Abstract
Nowadays, water pollution is one of the most dangerous environmental problems in the world. The presence of the so-called emerging pollutants in the different water bodies, impossible to eliminate through conventional biological and physical treatments used in wastewater treatment plants due to their persistent and recalcitrant nature, means that pollution continues growing throughout the world. The presence of these emerging pollutants involves serious risks to human and animal health for aquatic and terrestrial organisms. Therefore, in recent years, advanced oxidation processes (AOPs) have been postulated as a viable, innovative and efficient technology for the elimination of these types of compounds from water bodies. The oxidation/reduction reactions triggered in most of these processes require a suitable catalyst. The most recent research focuses on the use and development of different types of heterogeneous catalysts, which are capable of overcoming some of the operational limitations of homogeneous processes such as the generation of metallic sludge, difficult separation of treated water and narrow working pH. This review details the current advances in the field of heterogeneous AOPs, Fenton processes and photocatalysts for the removal of different types of emerging pollutants.
1. Introduction
In recent years, the indiscriminate use of raw materials, as well as industry itself, has generated numerous problems in terms of environmental contamination of soils and water bodies [1,2]. Many different types of highly toxic compounds, known as emerging pollutants, such as pesticides from the agri-food industry, persistent organic pollutants, food additives, pharmaceuticals (human and animal antibiotics, analgesics, chemotherapeutic agents, anxiolytics, etc.) and personal care products (PPCPs), have been detected in surface waters, river effluents, wastewater treatment plants (WWTPs), groundwater, sewage sludge and even in potable water [3,4].
Conventional biological treatments used in wastewater treatment plants are not able to eliminate these pollutants from water, leading to the uninterrupted entry of these pollutants into the environment, even at low concentrations. This fact results in a serious long-term risk, affecting the health and development of aquatic and/or terrestrial organisms and the availability of water around the world, aggravating the drinking water shortage in many countries [5,6,7]. According to the Food and Agriculture Organization (2013), the demand for drinking water in the world is expected to increase by more than 40% by 2050. Therefore, the development of innovative technologies for the decontamination of effluents represents an astonishing challenge for the scientific community since the current treatment plants are not completely effective for the removal of this broad range of organic pollutants [8,9].
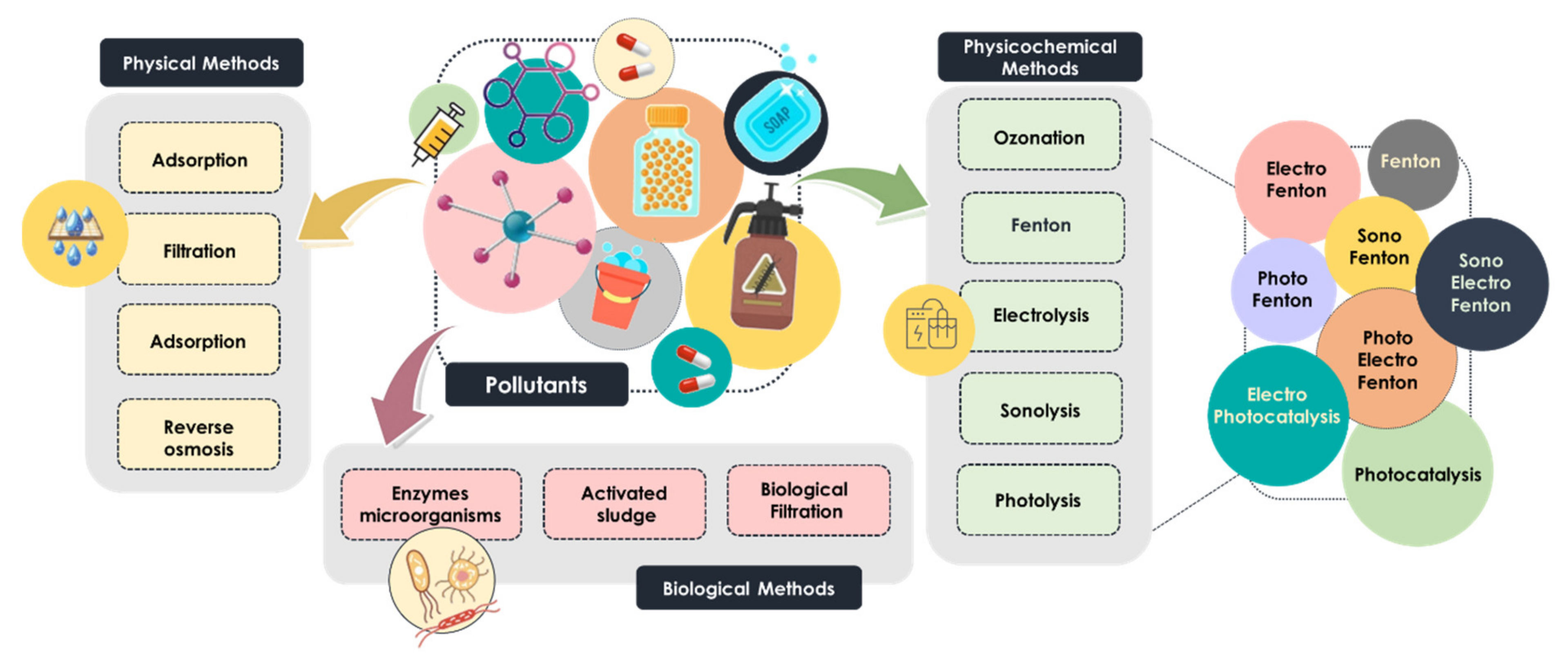
Nowadays, the most common technologies used in water treatment are classified as biological, physical and physicochemical processes (Figure 1). Biological methods, including enzymatic treatment, biological filtration and treatment with activated sludge, are widely applied for the removal of emerging pollutants. Despite their low cost, some persistent organic pollutants are not susceptible to direct biological treatment [6], and thus, physical and chemical methods are considered a preferable approach for removing heavy metals and recalcitrant pollutants [10]. Physical processes are categorized into adsorption, reverse osmosis, sedimentation and membrane-filtration-based processes, whereas the physicochemical methods mainly include electrolysis, Fenton-based processes, photolysis, ozonation and sonolysis [11,12]. These widely used technologies are commonly called advanced oxidation processes (AOPs). The aforementioned AOPs are considered very promising technologies due to their high efficiency, simplicity and good reproducibility, and consequently, they have received great attention in the search for water treatment solutions [13,14,15].
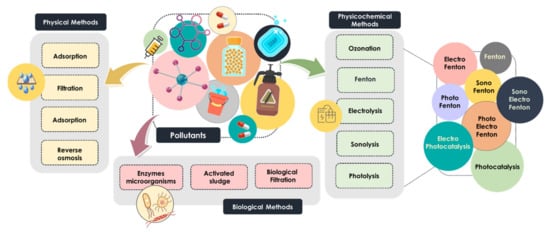
Figure 1.
Classification of technologies used in water treatment.
These processes are based on the in situ production of highly reactive free radicals such as hydroxyl radicals (•OH), sulfate radicals (SO4•−) and superoxide radical (O2•−) by the activation of the precursor oxidants (hydrogen peroxide, persulfate/peroxodisulfate, peroxymonosulfate and sodium percarbonate via oxidation/reduction reactions [16,17,18,19]. These radicals released in the main solution allow the degradation of organic pollutants followed by their partial/total mineralization to carbon dioxide, water and inorganic ions [20,21]. These oxidation processes include chemical (Fenton or Fenton-like), photochemical (photo-Fenton or photocatalysis), electrochemical (anodic oxidation, electro-Fenton, electrophotocatalysis) and sonochemical (sono-Fenton) processes and process combinations (photo-electro-Fenton, sono-electro-Fenton) [22,23,24]. The activation of the above-mentioned precursor compounds is carried out via multiple alternatives such as the use of transition-metal-based catalysts (Fe, Mn, Co, Cu, V, Ru, Mo, Cr, Ce), heat, UV radiation or visible light, ultrasound and/or alkalinity of the liquid medium [25,26,27]. However, AOPs based on the Fenton process possess some drawbacks hindering their large-scale application: the narrow working pH range, the complexity of the real water matrices favoring the precipitation of the transition metals present in the catalyst, leading to the formation of sludge, and undesirable by-products [19,20,28]. Some of these disadvantages related to Fenton-based and photocatalytic-based processes are alleviated using heterogeneous catalysts [10,29]. Despite homogeneous systems (liquid phase) being very efficient, the removal of the soluble iron salts from the environment requires extremely costly procedures. Therefore, the development of heterogeneous catalysts (solid-liquid phases) has been an essential approach towards improved catalysis. Although the efficiency of heterogeneous catalysts may be explained by different reaction pathways [30,31], the contaminants are generally adsorbed on the catalyst surface throughout the process, and then, chain reactions promote the breakdown and further formation of the bonds generating successive intermediate products until the final product which is finally desorbed from the solid [32,33,34]. The use of these heterogeneous catalysts has numerous advantages over homogeneous catalysts, allowing (i) catalyst regeneration and further utilization; (ii) a greater application scope due to some conversion reactions taking place on the heterogeneous catalyst; (iii) the use of a broader working pH range, even at natural pH value of the water matrix; (iv) easy recovery of catalysts without the formation of metallic sludge; (v) sustainable saving of the material and energy resources, helping to protect the environment; (vi) high specificity; and (vii) upgraded properties of active sites and reaction yield compared to homogeneous catalysts [31,33].
Within this context, this review focuses on the compilation of most relevant studies, from 2015 to the present, related to the application of heterogeneous catalysts in AOPs for the removal of emerging pollutants from water sources. It should be noted that as a result of recent reviews that have been accomplished in the field of heterogeneous ozonation, this issue is not evaluated in this review [35,36]. First of all, AOP studies based on heterogeneous Fenton and Fenton-like reactions and photocatalysts operating mainly at the laboratory scale are described, with a discussion of aspects relevant for improving the process effectiveness. Furthermore, several constructive suggestions are proposed for future research and applications in the field of heterogeneous catalysis for wastewater treatment.
2. Fenton Process
The Fenton reaction has been widely recognized as an efficient technology for the degradation of organic pollutants. It is based on the catalytic decomposition of hydrogen peroxide by the reaction between iron salts (ferrous ions) (Equation (1)) to produce hydroxyl radicals responsible for pollutant degradation [16].
Fe2+ + H2O2 + H+ → Fe3+ + H2O + •OH
Despite that, several studies have demonstrated high degradation and mineralization percentages with pharmaceuticals and other refractory compounds as target pollutants, multiple operational limitations have been associated with the homogeneous Fenton process, including narrow working pH, high chemical consumption, the production of iron sludge hindering the disposal and awkward catalyst reusability. These limitations inhibit its large-scale application as a one-step treatment; nevertheless, the homogeneous process has been used as an appropriate pretreatment to enhance the biodegradability of refractory compounds from the pharmaceutical industry and/or hospital effluents [37]. Consequently, addressing the disadvantages of the Fenton process represents an ongoing research effort in the field of wastewater treatment.
To overcome the aforementioned limitations, the search for a heterogeneous catalyst for potential application in the Fenton process and combined processes such as electrochemical treatment (electro-Fenton), photolysis (photo-Fenton), sonolysis (sono-Fenton) and their combinations (photo-electro-Fenton, sono-electro-Fenton) has been the focus of attention from the scientific community.
2.1. Heterogeneous Fenton Process
The proposed mechanism of the heterogeneous Fenton process takes place by the reaction between hydrogen peroxide and the active sites of the catalyst denoted by AS (Equations (2) and (3)) [38,39].
AS-Fe2+ + H2O2 → AS-Fe3+ + HO− + •OH
AS-Fe3+ + H2O2 → AS-Fe2+ + HO2• + H+
Additionally, low iron concentrations may be present in the bulk solution as a result of the leaching process from the solid heterogeneous catalyst. Therefore, hydroxyl radicals are generated by conventional Fenton reaction (Equation (1)), contributing slightly to the pollutant degradation rate [40,41].
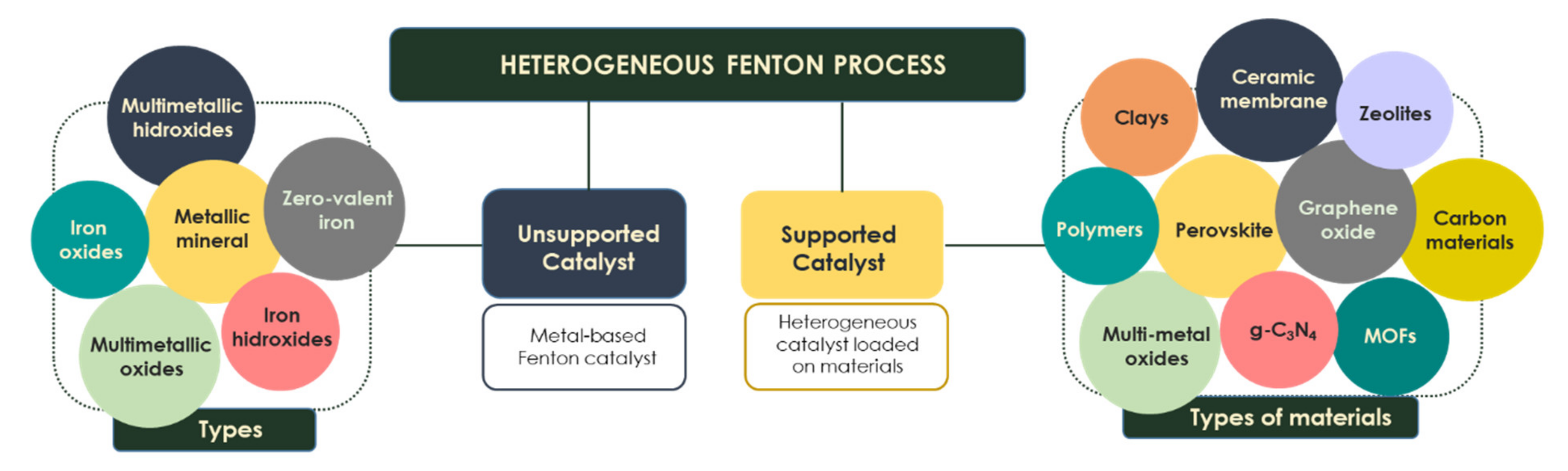
Over the last few years, many publications have proposed the use of different types of heterogeneous catalysts with upgraded characteristics such as activity under a wider pH range, low iron leaching and high stability, which allows their reuse for several cycles [39]. These catalysts allow achieving reactivity capacities comparable to the homogeneous process. They are mainly divided into two large groups: unsupported catalysts (zero-valent iron, metallic minerals, iron oxides/hydroxides or multimetallic catalysts) and catalyst loaded on materials (e.g., polymers, clays, zeolites) (Figure 2).
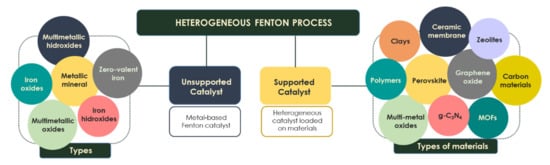
Figure 2.
Types of catalysts in the Fenton process.
2.1.1. Unsupported Heterogeneous Fenton Catalysts
The most abundant elements in the Earth’s crust have been evaluated as heterogeneous catalysts because of their easy recovery and negligible toxicity, making them a suitable alternative to the soluble salts used in the homogeneous process (He et al., 2016). These heterogeneous catalysts are mainly based on the zero-valent iron powders (ZVI or Fe0), iron/copper oxides, hydroxides, oxyhydroxides and insoluble iron and copper minerals. The copper system (Cu2+/Cu+) presents a similar behavior to the Fe3+/Fe2+ couple in the presence of hydrogen peroxide, even at a wider pH range (Equations (4) and (5)).
Cu2+ + H2O2 → Cu+ + HO2• + H+
Cu+ + H2O2 → Cu2+ + OH− + •OH
For this reason, the use of copper and its combination with other transition metals has garnered much attention in Fenton-like processes [42]. Zhang et al. [43] prepared a Cu/V bimetallic catalyst by a hydrothermal method for fluconazole degradation, confirming the as-prepared material possesses higher catalytic activity under a wider pH range and more satisfactory reusability than monometallic copper compounds.
Insoluble minerals such as pyrite (FeS2), chalcocite (Cu2S), bornite (Cu5FeS4), goethite (α-FeOOH), magnetite (Fe2O3), hematite (α-Fe2O3), ferrihydrite (Fh) and wüstite (FeO) have been successfully applied in the removal of organic pollutants [15,44]. More specifically, the use of pyrite as a heterogeneous catalyst in the Fenton process has been reported for the degradation of trichloroethylene, diclofenac, pyrene and toluene [45]. Kantar et al. [46] used pyrite as a catalyst packed in a column reactor for purification of real effluent from the pharmaceutical industry under dynamic conditions. They demonstrated that the pyrite-Fenton system effectively reduced the toxicity of the effluent, and the addition of citric acid to the contaminated effluent improved the effectiveness of the process and the useful lifespan of the pyrite column. Likewise, Muñoz et al. [47] evaluated natural magnetite as a heterogeneous catalyst for the degradation of sulfamethoxazole, achieving total elimination and more than 50% mineralization of the target antibiotic under optimal conditions (1 g/L catalyst concentration, 25 mg/L oxidant concentration, 25 °C and pH 5). Despite the minimal iron leaching from the magnetite, its application for sulfamethoxazole degradation in hospital effluents required longer reaction times to achieve its total elimination.
Hassani et al. [48] synthesized magnetite nanoparticles by high-energy planetary ball mill for their further application in ciprofloxacin degradation. The findings indicated 6 h ball-milled magnetite enhanced antibiotic removal by around 89% after 120 min treatment. They proposed the removal mechanism based on the adsorption and oxidation phases with a notable contribution of the hydroxyl radicals. In addition, Nie et al. [49] synthesized magnetite nanospheres via the solvothermal method for their application in tetracycline removal, achieving more than 80% removal after 110 min. The magnetic properties of the catalyst allowed an easy separation from the aqueous solution. They demonstrated the role of each reactive oxygen species, and hydroxyl radicals formed on the surface of the nanospheres played the main role in pharmaceutical degradation.
2.1.2. Supported Heterogeneous Fenton Catalysts
A heterogeneous catalyst may be incorporated into different solid matrices for the synthesis of the well-known supported Fenton catalyst (Figure 2). These support materials include clays, ceramic filtration membranes, polymers, multimetal oxides (Al, Ti, Si, Zr), metal–organic frameworks, perovskites, zeolites and carbon materials (graphene oxide, carbon nanotubes, biochar, activated carbon, g-C3N4 composites) [50,51,52]. The excellent assembling of the catalyst into the supported materials overcomes some operational limitations. This fact makes them more easily reusable and separable from the final effluent since traditional separation methods (sedimentation and filtration) are not able to separate nanosize particles [50,53].
Clay materials have been widely used as catalyst support in heterogeneous processes due to their high abundance, minimal toxicity and low cost [54]. These inorganic materials provide greater resistance to organic solvents and high thermal stability and prevent the agglomeration of nanoparticles [39]. Several methods have been proposed in the literature for the incorporation of catalysts into clays. Khankhasaeva et al. [55] synthesized Fe/Cu/Al-pillared clay for heterogeneous Fenton oxidative degradation using the antibiotic sulfanilamide as the target pollutant. The developed catalyst was active in the presence of hydrogen peroxide, significantly increasing the oxidation rate. Xu et al. [56] synthesized sepiolite-supported magnetite (Fe3O4-Sep) by chemical coprecipitation, achieving total bisphenol A removal after 15 min. Likewise, Sétifi et al. [57] incorporated iron oxyhydroxide particles on a K10 montmorillonite-clay surface for naproxen degradation. Almost total naproxen removal and around 90% mineralization were reached at pH 3 after 60 and 300 min, respectively. The authors demonstrated the reusability of the catalyst with no efficiency loss after four consecutive cycles.
Iron-based composites exhibit interesting applicability for the Fenton process [58]. Particularly, Titouhi and Belgaied [59] proposed the use of sodium alginate beads containing Fe as a heterogeneous catalyst for ofloxacin degradation through Fenton oxidation. Almost complete elimination of the antibiotic was achieved after 180 min reaction with negligible iron leaching and good stability after three successive oxidation processes. In the same way, Lyu et al. [60] synthesized copper-doped mesoporous silica microspheres by a hydrothermal method. The synthesized microspheres displayed outstanding performance for the removal of ibuprofen, phenytoin and diphenhydramine. Almost total pharmaceutical removal was achieved at neutral pH with copper leaching under the environmental regulations.
Furthermore, the application of multi-oxides, mainly cerium oxides, as catalyst support has been proposed by Zhang et al. [61], who synthesized Fe0/CeO2 catalyst for 90% tetracycline elimination through the heterogeneous Fenton process. The as-prepared catalyst showed applicability under pH values ranging from 3.0 to 7.0, excellent reusability (up to five successive cycles) and negligible iron leaching. Similarly, Hussain et al. [62] developed heterogeneous Fe and Cu catalysts supported on Zr (ZrFe and ZrCu) for testing the ibuprofen removal by Fenton and Fenton-like processes, respectively. Under the optimal conditions, ZrCu catalyst provided 98% degradation and 50% mineralization after 120 min. Ling et al. [63] carried out the catalytic detoxification of pharmaceutical wastewater by Fenton-like reaction with activated-alumina-supported metal-oxide-based catalyst CoMnAl. They concluded that transition metals on the composite surface revealed synergistic effects on the formation of hydroxyl radicals, which were the main reason for the effective detoxification of pharmaceutical wastewater.
Zeolites are interesting materials that have been widely used in the last few years as support materials in different fields because of their crystalline structure of aluminosilicates ensuring a uniform isostructural distribution of the catalyst [64]. Adityosulindro et al. [65] investigated the heterogeneous Fenton process using Fe supported on zeolite (Fe-Zeolite-ZSM5) as a catalyst in simulated and real wastewater containing ibuprofen. The results showed an ibuprofen elimination and TOC decay of around 88% and 27%, respectively, after 180 min reaction at natural pH. As was expected, they reported that the ibuprofen degradation rate was slower in wastewater effluent compared to simulated water because of the complex matrix. In the same way, Velichkova et al. [66] used Fe supported on zeolite as a heterogeneous catalyst (Fe/MFI-Zeolite-ZSM5) for paracetamol oxidation through the Fenton process. After 300 min treatment, paracetamol was completely eliminated, reaching up to 60% mineralization with minimal leaching, even working under continuous process coupling oxidation and membrane filtration.
Recently, the use of perovskite oxides as heterogeneous catalysts has attracted the attention of the scientific community due to their superior electronic, magnetic and electrochemical characteristics. These properties stem from a large number of ions included in the crystal structure, the high mobility of oxygen molecules and the stabilization of unusual oxidation states of the metals present in their structure. del Álamo et al. [67] synthesized LaCu0.5Mn0.5O3 perovskites for analyzing their catalytic activity in drug removal from hospital effluents using an up flow packed-bed reactor. This on-site pretreatment achieved an effective micropollutant removal in the real range of micrograms per liter. Likewise, Nie et al. [68] prepared nanoscale LaFeO3 perovskite via the sol–gel method for sulfamethoxazole elimination at neutral pH. The mechanism was based on the formation of a complex surface between the hydrogen peroxide and the perovskite, accelerating the Fe3+/Fe2+ cycle and the production of free oxygen radicals and thus enhancing the Fenton-like effectiveness. In the same way, perovskites doped with other transition metals such as copper (LaAl1−xCuxO3) have shown high degradation and mineralization percentages for persistent organic pollutants such as bisphenol A, phenol and phthalates through Fenton-like reactions [69].
Apart from perovskites, membrane-supported Fenton catalysts represent an interesting approach to remove many complex pollutants from the aqueous matrix, favoring the separation of the decontaminated fractions [70]. Accordingly, Plakas et al. [53] accomplished the removal of diclofenac at relatively low concentrations using a catalytic membrane reactor comprising a tubular porous alumina (α-Al2O3) membrane as support material for embedded iron oxide nanoparticles. Moderate degradation and mineralization were achieved under the optimal conditions; however, the authors proposed membrane modification for improving the adsorption capacity and the further oxidation within the catalytic membrane. In the same way, Zhang et al. [70] tested an ultrafiltration ceramic membrane as support for heterogeneous iron oxychloride catalysts, achieving 100% removal of p-chlorobenzoic acid and bisphenol A operating with only 10 s retention time.
Regarding the carbonaceous supports (carbon nanotubes, biochar, g-C3N4, activated carbon and graphene), graphene derivatives have been successfully used for catalyst immobilization due to catalytic activity, stability and reusability [11,71]. Graphene, a two-dimensional carbon allotrope, is characterized by an extremely large specific surface area (~2360 m2/g), high thermal/electrical conductivity, strong mechanical and chemical stability, strong electron transfer capacity and simple synthesis from graphite by chemical procedure [72]. Although several authors reported its agglomeration trend and restacking to form parent graphite due to strong interplanar interactions, these operational limitations may be overcome through external functionalization using other molecules and nanomaterials [73]. Therefore, these structures are considered as promising to perform nanotechnology-based processes. In this field, Yang et al. [74] synthesized nanoscale zero-valent iron encapsulated in a three-dimensional graphene network (3D-GN@nZVI) for the removal of an antibiotic, sulfadiazine. Likewise, Xu et al. [75] synthesized a heterogeneous catalyst based on three-dimensional macroporous nanoparticles of zero-valent copper self-assembled in graphene (3D-GN@Cu0). Their results showed high metronidazole removal at a wide pH range. Jiang et al. [76] developed Fe0 catalysts immobilized on mesoporous carbon (Fe0/MC) for tetracycline removal from an aqueous effluent by persulfate activation. These catalysts allowed for reducing the aggregation trend associated with the zero-valent iron nanoparticles, achieving 97.7% tetracycline removal under optimal conditions. The enhancement by the synthesis of manganese ferrite nanoparticles was described recently by Qin et al. [77]. They synthesized magnetic core-shell MnFe2O4@C-NH2 for the antibiotic degradation of ofloxacin, amoxicillin and tetracycline. The notable activity of the magnetic catalyst was attributed to the carbon shell due to the high specific surface area and the negligible metal leaching, and the incorporation of –NH2 improved the electron density of the carbon shell. These unique properties promote the metal regeneration cycles, thus increasing the process effectiveness. Metal-organic frameworks (MOFs) are constituted by the interaction of several metal ions coordinated to polyfunctional organic ligands with moderately strong bonds providing them with diverse properties such as high porosity, well-defined periodic network structure, abundant active sites and large surface area. In addition, their predesignable synthesis allows a stricter control of the structural and chemical characteristics with personalized functions for a specific objective function [76]. These distinctive properties suggest good performance as heterogeneous catalysts for Fenton or Fenton-like processes, attracting great attention from numerous researchers [78]. Notwithstanding, the addition of a supplementary active metal center involves an intensification in MOF performance due to the plausible synergistic effect [79].
Nonetheless, most of the studies reported in the literature investigated the use of MOFs as support for metallic nanoparticles [80]. Specifically, Tang and Wang [81] prepared bimetallic Fe and Cu MOFs (FexCu1−x(BDC)) as heterogeneous Fenton catalysts for the degradation of sulfamethoxazole. Complete antibiotic degradation was achieved in 120 min at natural solution pH. The removal efficiency was considerably higher than the efficiencies obtained using monometallic copper and iron catalysts, indicating a synergistic effect between both transition metals on the sulfamethoxazole degradation process. Furthermore, Tang and Wang [82] synthesized a three-dimensional “flower-like” MOF with Fe-Cu bimetallic nanoparticles incorporated in a mesoporous carbon layer (FeCu@C). This MOF was used as a heterogeneous Fenton catalyst for the degradation of sulfamethazine, achieving 100% degradation and 72.3% mineralization after 90 and 240 min, respectively. In addition, the magnetic properties of the catalyst ensured easy recovery and reuse in subsequent experimental runs without washing.
2.2. Photo-Fenton
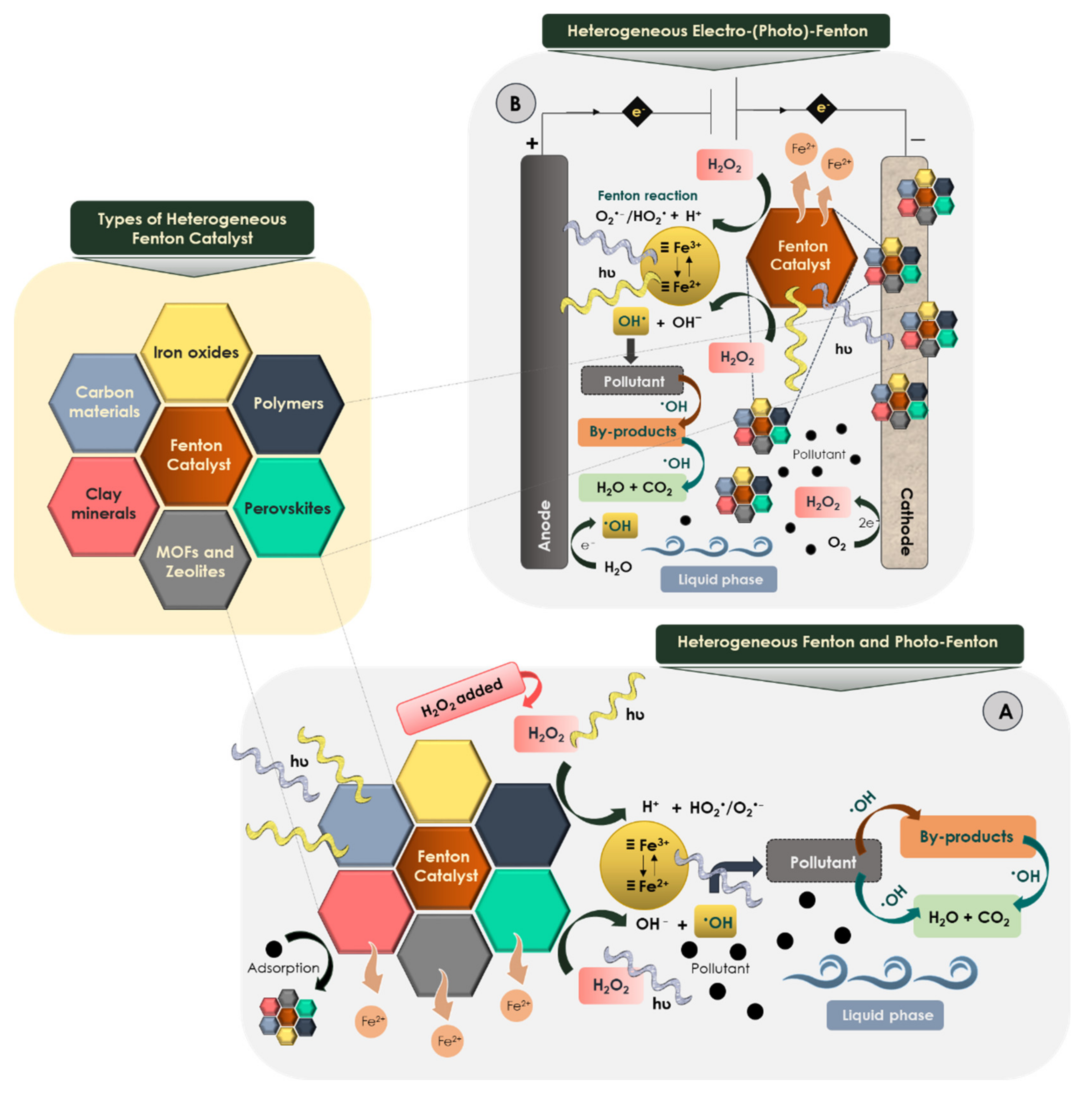
The enhancement of the Fenton reaction takes place by using ultraviolet-visible (UV-Vis) radiation (λ < 580 nm) (Figure 3A) generating additional hydroxyl radicals by (a) photoreduction of ferric ions to ferrous ions leading to the formation of iron complexes under acidic conditions (Equations (6) and (7)) and (b) hydrogen peroxide photolysis (Equation (8)) [54,83].
Fe3+ + H2O → Fe(OH)2 + H+
Fe(OH)2 + hν → Fe2+ + •OH (λ < 580 nm)
H2O2 + hν → 2•OH (λ < 310 nm)

Figure 3.
Mechanisms of heterogeneous Fenton processes. (A) Heterogeneous Fenton and photo-Fenton processes. (B) Heterogeneous electro-Fenton and electro-photo-Fenton processes.
This oxidation-based technology produces biodegradable intermediates and almost total mineralization under optimum operational conditions. Nonetheless, its higher energy consumption hinders its widespread industrial use, and thus, several strategies have been proposed in order to minimize operating cost and improve photo-Fenton efficiency by the application of heterogeneous catalysts and/or chelating agents [84].
In addition, photo-Fenton reactions may be driven by visible light, solar energy or alternative light (LED, fiber optic) instead of the use of simulated UV irradiation [85]. Different iron-based oxides have been widely applied as heterogeneous photo-Fenton catalysts due to their low cost, negligible toxicity, high semiconducting properties and easy recovery [84,86,87]. The semiconductors may be excited by light irradiation to produce photogenerated electrons; a plausible semiconducting mechanism of the iron oxides may be described by the following equations [39,88]:
Iron oxide + hν → Iron oxide (h+ + e−)
e− (conduction band) + O2 → O2•−
h+ (valence band) + H2O → H+ + •OH
h+ (valence band) + OH− → •OH
H2O2 + e− → •OH + OH−
Fe3+ + e− → Fe2+
Fe3+ + hν + OH− → Fe2+ + •OH
Among iron oxides used as heterogeneous photo-Fenton catalysts, ferrites (with general molecular formula MxFe3−xO4, where M is a bivalent transition metal ion) have attracted great interest due to their high stability and narrow bandgap leading to their potential active catalysis under visible light conditions. Likewise, hematite (narrow bandgap around 2.2 eV) may absorb light until 560 nm and collect around 40% solar spectrum energy, being considered as an adequate photo-Fenton catalyst. The regeneration step of ferrous ion (Equation (14)) represents the most important limiting factor in a Fenton-based process due to its relatively lower kinetic constant rate. Consequently, many studies have proposed the use of electron-rich materials combined with Fenton catalysts to accelerate the generation of ferrous ions through the electrons from these materials. As mentioned above, copper has been widely used as a heterogeneous catalyst because of its catalytic properties similar to iron. Gao et al. [89] reported the use of copper ferrites as the heterogeneous photo-Fenton catalyst for sulfamethoxazole degradation at circumneutral pH. Almost total degradation and around 32% mineralization were achieved under optimal conditions after 120 min treatment. Likewise, Emídio et al. [90] investigated the incorporation of copper in the magnetite lattice (Fe3−xCuxO4) in the photo-Fenton degradation of 5-fluorouracil and cyclophosphamide under UV-light irradiation. Modified magnetite showed higher catalytic activity compared to natural magnetite and a higher drug degradation rate, achieving 100% removal after 150 min on four successive runs. In the same way, Xu et al. [91] synthesized goethite doped with copper (α-(Fe-Cu)OOH) “nanoflowers” for diclofenac removal under visible light irradiation (>420 nm). The nanoflowers exhibited good stability after five cycles; the pollutant degradation rate was around 95% using 3% Cu-doped α-(Fe-Cu)OOH compared to 75% achieved by pure α-FeOOH. The catalytic activity was attributed to a two-way mechanism combining Fenton-like photocatalysis and the synergistic metal activation within the synthesized catalyst.
The use of the metallurgical industry waste as a heterogeneous catalyst has been postulated as an important solution for the harnessing of these toxic wastes [84]. These wastes, also called slag, are defined as different proportions of ferrous metal oxides such as magnetite, goethite and hematite combined with silicates, aluminum, calcium, magnesium, copper and titanium to a lesser or greater extent depending on the original industry [92]. Arzate-Salgado et al. [93] used two residues from the copper and steel industry as Fenton-type photocatalysts for diclofenac removal under simulated sunlight irradiation. Copper-based catalysts (2.2 eV) exhibited higher photocatalytic activity compared to steel material (2.95 eV). Total removal and around 87% mineralization of the anti-inflammatory drug were achieved at 90 and 300 min, respectively, at near-neutral pH. Similarly, recent studies have investigated biological-like catalysts in heterogeneous photo-Fenton processes. Some microorganisms may be used in the oxidation of metal ions in the presence of various enzymes [94]. These microbial processes involve nontoxic chemicals, making the process much more environmentally friendly at a lower cost. Du et al. [95] used biogenic Fe-Mn oxides produced by Pseudomonas sp. F2 as a heterogeneous catalyst in the photo-Fenton process for ofloxacin degradation under UV irradiation (<420 nm). The oxides mainly produced by the bacteria consisted of maghemite and mixed oxidation states of manganese. From their results, biogenic Fe-Mn oxide presents catalytic activity twice higher than that of the chemically synthesized Fe-Mn oxides, eliminating more than 95% of ofloxacin after 120 min under desirable conditions.
Regarding clay-based catalysts, pillared clays present good photocatalytic properties because of the addition of oxygenated-metal cations into the clay structure leading to increased porosity and surface area. More specifically, Hurtado et al. [96] assessed the use of Cu/Fe pillared clay as a catalyst in paracetamol mineralization by photo-Fenton process under circumneutral pH conditions. Around 80% TOC abatement was achieved under optimal conditions, showing iron leaching of about 3% after treatment and negligible loss of activity in the reusability cycles. Likewise, Sétifi et al. [57] synthesized goethite particles over K10 montmorillonite clay for naproxen photo-Fenton degradation. The use of UV-light radiation improved the process effectiveness by around 6 times, up to total naproxen degradation after 10 min. Furthermore, the incorporation of some semiconductor materials on clay-based catalysts combining photo-Fenton system and photocatalysis significantly improves iron regeneration and the formation of hydroxyl radicals with the consequent increase in the degradation efficiency of organic pollutants [97]. Molina et al. [54] reported the synthesis of supported Fe2O3-TiO2 heterostructures using commercial bentonite clay and their further application as photo-Fenton catalysts for the elimination of acetaminophen and antipyrine under simulated solar radiation. Complete degradation of acetaminophen and antipyrine was achieved in under 60 min with TOC conversions of around 40% and 25%, respectively. All the experiments revealed iron leaching of under 5 wt.%. In addition, Bansal et al. [98] also prepared heterogeneous catalysts based on Fe-TiO2 composite supporting a clay/foundry sand/ash mixture. These latter components are waste products from the metallurgical industry with high iron content. These heterogeneous catalysts were tested on the photo-Fenton process for the degradation of real effluents from the pharmaceutical industry under solar irradiation. Contaminated effluent DQO was reduced by 80% after 120 min, and the potential application of this catalyst on a large scale was demonstrated because of good reusability (>70 cycles).
Apart from clays materials, the use of membranes as heterogeneous Fenton-type catalyst support has also been investigated. Catalá et al. [99] used an iron-based mesoporous heterogeneous catalyst (crystalline iron oxides in hematite form) supported on mesostructured silica (Fe2O3/SBA-15) for the degradation of illicit drugs of abuse (benzodiazepines, cannabinoids, opioids, LSD, etc.) in a complex natural aqueous effluent using a photo-Fenton process under UV–visible irradiation. Removal efficiency was demonstrated after 6 h of treatment, reaching values higher than 75% in all cases under optimal conditions (catalyst = 0.6 g/L; pH = 3; H2O2 = 0.5 mg/L; T = 22 °C; 500 rpm). Subsequently, the effectiveness of the photo-Fenton treatment in the purification of the effluent was evaluated through phytotoxicity studies with Polystichum setiferum spores. Similarly, Liu et al. [100] used a ceramic membrane coated with iron oxychloride (FeOCl-M) to study the activation and degradation of nitrobenzene (NB) under UV irradiation. The complete elimination of NB was achieved after 7 min of treatment, thus verifying the effectiveness, recyclability and stability of this type of supported heterogeneous catalyst.
In line with the use of carbonaceous materials as support for photo-Fenton-type catalysts, biochar (material produced by combustion of biomass under minimal oxygen conditions) has also been postulated as an interesting support material due to its physicochemical properties, high catalytic potential, high availability and low cost compared to other carbonaceous materials [101,102]. Lai et al. [103] synthesized a biochar-supported MnFe2O4 compound and used it as a heterogeneous catalyst for a photo-Fenton process to degrade tetracycline under visible light irradiation. A 95% degradation was obtained under optimal conditions (tetracycline = 40 mg/L; H2O2 = 100 mmol/L; pH = 5.5), with low manganese and iron leaching (<0.2 mg/L). Likewise, Bocos et al. [104] used activated carbon with Fe as a heterogeneous catalyst of a photo-Fenton process to carry out the degradation of diatrizoic acid (DIA) under UV irradiation. The results showed a TOC removal of approximately 67% in 4 h without leaching of iron to the medium. On the other hand, Guo et al. [105] proposed a novel formula for the constitution of a heterogeneous catalyst of α-Fe2O3@g-C3N4 heterostructure: First, they carried out calcination of MIL-53 (Fe) and melamine and the anchoring of these particles in g-C3N4. This new catalyst was used in a photo-Fenton process for the degradation of TC (92% in 60 min), giving rise to degradation efficiencies up to 7–14 times higher than those obtained with α-Fe2O3/MIL-53, g-C3N4 and MIL-53 alone. Furthermore, these new catalysts performed well over a wide pH range and maintained good stability for five consecutive cycles.
Polymers as support for heterogeneous Fenton-type catalysts have not been widely studied in recent years. Among the different polymers, sodium alginate is perhaps the most studied in the photo-Fenton treatment. Contributing to this knowledge, Cuervo Lumbaque et al. [106] used three different materials as catalysts in a photo-Fenton process, namely mining waste (a mixture of goethite, magnetite and hematite), Fe3+/mining waste and Fe2+/mining waste included in sodium alginate spheres. The three catalysts were used to dose three different water matrices (distilled water, simulated wastewater and hospital wastewater) to degrade eight drugs. The results obtained showed almost complete degradation of the eight drugs in the different matrices after 116 min under the conditions studied (catalyst = 3 g of alginate spheres, H2O2 = 25 mg/L; pH = 5.0, simulated sunlight). However, their achievement demonstrated that the reason for the degradation of pharmaceuticals was the release of iron into the solution. So, their findings showed iron-alginate materials were homo/heterogeneous systems that provided iron dosage with enhanced performance in the removal of pharmaceuticals, compared to free systems. Similarly, Cruz et al. [107] synthesized an Fe3+ catalyst included in alginate spheres for the degradation of sulfamethoxazole through a photo-Fenton process under UV irradiation in two different water matrices, namely deionized water and bottled drinking water, and at different pH levels. After 30 min, the sulfamethoxazole was completely eliminated at pH 2 and 3, while at pH higher than 5, only 20% sulfamethoxazole degraded in 30 min. At pH 11, hardly any degradation was detected under the conditions tested (catalyst = 0.5 g/L; SMX = 20 mg/L; UV = 8 W, 365 nm; 700 rpm, T = 25 °C). Their results also confirmed that under studied conditions significant amounts of iron are released to the reaction media, turning the process into homogeneous with a minor heterogeneous contribution.
In the same way, Li et al. [108] used graphene oxide, which is a highly conductive and magnetic recovery component, as support for Fe3O4 and TiO2 nanoparticles to degrade amoxicillin by means of a photo-Fenton process in a photocatalytic submerged magnetic separation membrane reactor of their own elaboration. The results showed removal of amoxicillin of approximately 90% after 120 min of reaction under UV irradiation.
2.3. Electro-Fenton
Based on in situ electrogeneration of hydrogen peroxide by cathodic reduction of oxygen under acidic pH (Equation (16)) (Figure 3B), the electro-Fenton process minimizes operating costs by avoiding the continuous addition of hydrogen peroxide to the bulk solution [16]. Hydrogen peroxide concentration depends on the intensity of the applied current and the dissolved oxygen [15]. Hydroxyl radicals are generated by the reaction between hydrogen peroxide and the ferrous ions present in the solution. Simultaneously, the continuous regeneration of ferric ion to ferrous ion occurs at the cathode (Equation (17)) ensuring relatively constant catalyst concentration in the aqueous phase [109].
O2 + 2H+ + 2e− → H2O2
Fe3+ + e− → Fe2+
Fe2+ + •OH → Fe3+ + OH−
Furthermore, water molecules in bulk solution may be oxidized to oxygen at the anode (Equation (19)) to generate more hydrogen peroxide with the cogeneration of hydroxyl radicals (Figure 3B). In addition, by using some high-oxygen overvoltage anodes such as platinum and boron-doped diamond (BDD), hydroxyl radicals may be formed on the surfaces of these anodes according to Equation (20) [88].
H2O → 1/2O2 + 2H+ + 2e−
H2O → •OH + H+ + e−
Despite the stronger oxidation capacity of the electro-Fenton process, the homogeneous approach presents the same drawbacks as the aforementioned treatments: the need to operate under a narrow pH range limiting the precipitation of soluble iron as hydroxides and the inability to recover and further reuse the catalyst [110,111]. As mentioned above, these drawbacks may be overcome by using a solid heterogeneous catalyst classified as a function of the presence/absence of the support materials.
Iron minerals have been successfully used as catalysts in the electro-Fenton process. In particular, iron minerals such as pyrite (Equations (21)–(23)) and chalcopyrite (Equations (24)–(26)) are able to provide the iron required to the process and self-regulate the solution pH, as was demonstrated by Barhoumi et al. [112] and Droguett et al. [113].
2FeS2 + 7O2 + 2H2O → 2Fe2+ + 4SO42− + 4H+
2FeS2 + 15H2O2 → 2Fe3+ + 14H2O + 4SO42− + 2H+
FeS2 + 14Fe3+ + 8H2O → 15Fe2+ + 2SO42− + 16H+
CuFeS2 + 4O2 → Cu2+ + 2SO42− + Fe2+
CuFeS2 + 4H+ + O2 → Cu2+ + Fe2+ + 2S0 + 2H2O
CuFeS2 + 16Fe3+ + 8H2O → Cu2+ + 17Fe2+ + 2SO42− + 16H+
In the same way, Sanroman’s group used natural pyrite as the heterogeneous electro-Fenton catalyst for the 90% mineralization of vanillic acid, the main component of oil mill water. Similarly, Kalantary et al. [114] successfully degraded the antibiotic amoxicillin by electro-Fenton process using magnetite nanoparticles able to be separated from the aqueous phase by a magnetic field without production of iron sludge.
The emergence of cocatalysts offers a new perspective for the further development of AOPs [115]. The hydroxyl radical quantification carried out by Chumakov et al. [116] revealed chromium, cerium, copper and cobalt ions exhibiting a higher ability for the generation of hydroxyl radicals than ferrous ions. Thus, the selection of more active metals combined with iron allows improving the traditional electro-Fenton process. Nafcillin, a β-lactam antibiotic, was degraded by bimetallic nanoparticles of Fe/Cu by Cu incorporation [117]. These nanoparticles present a synergistic effect on the physicochemical properties of each metal, increasing its stability and reactivity [118]. The incorporation of Cu into LaCoO3 perovskite was analyzed by Xie et al. [119], who evaluated the use of this cocatalyst, achieving almost complete ciprofloxacin elimination after 120 min treatment. In addition, sulfamethazine degradation by zero-valent iron nanoparticles was enhanced by using molybdenum sulfide [120]. This metal sulfide has unsaturated sulfur atoms able to capture protons to form hydrogen sulfide, promoting iron regeneration. Higher hydroxyl radical concentration was detected in the bulk solution in the presence of a cocatalyst with the concomitant hydrogen peroxide abatement.
The use of chitosan–epichlorohydrin iron spheres as a supported heterogeneous catalyst was proposed by Rosales et al. [121], who evaluated the diclofenac degradation at neutral pH. Likewise, Hammouda et al. [122] tested immobilized iron alginate beads for indole removal.
The modification of the carbonaceous-based materials by introducing functional groups by heteroatom doping has been successfully applied over the last few years [123,124]. The functionalized cathodes may act as a heterogeneous catalyst initiating the Fenton reaction with electrogenerated H2O2 operating at lower pH [125]. Carbon-nanotube-incorporated CuFe nanolayered double hydroxide was immobilized on the surface of the graphite cathode for the degradation and mineralization of cefazolin antibiotic [126]. Despite alkaline pHs decreasing the removal efficiency, no significant modifications were detected ranging from 3.0 to 6.0, whereas increasing the current intensity quickened the diffusion rate of the reactants into the bulk solution, leading to an enhancement of the electro-Fenton effectiveness. Under optimized conditions, this functionalized cathode exhibited excellent stability after 10 runs, less than 4% difference in cefazolin removal compared to the levels after the first run. Inspired by the superb electron-transfer efficiency and prominent electrochemical activities of the composite oxides of manganese and nickel, Sun et al. [127] synthesized NiMn2O4 carbon felt cathode via a hard template method for ciprofloxacin degradation. The authors determined that a high concentration of the dissolved oxygen on carbon felt ensured the formation of hydroxyl radicals by a Fenton-like reaction via manganese and nickel ions (Equations (27) and (28)).
Mn3+ + H2O2 + H+ → Mn4+ + H2O + •OH
Ni2+ + H2O2 + H+ → Ni3+ + H2O + •OH
From their results, the meso-NiMn2O4 catalyst provided a higher specific area and more accessible pore channels for creating more radical-activated sites, being a good alternative to improve the electro-Fenton process. A similar effect was obtained on chloramphenicol using heteroatom N and O codoped porous carbon, previously synthesized using black soya bean (protein-rich biomass) as a precursor. The main advantages of this cathode are the large surface area and the presence of N and O facilitating the oxygen diffusion and thus the hydrogen peroxide production [128]. Within this context, the ferrocene-functionalized graphene-coated graphite felt cathode was used for ciprofloxacin degradation [129]. This composite was synthesized by the peptide link between the aminated graphene oxide and ferrocene. Due to the redox property of ferrocene, there was a high level of hydroxyl radical production, enhancing the removal of ciprofloxacin operating under a broader pH range and thus minimizing the sludge generation.
2.4. Photo-Electro-Fenton
The photo-electro-Fenton process consists of the combination of the electro-Fenton process with irradiation of ultraviolet light, visible light or sunlight (Figure 3B), leading to a photo-assisted improvement of the electro-Fenton process mediated by reactions (Equations (29)–(31)) [111]. This technology allows greater hydroxyl radical production due to synergistic effects between ferrous ions and light, improving pollutant degradation [130].
Fe3+ + H2O → Fe(OH)2+ + H+
Fe(OH)2+ + hν → Fe2+ + •OH
Fe(OOCR)2+ + hν → Fe2+ + CO2 + •R
The combination of photolysis and electro-Fenton process represents a promising option for treatment of the recalcitrant emerging pollutants due to the high degree of energy efficiency and increased generation of free radicals [130]. This assumption was explored by Skoumal et al. [131] in ibuprofen degradation. From their results, they concluded UV light irradiation affects the decomposition of complexes formed between Fe(III) and carboxylic acids. Fe(III)–oxalate complexes are slowly degraded by hydroxyl radicals and quickly photo-decarboxylate by UVA or solar irradiation. These results are in accordance with those of Thiam et al. [132], who demonstrated an analogous behavior in thiamphenicol mineralization by photo-electro-Fenton compared to that by pyrite-electro-Fenton. Likewise, Droguett et al. [113] suggested the use of chalcopyrite (Equations (24)–(26)) in cephalexin removal. The results showed significant improvement of TOC decay (around 88%) for the heterogeneous photo-electro-Fenton compared to the homogeneous process (63%) and heterogeneous electro-Fenton (29.7%).
Regarding supported heterogeneous catalysts, Bocos et al. [104] synthesized an iron-based catalyst supported on activated carbon for diatrizoic acid by the photo-electro-Fenton process. Complete mineralization of the pollutant was achieved after 120 min, whereas the photo-Fenton process only allowed obtaining 67% mineralization. Recently, the great potential of MOFs for industrial application was confirmed by Ye et al. [133], who tested the ability of a Fe-bpydc-catalyzed system for the degradation of bezafibrate, bisphenol A, fluoxetine and naproxen from urban wastewater. Numerous active sites on the surface of the MOF promote mass transport and charge transfer, increasing the degradation constant rate.
2.5. Sono-Fenton
Based on the “hot spot” theory, ultrasonic radiation consists of the collapse of cavitation bubbles under extreme conditions, therefore generating the thermal dissociation of water to form radical species (Equations (32)–(36)) [134].
H2O + ))) → •H + •OH
O2 + ))) → •O + •O
O• + H2O → 2•OH
•H + O2 → •OOH
H2O + O2 → •OH + •OOH
Hu et al. [135] degraded metronidazole in wide pH ranges (3.0–9.0) using Fe3O4 nanocomposites in the presence of ultrasonic radiation. Likewise, Abdili et al. [136] used zero-valent iron nanoparticles for sulfacetamide removal, achieving 91% degradation after 60 min treatment. The use of ultrasound greatly reduces the amount of deposits on the heterogeneous catalyst surface, ensuring catalyst reuse and regeneration [137]. Analogous to the other technologies, the use of the supported heterogeneous catalysts has been proposed by Dükkancı [138], who achieved total oxidation of bisphenol A by sono-Fenton process under visible light irradiation using iron-based perovskite LaFeO3 as the catalyst.
Sono-Fenton is considered as an eco-friendly and safe process that does not generate by-products or secondary contaminations; nevertheless, the high recombination of the reactive oxygen species and their high energy costs considerably limit the degradation of the pollutants and make it a good candidate for combination with other treatment technologies [139]. Despite Oturan et al. [140] combining the electro-Fenton process and ultrasound in a pioneering experiment for the degradation of organic pollutants, to date, few studies in this research field focus on the development of heterogeneous catalysts for the sono-electro-Fenton process. Ghanbari et al. [141] tested the use of hematite particles as the heterogeneous catalyst for paracetamol degradation, achieving almost 99% degradation after 60 min treatment under optimal conditions.
As was expected, the combination of ultrasound with UV/solar radiation increases the process effectiveness due to the presence of two simultaneous sources of oxidant radical production. Acoustic cavitation produces reactive oxygen species from the dissociation of water and oxygen and its further reaction with the bubbles, whereas the direct photolysis of hydrogen peroxide also produces hydroxyl radicals, accelerating the decomposition of recalcitrant organic pollutants [134].
3. Photocatalysis
Before the 1960s, metallic oxides were used for the reduction of carbon dioxide, synthesis of ammonia from nitrogen and generation of hydrogen. Since then, this research line has been oriented towards the use of these materials as active photocatalysts in water treatment [142]. Further studies using zinc oxide and titanium oxide for dye degradation elucidated the higher photocatalytic activity of the titanium. The mechanism of a photocatalyst involves the excitation of electrons from the valence band to the conduction band under light irradiation, followed by the formation of reactive oxygen species such as superoxide anion radicals and singlet oxygen or hydroxyl radicals [143,144]. The different contribution of the oxidant species allows predicting the mineralization pathways with notable precision [145]. Semiconducting photocatalytic materials including metal oxides such as TiO2, ZnO, WO3, ZrO2, V2O5, Nb2O5 and Fe2O3 have been successfully used for the degradation and further mineralization of organic compounds [146,147], Among them, titanium oxide represents an effective alternative in wastewater treatment. Despite their three different crystalline forms (anatase, brookite and rutile) in nature, anatase is considered the most photoactive form, and rutile is the most abundant and thermodynamically stable. Their excellent photocatalytic efficiency is likely due to the generation of electron–hole pairs [148,149]. The chemical reactions involved in the TiO2-mediated photocatalysis are detailed below (Equations (37)–(44)) [150].
TiO2 + hν → TiO2 (e−CB/h+VB)
TiO2 (h+VB) + H2O → TiO2 + H+ + •OH
TiO2 (h+VB) + HO− → TiO2 + •OH
O2•− + H+ → HO2•
HO2• + HO2• → H2O2 + O2
TiO2 (e−CB) + H2O2 → •OH + OH−
H2O2 + O2•− → •OH + OH− + O2
H2O2 + hν → 2•OH
Nevertheless, heterogeneous photocatalysis has multiple advantages such as easy separation and recycling of the catalysts compared to homogeneous photocatalysts [151]. Despite numerous researchers having reported excellent results at laboratory scale, their scale-up requires in-depth technical knowledge to overcome the operational drawbacks [152].
One underlying aspect of photocatalysis is the development of an appropriate catalyst able to overcome the operating limitations associated with titanium dioxide such as electron-hole recombination, fast backward reactions and ineffective photocatalytic activity under visible light irradiation [153,154]. Numerous researchers have demonstrated the high potential of solar-based techniques for the removal of emerging pollutants in water purification [155,156,157]. Nevertheless, the bandgap (above 3.0 eV) of TiO2 leading to only UV light absorption (wavelength less than 390 nm) hinders the solar-powered technology, since only about 5% of solar radiation is ultraviolet radiation [158,159]. At the present stage, achieving an efficient light response represents a challenging milestone in terms of energy effectiveness [160].
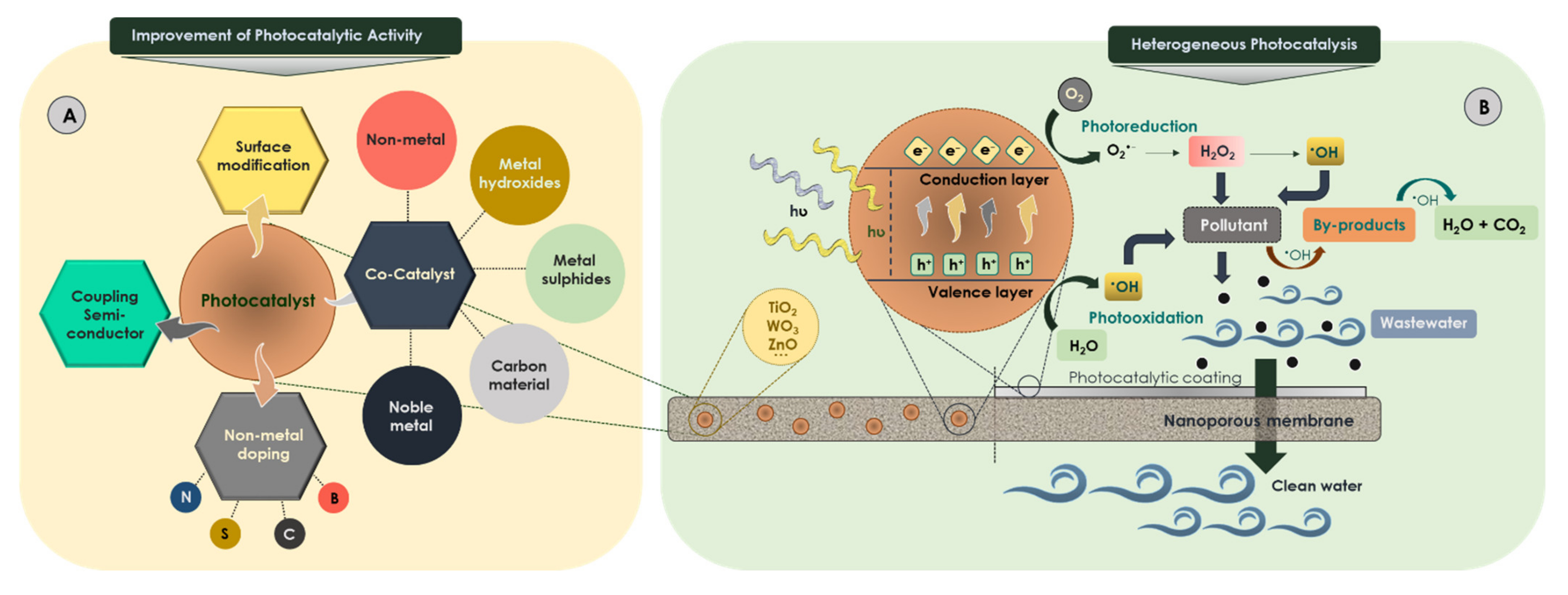
Based on the literature, the most effective strategies for improving the activation of photocatalysts are (i) the coupling of semiconductors [161,162,163]; (ii) nonmetal doping such as nitrogen, boron, sulfur, carbon and fluorine acting as superficial donors [164]; (iii) surface modification; and (iv) incorporation of dopant agents for the modification of the physical properties and surface activity enhancing their photo-response to visible light (Figure 4A). These dopant agents, called cocatalysts, are inactive materials able to improve catalytic activity and catalyst stability. These aforementioned strategies enhance the photocatalytic activity of titanium dioxide for various reasons related to modification of the electronic spectrum and charge separation [165,166].
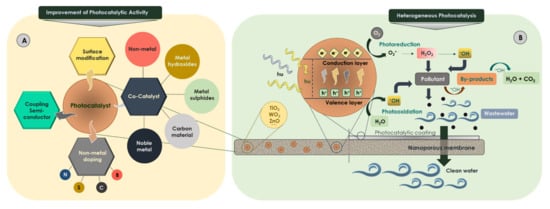
Figure 4.
(A) Improvement of photocatalyst and (B) heterogeneous membrane photocatalysis.
Huo et al. [167] and Wang et al. [168] synthesized the ZnWO4/CdS heterostructures and commercial TiO2-supported g-C3N4, respectively, for ciprofloxacin photodegradation under visible light irradiation. Li et al. [169] developed core-shell γ-Fe2O3@ZnO particles for ciprofloxacin removal under simulated solar irradiation, improving the photocatalytic activity of each bare material. From their results, these catalysts were stable, reusable and easily separable from the solution under a magnetic field. Despite most of the synthesized heterostructures generally involving the integration of two functional catalysts, Tahir et al. [170] prepared a ternary hybrid photocatalyst WO3/TiO2/g-C3N4 to enhance the catalyst activity for the removal of pharmaceuticals such as acetylsalicylate and methyl-theobromine.
The nonmetallic doping of photocatalysts reduces the bandgap of the photocatalyst, thus improving their activation under a wide wavelength range [171]. Shetty et al. [172] developed a nitrogen-doped TiO2 catalyst for the degradation of common drugs such as naproxen, paracetamol and ciprofloxacin. The doped catalyst was more efficient than commercial TiO2 under solar irradiation. This fact is likely due to the incorporation of nitrogen into the TiO2 lattice modifying the electronic structure of TiO2 [173]. In the same way, Kumar et al. [174] tested boron-doped TiO2 photocatalyst for the photodegradation of ibuprofen, flurbiprofen, 2,4-dichlorophenol and bisphenol A under UV–visible irradiation, achieving higher degradation percentages for all these pollutants compared to the bare photocatalyst. Heterostructures formed by coupling photocatalysts may be doped by nonmetallic elements, improving the resulting degradation rates. Pan et al. [175] synthesized a zirconium MOF doped with amino UiO-66 (NH2) and AgI for tetracycline removal under visible light. The doping of catalysts with carbonaceous materials has been extensively investigated to reduce the bandgap of the photocatalyst, thus allowing their performance under the visible spectrum. These carbon-based supports act as a bed for electron trapping, hindering the recombination of electron pairs and reducing the agglomeration trend of TiO2 nanoparticles [176]. Within this context, Ahmadi et al. [154] analyzed the use of TiO2 photocatalyst doped with multiwalled carbon nanotubes for the photocatalytic degradation of tetracycline and a pharmaceutical effluent under UV-C irradiation. Around 83% tetracycline removal was achieved, while COD of the pharmaceutical effluent decreased more than 84% under the same operating conditions. Likewise, Surenjan et al. [177] also evaluated carbon-doped TiO2 photocatalyst for degradation of diclofenac and carbamazepine under visible irradiation, achieving total removal of both pollutants under optimal conditions. In the same way, Darwish et al. [178] reported Ni/CdS supported on graphene nanolaminates for the photodegradation of sulfamethoxazole and cephalexin. Recently, graphitic carbon nitride (g-C3N4) has been investigated as a metal-free carbo-catalyst due to its large specific surface area and its potential activation by visible light [33]. Likewise, Ma et al. [179] evaluated the catalytic photodegradation of salicylic acid, reaching 62% removal after 180 min treatment under visible irradiation. The doping of TiO2 with F–N is anchored on a matrix of magnetic activated carbon with carbonized chitosan. Although they have been disclosed as eco-friendly remediation alternatives because the catalyst can be easily recovered from the bulk solution, further investigations are required for their widespread application. The immobilization of the TiO2 catalyst on chitosan-based composites was evaluated by Farhadian et al. [180], who reported their excellent performance in tetracycline degradation under visible light irradiation. The blending of chitosan increases the photocatalytic degradation rate 2-fold. Similarly, a new bio-based PET-TiO2 photocatalyst was developed by Malesic-Eleftheriadou et al. [181] for degrading a mixture of antibiotic compounds in simulated and real wastewater. The authors observed low adhesion between the polymeric matrix and inorganic oxide leading to a leaching process from the final composite films. Conducting polymers such as poly(o-phenylenediamine) (PoPD) may act as efficient electron donors and good hole transporters on visible-light irradiation; thus, Sandoval et al. [182] developed a ZnFe2O4/PoPD photocatalyst by a coupling strategy for increasing the poor photocatalytic activity of pure ZnFe2O4. More than 90% thimerosal degradation was achieved after 6 h light irradiation under optimal conditions. Their results suggested PoPD on the ZnFe2O4 surface enhanced the catalyst activity due to the charge separation ability of the PoPD on the metal oxide.
Similar to polymers, clays have been widely investigated as supports for photocatalysts. Belhouchet et al. [183] investigated the use of TiO2 nanocomposites supported on calcite (mainly calcium carbonate and palygorskite clay) to photocatalytically degrade tetracycline under UV and solar irradiation, reaching around 90% and 50% tetracycline and TOC removal, respectively. In the same research line, Akkari et al. [184] analyzed Fe3O4/ZnO nanoparticles on sepiolite for the degradation of ibuprofen, acetaminophen and antipyrine under sunlight irradiation. They are easily separated by an external magnetic field.
Regarding metal doping of photocatalysts, Durán-Álvarez et al. [185] deposited monometallic and bimetallic nanoparticles on TiO2 to improve their activation under visible irradiation. This photocatalyst was used in ciprofloxacin degradation under simulated solar radiation. Only partial degradation of the antibiotic was achieved after 360 min using monometallic alternatives, whereas total degradation and mineralization were obtained after 90 and 180 min, respectively, with bimetallic photocatalysts. Similar results were obtained by He et al. [186], who tested the doping of TiO2 with Fe-based organometallic structures synthesizing MIL-101 (Fe)/TiO2 magnetic MOFs for tetracycline degradation under sunlight. Almost 93% antibiotic degradation was achieved after only 10 min under solar irradiation. Alkaline earth metals, group II elements, may be used as doping elements; indeed, Al Abri et al. [187] developed Ce-doped ZnO photocatalysts for the degradation of nizatidine, levofloxacin and acetaminophen under UV irradiation. Around 95% removal was detected after 240 min, confirming that the metallic doping favored the trapping of electrons by the doping agent, decreasing the photo-corrosion of the catalyst. Heterostructures from the coupling of photocatalysts may be doped with metallic elements, in particular, Elhalil et al. [188] doped ZnO-Al2O3 photocatalyst with magnesium for further evaluating caffeine degradation under UV irradiation.
3.1. Photocatalytic-Based Membrane Filtration
Membranes have been successfully examined as additional technology for achieving the comprehensive remediation of water streams. Specifically, membrane processes based on the combination of photocatalysis and membrane filtration (Figure 4B) contribute to the breakdown of organic compounds present in the concentrate and reduce membrane fouling [189]. The membrane may act as a selective barrier avoiding the separation step, thus minimizing the energy consumption and operational costs [190]. Despite this, the use of photocatalytic membranes presents several drawbacks; for example, (i) the polymer membrane structure may be attacked by reactive oxygen species and/or light irradiation, (ii) the process effectiveness depends on the mass transfer rate limitation, (iii) the low effective specific surface area involves a relative loss of photocatalytic ability and (iv) the catalyst deactivation provokes a shorter lifespan of the membrane.
The main aspects of the two common configurations, photocatalysis with membrane filtration (two steps) or photocatalytic membrane reactor (PMR, single-step) were summarized by Iglesias et al. [158]. Although both alternatives were used in water purification, the single step requires a smaller spatial domain and lower maintenance costs and thus is highly recommended for industrial applications. Indeed, Espíndola et al. [191] have evaluated the performance of PMR in oxytetracycline degradation in the presence of suspended or immobilized TiO2. Despite the advantages of the nano-engineered photocatalytic membranes, the pharmaceutical removal effectiveness was lower than that of TiO2-P25 slurry conditions due to lower available surface area. The authors proposed the development of novel membranes with notable photoactivity and the addition of other oxidants (e.g., persulfate, ozone, hydrogen peroxide) for improving their performance.
Prior to the photodegradation studies, the compatibility of the membrane with the feed solution containing organic compounds should be evaluated using the Hansen solubility parameters. The Hansen approach relates the mixing energy and vaporization energies of the pure substances considering the interactions between molecules. This methodology represents a powerful tool for the assessment of interactions between the membrane components (polymer, solvent and additives) and aqueous feed throughout the filtration process [192].
Based on the aforementioned developments, the next-generation membranes involve the development of membranes with functional nanoparticles through the surface functionalization of existing membranes [193] or the incorporation of nanoparticles (e.g., metal, metal oxide, composites) into the polymeric or ceramic matrix (nano-engineered membranes) [194,195,196]. The addition of hydrophilic metal oxide nanoparticles such as alumina, silica, zeolite and titania to membranes increases the membrane surface hydrophilicity, permeability and fouling resistance. Furthermore, these inorganic nanomaterials improve the mechanical and thermal stability of polymeric membranes and also considerably reduce their compaction level [150]. Paredes et al. [197] analyzed a polyvinylidene fluoride double-layer hollow fiber polymeric membrane with immobilized TiO2 for the photocatalytic degradation of eight pharmaceutical products under UV irradiation. To promote the application of solar energy closer to the photocatalysis processes, Koe et al. [198] synthesized nanocomposites by coupling TiO2 with carbon quantum dots doped with different charges of nitrogen and sulfur. These nanoparticles were deposited on a polysulfone ultrafiltration membrane for the degradation of diclofenac from an aqueous effluent under visible irradiation, reaching around 62% drug degradation, more than 20-fold compared to pure TiO2. In this same way, Singh et al. [199] investigated the potential application of polysulfone ultrafiltration membrane with supported Cu2O as photocatalyst for ibuprofen removal under visible radiation, reaching 86% drug removal.
3.2. Photoelectrocatalysis
Photoelectrocatalytic oxidation consists of the application of an electric current intensity/voltage to the photoanode (semiconductor anode materials), which is able to act as photocatalyst under irradiation [200,201]. The photogenerated holes oxidize the organic compounds on the anode surface, and an external electrical circuit extracts the photoinduced electrons. In addition, the electrons generally move to the outside circuit around the cathode where they may participate in reductive reactions, including molecular hydrogen production from hydrogen ions [202]. The lack of electrons promotes the aggregation of the holes at the anode, leading to the desired decline of the recombination rate of photogenerated electron–hole pairs [201].
Numerous techniques have been developed for the synthesis of the photoanodes, including anodic oxidation, electrolytic plasma oxidation, sputtering, plasma spraying, evaporation and sol–gel [203]. Photoelectrocatalysis electrodes, mainly the photoanode, are based on one or more photocatalysts and/or cocatalysts supported on glass/metal mesh to enhance photocatalytic properties [204]. Most of the photoanodes used are composed of TiO2 due to its low cost, high photoactivity and excellent chemical and thermal stability.
The removal effectiveness depends on the structure and morphology of the photoanode, the solution pH, the electrical potential, the range of electrical intensity, the intensity of radiation and the pollutant concentration [201]. Recently, the application of photoelectrocatalysis has focused on wastewater treatment and the degradation of emerging pollutants, mainly pharmaceutical products and the treatment of industrial effluents [205,206,207]. Su et al. [208] analyzed sulfamethoxazole degradation using a TiO2/Ti photoanode under UV irradiation. Total antibiotic degradation was reached after 70 min at 0.5 V, confirming that a higher potential gradient reduces the recombination of holes and electrons, favoring the degradation process. Likewise, Mazierski et al. [209] synthesized photoanodes based on Ti/TiO2 nanotubes by the anodic oxidation technique for 5-fluorouracil degradation at 1.00 V. The ecotoxicity of the effluents after treatment was investigated by using Lemna minor. Around 30% TOC abatement and a higher growth rate of L. minor demonstrated that the degradation products are less toxic than the parent drug. The effect of a more complex matrix was checked by Collivignarelli et al. [203], who used TiO2 mesh photoanodes synthesized by plasma electrolytic oxidation for the treatment of effluent from the pharmaceutical industry, achieving 55% removal and 24% COD reduction after 120 min under UV irradiation. Furthermore, the development of photoanodes based on active catalysts under visible light is considered an astonishing challenge in this research field. Within this context, Kushwaha et al. [210] synthesized a photoactive third-generation catalyst CaCu3Ti4O12 under sunlight radiation. These third-generation catalysts, based on the union of low-bandgap photoactive materials and TiO2, were used for the photoelectrocatalytic degradation of various pharmaceutical products and derivatives such as erythrosine, ciprofloxacin and estriol, obtaining 81%, 72% and 78% degradation, respectively, after 40 min.
Bismuth-based semiconductors, mostly bismuth vanadate, have recently been investigated for their use in photoelectrocatalysis since they possess high photocatalytic activity mediated by visible spectrum radiation [204]. However, the yield of BiVO4 is relatively low due to the recombination rate of e−/h+ pairs [211]. To overcome this problem, the aforementioned strategies including metallic and nonmetallic doping and coupling with other semiconductors have been proposed. Within this framework, Orimolade et al. [212] developed a BiVO4/BiOI heterojunction electrodeposited on FTO glass as a photoanode for degradation of acetaminophen and ciprofloxacin under visible radiation. Their binary electrode achieved better degradation rates than either of the materials separately, showing almost 70% degradation of both pollutants after 120 min treatment at 1.50 V.
4. Future Perspectives
The problem of dealing with low concentrations of pollutants has stimulated several studies in the hunt for combined strategies that allow a prompt removal of these pollutants. Among them, adsorption/AOP is a good option in which the pollutants are adsorbed into a porous material that is removed by subsequent in situ AOP reactions that take place on the surface of the exhausted adsorbent or by desorption and treatment of concentrated aqueous solution. There are recent studies that combine both the adsorption processes of pollutants in different matrices and the catalytic degradation through the Fenton-based treatment of the adsorbed pollutants to regenerate the adsorbent. On this topic, Puga et al. [22] applied the latter in the removal of sulfamethizole by adsorption on pellets of carbonaceous nanogel following the heterogeneous electro-Fenton process using a novel synthesized iron perlite catalyst. This catalyst was synthesized by the iron fixation on a floating material such as perlite by hydrothermal treatment. The floating characteristics facilitate the separation of catalyst and the bulk solution, allowing the process to be performed in a flow system without any operational problems. In the same way, Shan et al. [213] constituted a three-dimensional hybrid hydrogel composed of reduced graphene oxide and Fe3O4 (3D-rGO-Fe3O4) to be used as an adsorbent for antibiotics. Subsequently, this hybrid hydrogel acted as a catalyst for a heterogeneous Fenton reaction in order to degrade the tetracycline and ciprofloxacin contained in the matrix, thus regenerating the adsorbent material. Regeneration was effective for 10 adsorption-oxidation cycles. Doping of graphene-derived compounds with nitrogen and sulfur heteroatoms significantly improves electrical conductivity and provides new electrocatalytic active sites [214,215]. Peng et al. [216] reported the synthesis of a new nanocomposite formed by nitrogen-modified reduced graphene oxide (N-rGO) as support for magnetic nanoparticles (Fe3O4) (N-rGO/Fe3O4). This nanocomposite was used for the adsorption of norfloxacin and ketoprofen with the subsequent catalytic degradation by activation of PMS through a Fenton-like oxidation process. Norfloxacin was completely degraded and 89% TOC removal efficiency was achieved in 210 min. Electron spin resonance proved that both •OH and SO4− radicals were produced during the treatment. In this field, the use of a photo-based process was also evaluated, and the photocatalytic adsorbent MIL-53(Fe) was successfully prepared by Gao et al. [217] by solvothermal method and used for the removal of two typical pharmaceuticals, clofibric acid and carbamazepine, from water. MIL-53(Fe) exhibited good adsorption performance, and the MIL-53(Fe)/H2O2/vis system showed significantly higher performance for the degradation of carbamazepine than the MIL-53 (Fe)/H2O2, Fe(II)/H2O2 and TiO2/vis systems. It was concluded that the direct excitation of the iron-oxo cluster mainly contributes to the photo-Fenton activity of MIL-53(Fe). Likewise, in photocatalysis processes, clays or clay materials such as montmorillonite, sepiolite, palygorskite, zeolite, kaolinite, calcite and diatomite have also been widely studied as support for photocatalysts due to their high adsorption capacity, large surface area and chemical and mechanical and stability [218].
Regarding heterogeneous catalysts for the catalytic degradation of pollutants, in recent years the use of heterogeneous nonmetallic catalysts, materials that have nonmetallic elements as active components, has been investigated in advanced oxidation processes for the degradation of emergent pollutants by Fenton-like process through activation of PS and PMS (radical SO4−) and photocatalysis technology. Nonmetallic catalysts, also called green catalysts or carbon catalysts, include graphene, graphitic carbon nitride, mesoporous carbon, activated carbon, nanodiamonds, fullerenes, carbon nanotubes and simple/multiwalled carbon nanotubes [219]. These nonmetallic catalysts are capable of overcoming some inherent defects of metallic catalysts, namely the inhibition of some functional groups, the need for additional impregnation steps in the supported catalysts and the easy detachment, thus reducing the catalytic activity and easy agglomeration [220]. Thus, Wang et al. [221] demonstrated that the efficiency of oxalic acid removal (100% removal in 80 min) was much higher using fluorine-doped carbon nanotubes (F-CNT) compared to four conventional metal-based catalysts (ZnO, Al2O3, Fe2O3 and MnO2). Similarly, Ma et al. [222] degraded bisphenol A in the presence of PMS using nitrogen-doped carbon nanotubes (NCNTF) as a heterogeneous catalyst. Among the advantages that carbon catalysts present over metallic catalysts are their low contamination capacity, low cost, easily adaptable porous surface and high chemical and thermal stability [33]. For all these reasons, these catalysts are successful, profitable, stable and environmentally friendly catalysts compared to traditional metallic ones, but their catalytic capacity still needs to be improved and their fabrication is still difficult; therefore, more research is needed in this field [223]. The so-called nanobiocatalysts (NBCs), which can be defined as encapsulated or supported enzymes in a multitude of materials, have also begun to be investigated in the field of bioremediation and degradation of emerging pollutants since the processes mediated by these NBCs use fewer products. The chemicals and catalysts are easily recoverable and prevent the use and manufacture of hazardous and toxic materials [224]. Among the materials used to produce NBCs, nanostructured materials such as nanofibers, hybrid nanoflowers, nanoporous substrates, nanotubes and magnetic nanoparticles stand out [225]. The crystalline structure, the functionalization of the surface, the size of the particle, the viscosity and the oxidation state influence the enzyme–catalyst interaction; therefore, it is important to highlight that the coordination strategy between the type of catalyst (enzyme) and the support material (nanoparticle) is vital for the stability and functioning of the system and must give rise to catalytically active systems [226]. Another key aspect of this type of conjugate is to determine the number of cycles that the enzyme can carry out without losing activity. However, on many occasions, the structure of the enzyme during immobilization is affected, thus causing a partial or total reduction in enzyme activity [225]. Therefore, there are few cases of systems that have achieved good stabilization for some processes. More research is still needed on the main mechanisms that control the activity of NBCs (physical entrapment and encapsulation) to specify the scope and usefulness of these biocatalysts in different environmental remediation processes.
In recent years, hybrid microbial fuel cells (MFCs) integrated with other technologies have been postulated as very promising techniques for the degradation of refractory organic compounds present in wastewater [227]. Classic microbial fuel cells involve the conversion of chemical energy into electrical energy by oxidizing organic compounds using electrogenic microorganisms [228]. The cells are composed of anode and cathode, usually with carbon felt, and can be single-chamber or double-chamber. In addition, a substrate is needed for their operation, which is a simple organic compound such as glucose or acetate; also needed are molecular oxygen, a fuel that is the refractory organic compound itself, a proton exchange membrane (PEM) and the electrogenic microorganisms that are responsible for the generation of electrons [229]. However, although the classic MFC system provides good results, some contaminants present in the water cannot be used as fuels because they show high toxicity towards the microorganisms that are present at the anode of the MFCs, thus reducing the degradation efficiency [227,230]. It is, therefore, this fact that has led current MFC research efforts to focus on addressing this problem by integrating this technology with other treatment technologies such as photocatalysis, photo-electro-catalysis and Fenton processes (MFC-photocatalysis and MFC-Fenton) [228]. Likewise, through an MFC-photocatalysis treatment system, the efficient removal of metronidazole from wastewater was achieved. Wang et al. [231], while using an MFC-Fenton system, achieved a removal and mineralization efficiency of metronidazole of 94.5% and 89.5%, respectively.
The presence of pharmaceutical products in the water cycle may cause harmful effects such as induction resistance in pathogenic microorganisms or morphological, metabolic and sex alterations in aquatic organisms [232]. Therefore, the World Health Organization (WHO) has recently published a list of antibiotic-resistant “priority pathogens”, which are considered a hazardous threat to human health [233]. For this reason, the development of novel technologies able to mitigate the antibiotic-resistance spread represents an astonishing challenge for the scientific community. To date, only a few publications are related to the simultaneous antibiotic-resistant microorganism inactivation and antibiotic removal by AOPs [234,235,236]. Rizzo et al. [237] evaluated photo-driven AOPs with peracetic acid in the inactivation of sulfamethoxazole-resistant E. coli and the degradation of three pharmaceutical compounds (sulfamethoxazole, diclofenac and carbamazepine) under different light sources. In the same way, Ioannou-Ttofa et al. [234] reported total ampicillin degradation and complete inactivation of ampicillin-resistant E. coli bacteria by solar photo-Fenton process under optimal experimental conditions. In addition, no post-treatment bacteria regrowth was observed, even after 48 h incubation, demonstrating the permanent damage to bacterial cellular functions caused by UV irradiation. The obtained results open up a novel route for the tertiary treatment of urban effluents; nonetheless, further studies are required to determine a compromise solution able to deactivate pathogens and remove emerging pollutants simultaneously with higher process effectiveness.
5. Conclusions
This review has shown the present trends in the use of heterogeneous AOPs for the treatment of aquatic environments, discussing those aspects relevant for each available alternative. The main drawbacks of these advanced oxidation processes are associated with higher operating costs related to energy consumption (ultraviolet-light irradiation); to date, they are not considered economically viable processes at a large scale. Consequently, renewable energies such as solar radiation open up a promising route for the development of efficient and affordable technology.
The effectiveness of these novel wastewater treatment technologies should be assessed in continuous-flow systems for real industrial effluent in the presence of organic matter and other contaminants. Most of the studies are focused on the removal of emerging pollutants from simulated water under batch conditions; nevertheless, recently, more researchers have investigated the operation of these technologies using real matrices.
Despite their excellent degradation and mineralization percentage results at the bench scale, the main drawbacks of these technologies are the relatively high energy consumption and the concomitant elevated operating costs. Therefore, the main challenges facing these technologies are the enhancement of their performance and stability to achieve eco-friendlier and more cost-effective wastewater treatment on pilot and large scales.
Author Contributions
G.L.: conceptualization, investigation, writing—original draft. J.M.: investigation, writing—original draft. A.S.: funding acquisition, writing—review and editing. M.P.: conceptualization, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been financially supported Project PID2020-113667GB-I00 funded by MCIN/AEI/10.13039/501100011033 and PDC2021-121394-I00 funded by MCIN/AEI/10.13039/501100011033 and by the European Union Next Generation EU/PRTR. The authors are grateful to the Xunta de Galicia for the financial support of Jessica Meijide under her postdoctoral fellowship (ED481B 2018/096).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Fdez-Sanromán, A.; Pazos, M.; Rosales, E.; Sanromán, M.Á. Prospects on integrated electrokinetic systems for decontamination of soil polluted with organic contaminants. Curr. Opin. Electrochem. 2021, 27, 100692. [Google Scholar] [CrossRef]
- Cheng, M.; Zeng, G.; Huang, D.; Lai, C.; Xu, P.; Zhang, C.; Liu, Y. Hydroxyl radicals based advanced oxidation processes (AOPs) for remediation of soils contaminated with organic compounds: A review. Chem. Eng. J. 2016, 284, 582–598. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sarmah, A.K.; Padhye, L.P. Fate of pharmaceuticals and personal care products in a wastewater treatment plant with parallel secondary wastewater treatment train. J. Environ. Manag. 2019, 233, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Gosset, A.; Ferro, Y.; Durrieu, C. Methods for evaluating the pollution impact of urban wet weather discharges on biocenosis: A review. Water Res. 2016, 89, 330–354. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Li, X.; Xia, Q.; Wu, J.; Li, Y.; Xiao, J.; Li, Z. Adsorptive and photocatalytic removal of Persistent Organic Pollutants (POPs) in water by metal-organic frameworks (MOFs). Chem. Eng. J. 2018, 337, 351–371. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Casado, J. Towards industrial implementation of Electro-Fenton and derived technologies for wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 102823. [Google Scholar] [CrossRef]
- Du, X.; Zhou, M. Strategies to enhance catalytic performance of metal-organic frameworks in sulfate radical-based advanced oxidation processes for organic pollutants removal. Chem. Eng. J. 2021, 403, 126346. [Google Scholar] [CrossRef]
- Escudero-Curiel, S.; Penelas, U.; Sanromán, M.Á.; Pazos, M. An approach towards Zero-Waste wastewater technology: Fluoxetine adsorption on biochar and removal by the sulfate radical. Chemosphere 2021, 268, 129318. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; He, D.; Pan, X. Application of iron-based materials in heterogeneous advanced oxidation processes for wastewater treatment: A review. Chem. Eng. J. 2021, 407, 127191. [Google Scholar] [CrossRef]
- Kumar, A.; Rana, A.; Sharma, G.; Naushad, M.; Dhiman, P.; Kumari, A.; Stadler, F.J. Recent advances in nano-Fenton catalytic degradation of emerging pharmaceutical contaminants. J. Mol. Liq. 2019, 290, 111177. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current advances and trends in electro-Fenton process using heterogeneous catalysts—A review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-fenton process and related electrochemical technologies based on fenton’s reaction chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Arellano, M.; Sanromán, M.A.; Pazos, M. Electro-assisted activation of peroxymonosulfate by iron-based minerals for the degradation of 1-butyl-1-methylpyrrolidinium chloride. Sep. Purif. Technol. 2019, 208, 34–41. [Google Scholar] [CrossRef]
- Kang, J.; Duan, X.; Zhou, L.; Sun, H.; Tadé, M.O.; Wang, S. Carbocatalytic activation of persulfate for removal of antibiotics in water solutions. Chem. Eng. J. 2016, 288, 399–405. [Google Scholar] [CrossRef]
- Sablas, M.M.; de Luna, M.D.G.; Garcia-Segura, S.; Chen, C.W.; Chen, C.F.; Dong, C. Di Percarbonate mediated advanced oxidation completely degrades recalcitrant pesticide imidacloprid: Role of reactive oxygen species and transformation products. Sep. Purif. Technol. 2020, 250, 117269. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.F.R.; Silva, A.M.T. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 33–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Puga, A.; Rosales, E.; Pazos, M.; Sanromán, M.A. Prompt removal of antibiotic by adsorption/electro-Fenton degradation using an iron-doped perlite as heterogeneous catalyst. Process Saf. Environ. Prot. 2020, 144, 100–110. [Google Scholar] [CrossRef]
- Wang, C.; Shih, Y. Degradation and detoxification of diazinon by sono-Fenton and sono-Fenton-like processes. Sep. Purif. Technol. 2015, 140, 6–12. [Google Scholar] [CrossRef]
- Giannakis, S.; Papoutsakis, S.; Darakas, E.; Escalas-Cañellas, A.; Pétrier, C.; Pulgarin, C. Ultrasound enhancement of near-neutral photo-Fenton for effective E. coli inactivation in wastewater. Ultrason. Sonochem. 2015, 22, 515–526. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Huang, J.; Hu, X.; Zhang, S.; Dai, Q.; Chai, H.; Gu, L. Activation of sodium percarbonate by vanadium for the degradation of aniline in water: Mechanism and identification of reactive species. Chemosphere 2019, 215, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.; Fang, C.; Geng, Z.; Jin, Y.; Xiao, D.; Wang, Z.; Liu, J.; Guo, Y. Significantly enhanced base activation of peroxymonosulfate by polyphosphates: Kinetics and mechanism. Chemosphere 2017, 173, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Xie, W.; Fan, Y.; Shi, Y.; Kong, D.; Lu, J. Degradation of trimethoprim by thermo-activated persulfate oxidation: Reaction kinetics and transformation mechanisms. Chem. Eng. J. 2016, 286, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Poza-Nogueiras, V.; Moratalla, Á.; Pazos, M.; Sanromán, Á.; Sáez, C.; Rodrigo, M.A. Towards a more realistic heterogeneous electro-Fenton. J. Electroanal. Chem. 2021, 895, 115475. [Google Scholar] [CrossRef]
- Cihanoğlu, A.; Gündüz, G.; Dükkanci, M. Degradation of acetic acid by heterogeneous Fenton-like oxidation over iron-containing ZSM-5 zeolites. Appl. Catal. B Environ. 2015, 165, 687–699. [Google Scholar] [CrossRef]
- Thomas, A. Functional materials: From hard to soft porous frameworks. Angew. Chem. Int. Ed. 2010, 49, 8328–8344. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, R.; Bhanja, P.; Bhaumik, A. The design and synthesis of heterogeneous catalysts for environmental applications. Dalton Trans. 2021, 50, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015, 176–177, 249–265. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Mao, Q.; Zhou, Y.; Wei, J.; Liu, X.; Yang, J.; Luo, L.; Zhang, J.; Chen, H.; Chen, H.; et al. Metal-free carbon materials-catalyzed sulfate radical-based advanced oxidation processes: A review on heterogeneous catalysts and applications. Chemosphere 2017, 189, 224–238. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Wang, F.; Xiong, X.; Tian, K.; Sun, Y.; Yu, T. Application of heterogeneous catalytic ozonation for Refractory Organics in Wastewater. Catalysts 2019, 9, 241. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Xu, L.; Fu, K.; Zhu, F.; Yang, T.; Yang, T.; Luo, J.; Wu, M.; Yu, D. Ultrastable MOF-based foams for versatile applications. Nano Res. 2021, 1–10, in press. [Google Scholar] [CrossRef]
- Tekin, H.; Bilkay, O.; Ataberk, S.S.; Balta, T.H.; Ceribasi, I.H.; Sanin, F.D.; Dilek, F.B.; Yetis, U. Use of Fenton oxidation to improve the biodegradability of a pharmaceutical wastewater. J. Hazard. Mater. 2006, 136, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Dinarvand, M.; Sohrabi, M.; Royaee, S.J.; Zeynali, V. Degradation of phenol by heterogeneous Fenton process in an impinging streams reactor with catalyst bed. Asia-Pac. J. Chem. Eng. 2017, 12, 631–639. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, X.; Men, B.; Wang, D. Interfacial mechanisms of heterogeneous Fenton reactions catalyzed by iron-based materials: A review. J. Environ. Sci. 2016, 39, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.C.; Chang, S.Y.; Schroeder, S.L.M. Fenton-like oxidation of 4-chlorophenol: Homogeneous or heterogeneous? Ind. Eng. Chem. Res. 2015, 54, 8122–8129. [Google Scholar] [CrossRef] [Green Version]
- Carrasco-Díaz, M.R.; Castillejos-López, E.; Cerpa-Naranjo, A.; Rojas-Cervantes, M.L. Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chem. Eng. J. 2016, 304, 408–418. [Google Scholar] [CrossRef]
- Zhang, N.; Xue, C.; Wang, K.; Fang, Z. Efficient oxidative degradation of fluconazole by a heterogeneous Fenton process with Cu-V bimetallic catalysts. Chem. Eng. J. 2020, 380, 122516. [Google Scholar] [CrossRef]
- Usman, M.; Byrne, J.M.; Chaudhary, A.; Orsetti, S.; Hanna, K.; Ruby, C.; Kappler, A.; Haderlein, S.B. Magnetite and Green Rust: Synthesis, Properties, and Environmental Applications of Mixed-Valent Iron Minerals. Chem. Rev. 2018, 118, 3251–3304. [Google Scholar] [CrossRef]
- Ammar, S.; Oturan, M.A.; Labiadh, L.; Guersalli, A.; Abdelhedi, R.; Oturan, N.; Brillas, E. Degradation of tyrosol by a novel electro-Fenton process using pyrite as heterogeneous source of iron catalyst. Water Res. 2015, 74, 77–87. [Google Scholar] [CrossRef]
- Kantar, C.; Oral, O.; Oz, N.A. Ligand enhanced pharmaceutical wastewater treatment with Fenton process using pyrite as the catalyst: Column experiments. Chemosphere 2019, 237, 124440. [Google Scholar] [CrossRef]
- Muñoz, M.; Conde, J.; de Pedro, Z.M.; Casas, J.A. Antibiotics abatement in synthetic and real aqueous matrices by H2O2/natural magnetite. Catal. Today 2018, 313, 142–147. [Google Scholar] [CrossRef]
- Hassani, A.; Karaca, M.; Karaca, S.; Khataee, A.; Açışlı, Ö.; Yılmaz, B. Preparation of magnetite nanoparticles by high-energy planetary ball mill and its application for ciprofloxacin degradation through heterogeneous Fenton process. J. Environ. Manag. 2018, 211, 53–62. [Google Scholar] [CrossRef]
- Nie, M.; Li, Y.; He, J.; Xie, C.; Wu, Z.; Sun, B.; Zhang, K.; Kong, L.; Liu, J. Degradation of tetracycline in water using Fe3O4 nanospheres as Fenton-like catalysts: Kinetics, mechanisms and pathways. New J. Chem. 2020, 44, 2847–2857. [Google Scholar] [CrossRef]
- Scaria, J.; Gopinath, A.; Nidheesh, P.V. A versatile strategy to eliminate emerging contaminants from the aqueous environment: Heterogeneous Fenton process. J. Clean. Prod. 2021, 278, 124014. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J. Metal Organic Framework with Coordinatively Unsaturated Sites as Efficient Fenton-like Catalyst for Enhanced Degradation of Sulfamethazine. Environ. Sci. Technol. 2018, 52, 5367–5377. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V. Heterogeneous Fenton catalysts for the abatement of organic pollutants from aqueous solution: A review. RSC Adv. 2015, 5, 40552–40577. [Google Scholar] [CrossRef]
- Plakas, K.V.; Mantza, A.; Sklari, S.D.; Zaspalis, V.T.; Karabelas, A.J. Heterogeneous Fenton-like oxidation of pharmaceutical diclofenac by a catalytic iron-oxide ceramic microfiltration membrane. Chem. Eng. J. 2019, 373, 700–708. [Google Scholar] [CrossRef]
- Molina, C.B.; Sanz-Santos, E.; Boukhemkhem, A.; Bedia, J.; Belver, C.; Rodriguez, J.J. Removal of emerging pollutants in aqueous phase by heterogeneous Fenton and photo-Fenton with Fe2O3-TiO2-clay heterostructures. Environ. Sci. Pollut. Res. 2020, 27, 38434–38445. [Google Scholar] [CrossRef] [PubMed]
- Khankhasaeva, S.T.; Dashinamzhilova, E.T.; Dambueva, D.V. Oxidative degradation of sulfanilamide catalyzed by Fe/Cu/Al-pillared clays. Appl. Clay Sci. 2017, 146, 92–99. [Google Scholar] [CrossRef]
- Xu, X.; Chen, W.; Zong, S.; Ren, X.; Liu, D. Magnetic clay as catalyst applied to organics degradation in a combined adsorption and Fenton-like process. Chem. Eng. J. 2019, 373, 140–149. [Google Scholar] [CrossRef]
- Sétifi, N.; Debbache, N.; Sehili, T.; Halimi, O. Heterogeneous Fenton-like oxidation of naproxen using synthesized goethite-montmorillonite nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 370, 67–74. [Google Scholar] [CrossRef]
- Iglesias, O.; Gómez, J.; Pazos, M.; Sanromán, M.Á. Electro-Fenton oxidation of imidacloprid by Fe alginate gel beads. Appl. Catal. B Environ. 2014, 144, 416–424. [Google Scholar] [CrossRef]
- Titouhi, H.; Belgaied, J.E. Heterogeneous Fenton oxidation of ofloxacin drug by iron alginate support. Environ. Technol. 2016, 37, 2003–2015. [Google Scholar] [CrossRef]
- Lyu, L.; Zhang, L.; Hu, C. Enhanced Fenton-like degradation of pharmaceuticals over framework copper species in copper-doped mesoporous silica microspheres. Chem. Eng. J. 2015, 274, 298–306. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, J.; Fang, Z.; Tsang, E.P. Ceria accelerated nanoscale zerovalent iron assisted heterogenous Fenton oxidation of tetracycline. Chem. Eng. J. 2019, 369, 588–599. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Briguglio, S.; Mattiussi, M.; Gelao, V.; Cabras, I.; Zorzenon, L.; Trovarelli, A.; Goi, D. Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J. Environ. Chem. Eng. 2020, 8, 103586. [Google Scholar] [CrossRef]
- Ling, L.; Liu, Y.; Pan, D.; Lyu, W.; Xu, X.; Xiang, X.; Lyu, M.; Zhu, L. Catalytic detoxification of pharmaceutical wastewater by Fenton-like reaction with activated alumina supported CoMnAl composite metal oxides catalyst. Chem. Eng. J. 2020, 381, 122607. [Google Scholar] [CrossRef]
- Martinez-Macias, C.; Serna, P.; Gates, B.C. Isostructural Zeolite-Supported Rhodium and Iridium Complexes: Tuning Catalytic Activity and Selectivity by Ligand Modification. ACS Catal. 2015, 5, 5647–5656. [Google Scholar] [CrossRef]
- Adityosulindro, S.; Julcour, C.; Barthe, L. Heterogeneous Fenton oxidation using Fe-ZSM5 catalyst for removal of ibuprofen in wastewater. J. Environ. Chem. Eng. 2018, 6, 5920–5928. [Google Scholar] [CrossRef] [Green Version]
- Velichkova, F.; Delmas, H.; Julcour, C.; Koumanova, B. Heterogeneous fenton and photo-fenton oxidation for paracetamol removal using iron containing ZSM-5 zeolite as catalyst. AlChE J. 2017, 63, 669–679. [Google Scholar] [CrossRef] [Green Version]
- del Álamo, A.C.; González, C.; Pariente, M.I.; Molina, R.; Martínez, F. Fenton-like catalyst based on a reticulated porous perovskite material: Activity and stability for the on-site removal of pharmaceutical micropollutans in a hospital wastewater. Chem. Eng. J. 2020, 401, 126113. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, L.; Li, Y.Y.; Hu, C. Enhanced Fenton-like degradation of refractory organic compounds by surface complex formation of LaFeO3 and H2O2. J. Hazard. Mater. 2015, 294, 195–200. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Hu, C.; Wang, X.; Lyu, L.; Sheng, G. Enhanced degradation of organic pollutants over Cu-doped LaAlO3 perovskite through heterogeneous Fenton-like reactions. Chem. Eng. J. 2018, 332, 572–581. [Google Scholar] [CrossRef]
- Zhang, S.; Hedtke, T.; Zhu, Q.; Sun, M.; Weon, S.; Zhao, Y.; Stavitski, E.; Elimelech, M.; Kim, J.H. Membrane-Confined Iron Oxychloride Nanocatalysts for Highly Efficient Heterogeneous Fenton Water Treatment. Environ. Sci. Technol. 2021, 55, 9266–9275. [Google Scholar] [CrossRef]
- Nidheesh, P.V. Graphene-based materials supported advanced oxidation processes for water and wastewater treatment: A review. Environ. Sci. Pollut. Res. 2017, 24, 27047–27069. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Gan, D.; Guo, L.; Liu, M.; Chen, J.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A facile strategy for preparation of magnetic graphene oxide composites and their potential for environmental adsorption. Ceram. Int. 2018, 44, 18571–18577. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Recent advances in the use of graphene-family nanoadsorbents for removal of toxic pollutants from wastewater. Adv. Colloid Interface Sci. 2014, 204, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, L.; Li, W.; Fan, W.; Song, S.; Yang, J. Adsorption and degradation of sulfadiazine over nanoscale zero-valent iron encapsulated in three-dimensional graphene network through oxygen-driven heterogeneous Fenton-like reactions. Appl. Catal. B Environ. 2019, 259, 118057. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Li, W.; Tao, Y.; Sui, Z.; Song, S.; Yang, J. Three-dimensional macroporous graphene-wrapped zero-valent copper nanoparticles as efficient micro-electrolysis-promoted Fenton-like catalysts for metronidazole removal. Sci. Total Environ. 2019, 658, 219–233. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, P.; Wang, H.; Zeng, G.; Huang, D.; Chen, M.; Lai, C.; Zhang, C.; Wan, J.; Xue, W. Strategies to improve metal organic frameworks photocatalyst’s performance for degradation of organic pollutants. Coord. Chem. Rev. 2018, 376, 449–466. [Google Scholar] [CrossRef]
- Qin, H.; Cheng, H.; Li, H.; Wang, Y. Degradation of ofloxacin, amoxicillin and tetracycline antibiotics using magnetic core–shell MnFe2O4@C-NH2 as a heterogeneous Fenton catalyst. Chem. Eng. J. 2020, 396, 125304. [Google Scholar] [CrossRef]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, thermal and mechanical stabilities of metal-organic frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Cheng, M.; Lai, C.; Liu, Y.; Zeng, G.; Huang, D.; Zhang, C.; Qin, L.; Hu, L.; Zhou, C.; Xiong, W. Metal-organic frameworks for highly efficient heterogeneous Fenton-like catalysis. Coord. Chem. Rev. 2018, 368, 80–92. [Google Scholar] [CrossRef]
- Bavykina, A.; Kolobov, N.; Khan, I.S.; Bau, J.A.; Ramirez, A.; Gascon, J. Metal-Organic Frameworks in Heterogeneous Catalysis: Recent Progress, New Trends, and Future Perspectives. Chem. Rev. 2020, 120, 8468–8535. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, J. Iron-copper bimetallic metal-organic frameworks for efficient Fenton-like degradation of sulfamethoxazole under mild conditions. Chemosphere 2020, 241, 125002. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, J. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation. Chem. Eng. J. 2019, 375, 122007. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Rahim Pouran, S.; Abdul Raman, A.A.; Wan Daud, W.M.A. Review on the application of modified iron oxides as heterogeneous catalysts in Fenton reactions. J. Clean. Prod. 2014, 64, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Yao, H.; Fu, W.; Hua, L.; Zhang, G.; Zhang, W. Reactive Photo-Fenton ceramic membranes: Synthesis, characterization and antifouling performance. Water Res. 2018, 144, 690–698. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Oliveira, L.C.A.; Murad, E. Iron oxide catalysts: Fenton and Fentonlike reactions—A review. Clay Miner. 2012, 47, 285–302. [Google Scholar] [CrossRef]
- Serpone, N.; Artemev, Y.M.; Ryabchuk, V.K.; Emeline, A.V.; Horikoshi, S. Light-driven advanced oxidation processes in the disposal of emerging pharmaceutical contaminants in aqueous media: A brief review. Curr. Opin. Green Sustain. Chem. 2017, 6, 18–33. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous fenton catalytic reactivity: A review. Appl. Catal. B Environ. 2019, 255, 117739. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Han, Y.; Tan, F.; Shi, Y.; Liu, M.; Li, X. 3D mesoporous CuFe2O4 as a catalyst for photo-Fenton removal of sulfonamide antibiotics at near neutral pH. J. Colloid Interface Sci. 2018, 524, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Emídio, E.S.; Hammer, P.; Nogueira, R.F.P. Simultaneous degradation of the anticancer drugs 5-fluorouracil and cyclophosphamide using a heterogeneous photo-Fenton process based on copper-containing magnetites (Fe3−xCuxO4). Chemosphere 2020, 241, 124990. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Yuan, B.; Shen, C.; Fu, M.; Cui, H.; Sun, W. Large scale preparation of Cu-doped α-FeOOH nanoflowers and their photo-Fenton-like catalytic degradation of diclofenac sodium. Chem. Eng. J. 2016, 291, 174–183. [Google Scholar] [CrossRef]
- Solís-López, M.; Durán-Moreno, A.; Rigas, F.; Morales, A.A.; Navarrete, M.; Ramírez-Zamora, R.M. Assessment of Copper Slag as a Sustainable Fenton-Type Photocatalyst for Water Disinfection. In Water Reclamation and Sustainability; Elsevier: Amsteradm, The Netherlands, 2014; pp. 199–227. [Google Scholar] [CrossRef]
- Arzate-Salgado, S.Y.; Morales-Pérez, A.A.; Solís-López, M.; Ramírez-Zamora, R.M. Evaluation of metallurgical slag as a Fenton-type photocatalyst for the degradation of an emerging pollutant: Diclofenac. Catal. Today 2016, 266, 126–135. [Google Scholar] [CrossRef]
- Xie, Y.; Li, P.; Zeng, Y.; Li, X.; Xiao, Y.; Wang, Y.; Zhang, Y. Thermally treated fungal manganese oxides for bisphenol A degradation using sulfate radicals. Chem. Eng. J. 2018, 335, 728–736. [Google Scholar] [CrossRef]
- Du, Z.; Li, K.; Zhou, S.; Liu, X.; Yu, Y.; Zhang, Y.; He, Y.; Zhang, Y. Degradation of ofloxacin with heterogeneous photo-Fenton catalyzed by biogenic Fe-Mn oxides. Chem. Eng. J. 2020, 380, 122427. [Google Scholar] [CrossRef]
- Hurtado, L.; Romero, R.; Mendoza, A.; Brewer, S.; Donkor, K.; Gómez-Espinosa, R.M.; Natividad, R. Paracetamol mineralization by Photo Fenton process catalyzed by a Cu/Fe-PILC under circumneutral pH conditions. J. Photochem. Photobiol. A Chem. 2019, 373, 162–170. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, R.; Zhu, G.; Zhu, J.; Liang, X.; Zhu, Y.; He, H. Mechanisms for the enhanced photo-Fenton activity of ferrihydrite modified with BiVO4 at neutral pH. Appl. Catal. B Environ. 2017, 212, 50–58. [Google Scholar] [CrossRef]
- Bansal, P.; Verma, A.; Talwar, S. Detoxification of real pharmaceutical wastewater by integrating photocatalysis and photo-Fenton in fixed-mode. Chem. Eng. J. 2018, 349, 838–848. [Google Scholar] [CrossRef]
- Catalá, M.; Domínguez-Morueco, N.; Migens, A.; Molina, R.; Martínez, F.; Valcárcel, Y.; Mastroianni, N.; López de Alda, M.; Barceló, D.; Segura, Y. Elimination of drugs of abuse and their toxicity from natural waters by photo-Fenton treatment. Sci. Total Environ. 2015, 520, 198–205. [Google Scholar] [CrossRef]
- Liu, F.; Yao, H.; Sun, S.; Tao, W.; Wei, T.; Sun, P. Photo-Fenton activation mechanism and antifouling performance of an FeOCl-coated ceramic membrane. Chem. Eng. J. 2020, 402, 125477. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Yan, J.; Han, L.; Gao, W.; Xue, S.; Chen, M. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 2015, 175, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Huang, F.; Zeng, G.; Huang, D.; Qin, L.; Cheng, M.; Zhang, C.; Li, B.; Yi, H.; Liu, S.; et al. Fabrication of novel magnetic MnFe2O4/bio-char composite and heterogeneous photo-Fenton degradation of tetracycline in near neutral pH. Chemosphere 2019, 224, 910–921. [Google Scholar] [CrossRef] [PubMed]
- Bocos, E.; Oturan, N.; Pazos, M.; Sanromán, M.Á.; Oturan, M.A. Elimination of radiocontrast agent diatrizoic acid by photo-Fenton process and enhanced treatment by coupling with electro-Fenton process. Environ. Sci. Pollut. Res. 2016, 23, 19134–19144. [Google Scholar] [CrossRef]
- Guo, T.; Wang, K.; Zhang, G.; Wu, X. A novel α-Fe2O3@g-C3N4 catalyst: Synthesis derived from Fe-based MOF and its superior photo-Fenton performance. Appl. Surf. Sci. 2019, 469, 331–339. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Wielens Becker, R.; Salmoria Araújo, D.; Dallegrave, A.; Ost Fracari, T.; Lavayen, V.; Sirtori, C. Degradation of pharmaceuticals in different water matrices by a solar homo/heterogeneous photo-Fenton process over modified alginate spheres. Environ. Sci. Pollut. Res. 2019, 26, 6532–6544. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.; Couto, L.; Esplugas, S.; Sans, C. Study of the contribution of homogeneous catalysis on heterogeneous Fe(III)/alginate mediated photo-Fenton process. Chem. Eng. J. 2017, 318, 272–280. [Google Scholar] [CrossRef]
- Li, Q.; Kong, H.; Jia, R.; Shao, J.; He, Y. Enhanced catalytic degradation of amoxicillin with TiO2-Fe3O4 composites: Via a submerged magnetic separation membrane photocatalytic reactor (SMSMPR). RSC Adv. 2019, 9, 12538–12546. [Google Scholar] [CrossRef] [Green Version]
- Meijide, J.; Dunlop, P.S.M.; Pazos, M.; Sanromán, M.A. Heterogeneous electro-fenton as “Green” technology for pharmaceutical removal: A review. Catalysts 2021, 11, 85. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Zhou, M.; Martínez-Huitle, C.A. Heterogeneous electro-Fenton and photoelectro-Fenton processes: A critical review of fundamental principles and application for water/wastewater treatment. Appl. Catal. B Environ. 2018, 235, 103–129. [Google Scholar] [CrossRef]
- Barhoumi, N.; Labiadh, L.; Oturan, M.A.; Oturan, N.; Gadri, A.; Ammar, S.; Brillas, E. Electrochemical mineralization of the antibiotic levofloxacin by electro-Fenton-pyrite process. Chemosphere 2015, 141, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Droguett, C.; Salazar, R.; Brillas, E.; Sirés, I.; Carlesi, C.; Marco, J.F.; Thiam, A. Treatment of antibiotic cephalexin by heterogeneous electrochemical Fenton-based processes using chalcopyrite as sustainable catalyst. Sci. Total Environ. 2020, 740, 140154. [Google Scholar] [CrossRef] [PubMed]
- Kalantary, R.R.; Farzadkia, M.; Kermani, M.; Rahmatinia, M. Heterogeneous electro-Fenton process by Nano-Fe3O4 for catalytic degradation of amoxicillin: Process optimization using response surface methodology. J. Environ. Chem. Eng. 2018, 6, 4644–4652. [Google Scholar] [CrossRef]
- Xing, M.; Xu, W.; Dong, C.; Bai, Y.; Zeng, J.; Zhou, Y.; Zhang, J.; Yin, Y. Metal Sulfides as Excellent Co-catalysts for H2O2 Decomposition in Advanced Oxidation Processes. Chem 2018, 4, 1359–1372. [Google Scholar] [CrossRef] [Green Version]
- Chumakov, A.; Batalova, V.; Slizhov, Y. Electro-Fenton-like reactions of transition metal ions with electrogenerated hydrogen peroxide. AIP Conf. Proc. 2016, 1772, 040004. [Google Scholar] [CrossRef]
- Campos, S.; Salazar, R.; Arancibia-Miranda, N.; Rubio, M.A.; Aranda, M.; García, A.; Sepúlveda, P.; Espinoza, L.C. Nafcillin degradation by heterogeneous electro-Fenton process using Fe, Cu and Fe/Cu nanoparticles. Chemosphere 2020, 247, 125813. [Google Scholar] [CrossRef] [PubMed]
- Tabrizian, P.; Ma, W.; Bakr, A.; Rahaman, M.S. pH-sensitive and magnetically separable Fe/Cu bimetallic nanoparticles supported by graphene oxide (GO) for high-efficiency removal of tetracyclines. J. Colloid Interface Sci. 2019, 534, 549–562. [Google Scholar] [CrossRef]
- Xie, L.; Liu, X.; Chang, J.; Zhang, C.; Li, Y.; Zhang, H.; Zhan, S.; Hu, W. Enhanced redox activity and oxygen vacancies of perovskite triggered by copper incorporation for the improvement of electro-Fenton activity. Chem. Eng. J. 2022, 428, 131352. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, M.; Pan, Y.; Du, X.; Wang, Q. MoS2 as highly efficient co-catalyst enhancing the performance of Fe0 based electro-Fenton process in degradation of sulfamethazine: Approach and mechanism. Chem. Eng. J. 2021, 403, 126361. [Google Scholar] [CrossRef]
- Rosales, E.; Diaz, S.; Pazos, M.; Sanromán, M.A. Comprehensive strategy for the degradation of anti-inflammatory drug diclofenac by different advanced oxidation processes. Sep. Purif. Technol. 2019, 208, 130–141. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Fourcade, F.; Assadi, A.; Soutrel, I.; Adhoum, N.; Amrane, A.; Monser, L. Effective Heterogeneous Electro-Fenton Process for The Degradation of a Malodorous Compound, Indole, Using Iron Loaded Alginate Beads as A Reusable Catalyst; Elsevier: Amsterdam, The Netherlands, 2016; Volume 182, ISBN 2167170432. [Google Scholar]
- Yang, W.; Zhou, M.; Cai, J.; Liang, L.; Ren, G.; Jiang, L. Ultrahigh yield of hydrogen peroxide on graphite felt cathode modified with electrochemically exfoliated graphene. J. Mater. Chem. A 2017, 5, 8070–8080. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Acevedo-García, V.; Pazos, M.; Sanromán, M.Á.; Rosales, E. Iron-doped cathodes for electro-Fenton implementation: Application for pymetrozine degradation. Electrochim. Acta 2020, 338, 135768. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Huong Le, T.X.; Bechelany, M.; Esposito, G.; Van Hullebusch, E.D.; Oturan, M.A.; Cretin, M. A hierarchical CoFe-layered double hydroxide modified carbon-felt cathode for heterogeneous electro-Fenton process. J. Mater. Chem. A 2017, 5, 3655–3666. [Google Scholar] [CrossRef]
- Ghasemi, M.; Khataee, A.; Gholami, P.; Soltani, R.D.C.; Hassani, A.; Orooji, Y. In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Mi, X.; Zhan, S.; Hu, W. Evaluation of ciprofloxacin destruction between ordered mesoporous and bulk NiMn2O4/CF cathode: Efficient mineralization in a heterogeneous electro-Fenton-like process. Environ. Sci. Nano 2019, 6, 661–671. [Google Scholar] [CrossRef]
- Hu, X.; Deng, Y.; Zhou, J.; Liu, B.; Yang, A.; Jin, T.; Fai Tsang, Y. N- and O self-doped biomass porous carbon cathode in an electro-Fenton system for Chloramphenicol degradation. Sep. Purif. Technol. 2020, 251, 117376. [Google Scholar] [CrossRef]
- Divyapriya, G.; Nambi, I.; Senthilnathan, J. Ferrocene functionalized graphene based electrode for the electro–Fenton oxidation of ciprofloxacin. Chemosphere 2018, 209, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Skoumal, M.; Rodríguez, R.M.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Arias, C.; Brillas, E. Electro-Fenton, UVA photoelectro-Fenton and solar photoelectro-Fenton degradation of the drug ibuprofen in acid aqueous medium using platinum and boron-doped diamond anodes. Electrochim. Acta 2009, 54, 2077–2085. [Google Scholar] [CrossRef]
- Thiam, A.; Salazar, R.; Brillas, E.; Sirés, I. In-situ dosage of Fe2+ catalyst using natural pyrite for thiamphenicol mineralization by photoelectro-Fenton process. J. Environ. Manag. 2020, 270, 110835. [Google Scholar] [CrossRef]
- Ye, Z.; Schukraft, G.E.M.; L’Hermitte, A.; Xiong, Y.; Brillas, E.; Petit, C.; Sirés, I. Mechanism and stability of an Fe-based 2D MOF during the photoelectro-Fenton treatment of organic micropollutants under UVA and visible light irradiation. Water Res. 2020, 184, 115986. [Google Scholar] [CrossRef]
- Moradi, M.; Elahinia, A.; Vasseghian, Y.; Dragoi, E.N.; Omidi, F.; Mousavi Khaneghah, A. A review on pollutants removal by Sono-photo -Fenton processes. J. Environ. Chem. Eng. 2020, 8, 104330. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, G.; Huang, M.; Lin, K.; Yi, Y.; Fang, Z.; Li, P.; Wang, K. Enhanced degradation of metronidazole by heterogeneous sono-Fenton reaction coupled ultrasound using Fe3O4 magnetic nanoparticles. Environ. Technol. 2017, 3330, 1–22. [Google Scholar] [CrossRef]
- Abdili, T.; Rahmani, A.; Rahmani, H.; Alighadri, M.; Rahmani, K. Heterogeneous oxidation of sulfacetamide in aquatic environment using ultrasonic and nano-fenton: Kinetics intermediates and bioassay test. Desalination Water Treat. 2019, 166, 158–167. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Royer, S.; Zhang, H. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst. J. Hazard. Mater. 2016, 302, 458–467. [Google Scholar] [CrossRef]
- Dükkancı, M. Sono-photo-Fenton oxidation of bisphenol-A over a LaFeO3 perovskite catalyst. Ultrason. Sonochem. 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, X.; Han, J.; Gong, H.; Meng, L.; Mei, X.; Sun, Y.; Qi, L.; Gan, L. Degradation of emerging contaminants by sono-Fenton process with in situ generated H2O2 and the improvement by P25-mediated visible light irradiation. J. Hazard. Mater. 2020, 391, 122229. [Google Scholar] [CrossRef] [PubMed]
- Oturan, M.A.; Sirés, I.; Oturan, N.; Pérocheau, S.; Laborde, J.L.; Trévin, S. Sonoelectro-Fenton process: A novel hybrid technique for the destruction of organic pollutants in water. J. Electroanal. Chem. 2008, 624, 329–332. [Google Scholar] [CrossRef]
- Ghanbari, F.; Hassani, A.; Wacławek, S.; Wang, Z.; Matyszczak, G.; Lin, K.Y.A.; Dolatabadi, M. Insights into paracetamol degradation in aqueous solutions by ultrasound-assisted heterogeneous electro-Fenton process: Key operating parameters, mineralization and toxicity assessment. Sep. Purif. Technol. 2021, 266, 118533. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Can the photocatalyst TiO2 be incorporated into a wastewater treatment method? Background and prospects. Catal. Today 2020, 340, 334–346. [Google Scholar] [CrossRef]
- Cruz-Ortiz, B.R.; Hamilton, J.W.J.; Pablos, C.; Díaz-Jiménez, L.; Cortés-Hernández, D.A.; Sharma, P.K.; Castro-Alférez, M.; Fernández-Ibañez, P.; Dunlop, P.S.M.; Byrne, J.A. Mechanism of photocatalytic disinfection using titania-graphene composites under UV and visible irradiation. Chem. Eng. J. 2017, 316, 179–186. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, J.C.; Luque, R. Heterogeneous photocatalytic nanomaterials: Prospects and challenges in selective transformations of biomass-derived compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Aware, D.V.; Jadhav, S.S. Synthesis, characterization and photocatalytic applications of Zn-doped TiO2 nanoparticles by sol–gel method. Appl. Nanosci. 2016, 6, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review; Elsevier: Amsterdam, The Netherlands, 2016; Volume 88, ISBN 6037967695. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Escudero, C.J.; Iglesias, O.; Dominguez, S.; Rivero, M.J.; Ortiz, I. Performance of electrochemical oxidation and photocatalysis in terms of kinetics and energy consumption. New insights into the p-cresol degradation. J. Environ. Manag. 2017, 195, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rosman, N.; Salleh, W.N.W.; Mohamed, M.A.; Jaafar, J.; Ismail, A.F.; Harun, Z. Hybrid membrane filtration-advanced oxidation processes for removal of pharmaceutical residue. J. Colloid Interface Sci. 2018, 532, 236–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Ravi Anusuyadevi, P.; Aymonier, C.; Luque, R.; Marre, S. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 2019, 48, 3868–3902. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, T.; Mul, G.; Moulijn, J.; Stankiewicz, A. A review of intensification of photocatalytic processes. Chem. Eng. Process. Process Intensif. 2007, 46, 781–789. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 surface: Principles, mechanisms, and selected results. Chem. Rev 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ramezani Motlagh, H.; Jaafarzadeh, N.; Mostoufi, A.; Saeedi, R.; Barzegar, G.; Jorfi, S. Enhanced photocatalytic degradation of tetracycline and real pharmaceutical wastewater using MWCNT/TiO2 nano-composite. J. Environ. Manag. 2017, 186, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.M.; Macedo, G.; Pedrosa, M.; Becerra-Castro, C.; Castro-Silva, S.; Pereira, M.F.R.; Silva, A.M.T.; Nunes, O.C.; Manaia, C.M. Ozonation and UV254nm radiation for the removal of microorganisms and antibiotic resistance genes from urban wastewater. J. Hazard. Mater. 2017, 323, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Miralles-Cuevas, S.; Oller, I.; Agüera, A.; Sánchez Pérez, J.A.; Malato, S. Strategies for reducing cost by using solar photo-Fenton treatment combined with nanofiltration to remove microcontaminants in real municipal effluents: Toxicity and economic assessment. Chem. Eng. J. 2017, 318, 161–170. [Google Scholar] [CrossRef]
- Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, O.; Rivero, M.J.; Urtiaga, A.M.; Ortiz, I. Membrane-based photocatalytic systems for process intensification. Chem. Eng. J. 2016, 305, 136–148. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Ye, J.; Sayama, K.; Arakawa, H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Mater. Sustain. Energy A Collect. Peer-Rev. Res. Rev. Artic. Nat. Publ. Gr. 2001, 704, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Amini, A.; Zhu, C.; Xu, Z.; Song, H.; Wang, N. Enhanced photocatalytic performance of TiO2-ZnO hybrid nanostructures. Sci. Rep. 2014, 4, 4181. [Google Scholar] [CrossRef] [PubMed]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; Del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO-TiO2composites. Bol. Soc. Esp. Ceram. Vidr. 2016, 55, 55–62. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Yu, J.; Jiang, C. Principle and Surface Science of Photocatalysis; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, ISBN 9780081028902. [Google Scholar]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, R.M.; Martins, R.C. N-TiO2 photocatalysts: A review of their characteristics and capacity for emerging contaminants removal. Water 2019, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Naldoni, A.; Altomare, M.; Zoppellaro, G.; Liu, N.; Kment, Š.; Zbořil, R.; Schmuki, P. Photocatalysis with reduced TiO2: From black TiO2 to cocatalyst-free hydrogen production. ACS Catal. 2019, 9, 345–364. [Google Scholar] [CrossRef] [Green Version]
- Lettieri, S.; Pavone, M.; Fioravanti, A.; Amato, L.S.; Maddalena, P. Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: A review. Materials 2021, 14, 1645. [Google Scholar] [CrossRef] [PubMed]
- Huo, P.; Tang, Y.; Zhou, M.; Li, J.; Ye, Z.; Ma, C.; Yu, L.; Yan, Y. Fabrication of ZnWO4-CdS heterostructure photocatalysts for visible light induced degradation of ciprofloxacin antibiotics. J. Ind. Eng. Chem. 2016, 37, 340–346. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Ma, C.; Guan, Q.; Lu, Z.; Huo, P.; Yan, Y. Melamine modified P25 with heating method and enhanced the photocatalytic activity on degradation of ciprofloxacin. Appl. Surf. Sci. 2015, 329, 17–22. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W. Precisely controlled fabrication of magnetic 3D γ-Fe2O3@ZnO core-shell photocatalyst with enhanced activity: Ciprofloxacin degradation and mechanism insight. Chem. Eng. J. 2017, 308, 377–385. [Google Scholar] [CrossRef]
- Tahir, M.B.; Sagir, M.; Shahzad, K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2019, 363, 205–213. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-doped titanium dioxide as visible-light-sensitive photocatalyst: Designs, developments, and prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Chavan, V.B.; Kulkarni, P.S.; Kulkarni, B.D.; Kamble, S.P. Photocatalytic Degradation of Pharmaceuticals Pollutants Using N-Doped TiO2 Photocatalyst: Identification of CFX Degradation Intermediates. Indian Chem. Eng. 2016, 59, 177–199. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; Fang, L.; Lo, I.M.C. Visible-light-driven N-TiO2@SiO2@Fe3O4 magnetic nanophotocatalysts: Synthesis, characterization, and photocatalytic degradation of PPCPs. J. Hazard. Mater. 2019, 370, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Bilgin Simsek, E. Solvothermal synthesized boron doped TiO2 catalysts: Photocatalytic degradation of endocrine disrupting compounds and pharmaceuticals under visible light irradiation. Appl. Catal. B Environ. 2017, 200, 309–322. [Google Scholar] [CrossRef]
- Pan, Y.; Yuan, X.; Jiang, L.; Wang, H.; Yu, H.; Zhang, J. Stable self-assembly AgI/UiO-66(NH2) heterojunction as efficient visible-light responsive photocatalyst for tetracycline degradation and mechanism insight. Chem. Eng. J. 2020, 384, 123310. [Google Scholar] [CrossRef]
- Yuan, C.; Hung, C.H.; Li, H.W.; Chang, W.H. Photodegradation of ibuprofen by TiO2 co-doping with urea and functionalized CNT irradiated with visible light—Effect of doping content and pH. Chemosphere 2016, 155, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Surenjan, A.; Sambandam, B.; Pradeep, T.; Philip, L. Synthesis, characterization and performance of visible light active C-TiO2 for pharmaceutical photodegradation. J. Environ. Chem. Eng. 2017, 5, 757–767. [Google Scholar] [CrossRef]
- Darwish, M.; Mohammadi, A.; Assi, N. Integration of nickel doping with loading on graphene for enhanced adsorptive and catalytic properties of CdS nanoparticles towards visible light degradation of some antibiotics. J. Hazard. Mater. 2016, 320, 304–314. [Google Scholar] [CrossRef]
- Ma, R.; Wang, X.; Huang, J.; Song, J.; Zhang, J.; Wang, X. Photocatalytic degradation of salicylic acid with magnetic activated carbon-supported F-N codoped TiO2 under visible light. Vacuum 2017, 141, 157–165. [Google Scholar] [CrossRef]
- Farhadian, N.; Akbarzadeh, R.; Pirsaheb, M.; Jen, T.C.; Fakhri, Y.; Asadi, A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019, 132, 360–373. [Google Scholar] [CrossRef]
- Malesic-Eleftheriadou, N.; Evgenidou, E.; Kyzas, G.Z.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of antibiotics in aqueous media by using new synthesized bio-based poly(ethylene terephthalate)-TiO2 photocatalysts. Chemosphere 2019, 234, 746–755. [Google Scholar] [CrossRef]
- Sandoval, C.; Ranganathan, S.; Ramírez, E.; Mansilla, H.D.; Dinamarca, R.; Pecchi, G.; Yáñez, J. Visible light assisted photodegradation of thimerosal by high performance ZnFe2O4/poly(o-phenylenediamine) composite. Mater. Res. Bull. 2019, 116, 8–15. [Google Scholar] [CrossRef]
- Belhouchet, N.; Hamdi, B.; Chenchouni, H.; Bessekhouad, Y. Photocatalytic degradation of tetracycline antibiotic using new calcite/titania nanocomposites. J. Photochem. Photobiol. A Chem. 2019, 372, 196–205. [Google Scholar] [CrossRef]
- Akkari, M.; Aranda, P.; Belver, C.; Bedia, J.; Ben Haj Amara, A.; Ruiz-Hitzky, E. ZnO/sepiolite heterostructured materials for solar photocatalytic degradation of pharmaceuticals in wastewater. Appl. Clay Sci. 2018, 156, 104–109. [Google Scholar] [CrossRef]
- Durán-Álvarez, J.C.; Avella, E.; Ramírez-Zamora, R.M.; Zanella, R. Photocatalytic degradation of ciprofloxacin using mono- (Au, Ag and Cu) and bi- (Au-Ag and Au-Cu) metallic nanoparticles supported on TiO2 under UV-C and simulated sunlight. Catal. Today 2016, 266, 175–187. [Google Scholar] [CrossRef]
- He, L.; Dong, Y.; Zheng, Y.; Jia, Q.; Shan, S.; Zhang, Y. A novel magnetic MIL-101(Fe)/TiO2 composite for photo degradation of tetracycline under solar light. J. Hazard. Mater. 2019, 361, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Al Abri, R.; Al Marzouqi, F.; Kuvarega, A.T.; Meetani, M.A.; Al Kindy, S.M.Z.; Karthikeyan, S.; Kim, Y.; Selvaraj, R. Nanostructured cerium-doped ZnO for photocatalytic degradation of pharmaceuticals in aqueous solution. J. Photochem. Photobiol. A Chem. 2019, 384, 112065. [Google Scholar] [CrossRef]
- Elhalil, A.; Elmoubarki, R.; Farnane, M.; Machrouhi, A.; Sadiq, M.; Mahjoubi, F.Z.; Qourzal, S.; Barka, N. Photocatalytic degradation of caffeine as a model pharmaceutical pollutant on Mg doped ZnO-Al2O3 heterostructure. Environ. Nanotechnol. Monit. Manag. 2018, 10, 63–72. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Van Hullebusch, E.D.; Cretin, M.; Esposito, G.; Oturan, M.A. Coupling of membrane filtration and advanced oxidation processes for removal of pharmaceutical residues: A critical review. Sep. Purif. Technol. 2015, 156, 891–914. [Google Scholar] [CrossRef]
- Shi, L.; Yin, Y.; Zhang, L.C.; Wang, S.; Sillanpää, M.; Sun, H. Design and engineering heterojunctions for the photoelectrochemical monitoring of environmental pollutants: A review. Appl. Catal. B Environ. 2019, 248, 405–422. [Google Scholar] [CrossRef]
- Espíndola, J.C.; Cristóvão, R.O.; Mendes, A.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic membrane reactor performance towards oxytetracycline removal from synthetic and real matrices: Suspended vs. immobilized TiO2-P25. Chem. Eng. J. 2019, 378, 122114. [Google Scholar] [CrossRef]
- Chakraborty, S.; Loutatidou, S.; Palmisano, G.; Kujawa, J.; Mavukkandy, M.O.; Al-Gharabli, S.; Curcio, E.; Arafat, H.A. Photocatalytic hollow fiber membranes for the degradation of pharmaceutical compounds in wastewater. J. Environ. Chem. Eng. 2017, 5, 5014–5024. [Google Scholar] [CrossRef]
- Dzinun, H.; Othman, M.H.D.; Ismail, A.F. Photocatalytic performance of TiO2/Clinoptilolite: Comparison study in suspension and hybrid photocatalytic membrane reactor. Chemosphere 2019, 228, 241–248. [Google Scholar] [CrossRef]
- Rahimpour, A.; Jahanshahi, M.; Rajaeian, B.; Rahimnejad, M. TiO2 entrapped nano-composite PVDF/SPES membranes: Preparation, characterization, antifouling and antibacterial properties. Desalination 2011, 278, 343–353. [Google Scholar] [CrossRef]
- Méricq, J.P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.W.N.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.M.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2 nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Paredes, L.; Murgolo, S.; Dzinun, H.; Dzarfan Othman, M.H.; Ismail, A.F.; Carballa, M.; Mascolo, G. Application of immobilized TiO2 on PVDF dual layer hollow fibre membrane to improve the photocatalytic removal of pharmaceuticals in different water matrices. Appl. Catal. B Environ. 2019, 240, 9–18. [Google Scholar] [CrossRef]
- Koe, W.S.; Chong, W.C.; Pang, Y.L.; Koo, C.H.; Ebrahim, M.; Mohammad, A.W. Novel nitrogen and sulphur co-doped carbon quantum dots/titanium oxide photocatalytic membrane for in-situ degradation and removal of pharmaceutical compound. J. Water Process Eng. 2020, 33, 101068. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.S.K.; Purkait, M.K. Cu2O photocatalyst modified antifouling polysulfone mixed matrix membrane for ultrafiltration of protein and visible light driven photocatalytic pharmaceutical removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Zarei, E.; Ojani, R. Fundamentals and some applications of photoelectrocatalysis and effective factors on its efficiency: A review. J. Solid State Electrochem. 2017, 21, 305–336. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Antoniadou, M.; Daskalaki, V.M.; Balis, N.; Kondarides, D.I.; Kordulis, C.; Lianos, P. Photocatalysis and photoelectrocatalysis using (CdS-ZnS)/TiO2 combined photocatalysts. Appl. Catal. B Environ. 2011, 107, 188–196. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Carnevale Miino, M.; Arab, H.; Bestetti, M.; Franz, S. Decolorization and biodegradability of a real pharmaceutical wastewater treated by H2O2-assisted photoelectrocatalysis on TiO2 meshes. J. Hazard. Mater. 2020, 387, 121668. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Two dimensional graphitic materials for photoelectrocatalysis: A short review. Catal. Today 2018, 315, 2–8. [Google Scholar] [CrossRef]
- Ensaldo-Rentería, M.K.; Ramírez-Robledo, G.; Sandoval-González, A.; Pineda-Arellano, C.A.; Álvarez-Gallegos, A.A.; Zamudio-Lara, Á.; Silva-Martínez, S. Photoelectrocatalytic oxidation of acid green 50 dye in aqueous solution using Ti/TiO2-NT electrode. J. Environ. Chem. Eng. 2018, 6, 1182–1188. [Google Scholar] [CrossRef]
- Papagiannis, I.; Koutsikou, G.; Frontistis, Z.; Konstantinou, I.; Avgouropoulos, G.; Mantzavinos, D.; Lianos, P. Photoelectrocatalytic vs. photocatalytic degradation of organic water born pollutants. Catalysts 2018, 8, 455. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhou, S.; Feng, X. Optimization of the photoelectrocatalytic oxidation of landfill leachate using copper and nitrate co-doped TiO2 (Ti) by response surface methodology. PLoS ONE 2017, 12, e0171234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.F.; Wang, G.B.; Kuo, D.T.F.; Chang, M.L.; Shih, Y. hsin Photoelectrocatalytic degradation of the antibiotic sulfamethoxazole using TiO2/Ti photoanode. Appl. Catal. B Environ. 2016, 186, 184–192. [Google Scholar] [CrossRef]
- Mazierski, P.; Fiszka, A.; Wilczewska, P.; Białk-Bielinska, A.; Zaleska-Medynska, A.; Siedlecka, E.; Pieczynska, A. Removal of 5- fl uorouracil by solar-driven photoelectrocatalytic oxidation using Ti/TiO2(NT) photoelectrodes. Water Res. 2019, 157, 610–620. [Google Scholar] [CrossRef]
- Kushwaha, H.S.; Madhar, N.A.; Ilahi, B.; Thomas, P.; Halder, A.; Vaish, R. Efficient Solar Energy Conversion Using CaCu3Ti4O12 Photoanode for Photocatalysis and Photoelectrocatalysis. Sci. Rep. 2016, 6, 18557. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Li, J.; Bai, J.; Li, L.; Chen, S.; Zhou, B. Bivo4 photoanode with exposed (040) facets for enhanced photoelectrochemical performance. Nano-Micro Lett. 2018, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Orimolade, B.O.; Koiki, B.A.; Peleyeju, G.M.; Arotiba, O.A. Visible light driven photoelectrocatalysis on a FTO/BiVO4/BiOI anode for water treatment involving emerging pharmaceutical pollutants. Electrochim. Acta 2019, 307, 285–292. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Jiang, C.; Chen, Y.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Wiesner, M.R. Hydrophilic and strengthened 3D reduced graphene oxide/nano-Fe3O4 hybrid hydrogel for enhanced adsorption and catalytic oxidation of typical pharmaceuticals. Environ. Sci. Nano 2018, 5, 1650–1660. [Google Scholar] [CrossRef]
- Duan, X.; O’Donnell, K.; Sun, H.; Wang, Y.; Wang, S. Sulfur and Nitrogen Co-Doped Graphene for Metal-Free Catalytic Oxidation Reactions. Small 2015, 11, 3036–3044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Wang, Y.; Liu, S.; Ge, L.; Wang, L.; Zhu, Z.; Wang, S. Facile synthesis of nitrogen doped reduced graphene oxide as a superior metal-free catalyst for oxidation. Chem. Commun. 2013, 49, 9914–9916. [Google Scholar] [CrossRef]
- Peng, G.; Zhang, M.; Deng, S.; Shan, D.; He, Q.; Yu, G. Adsorption and catalytic oxidation of pharmaceuticals by nitrogen-doped reduced graphene oxide/Fe3O4 nanocomposite. Chem. Eng. J. 2018, 341, 361–370. [Google Scholar] [CrossRef]
- Gao, Y.; Li, S.; Li, Y.; Yao, L.; Zhang, H. Accelerated photocatalytic degradation of organic pollutant over metal-organic framework MIL-53(Fe) under visible LED light mediated by persulfate. Appl. Catal. B Environ. 2017, 202, 165–174. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Zhang, J.; Xie, S.; Wang, X.; Ji, Z. Honeycomb-like micro-mesoporous structure TiO2/sepiolite composite for combined chemisorption and photocatalytic elimination of formaldehyde. Microporous Mesoporous Mater. 2017, 248, 234–245. [Google Scholar] [CrossRef]
- Dai, L.; Xue, Y.; Qu, L.; Choi, H.J.; Baek, J.B. Metal-Free Catalysts for Oxygen Reduction Reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, Y.P.; Su, M.; Yuan, Z.Y. Metal-Free Carbonaceous Materials as Promising Heterogeneous Catalysts. ChemCatChem 2015, 7, 2765–2787. [Google Scholar] [CrossRef]
- Wang, J.; Chen, S.; Quan, X.; Yu, H. Fluorine-doped carbon nanotubes as an efficient metal-free catalyst for destruction of organic pollutants in catalytic ozonation. Chemosphere 2018, 190, 135–143. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Dai, Z.; Li, D.; Ao, Z.; Wang, S.; An, T. Theoretical exploration of VOCs removal mechanism by carbon nanotubes through persulfate-based advanced oxidation processes: Adsorption and catalytic oxidation. J. Hazard. Mater. 2021, 405, 124684. [Google Scholar] [CrossRef] [PubMed]
- Reshmy, R.; Philip, E.; Sirohi, R.; Tarafdar, A.; Arun, K.B.; Madhavan, A.; Binod, P.; Kumar Awasthi, M.; Varjani, S.; Szakacs, G.; et al. Nanobiocatalysts: Advancements and applications in enzyme technology. Bioresour. Technol. 2021, 337, 125491. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, G.; Sreeram, K.J. Nano-biocatalyst: Bi-functionalization of protease and amylase on copper oxide nanoparticles. Colloids Surfaces B Biointerfaces 2021, 197, 111386. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Suresh, R.; Rajendran, S.; Kumar, P.S.; Dutta, K.; Vo, D.V.N. Current advances in microbial fuel cell technology toward removal of organic contaminants—A review. Chemosphere 2022, 287, 132186. [Google Scholar] [CrossRef] [PubMed]
- Peera, S.G.; Maiyalagan, T.; Liu, C.; Ashmath, S.; Lee, T.G.; Jiang, Z.; Mao, S. A review on carbon and non-precious metal based cathode catalysts in microbial fuel cells. Int. J. Hydrogen Energy 2021, 46, 3056–3089. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Liang, P.; Zhao, X.; Liang, M.; Zhou, B. Combined photoelectrocatalytic microbial fuel cell (PEC-MFC) degradation of refractory organic pollutants and in-situ electricity utilization. Chemosphere 2019, 214, 669–678. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, Z.; Huang, L.; Pan, Y.; Quan, X.; Li Puma, G. Intensified degradation and mineralization of antibiotic metronidazole in photo-assisted microbial fuel cells with Mo-W catalytic cathodes under anaerobic or aerobic conditions in the presence of Fe(III). Chem. Eng. J. 2019, 376, 119566. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, P.; Caroli, S.; Caracciolo, A.B. Pharmaceuticals as priority water contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- WHO. Public Health Governance and Regulation of Drinking—Water and Sanitation Services; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Ioannou-Ttofa, L.; Raj, S.; Prakash, H.; Fatta-Kassinos, D. Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem. Eng. J. 2019, 355, 91–102. [Google Scholar] [CrossRef]
- Karaolia, P.; Michael-Kordatou, I.; Hapeshi, E.; Drosou, C.; Bertakis, Y.; Christofilos, D.; Armatas, G.S.; Sygellou, L.; Schwartz, T.; Xekoukoulotakis, N.P.; et al. Removal of antibiotics, antibiotic-resistant bacteria and their associated genes by graphene-based TiO2 composite photocatalysts under solar radiation in urban wastewaters. Appl. Catal. B Environ. 2018, 224, 810–824. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs. new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Agovino, T.; Nahim-Granados, S.; Castro-Alférez, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Tertiary treatment of urban wastewater by solar and UV-C driven advanced oxidation with peracetic acid: Effect on contaminants of emerging concern and antibiotic resistance. Water Res. 2019, 149, 272–281. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).