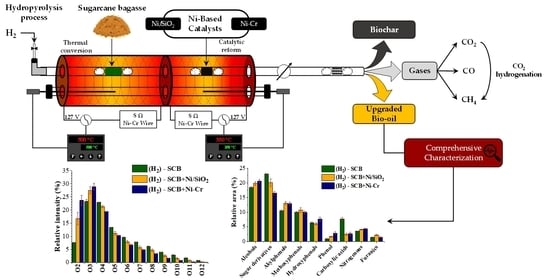
Thermal Conversion of Sugarcane Bagasse Coupled with Vapor Phase Hydrotreatment over Nickel-Based Catalysts: A Comprehensive Characterization of Upgraded Products
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of the Processes on Product Distribution and Elemental Composition of Biochar
2.2. Effect of the Processes upon the Composition of the Gaseous Products
2.3. Influence of the Processes on the Composition of the Liquid Products
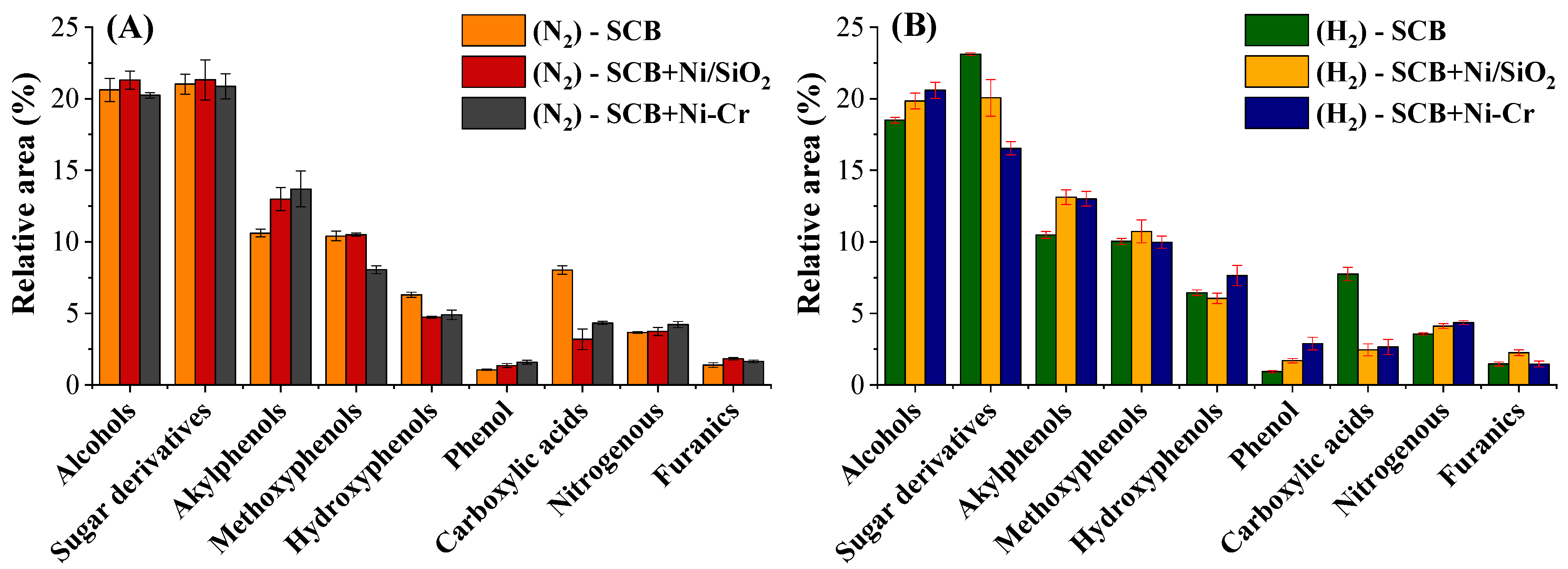
2.3.1. Chemical Characterization of Volatilizable Compounds by GC/MS
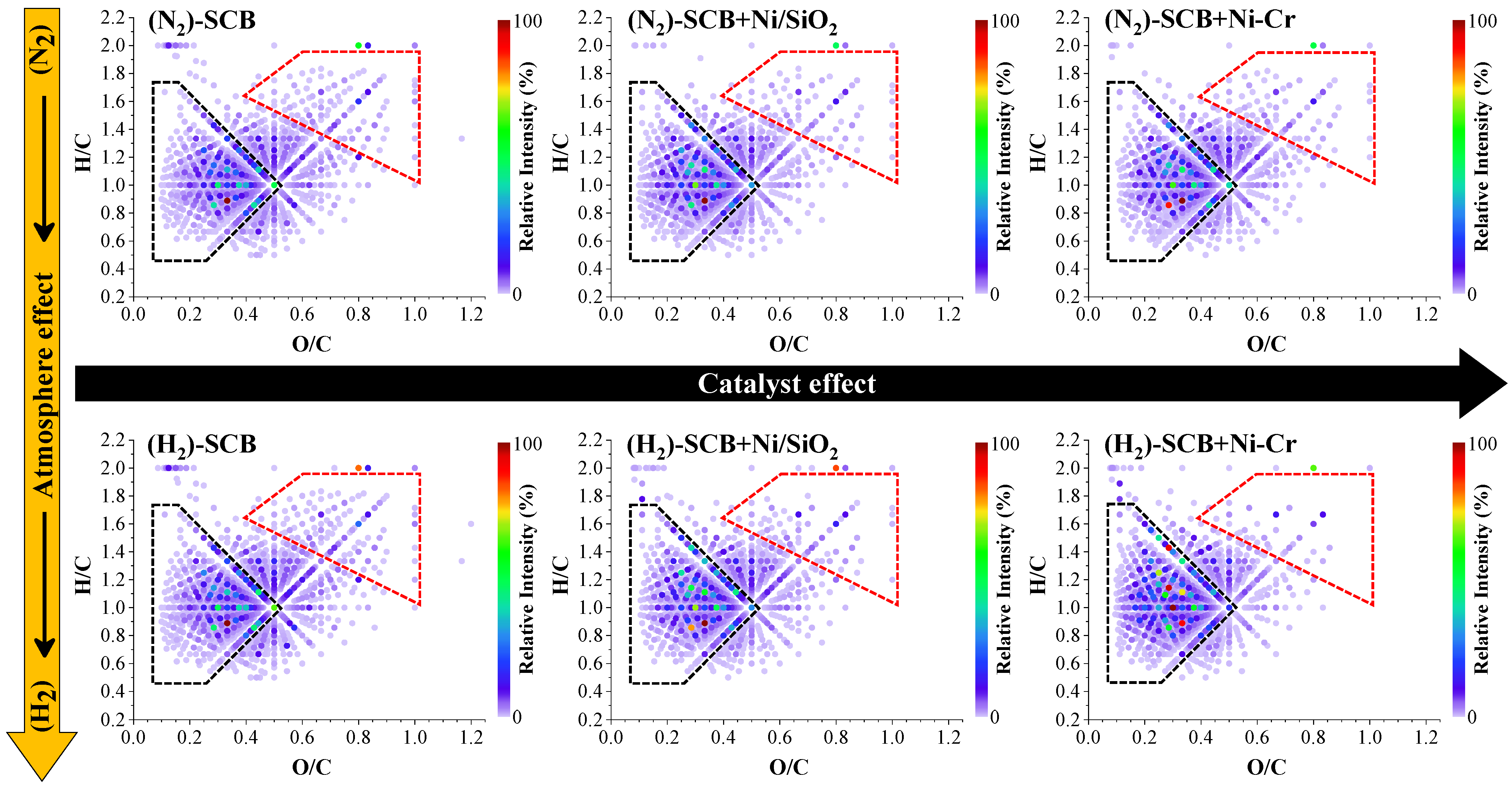
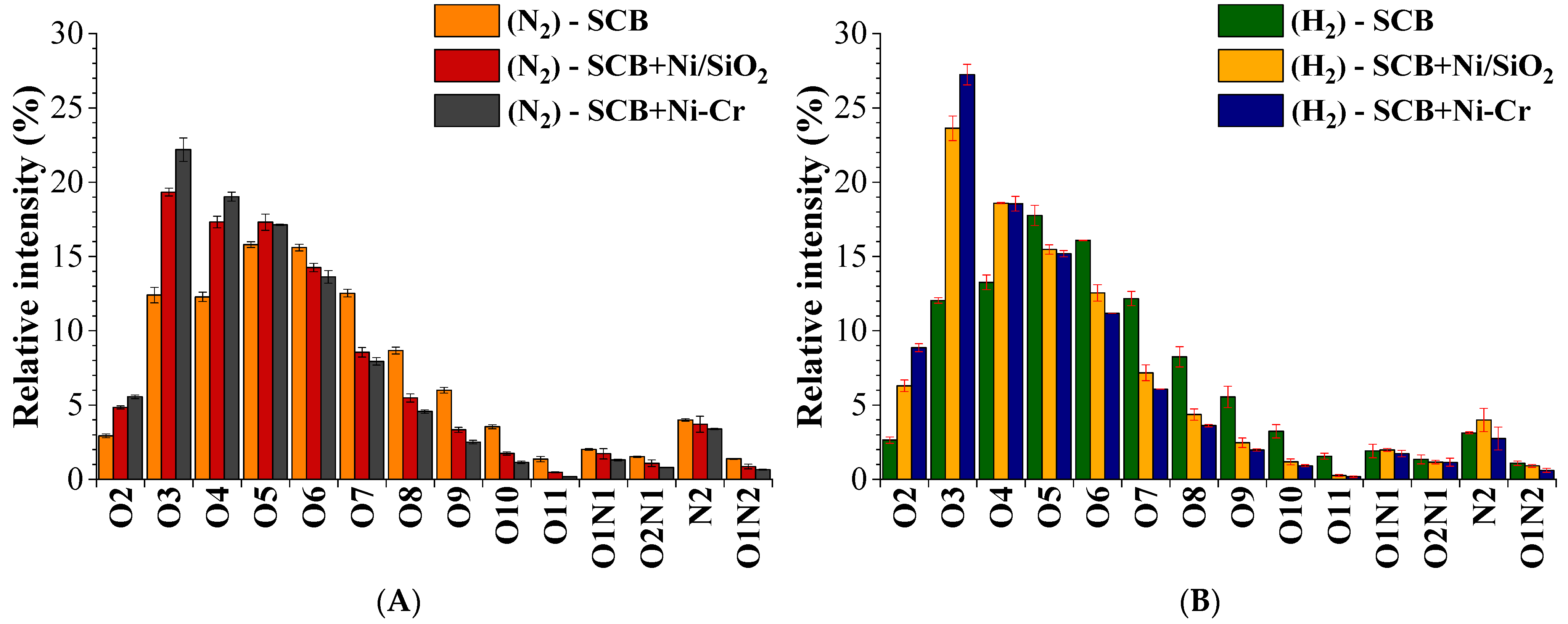
2.3.2. Molecular Characterization of Polar Compounds by UHRMS
Negative-Mode (HESI(−)-FT-Orbitrap MS)
Positive-Mode (HESI(+)-FT-Orbitrap MS)
3. Materials and Methods
3.1. Materials
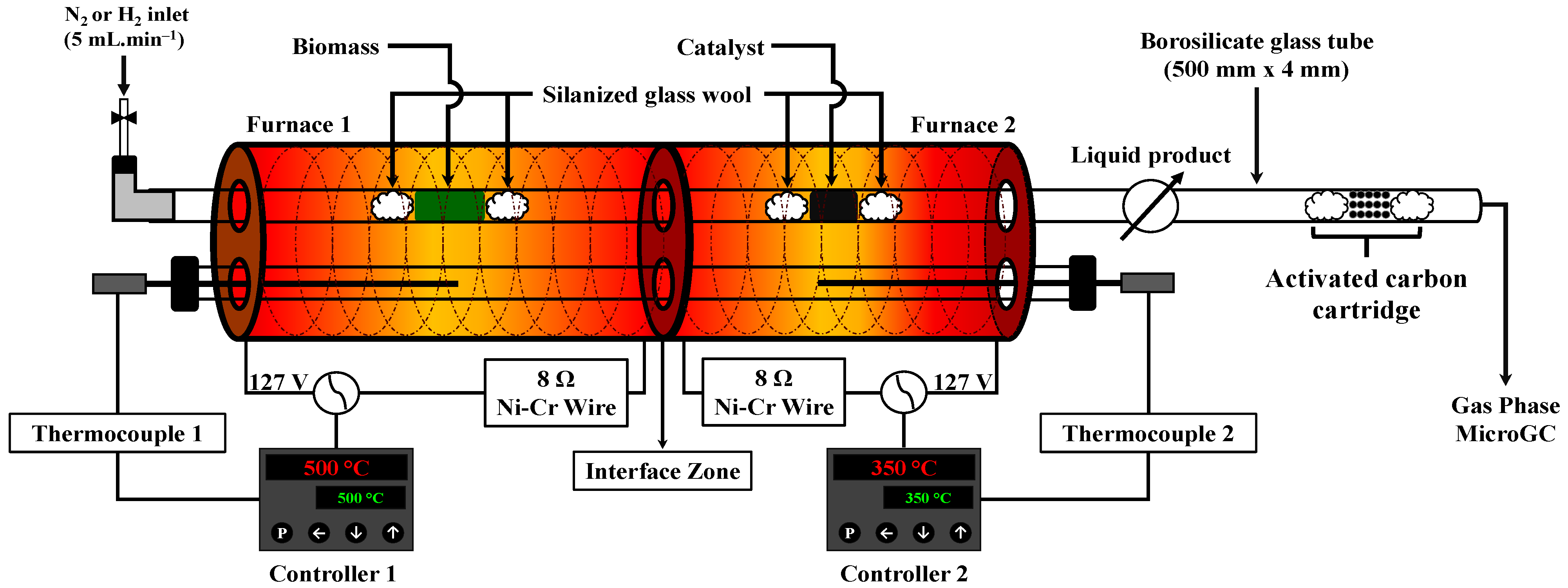
3.2. Experimental Procedure
3.2.1. Micro-Scale Non-Catalytic and Hydropyrolysis Experiments
3.2.2. Gravimetric Yields
3.3. Analytical Methods
3.3.1. Biochar Characterization
3.3.2. Characterization of the Gaseous Products by μGC-TCD
3.3.3. Characterization of the Liquid Products by Gas Chromatographic/Mass Spectrometry (GC/MS)
3.3.4. Characterization of the Liquid Products by Heated Electrospray Ionization Coupled to a Fourier Transform Orbitrap Mass Spectrometer (HESI(±)-FT-Orbitrap MS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, P.; Zheng, Y.; Liang, X.; Jia, Z.; Wang, X.; Guo, Y.; Ren, L. Pyrolysis of sugarcane bagasse for bio-chemicals production catalyzed by micro-mesoporous composite molecular sieves. Chem. Pap. 2021, 75, 3283–3293. [Google Scholar] [CrossRef]
- Nunes, V.O.; Fraga, A.C.; Silva, R.V.S.; Pontes, N.S.; Pinho, A.R.; Sousa-Aguiar, E.F.; Azevedo, D.A. Chemical characterisation of sugarcane bagasse bio-oils from hydrothermal liquefaction: Effect of reaction conditions on products distribution and composition. J. Environ. Chem. Eng. 2021, 9, 106513. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- CONAB-Companhia Nacional de Abastecimento Acompanhamento da Safra Brasileira de Cana-de-Açúcar. Available online: https://www.conab.gov.br/component/k2/item/download/38841_c46487b7985626b6b41f7083ce9336c5 (accessed on 27 December 2021).
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Ordonez-Loza, J.; Chejne, F.; Jameel, A.G.A.; Telalovic, S.; Arrieta, A.A.; Sarathy, S.M. An investigation into the pyrolysis and oxidation of bio-oil from sugarcane bagasse: Kinetics and evolved gases using TGA-FTIR. J. Environ. Chem. Eng. 2021, 9, 106144. [Google Scholar] [CrossRef]
- Schmitt, C.C.; Moreira, R.; Neves, R.C.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Biomass waste conversion into value-added products via microwave-assisted Co-Pyrolysis platform. Renew. Energy 2021, 170, 400–409. [Google Scholar] [CrossRef]
- Shirazi, Y.; Viamajala, S.; Varanasi, S. In situ and Ex situ Catalytic Pyrolysis of Microalgae and Integration with Pyrolytic Fractionation. Front. Chem. 2020, 8, 786. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Yang, Q.; Ben, H.; Seufitelli, G.V.S.; Resende, F.L.P. Parametric study of catalytic hydropyrolysis of rice husk over a hierarchical micro-mesoporous composite catalyst for production of light alkanes, alkenes, and liquid aromatic hydrocarbons. Fuel 2022, 310, 122457. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Z.; Wang, X.; Guo, H.; Cui, M.; Yang, Y. Catalytic Fast Pyrolysis of Biomass Impregnated with Potassium Phosphate in a Hydrogen Atmosphere for the Production of Phenol and Activated Carbon. Front. Chem. 2018, 6, 32. [Google Scholar] [CrossRef]
- Dyer, A.C.; Nahil, M.A.; Williams, P.T. Catalytic co-pyrolysis of biomass and waste plastics as a route to upgraded bio-oil. J. Energy Inst. 2021, 97, 27–36. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Høj, M.; Gabrielsen, J.; Clausen, L.R.; Jensen, P.A.; Jensen, A.D. A perspective on catalytic hydropyrolysis of biomass. Renew. Sustain. Energy Rev. 2021, 143, 110960. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total Environ. 2020, 749, 142386. [Google Scholar] [CrossRef]
- Aparecida da Silveira Rossi, R.; Barbosa, J.M.; Antonio de Souza Barrozo, M.; Martins Vieira, L.G. Solar assisted catalytic thermochemical processes: Pyrolysis and hydropyrolysis of Chlamydomonas reinhardtii microalgae. Renew. Energy 2021, 170, 669–682. [Google Scholar] [CrossRef]
- Santana Junior, J.A.; Menezes, A.L.; Ataíde, C.H. Catalytic upgrading of fast hydropyrolysis vapors from industrial Kraft lignins using ZSM-5 zeolite and HY-340 niobic acid. J. Anal. Appl. Pyrolysis 2019, 144, 104720. [Google Scholar] [CrossRef]
- Ding, Y.-L.; Wang, H.-Q.; Xiang, M.; Yu, P.; Li, R.-Q.; Ke, Q.-P. The Effect of Ni-ZSM-5 Catalysts on Catalytic Pyrolysis and Hydro-Pyrolysis of Biomass. Front. Chem. 2020, 8, 790. [Google Scholar] [CrossRef]
- Hu, M.; Cui, B.; Xiao, B.; Luo, S.; Guo, D. Insight into the Ex Situ Catalytic Pyrolysis of Biomass over Char Supported Metals Catalyst: Syngas Production and Tar Decomposition. Nanomaterials 2020, 10, 1397. [Google Scholar] [CrossRef]
- Ren, X.-Y.; Cao, J.-P.; Zhao, S.-X.; Zhao, X.-Y.; Liu, T.-L.; Feng, X.-B.; Li, Y.; Zhang, J.; Bai, H.-C. Encapsulation Ni in HZSM-5 for catalytic hydropyrolysis of biomass to light aromatics. Fuel Process. Technol. 2021, 218, 106854. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, X.; Resende, F.L.P.; Zhou, J.; Wang, M.; Dichiara, A.B. Hydropyrolysis of Residual Camellia sinensis and Its Cellulose and Lignin Fractions over Nickel Nanoparticles Confined Inside Carbon Nanotube Microreactors at Atmospheric Pressure. ACS Sustain. Chem. Eng. 2021, 9, 10827–10836. [Google Scholar] [CrossRef]
- Eykelbosh, A.J.; Johnson, M.S.; Santos de Queiroz, E.; Dalmagro, H.J.; Guimarães Couto, E. Biochar from Sugarcane Filtercake Reduces Soil CO2 Emissions Relative to Raw Residue and Improves Water Retention and Nutrient Availability in a Highly-Weathered Tropical Soil. PLoS ONE 2014, 9, e98523. [Google Scholar] [CrossRef] [PubMed]
- Vecino Mantilla, S.; Gauthier-Maradei, P.; Álvarez Gil, P.; Tarazona Cárdenas, S. Comparative study of bio-oil production from sugarcane bagasse and palm empty fruit bunch: Yield optimization and bio-oil characterization. J. Anal. Appl. Pyrolysis 2014, 108, 284–294. [Google Scholar] [CrossRef]
- Resende, F.L.P. Recent advances on fast hydropyrolysis of biomass. Catal. Today 2016, 269, 148–155. [Google Scholar] [CrossRef]
- Jan, O.; Marchand, R.; Anjos, L.C.A.; Seufitelli, G.V.S.; Nikolla, E.; Resende, F.L.P. Hydropyrolysis of Lignin Using Pd/HZSM-5. Energy Fuels 2015, 29, 1793–1800. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Jiang, L.; Wang, Y.; Li, Y.; Ke, L.; Yang, Q.; Peng, Y.; Xu, J.; Dai, L.; Wu, Q.; et al. Catalytic pyrolysis of woody oil over SiC foam-MCM41 catalyst for aromatic-rich bio-oil production in a dual microwave system. J. Clean. Prod. 2020, 255, 120179. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Z.; Gong, H.; Wang, X.; Yu, H. A strategy of using recycled char as a co-catalyst in cyclic in-situ catalytic cattle manure pyrolysis for increasing gas production. Waste Manag. 2020, 107, 74–81. [Google Scholar] [CrossRef]
- Yung, M.M.; Starace, A.K.; Mukarakate, C.; Crow, A.M.; Leshnov, M.A.; Magrini, K.A. Biomass Catalytic Pyrolysis on Ni/ZSM-5: Effects of Nickel Pretreatment and Loading. Energy & Fuels 2016, 30, 5259–5268. [Google Scholar] [CrossRef]
- Persson, H.; Duman, I.; Wang, S.; Pettersson, L.J.; Yang, W. Catalytic pyrolysis over transition metal-modified zeolites: A comparative study between catalyst activity and deactivation. J. Anal. Appl. Pyrolysis 2019, 138, 54–61. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.; Zhang, X.; Liu, Z.; Cao, Q.; Xie, X.; Yuan, S. Experimental study on catalytic pyrolysis of biomass over a Ni/Ca-promoted Fe catalyst. Fuel 2020, 263, 116690. [Google Scholar] [CrossRef]
- Kamali, M.; Sweygers, N.; Al-Salem, S.; Appels, L.; Aminabhavi, T.M.; Dewil, R. Biochar for soil applications-sustainability aspects, challenges and future prospects. Chem. Eng. J. 2022, 428, 131189. [Google Scholar] [CrossRef]
- Tafti, N.; Wang, J.; Gaston, L.; Park, J.; Wang, M.; Pensky, S. Agronomic and environmental performance of biochar amendment in alluvial soils under subtropical sugarcane production. Agrosystems, Geosci. Environ. 2021, 4, e20209. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Lovell, E.; Kan, T.; Weldekidan, H.; He, J.; Dastjerdi, B.; Scott, J. Bio-oil upgrading with catalytic pyrolysis of biomass using Copper/zeolite-Nickel/zeolite and Copper-Nickel/zeolite catalysts. Bioresour. Technol. 2019, 279, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Jin, F.; Wu, G.; Ding, Y. The synergetic effect of acid and nickel sites on bifunctional MWW zeolite catalysts for ethylene oligomerization and aromatization. Sustain. Energy Fuels 2019, 3, 3569–3581. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, H.; Hu, X.; Wang, R.; Dong, W. Productions of phenolic rich bio-oil using waste chilli stem biomass by catalytic pyrolysis: Evaluation of reaction parameters on products distributions. J. Energy Inst. 2021, 97, 233–239. [Google Scholar] [CrossRef]
- Martin, N.M.; Velin, P.; Skoglundh, M.; Bauer, M.; Carlsson, P.-A. Catalytic hydrogenation of CO2 to methane over supported Pd, Rh and Ni catalysts. Catal. Sci. Technol. 2017, 7, 1086–1094. [Google Scholar] [CrossRef] [Green Version]
- Pieta, I.S.; Lewalska-Graczyk, A.; Kowalik, P.; Antoniak-Jurak, K.; Krysa, M.; Sroka-Bartnicka, A.; Gajek, A.; Lisowski, W.; Mrdenovic, D.; Pieta, P.; et al. CO2 Hydrogenation to Methane over Ni-Catalysts: The Effect of Support and Vanadia Promoting. Catalysts 2021, 11, 433. [Google Scholar] [CrossRef]
- Li, T.; Li, Y.; Cheng, Y.; Li, X.; Shen, Y.; Yan, L.; Wang, M.; Chang, L.; Bao, W. Effect of hydrogen-rich gas from char gasification on rapid pyrolysis products of low rank coal in a downer pyrolyzer. RSC Adv. 2021, 11, 38537–38546. [Google Scholar] [CrossRef]
- Gupta, S.; Lanjewar, R.; Mondal, P. Enhancement of hydrocarbons and phenols in catalytic pyrolysis bio-oil by employing aluminum hydroxide nanoparticle based spent adsorbent derived catalysts. Chemosphere 2022, 287, 132220. [Google Scholar] [CrossRef]
- Lu, Q.; Ye, X.; Zhang, Z.; Wang, Z.; Cui, M.; Yang, Y. Catalytic fast pyrolysis of sugarcane bagasse using activated carbon catalyst in a hydrogen atmosphere to selectively produce 4-ethyl phenol. J. Anal. Appl. Pyrolysis 2018, 136, 125–131. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Garcia-Perez, M. Quantification of Bio-Oil Functional Groups and Evidences of the Presence of Pyrolytic Humins. Energy Fuels 2016, 30, 6505–6524. [Google Scholar] [CrossRef]
- Zhang, Y.; Lei, H.; Yang, Z.; Duan, D.; Villota, E.; Ruan, R. From glucose-based carbohydrates to phenol-rich bio-oils integrated with syngas production via catalytic pyrolysis over an activated carbon catalyst. Green Chem. 2018, 20, 3346–3358. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Zhu, Y.; Chen, J.; Lu, Y.; Li, S.; Zheng, Y.; Zheng, Z. Efficient ex-situ catalytic upgrading of biomass pyrolysis vapors to produce methylfurans and phenol over bio-based activated carbon. Biomass Bioenergy 2020, 142, 105794. [Google Scholar] [CrossRef]
- Carriel Schmitt, C.; Zimina, A.; Fam, Y.; Raffelt, K.; Grunwaldt, J.-D.; Dahmen, N. Evaluation of High-Loaded Ni-Based Catalysts for Upgrading Fast Pyrolysis Bio-Oil. Catalysts 2019, 9, 784. [Google Scholar] [CrossRef] [Green Version]
- He, T.; Liu, X.; Ge, Y.; Han, D.; Li, J.; Wang, Z.; Wu, J. Gas phase hydrodeoxygenation of anisole and guaiacol to aromatics with a high selectivity over Ni-Mo/SiO2. Catal. Commun. 2017, 102, 127–130. [Google Scholar] [CrossRef]
- Ambursa, M.M.; Juan, J.C.; Yahaya, Y.; Taufiq-Yap, Y.H.; Lin, Y.-C.; Lee, H.V. A review on catalytic hydrodeoxygenation of lignin to transportation fuels by using nickel-based catalysts. Renew. Sustain. Energy Rev. 2021, 138, 110667. [Google Scholar] [CrossRef]
- Ramos, M.L.N.S.; Carregosa, I.S.C.; Carvalho, M.P.; da Costa, M.T.; Gagliardi, P.R.; Wisniewski, A. Potential of cattle manure pyrolysis liquid as an alternative environmentally friendly source of agricultural fungicides. J. Anal. Appl. Pyrolysis 2020, 149, 104862. [Google Scholar] [CrossRef]
- Hertzog, J.; Carré, V.; Jia, L.; Mackay, C.L.; Pinard, L.; Dufour, A.; Mašek, O.; Aubriet, F. Catalytic Fast Pyrolysis of Biomass over Microporous and Hierarchical Zeolites: Characterization of Heavy Products. ACS Sustain. Chem. Eng. 2018, 6, 4717–4728. [Google Scholar] [CrossRef]
- Santos, T.M.; da Silva, W.R.; Carregosa, J.d.C.; Wisniewski, A. Comprehensive characterization of cattle manure bio-oil for scale-up assessment comparing non-equivalent reactor designs. J. Anal. Appl. Pyrolysis 2022, 162, 105465. [Google Scholar] [CrossRef]
- Michailof, C.M.; Kalogiannis, K.G.; Sfetsas, T.; Patiaka, D.T.; Lappas, A.A. Advanced analytical techniques for bio-oil characterization. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 614–639. [Google Scholar] [CrossRef]
- Zhong, D.; Zeng, K.; Li, J.; Qiu, Y.; Flamant, G.; Nzihou, A.; Vladimirovich, V.S.; Yang, H.; Chen, H. Characteristics and evolution of heavy components in bio-oil from the pyrolysis of cellulose, hemicellulose and lignin. Renew. Sustain. Energy Rev. 2022, 157, 111989. [Google Scholar] [CrossRef]
- Hertzog, J.; Carré, V.; Le Brech, Y.; Dufour, A.; Aubriet, F. Toward Controlled Ionization Conditions for ESI-FT-ICR-MS Analysis of Bio-Oils from Lignocellulosic Material. Energy Fuels 2016, 30, 5729–5739. [Google Scholar] [CrossRef]
- Hita, I.; Cordero-Lanzac, T.; Kekäläinen, T.; Okafor, O.; Rodríguez-Mirasol, J.; Cordero, T.; Bilbao, J.; Jänis, J.; Castano, P. In-Depth Analysis of Raw Bio-Oil and Its Hydrodeoxygenated Products for a Comprehensive Catalyst Performance Evaluation. ACS Sustain. Chem. Eng. 2020, 8, 18433–18445. [Google Scholar] [CrossRef]
- Palacio Lozano, D.C.; Jones, H.E.; Ramirez Reina, T.; Volpe, R.; Barrow, M.P. Unlocking the potential of biofuels via reaction pathways in van Krevelen diagrams. Green Chem. 2021, 23, 8949–8963. [Google Scholar] [CrossRef]
- Benés, M.; Bilbao, R.; Santos, J.M.; Alves Melo, J.; Wisniewski, A.; Fonts, I. Hydrodeoxygenation of Lignocellulosic Fast Pyrolysis Bio-Oil: Characterization of the Products and Effect of the Catalyst Loading Ratio. Energy Fuels 2019, 33, 4272–4286. [Google Scholar] [CrossRef]
- Reymond, C.; Dubuis, A.; Le Masle, A.; Colas, C.; Chahen, L.; Destandau, E.; Charon, N. Characterization of liquid–liquid extraction fractions from lignocellulosic biomass by high performance liquid chromatography hyphenated to tandem high-resolution mass spectrometry. J. Chromatogr. A 2020, 1610, 460569. [Google Scholar] [CrossRef]
- Staš, M.; Auersvald, M.; Kejla, L.; Vrtiška, D.; Kroufek, J.; Kubička, D. Quantitative analysis of pyrolysis bio-oils: A review. TrAC Trends Anal. Chem. 2020, 126, 115857. [Google Scholar] [CrossRef]
- Santos, L.; Silva, F.; Santos, L.; Carregosa, I.; Wisniewski Jr., A. Potential Bio-Oil Production from Invasive Aquatic Plants by Microscale Pyrolysis Studies. J. Braz. Chem. Soc. 2017, 29, 151–158. [Google Scholar] [CrossRef]
- Ohno, T.; Ohno, P.E. Influence of heteroatom pre-selection on the molecular formula assignment of soil organic matter components determined by ultrahigh resolution mass spectrometry. Anal. Bioanal. Chem. 2013, 405, 3299–3306. [Google Scholar] [CrossRef]




| Sample | LLF (wt %) | Gases (wt %) | HLF (wt %) | Biochar (wt %) |
|---|---|---|---|---|
| (N2)—SCB | 61.83 ± 1.57 | 16.15 ± 1.29 | 1.68 ± 0.36 | 20.34 ± 0.92 |
| (H2)—SCB | 60.06 ± 0.96 | 17.22 ± 0.52 | 1.43 ± 0.06 | 21.29 ± 1.00 |
| (N2)—SCB+Ni/SiO2 | 56.25 ± 0.69 | 20.88 ± 1.04 | 1.82 ± 0.32 | 21.04 ± 0.61 |
| (H2)—SCB+Ni/SiO2 | 56.53 ± 0.35 | 19.58 ± 0.39 | 1.57 ± 0.16 | 22.32 ± 0.66 |
| (N2)—SCB+Ni-Cr | 55.13 ± 1.51 | 21.78 ± 0.65 | 1.96 ± 0.20 | 21.13 ± 1.00 |
| (H2)—SCB+Ni-Cr | 54.93 ± 0.41 | 22.39 ± 0.82 | 1.49 ± 0.13 | 21.19 ± 1.34 |
| Sample | C (wt %) | H (wt %) | N (wt %) | O (wt %) a | Ash (wt %) |
|---|---|---|---|---|---|
| (N2)—SCB | 69.48 | 3.08 | 0.92 | 15.74 | 10.78 |
| (H2)—SCB | 69.28 | 2.98 | 0.85 | 17.07 | 10.00 |
| (N2)—SCB+Ni/SiO2 | 69.55 | 2.77 | 0.84 | 16.64 | 9.97 |
| (H2)—SCB+Ni/SiO2 | 68.91 | 2.82 | 0.84 | 18.01 | 9.90 |
| (N2)—SCB+Ni-Cr | 69.93 | 2.64 | 0.99 | 16.37 | 9.43 |
| (H2)—SCB+Ni-Cr | 69.33 | 3.08 | 0.85 | 16.78 | 9.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, T.M.; Silva, W.R.d.; Carregosa, J.d.C.; Schmitt, C.C.; Moreira, R.; Raffelt, K.; Dahmen, N.; Wisniewski, A., Jr. Thermal Conversion of Sugarcane Bagasse Coupled with Vapor Phase Hydrotreatment over Nickel-Based Catalysts: A Comprehensive Characterization of Upgraded Products. Catalysts 2022, 12, 355. https://doi.org/10.3390/catal12040355
Santos TM, Silva WRd, Carregosa JdC, Schmitt CC, Moreira R, Raffelt K, Dahmen N, Wisniewski A Jr. Thermal Conversion of Sugarcane Bagasse Coupled with Vapor Phase Hydrotreatment over Nickel-Based Catalysts: A Comprehensive Characterization of Upgraded Products. Catalysts. 2022; 12(4):355. https://doi.org/10.3390/catal12040355
Chicago/Turabian StyleSantos, Tarcísio Martins, Wenes Ramos da Silva, Jhonattas de Carvalho Carregosa, Caroline Carriel Schmitt, Renata Moreira, Klaus Raffelt, Nicolaus Dahmen, and Alberto Wisniewski, Jr. 2022. "Thermal Conversion of Sugarcane Bagasse Coupled with Vapor Phase Hydrotreatment over Nickel-Based Catalysts: A Comprehensive Characterization of Upgraded Products" Catalysts 12, no. 4: 355. https://doi.org/10.3390/catal12040355
APA StyleSantos, T. M., Silva, W. R. d., Carregosa, J. d. C., Schmitt, C. C., Moreira, R., Raffelt, K., Dahmen, N., & Wisniewski, A., Jr. (2022). Thermal Conversion of Sugarcane Bagasse Coupled with Vapor Phase Hydrotreatment over Nickel-Based Catalysts: A Comprehensive Characterization of Upgraded Products. Catalysts, 12(4), 355. https://doi.org/10.3390/catal12040355