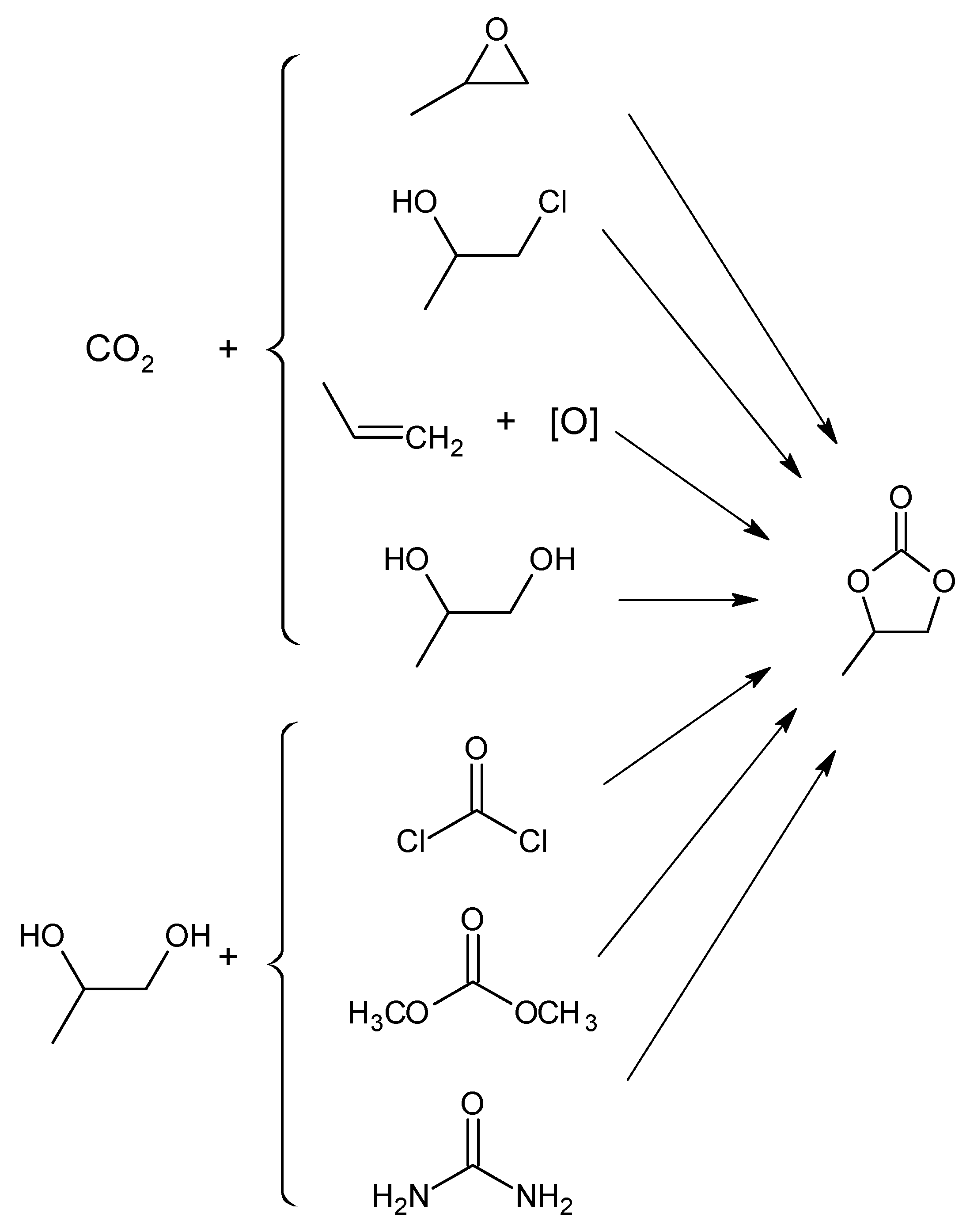
2.1. Metal Oxides
Most scientific reports focusing on the production of propylene carbonate from urea and PG refer synthetic methods involving metal oxide catalysts, which are often used as catalysts owing to their simple synthesis, availability, and various properties [
61].
Zinc-based oxides are the most common catalysts used for PC synthesis from propylene glycol and urea [
62,
63,
64,
65,
66,
67,
68,
69,
70,
71,
72,
73,
74,
75,
76,
77,
78,
79,
80,
81,
82,
83,
84,
85,
86,
87,
88,
89]. ZnO has favorable acid-base properties, which make it an active and selective catalyst in the synthesis of PC. The proposed mechanism of the ZnO-catalyzed reaction is shown in
Scheme 2 [
85].
The urea molecule is stable because of its resonance structures [
90]. The zinc atom activates the urea by coordinating with the oxygen atom of its carbonyl group. Electron transfer leads to the formation of zinc isocyanate complexes, which are soluble in the reaction mixture and react further with the propylene glycol molecule. This results in the generation of an intermediate product (i.e., 2-hydroxypropyl carbamate (HPC)), which cyclizes to form propylene carbonate. The cyclization of HPC is also catalyzed by ZnO through the formation of a coordination bond with the carbonyl oxygen of HPC.
Li et al. [
85] synthesized propylene carbonate from urea and PG using various metal oxides and zinc sulfide (
Table 2). The best results were obtained with ZnO (yield = 98.9%). Furthermore, researchers determined the acidity and basicity of the catalysts based on temperature-programmed desorption (TPD) tests (
Table 3). These measurements indicated that the excellent results of the zinc oxide-catalyzed synthesis were due to low acidity and basicity. According to the authors, the acidic nature of certain catalysts (e.g., Al
2O
3, ZrO
2, ZnS) led to low catalytic activity, which resulted in large amounts of the intermediate product, HPC, in the post-reaction mixture. In contrast, catalysts with low acidity (e.g., ZnO, MgO, CaO, La
2O
3) were active, and as a result, the HPC and urea contents in the product were low. This work also revealed that catalysts dominated by basic character (i.e., A/B < 1) were less selective. After using CaO, MgO, or La
2O
3 as the catalyst, 4-methyl-2-oxazolidone (MOD) was present in the post-reaction mixture. The authors explained that the strongly basic catalyst active sites tended to interact with the hydrogen of the HPC hydroxyl group, which increased the electrophilicity of the carbon atom bound to the hydroxyl group. Subsequently, HPC cyclization, followed by the elimination of water when the nitrogen atom’s electron pair attacked the carbon bound to the hydroxyl group, resulted in the formation of the MOD molecule (
Scheme 3).
Table 4 presents the results of the ZnO-catalyzed synthesis of propylene carbonate, where the reactions were carried out with an excess of PG relative to urea. This was due to the minimization of the formation of nitrogen-containing byproducts, e.g., the biuret reagent and its homologues, and MOD. These reactions were carried out for 2–5 h. Zhao et al. [
88] found that extending the reaction time reduced the yield of PC synthesis resulting from its polymerization. The syntheses of propylene carbonate were carried out at temperatures between 138–170 °C; higher reaction temperatures favored both PC polymerization [
89] and MOD formation [
78].
The problem with dissolving the zinc (ZnO) catalyst in the reaction mixture was noticed by Hao et al. [
77], who reported that during the urea alcoholysis process, a Zn(NCO)
2(NH
3)
2 complex soluble in the reaction mixture was formed. Losses resulting from catalyst dissolution prompted scientists to conduct research on the effective immobilization of active ingredients on appropriate carriers.
Yu et al. [
84] synthesized PC using ZnO immobilized on NaY zeolite (ZnO/NaY). The process was carried out in a tubular reactor with a fixed catalyst bed. The catalyst was obtained by impregnating the support in an aqueous solution of Zn(NO
3)
2. The precatalyst obtained after the impregnation process was then dried and calcined in air for 3 h at 500 °C. The obtained catalyst was highly active and selective at 150 °C, achieving a PC yield of 82.3%, corresponding to a urea conversion of 86.2%. A four-fold molar excess of PG relative to urea was used for this reaction, and the catalyst was stable under the applied conditions; there was no significant decrease in the yield of PC after 35 h of running the synthetic process (the yield remained >80%). The authors determined that the properties and activity of the catalyst depended strongly on the amount of immobilized ZnO. The best results were obtained when ZnO accounted for 5 wt.% of the catalyst, whereas higher or lower ZnO contents resulted in lower catalytic activity and lower selectivity. This study also showed that ZnO/NaY containing 5 wt.% ZnO exhibited the most balanced strength of the acidic and basic sites, which was crucial for high PC production performance.
Researchers have investigated numerous catalytic systems comprising ZnO and other oxides (
Table 5). Zhang et al. [
81] used a catalyst composed of zinc and magnesium oxides (ZnO-MgO), which was prepared by co-precipitation from an aqueous solution of zinc and magnesium nitrates using urea as a precipitating agent [
91]. The obtained precipitate was then washed and calcined at 600 °C for 6 h. Experimental results showed that the increased basicity and specific surface area positively impacted the final reaction yield. The authors evaluated the catalytic properties of systems containing various ZnO:MgO molar ratios. For catalysts with a molar ratio in the range from 4:1 to 1:4, an increase in specific surface areas (from 3.49 m
2/g to 15.06 m
2/g, respectively) was observed along with an increase in MgO content. Higher content of magnesium caused a reduction in ZnO coalescence. The highest yield of PC (94.8%) was obtained when the ratio was 1:4. However, a further increase in the MgO content (Zn/Mg 0.1) led to a lower area (3.83 m
2/g) and lower PC yield (80.4%) as an aggregation of the crystals was observed. According to the authors, the higher catalytic activity exhibited by the two-component oxide system could be attributed to the modification of the catalysts’ electronic properties and porosities. Experimental results showed that the increased basicity and specific surface area positively impacted the final reaction yield.
Liu et al. [
79] investigated a three-component catalytic system comprising zinc, calcium, and aluminum oxides (i.e., ZnO-CaO-Al
2O
3). The CaO-Al
2O
3 system was obtained by mixing powdered CaO and γ-Al
2O
3 in a 10% HNO
3 solution. The resulting paste was then extruded and dried, and the extrudates were calcined at 540 °C for 4 h. The obtained CaO-Al
2O
3 was then impregnated in an ethanolic Zn(NO
3)
2 solution. After the impregnation process, the precatalyst was dried and calcined at 540 °C for 4 h. The authors evaluated the influence of the catalyst composition on the efficiency of the process. Their results confirmed that the catalyst should embody balanced acid-base properties. Based on catalyst composition screening tests, they determined that the mass percentage of CaO should be 30% (in relation to γ-Al
2O
3), and the mass percentage of ZnO should be 20% (in relation to the CaO-Al
2O
3 system). The highest reaction yield (90.8%) was obtained in the synthesis of PC over 3 h at 184 °C using a three-fold molar excess of PG relative to urea. The authors reported that it was not possible to obtain a higher yield for propylene carbonate synthesis owing to the presence of CaO, the basic nature of which caused HPC dehydration to form MOD, as well as PC decomposition.
Zinc-free oxide catalysts have also been described in the literature (
Table 6) [
66,
73,
74,
75,
76,
85,
88]. Many studies in this group describe catalytic systems containing magnesium oxide (MgO), which has specific properties that make it an active catalyst for PC synthesis via urea alcoholysis; however, this compound can promote the formation of MOD [
85]. Lead-based catalysts also have favorable catalytic properties. For example, high selectivity for PC synthesis (93.9%) was observed when using lead dioxide (PbO
2) as a catalyst [
76]. Unfortunately, the significant disadvantage of such catalysts lies in their toxicity. Other oxides, such as Al
2O
3, ZrO
2 [
85], or dubityl tin oxide (Bu
2SnO) [
88] were not active catalysts for this synthesis.
Synthetic compounds with perovskite structures represent a special type of oxides that have been tested for their activity as catalysts for the synthesis of propylene carbonate by urea alcoholysis (
Table 7) [
93,
94]. These oxides have the general formula, ABO
3, where A is a large-diameter metal cation, and B is a small-diameter metal cation [
95]. Du et al. [
94] investigated how the MgTiO
3 preparation method influenced its catalytic properties in terms of PC synthesis. The catalyst was prepared using a sol-gel method; specifically, a solution of magnesium nitrate (Mg(NO
3)
2) was added dropwise to a solution of tetrabutoxy titanium (IV) (Ti(OBu)
4) in acetic acid. Anhydrous ethanol was added to this mixture, which was then concentrated in a vacuum evaporator, and the resulting gel was calcined at various temperatures (600, 700, 800, or 900 °C). The MgTiO
3 catalyst with the highest activity was obtained when the Mg:Ti molar ratio was 1:1 and the gel calcination was carried out at 700 °C for 3 h. Under these conditions, MgTiO
3 was obtained with the highest quantity of basic centers, which resulted in an increase in catalytic activity and selectivity. However, it was also shown that MgO aggregated on the catalyst surface when Ti(OBu)
4 and Mg(NO
3)
2 were used in a 1:1 molar ratio during catalyst preparation. As a result, after the fourth catalytic cycle, the yield of propylene carbonate decreased from 93.5% to approx. 60% because of the leaching of MgO from the catalyst surface. XRD tests showed that the intensity of the MgO diffraction peak (2Φ = 42.9°) decreased with a decrease in the molar ratio of Mg:Ti, whereas for the MTO-0.8 catalyst (with a molar ratio of Mg:Ti = 0.8:1) no peaks corresponding to MgO were detected. Researchers also showed that in the case of the MTO-1.5 catalyst (Mg:Ti = 1.5:1), the peaks corresponding to MgTiO
3 were shifted to the right, which may be due to distortion of the structure.
To date, there are few publications in the scientific literature describing the kinetics of PC synthesis from urea and PG. To implement this method into industrial practice, it is necessary to determine the kinetic equation corresponding to the reaction where propylene carbonate is obtained through urea alcoholysis.
Shi et al. [
96] designed a two-step process for the preparation of DMC. The first stage of the process involved the synthesis of propylene carbonate via urea alcoholysis, and the second stage involved PC transesterification with methanol. The authors considered Wang’s previous findings [
97], and on that basis, they proposed the kinetic Equation (1) for the synthesis of PC from urea and PG catalyzed by 2 wt.% MgO:
r
PC—reaction rate of propylene carbonate synthesis, mol/(L·min)
T—reaction temperature, K
Curea—urea concentration, mol/L
CPG—concentration of propylene glycol in the reaction mixture, mol/L.
The authors also determined the total annual operating cost of the designed installation for several synthetic variants. On the basis of calculations, they showed that obtaining propylene carbonate by reactive distillation was more profitable than using a cascade of three continuous stirred-tank reactors (CSTRs). Analogous simulations were carried out by Patraşcu et al. [
98] using the same kinetic equation for the PC synthesis reaction. Overall, studies have shown that the synthesis of DMC from propylene carbonate and methanol can proceed with excess PC, which facilitates the preparation of high-purity DMC.
In the patent literature, there are several examples of PC synthesis catalyzed by metal oxides in the CSTR cascade [
64,
70,
99]. One innovative solution involves the use of a horizontal reactor with partitions increasing the turbulence of the flow and helping the reaction system imitate a cascade of reactors [
67]. Another method of PC synthesis in a reactive column with a metal oxide catalyst was also patented [
100]. The reports discussed herein suggest that the preparation of PC through urea alcoholysis catalyzed by metal oxides is relatively advanced.
2.2. Metal Salts
Metal salts have also been tested for their potential applications as catalysts for PC synthesis from urea and PG. As in the case of metal oxides, most of the salts used as catalysts are advantageous because they are inexpensive and readily available.
Among the salts used for this reaction, compounds of zinc [
76,
88,
101,
102], magnesium [
76,
102], lead [
101], and tin [
103] exhibited the best catalytic properties. Doya et al. [
76] used various zinc salts (e.g., ZnCO
3, ZnCl
2, Zn(NO
3)
2, and Zn(OAc)
2) and magnesium acetate (Mg(OAc)
2) and compared their catalytic properties with those of Bu
2Sn(C
11H
23COO)
2, which was employed by Su and Speranza in the first published studies on cyclic alkylene carbonate synthesis [
58]. The results showed that the zinc and magnesium salts had similar catalytic properties (PC selectivities ranged from 88.1% to 89.5%) and were more selective than Bu
2Sn(C
11H
23COO)
2, which only achieves up to 64.6% selectivity for PC synthesis. In the patent, Sun et al. [
103] presented examples of PC synthesis using various salts, among which the highest yield (93.47%) was obtained for tin(II) chloride; however, it should be noted that the reactions with other catalysts were carried out under conditions that may not be optimal for the desired process.
The highest yield of PC (96.5%) was achieved by Gao et al. [
102] using magnesium chloride (MgCl
2) as a catalyst. Zhou et al. [
101] obtained a 93.5% yield using a mixture of basic zinc carbonate and lead carbonate prepared using a co-precipitation method. According to the authors, the most active catalytic system contained a 1:2 molar ratio of PbCO
3:Zn
5(CO
3)
2(OH)
6.
Zhao et al. [
88] conducted research on the selection of an active catalyst for the synthesis of propylene carbonate. Among the investigated compounds, anhydrous zinc acetate (Zn(OAc)
2) exhibited the best activity; therefore, further studies were carried out to evaluate the influence of certain parameters on the efficiency of the process. Under the optimized conditions (mole ratio of urea:PG = 1:4; reaction time = 3 h; temperature = 170 °C), a 94% yield of PC was obtained. The authors also studied the effect of Zn(OAc)
2 immobilization on various supports, e.g., activated carbon (AC), zeolite (zeo), and γ-alumina (γ-Al
2O
3). The Zn(OAc)
2 was immobilized on these supports by an incipient impregnation process. The catalytic activity of the catalyst was particularly influenced by the physical properties of the support. The best results were achieved for catalysts immobilized on AC, which showed the highest specific surface among the tested carriers, and on γ-Al
2O
3 with the highest average pore size. Using AC and γ-Al
2O
3 as the carrier, the yields of PC synthesis were 77.5 and 77.9%, respectively. Unfortunately, the applied immobilization method was not effective because Zn(OAc)
2 easily leached from the support. According to the authors, the fresh Zn(OAc)
2/AC catalyst contained 3 wt.% zinc, and after PC synthesis, the Zn content dropped to 2.3 wt.%. After the zinc acetate was washed off of the catalyst, the yield of PC was only 66% once the catalyst was recycled.
Additional studies on the synthesis of propylene carbonate have used potassium silicate [
104] and zinc sulfide as catalysts [
85], but these compounds were characterized by low catalytic activity. The relevant patent [
105] describes a method for obtaining organic carbonates by urea alcoholysis using silicates as catalysts.
Table 8 shows the results of studies probing the catalytic activity of these salts in the synthesis of propylene carbonate.
Polyoxometalates represent a special type of metal salts. Their catalytic activity for the synthesis of propylene carbonate was patented by Liu et al. [
106]. The catalysts were obtained by mixing a heteropolyacid with a suitable metal carbonate in water, and then evaporating the water and calcining the precipitated precatalyst at 200–400 °C. The authors emphasized that the separation of the precatalyst from the aqueous mixture was also possible via partial evaporation of the water, cooling to 0–5 °C, and centrifuging to isolate the precipitate. Phosphomolybdic acid (H
3PMo
12O
40), phosphotungstic acid (H
3PW
12O
40), or silicon tungsten acid (H
4SiW
12O
40) could be applied with an appropriate carbonate or hydroxycarbonate (e.g., ZnCO
3, MgCO
3, 4MgCO
3 × Mg(OH)
2, CaCO
3, K
2CO
3) to synthesize these catalysts. The catalytic activities of heteropolyoxometalates are presented in
Table 9. In general, these heteropolyoxometalates do not have high catalytic activity; the highest PC yield (78.71%) was obtained using Zn
3(PMo
12O
40)
2.
2.4. Ionic Liquids
Ionic liquids (ILs) are chemical compounds composed of ions with a melting point lower than 100 °C [
111]. Gabriel and Weiner (1888) were the first to document the synthesis of an ionic liquid, specifically, ethanol-ammonium nitrate (mp = 52–55 °C) [
112], although Walden is often considered the father of ILs, following the reported synthesis of nitrate ethylammonium liquid at room temperature (mp = 12 °C) in 1914 [
113]. However, scientific interest in ionic liquids increased significantly around the beginning of the 21st century and has continued to this day [
114].
Because ionic liquids can be designed by selecting the appropriate cations and anions, it is possible to obtain a chemical compound with the desired properties. This advantage means that ionic liquids can be used in many fields of science; however, only some of them are currently produced on an industrial scale [
115]. In general, the low availability of starting materials limits their production possibilities. The low cost of key reagents and abundance of their manufacturers are essential for a given ionic liquid to be produced on a commercial scale.
The scientific literature contains thousands of publications discussing the use of ionic liquids as catalysts in chemical synthesis. However, to date, few scientific reports have detailed the synthesis of propylene carbonate by ionic liquid-catalyzed urea alcoholysis. Kuznetsov et al. [
116] synthesized cyclic alkylene carbonates using a two-phase reaction system comprising a deep eutectic solvent (DES) [
117,
118] and a chlorinated organic solvent. The DES was an equimolar mixture of zinc chloride, urea, and glycol, which formed a transparent colorless solution when heated to above 50 °C. The synthesis of propylene carbonate was carried out at low temperature (84 °C) and required a long reaction time (24 h) to ultimately reach a maximum yield of 70%.
A patent from 2016 [
119] describes a method for obtaining PC through a reactive distillation method, in which the catalytic system consisted of zinc oxide, a zinc salt, and a quaternary ammonium salt. Another patent [
120] details the synthesis of cyclic alkylene carbonates using a catalytic system containing a metal salt and an ionic liquid with an imidazolium cation. The preparation of PC by urea alcoholysis at 160 °C for 3 h under reduced pressure (150 kPa) using a catalytic system containing 1-hexadecyl-3-methylimidazolium chloride and zinc chloride ([C
16mim]Cl-ZnCl
2) led to a 94.1% yield of PC. The catalytic system did not lose significant activity in this case; after its 5th cycle, the PC yield was 90.1%. The proposed mechanism of the reaction catalyzed by this system (
Scheme 4) [
121] assumes an increase in urea reactivity due to (i) the interaction between the oxygen of the urea carbonyl group and Zn
2+ and (ii) the formation of a hydrogen bond between this oxygen and the hydrogen of the imidazole ring at the C(2) position.
Owing to the relative novelty of PC synthesis via urea alcoholysis using ionic liquid catalysts, it can be expected that there will be more publications on this subject in the coming years.