A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue
Abstract
:1. Introduction
2. Experimental
2.1. Reagent and Materials
2.2. Electrode Modification
2.3. Experimental Procedure
3. Results and Discussion
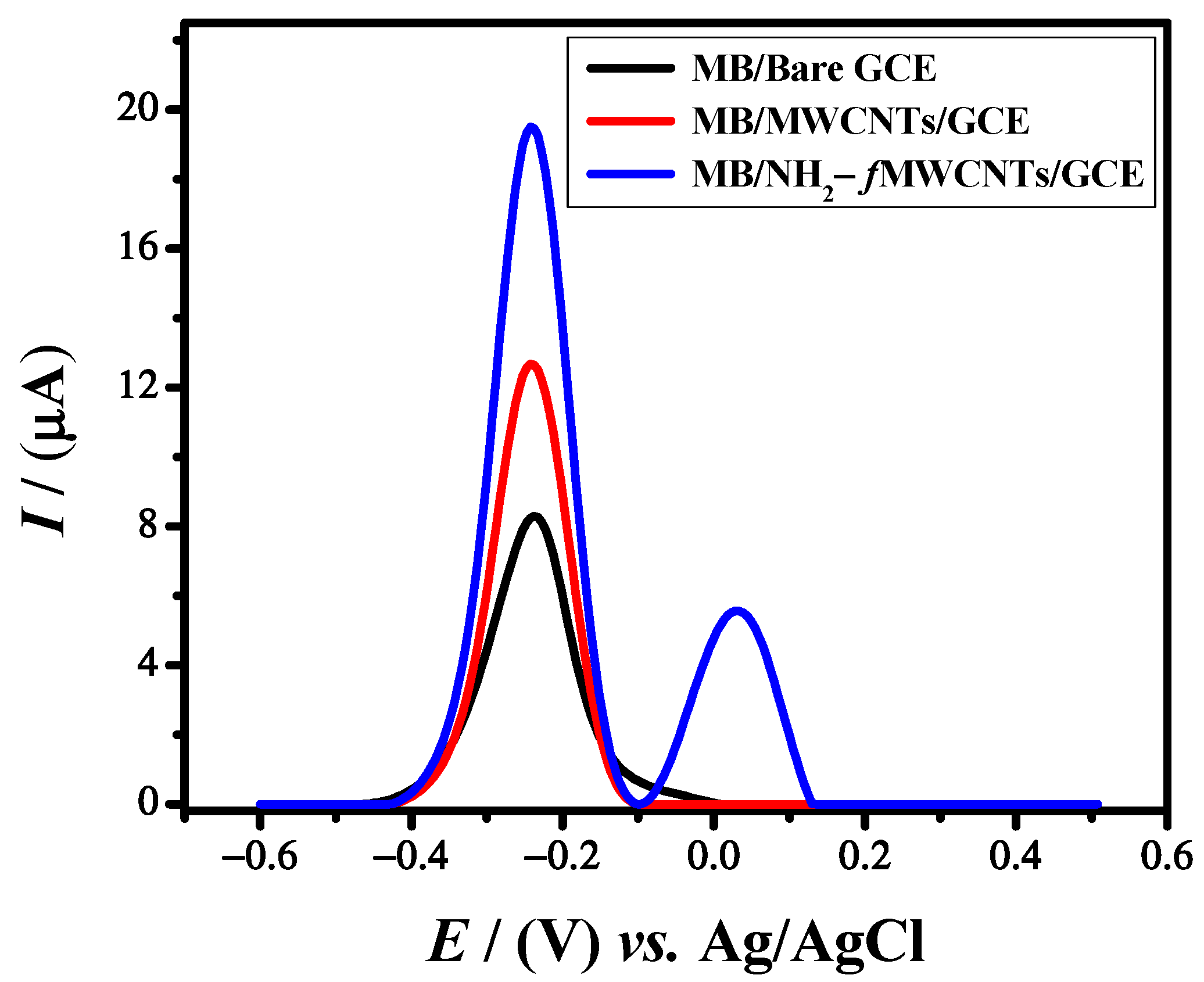
3.1. Electrochemical Characterization
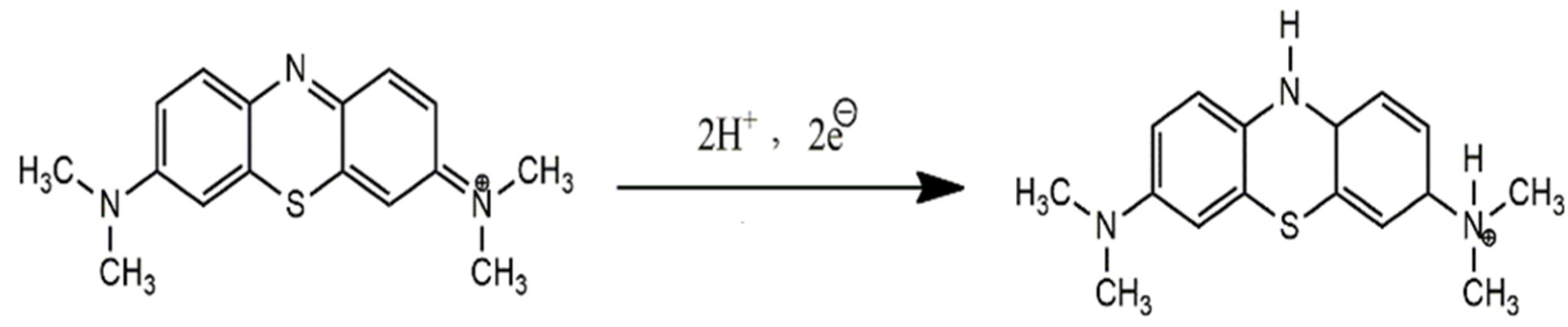
3.2. Voltammetric Analysis of the Targeted Analyte
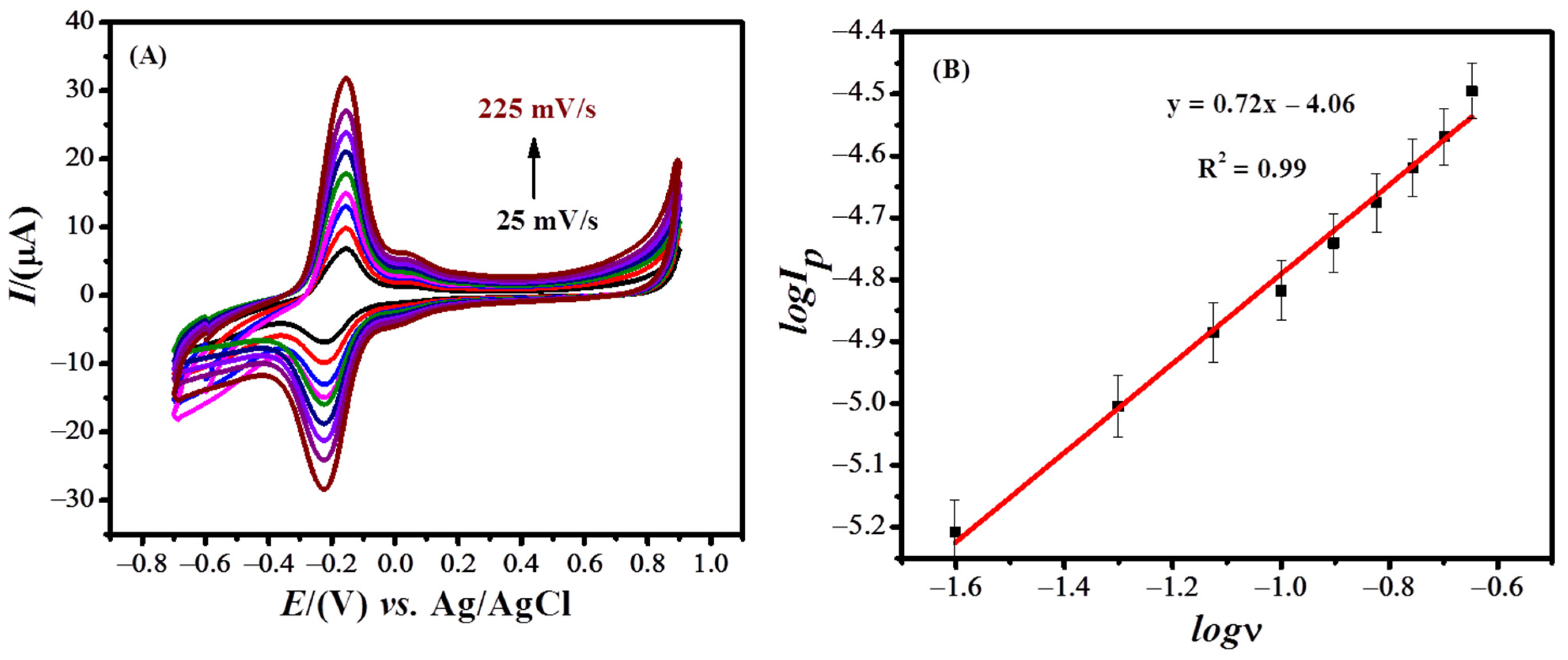
3.3. Effect of Scan Rate
3.4. Optimization of Experimental Parameters. Influence of Supporting Electrolyte, pH, Deposition Potential, and Deposition Time
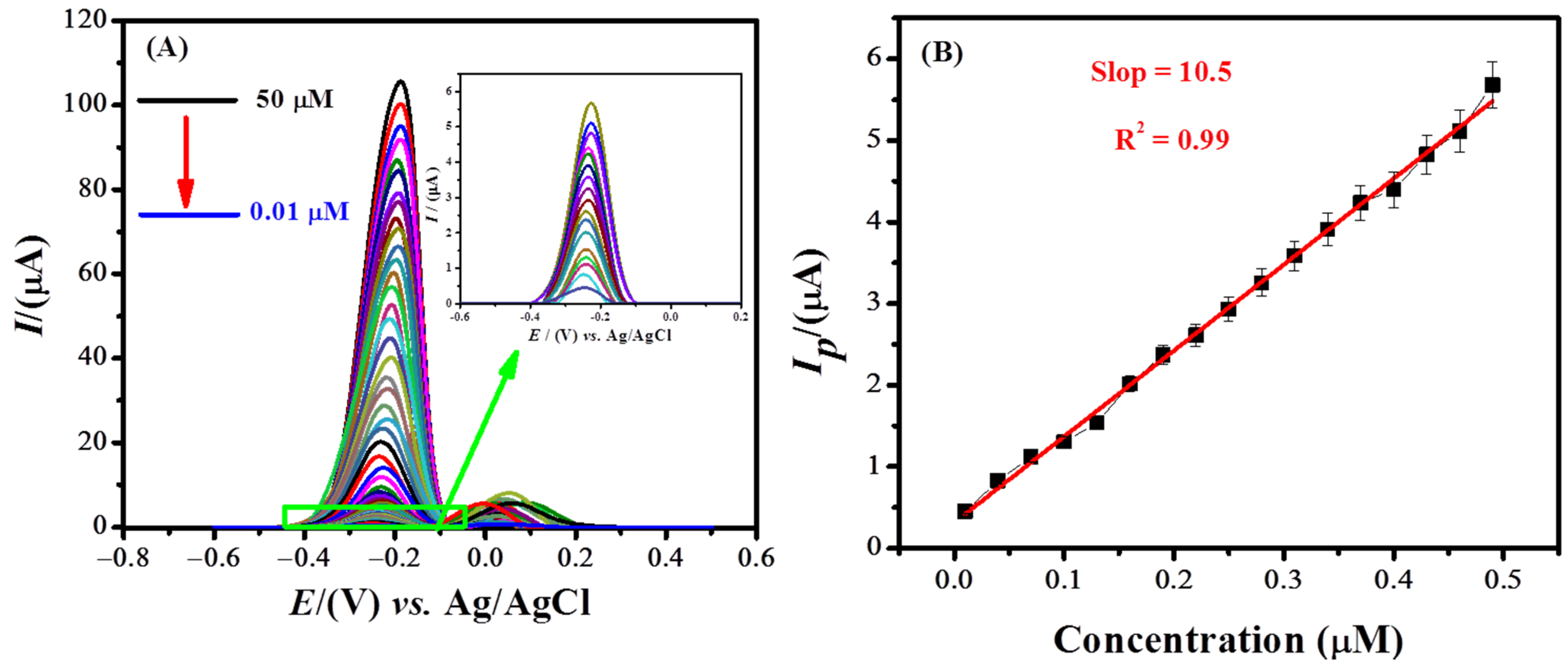
3.5. Analytical Characterization
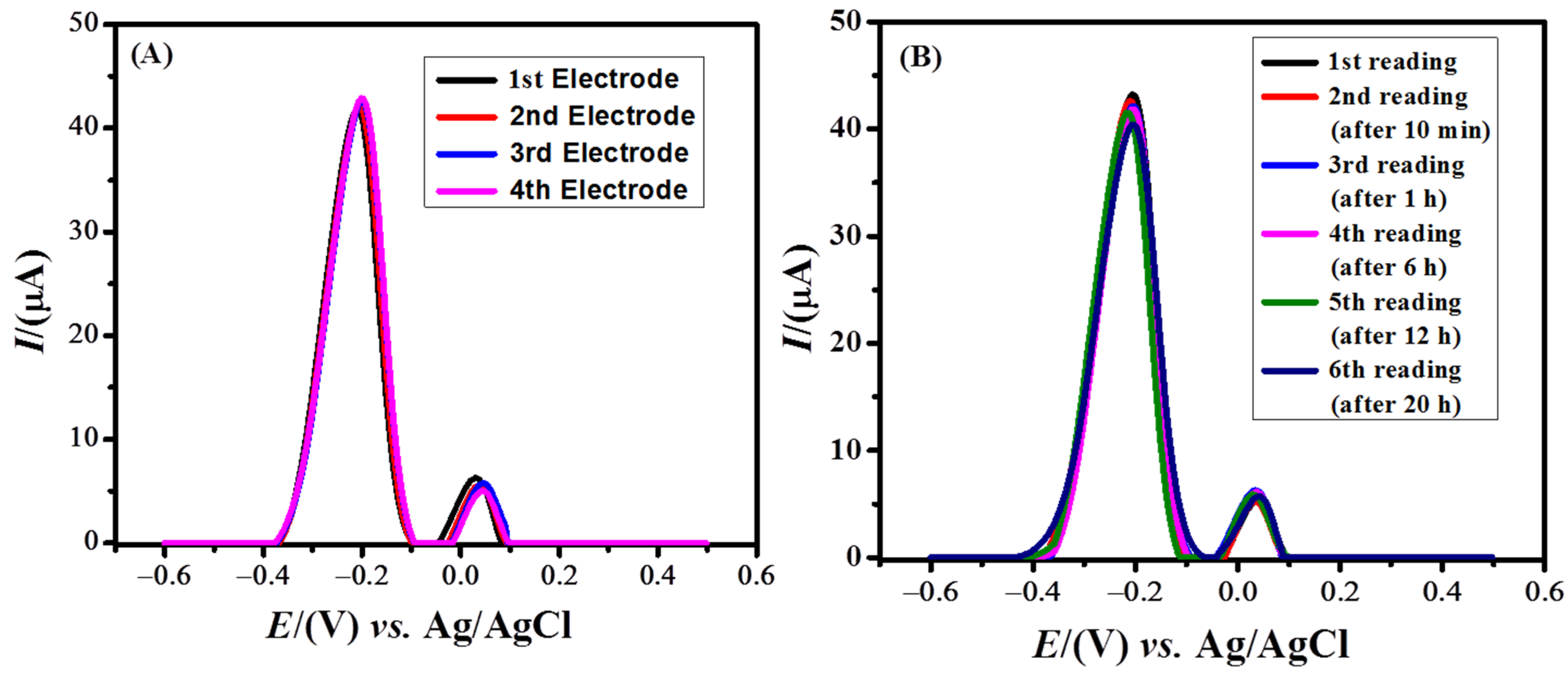
3.6. Validity of the Designed Sensor
3.7. Degradation Study of MB on the Designed Electrochemical Sensor
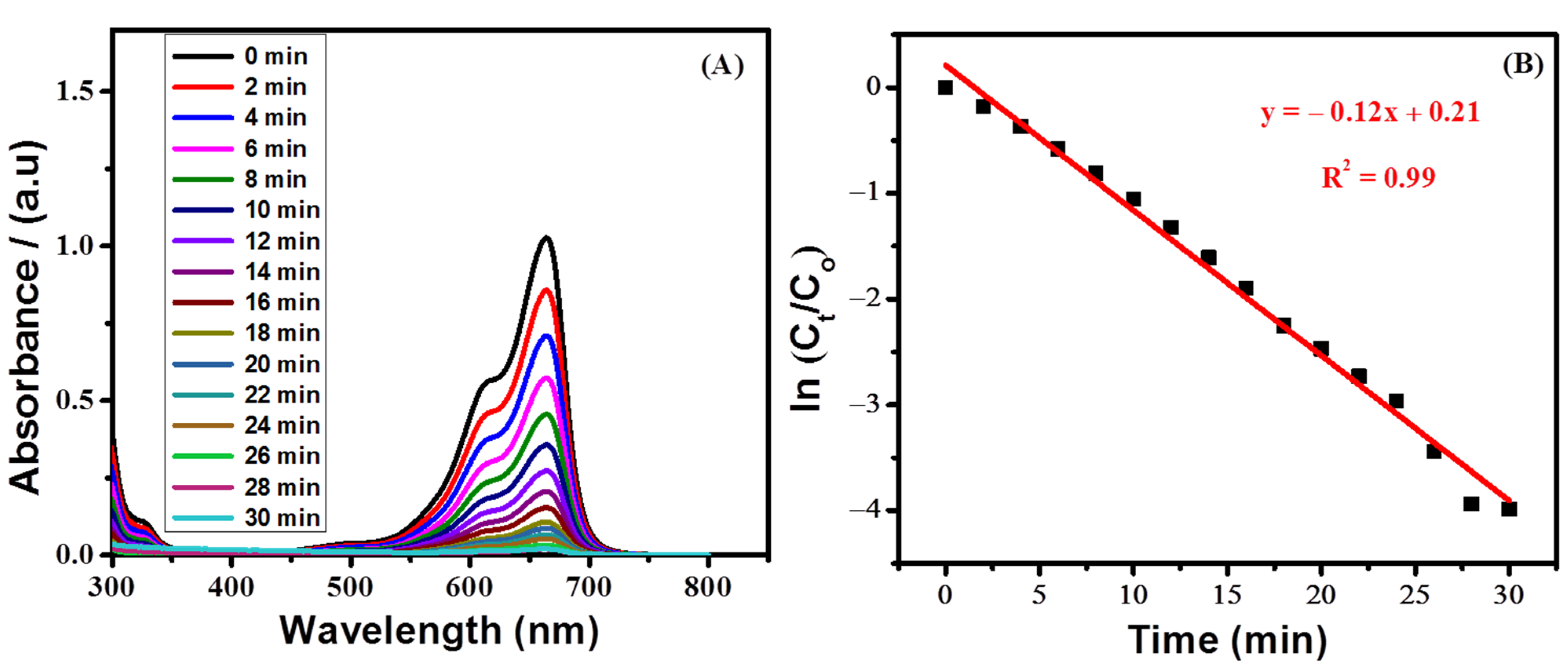
3.8. Spectrophotometric Study of the Degradation of MB
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Veisi, H.; Razeghi, S.; Mohammadi, P.; Hemmati, S. Silver nanoparticles decorated on thiol-modified magnetite nanoparticles (Fe3O4/SiO2-Pr-S-Ag) as a recyclable nanocatalyst for degradation of organic dyes. Mater. Sci. Eng. C 2019, 97, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, V.; Kim, K.H.; Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019, 177, 108569. [Google Scholar] [CrossRef] [PubMed]
- Anwer, H.; Mahmood, A.; Lee, J.; Kim, K.H.; Park, J.-W.; Yip, A.C. Photocatalysts for degradation of dyes in industrial effluents: Opportunities and challenges. J. Nano Res. 2019, 12, 955–972. [Google Scholar] [CrossRef]
- Pandey, S.; Do, J.Y.; Kim, J.; Kang, M. Fast and highly efficient catalytic degradation of dyes using κ-carrageenan stabilized silver nanoparticles nanocatalyst. Carbohydr. Polym. 2020, 230, 115597. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Nasar, A. Adsorptive treatment of hazardous methylene blue dye from artificially contaminated water using cucumis sativus peel waste as a low-cost adsorbent. Groundw. Sustain. Dev. 2017, 5, 152–159. [Google Scholar] [CrossRef]
- Pacheco-Álvarez, M.O.; Picos, A.; Pérez-Segura, T.; Peralta-Hernández, J.M. Proposal for highly efficient electrochemical discoloration and degradation of azo dyes with parallel arrangement electrodes. J. Electroanal. Chem. 2019, 838, 195–203. [Google Scholar] [CrossRef]
- Hossain, M.A.; Ali, M.M.; Islam, T.S.A. Comparative Adsorption of Methylene Blue on Different Low Cost Adsorbents by Continuous Column Process. Phys. Astron. 2018, 77, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H.; Ihm, S.K. Heterogeneous catalytic wet air oxidation of refractory organic pollutants in industrial wastewaters: A review. J. Hazard. Mater. 2011, 186, 16–34. [Google Scholar] [CrossRef]
- Berneth, H. Azine dyes. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Mathelin, C.; Croce, S.; Brasse, D.; Gairard, B.; Gharbi, M.; Andriamisandratsoa, N.; Bekaert, V.; Francis, Z.; Guyonnet, J.-L.; Huss, D. Methylene blue dye, an accurate dye for sentinel lymph node identification in early breast cancer. Anticancer Res. 2009, 29, 4119–4125. [Google Scholar]
- Chen, W.; Chen, L.; Yang, S.; Chen, Z.; Qian, G.; Zhang, S.; Jing, J. A novel technique for localization of small pulmonary nodules. J. Chest. 2007, 131, 1526–1531. [Google Scholar] [CrossRef]
- Hamel, J. A review of acute cyanide poisoning with a treatment update. Crit. Care Nurse 2011, 31, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.E.; Kirgan, D.M.; Guenther, J.M.; Morton, D.L. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann. Surg. 1994, 220, 391. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B. Pharmacokinetics and organ distribution of intravenous and oral methylene blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Salhab, M.; Mokbel, K. Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. Int. Semin. Surg. Oncol. 2005, 2, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.W.; Huh, E.H.; Park, J.H.; Rha, K.H.; Kim, S.K.; Park, S.W. Urinary tract injuries during pelvic surgery: Incidence rates and predisposing factors. Int. Urogynecol. J. 2006, 17, 360–364. [Google Scholar] [CrossRef]
- Lipskikh, O.; Korotkova, E.; Khristunova, Y.P.; Barek, J.; Kratochvil, B. Sensors for voltammetric determination of food azo dyes-A critical review. Electrochim. Acta 2018, 260, 974–985. [Google Scholar] [CrossRef]
- Tang, B.; Xi, C.; Zou, Y.; Wang, G.; Li, X.; Zhang, L.; Chen, D.; Zhang, J. Simultaneous determination of 16 synthetic colorants in hotpot condiment by high performance liquid chromatography. J. Chromatogr. B 2014, 960, 87–91. [Google Scholar] [CrossRef]
- Alesso, M.; Bondioli, G.; Talío, M.C.; Luconi, M.O.; Fernández, L.P. Micelles mediated separation fluorimetric methodology for Rhodamine B determination in condiments, snacks and candies. Food Chem. 2012, 134, 513–517. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, J.; Zhang, Z.; Lu, N.; Yuan, X.; Wu, D. Green synthesis of a bromocresol purple/graphene composite and its application in electrochemical determination of 2, 4, 6-trichlorophenol. J. Anal. Methods Chem. 2015, 7, 3178–3184. [Google Scholar] [CrossRef]
- Terbouche, A.; Lameche, S.; Ait-Ramdane Terbouche, C.; Guerniche, D.; Lerari, D.; Bachari, K.; Hauchard, D. A new electrochemical sensor based on carbon paste electrode/Ru (III) complex for determination of nitrite: Electrochemical impedance and cyclic voltammetry measurements. Measurement 2016, 92, 524–533. [Google Scholar] [CrossRef]
- Yadav, S.; Carrascosa, L.G.; Sina, A.A.; Shiddiky, M.J.; Hill, M.M.; Trau, M. Electrochemical detection of protein glycosylation using lectin and protein–gold affinity interactions. Analyst 2016, 141, 2356–2361. [Google Scholar] [CrossRef] [PubMed]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Randhawa, A.; Lim, K.T. Carbon Nanotubes-Based Hydrogels for Bacterial Eradiation and Wound-Healing Applications. Appl. Sci. 2021, 11, 9550. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Dong, T.; Xu, Y.; Shang, Y. Application of electrochemical methods for the detection of abiotic stress biomarkers in plants. Biosens. Bioelectron. 2021, 182, 113105. [Google Scholar] [CrossRef] [PubMed]
- Hierold, C.; Jungen, A.; Stampfer, C.; Helbling, T. Nano electromechanical sensors based on carbon nanotubes. Sens. Actuator A Phys. 2007, 136, 51–61. [Google Scholar] [CrossRef]
- Li, C.; Thostenson, E.T.; Chou, T.W. Sensors and actuators based on carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2008, 68, 1227–1249. [Google Scholar] [CrossRef]
- Metters, J.P.; Banks, C.E. Banks, Electrochemical utilisation of chemical vapour deposition grown carbon nanotubes as sensors. J. Vac. 2012, 86, 507–519. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Giannaris, C.; Rubio, A. Single-walled carbon nanotube–polymer composites: Strength and weakness. J. Adv. Mater. 2000, 12, 750–753. [Google Scholar] [CrossRef]
- Njuguna, J.; Pielichowski, K.; Alcock, J.R. Epoxy-based fibre reinforced nanocomposites. Adv. Eng. Mater. 2007, 9, 835–847. [Google Scholar] [CrossRef] [Green Version]
- Holzinger, M.; Abraham, J.; Whelan, P.; Graupner, R.; Ley, L.; Hennrich, F.; Kappes, M.; Hirsch, A. Functionalization of single-walled carbon nanotubes with (R-) oxycarbonyl nitrenes. J. Am. Chem. Soc. 2003, 125, 8566–8580. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. J. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Mahajan, A.; Kingon, A.; Kukovecz, A.; Konya, Z.; Vilarinho, P.M. Studies on the thermal decomposition of multiwall carbon nanotubes under different atmospheres. Mater. Lett. 2013, 90, 165–168. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Wei, J.; Lin, J.; Li, Q.; KuaYou, J. A glucose biosensor based on electrodeposition of palladium nanoparticles and glucose oxidase onto Nafion-solubilized carbon nanotube electrode. Biosens. Bioelectron. 2005, 20, 2341–2346. [Google Scholar] [CrossRef] [PubMed]
- De Volder, M.F.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon nanotubes: Present and future commercial applications. J. Sci. 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, J.; Franklin, N.R.; Zhou, C.; Chapline, M.G.; Peng, S.; Cho, K.; Dai, H. Nanotube molecular wires as chemical sensors. J. Sci. 2000, 287, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Nandhini, N.; Rajeshkumar, S.; Mythili, S. The possible mechanism of eco-friendly synthesized nanoparticles on hazardous dyes degradation. Biocatal. Agric. Biotechnol. 2019, 19, 101138. [Google Scholar] [CrossRef]
- Ollis, D.F. Photocatalytic purification and remediation of contaminated air and water. J. C R. Acad. Sci. IIC-Chem. 2000, 3, 405–411. [Google Scholar] [CrossRef]
- Shah, A.H.; Zaid, W.; Shah, A.; Rana, U.A.; Hussain, H.; Ashiq, M.N.; Qureshi, R.; Badshah, A.; Zia, M.A.; Kraatz, H.B. pH Dependent electrochemical characterization, computational studies and evaluation of thermodynamic, kinetic and analytical parameters of two phenazines. J. Electrochem. Soc. 2014, 162, 115–123. [Google Scholar] [CrossRef]
- Ahmad, K.; Shah, A.H.; Adhikari, B.; Rana, U.A.; Vijayaratnam, C.; Muhammad, N.; Shujah, S.; Rauf, A.; Hussain, H.; Badshah, A. pH-dependent redox mechanism and evaluation of kinetic and thermodynamic parameters of a novel anthraquinone. RSC Adv. 2014, 4, 31657–31665. [Google Scholar] [CrossRef]
- Sacco, A. Electrochemical impedance spectroscopy: Fundamentals and application in dye-sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 79, 814–829. [Google Scholar] [CrossRef]
- Macdonald, J.R.; Johnson, W.B. Chapter 1: Theory, experiment, and applications, Fundamentals of Impedance Spectroscopy. In Impedance Spectroscopy, 3rd ed.; Barsoukov, E., Macdonald, J.R., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- Randviir, E.P.; Banks, C.E. Electrochemical impedance spectroscopy: An overview of bioanalytical applications. Anal. Methods 2013, 5, 1098–1115. [Google Scholar] [CrossRef]
- Mohajeri, S.; Dolati, A.; Rezaie, S.S. Electrochemical sensors based on functionalized carbon nanotubes modified with platinum nanoparticles for the detection of sulfide ions in aqueous media. J. Chem. Sci. 2019, 131, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Zahid, A.; Lashin, A.; Rana, U.A.; Al-Arifi, N.; Ullah, I.; Dionysiou, D.D.; Qureshi, R.; Waseem, A.; Kraatz, H.-B.; Shah, A.J.E.A. Development of surfactant based electrochemical sensor for the trace level detection of mercury. Electrochim. Acta 2016, 190, 1007–1014. [Google Scholar] [CrossRef]
- Belter, M.; Sajnóg, A.; Barałkiewicz, D. Over a century of detection and quantification capabilities in analytical chemistry—Historical overview and trends. Talanta 2014, 129, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Kokab, T.; Shah, A.; Nisar, J.; Khan, A.M.; Khan, S.B.; Shah, A.H. Tripeptide Derivative-Modified Glassy Carbon Electrode: A Novel Electrochemical Sensor for Sensitive and Selective Detection of Cd2+ Ions. ACS Omega 2020, 5, 10123–10132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, P.; Salamanca Neto, C.; Moraes, J.; Sartori, E.; Bessegato, G.G.; Lopes, F.; Almeida, L. A novel sensing platform based on self-doped TiO2 nanotubes for methylene blue dye electrochemical monitoring during its electro-Fenton degradation. J. Solid State Electrochem. 2020, 24, 1951–1959. [Google Scholar] [CrossRef]
- Tonlé, I.K.; Ngameni, E.; Tcheumi, H.L.; Tchiéda, V.; Carteret, C.; Walcarius, A. Sorption of methylene blue on an organoclay bearing thiol groups and application to electrochemical sensing of the dye. Talanta 2008, 74, 489–497. [Google Scholar] [CrossRef]
- Hassan, S.S.; Nafady, A.; Solangi, A.R.; Kalhoro, M.S.; Abro, M.I.; Sherazi, S.T.H. Ultra-trace level electrochemical sensor for methylene blue dye based on nafion stabilized ibuprofen derived gold nanoparticles. Sens. Actuators B Chem. 2015, 208, 320–326. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, C.; Hou, B.; Lin, C.; Yan, X.; Feng, J.; Yana, W. Secondary particle size determining sedimentation and adsorption kinetics of titanate-based materials for ammonia nitrogen and methylene blue removal. J. Mol. Liq. 2021, 343, 117026. [Google Scholar] [CrossRef]




| Methylene Blue | Electrode Modifier | Linearity Range/(μM) | LOD/nM | Reference |
|---|---|---|---|---|
| Oxidation | Self-doped TiO2 nanotubes | 1.0–7.94 | 475 | [49] |
| Oxidation | Thiol functionalized Clay | 1.0–14 | 400 | [50] |
| Oxidation | Ibu-AuNps | 0.01–1.1 | 3.9 | [51] |
| Oxidation | NH2-fMWCNTs | 0.01–0.5 | 0.21 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, M.; Shah, A.; Nisar, J.; Shah, I.; Haleem, A.; Ashiq, M.N. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts 2022, 12, 306. https://doi.org/10.3390/catal12030306
Hayat M, Shah A, Nisar J, Shah I, Haleem A, Ashiq MN. A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts. 2022; 12(3):306. https://doi.org/10.3390/catal12030306
Chicago/Turabian StyleHayat, Mazhar, Afzal Shah, Jan Nisar, Iltaf Shah, Abdul Haleem, and Muhammad Naeem Ashiq. 2022. "A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue" Catalysts 12, no. 3: 306. https://doi.org/10.3390/catal12030306
APA StyleHayat, M., Shah, A., Nisar, J., Shah, I., Haleem, A., & Ashiq, M. N. (2022). A Novel Electrochemical Sensing Platform for the Sensitive Detection and Degradation Monitoring of Methylene Blue. Catalysts, 12(3), 306. https://doi.org/10.3390/catal12030306








