO2-rGO | Hydrothermal route | TCS (100 mg/L) | Solar light | 85/100% removal 0.251 h−1 | [137] |
| CdS@TiO2-rGO nanocomposite | Hydrothermal route | TCS (40ppm) | visible light | 100% removal 2.7 × 10−3 min−1 | [138] |
| Ag (NPs)/TiO2 film | Template assisted synthesis | TCS (1.0 mg/L) | UV-A | 75% removal 0.992 mg/L/min | [142] |
| Co,N-codoped TiO2 nanoparticles | Hydrothermal route | TCS (10mg/L) | UV/Vis LED lights irradiation | >99% removal 0.2340 ± 0.006 min−1 | [143] |
| Fe2O3-TiO2/PVP composite | Spray coating | TCS (1–10 mg/L) | Solar light | 83% removal 0.3405–0.0687 min−1 | [139] |
| Fe3O4/C3N4 | Microwave-assisted hydrothermal route | TCS (4 × 10−5 M) | Visible-light | 46% removal 2.3 × 10−5 s−1 | [140] |
| Fe/Nb2O5 | Impregnation | TCS | Solar/artificial irradiation | 80% removal | [136] |
| CuO-loaded halloysite nanotubes | Hydrothermal route | TCS (2 mg/L) | activated PS | 100% removal | [147] |
| CoxMn2-xO4 | Solution-based oxidation/precipitation process | TCS (10 mg/L) | activated PMS | 96.4% removal 0.106 min−1 | [144] |
| Fe3O4 | commercial | TCS (0.03mM) | activated PMS | 100% removal | [145] |
| MnFe2O4 | commercial | TCS (0.03 mM) | activated PMS | 100% removal | [146] |
| Fe3O4 | Ultrasonic-assisted reverse coprecipitation | TCS (5 mg/L) | activated PS | ~88% removal 0.022 min−1 | [148] |
| Material | Preparation | Target | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| Ag/Pd/Au/Pt-doped TiO2 | photodeposition/sol–gel method | Paraben mixture MP, EP, PP, BuP, BeP (10 mg/L) | Sunlight | 90% removal | [152] |
| TiO2 supported nanotubes | one-step anodic oxidation method | Paraben mixture MP, EP, PP (1 mg/L) | UV/Sunlight | 35%r emoval | [153] |
| TiO2 supported nanotubes | one-step anodic oxidation method | Paraben mixture MP, EP, PP (1 mg/L) | UV/ozone | 100% removal | [154] |
| TiO2 nanopowder | commercial | MP, EP (300 g/L) | PS, natural sunlight | 90% removal (0.006 ± 0.005 min−1) | [155] |
| CoOx/BiVO4 | wet impregnation | PP (200–400 μg/L) | simulated sunlight | 97% removal (0.025 ± 0.001 min−1) | [156] |
| CoxNi1-xTiO3 nanorods | solution-based method | EP (250 g/L) | simulated solar/visible light | 92% removal | [157] |
| nano-ZnO | commercial | BzP (15 mg/L) | UV/H2O2 | 100% removal (0.3305 min−1) | [158] |
| nano-ZnO | commercial | MP, EP, PP, BuP and BeP (10 mg/L) | UV/ozone | 100%, 100%, 100%, 100% and 94% removal | [159] |
| Material | Preparation | Target | Conditions | Results | Ref. |
|---|---|---|---|---|---|
| MnO2 NPs decorated titanate nanotubes | hydrothermal method | E2 (4 μM) | simulated solar light | 82.6% removal (0.198 min−1) | [161] |
| MnO2 nanozyme | commercial | E2 (1 mg/L) | Enzyme-like activity | 97.3% removal (0.0131 min−1) | [162] |
| TiO2-Fe zeolite/SnS2 | Low T/solution method | E2 (5 μM) | Solar light/H2O2 | 78.1% removal (0.01539 min−1) | [163] |
| Al-polythiophene doped ZnO | Co-precipitation | E2 (1 mg/L) | UV-A irradiation | 96% removal (0.4451 h−1) | [164] |
| Ag, Sr-modified TiO2 | Sol–gel | EE2 (10 mg/L) | Visible light | 94% removal (0.1699 min−1) | [165] |
| Nb2O5 | commercial | EE2 (10 mg/L) | UV irradiation | 85% removal | [167] |
| a-FeOOH doped MnO2@MnCO3 microsphere | surface oxidation/hydrothermal reaction | E3 (0.5 mg/L) | simulated solar irradiation | 90% removal | [168] |
| Fe-doped TiO2 | Hydrothermal/sol–gel | E3 (10 μM) | High/low UV irradiation | 80% removal (0.009–0.003 min−1/ 0.005–0.016 min−1) | [170] |
| Bi/Bi2O3 | Impregnation method | E1 (10.4 μmol/L) | UV–vis light irradiation | ~95% removal (0.045 min−1) | [169] |
| TiO2–ZnO nanocomposite | non-aqueous sol–gel process | E2, EE2 (0.05–10 mg/L) | UV and visible irradiation | ~100% removal (E2: 0.022 min−1, EE2: 0.013 min−1) | [166] |
| W-doped nanotubular TiO2 | Electrochemical synthesis | E1, EE2 (10 mg/L) | UV and visible light | 53.4%, 66% removal (EE2: 0.001215 min−1) | [171] |
| Fe3O4 @ SBA15 | water-in-oil microemulsion/sol–gel techniques | E1, E2, EE2 (100–500 μg/L) | Fenton-like | ~90% removal (E1: 0.160–2.708 h−1 E2: 0.228–2.713 h−1 EE2: 0.214–3.211 h−1) | [172] |
| (GO)-Carbon-ZnO nanostructures | microwave assisted technique | E1, E2, E3, EE2 (5 mg/L) | visible light | 89–98% removal (E1: 0.01019 min−1 E2: 0.01236 min−1 E3: 0.01286 min−1 EE2: 0.01567 min−1) | [173] |
| Ag/Pt functionalized WO3 films | Drop casting | Progesterone (0.35 mg/L) | polychromatic irradiation, bias +0.7 V vs. Ag/AgCl | ~27% removal (Ag/WO3: 0.001061 min−1 Pt/WO3: 0.001086 min−1) | [174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imparato, C.; Bifulco, A.; Silvestri, B.; Vitiello, G. Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials. Catalysts 2022, 12, 289. https://doi.org/10.3390/catal12030289
Imparato C, Bifulco A, Silvestri B, Vitiello G. Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials. Catalysts. 2022; 12(3):289. https://doi.org/10.3390/catal12030289
Chicago/Turabian StyleImparato, Claudio, Aurelio Bifulco, Brigida Silvestri, and Giuseppe Vitiello. 2022. "Recent Advances in Endocrine Disrupting Compounds Degradation through Metal Oxide-Based Nanomaterials" Catalysts 12, no. 3: 289. https://doi.org/10.3390/catal12030289








