Cerium-Copper Oxides Synthesized in a Multi-Inlet Vortex Reactor as Effective Nanocatalysts for CO and Ethene Oxidation Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CuCeOx Catalysts
2.2. Characterization Techniques
2.3. Catalytic Activity Tests
2.3.1. CO Oxidation Reaction
2.3.2. Ethene Oxidation Reaction
3. Results
3.1. Structural and Textural Properties
3.2. Chemical Properties
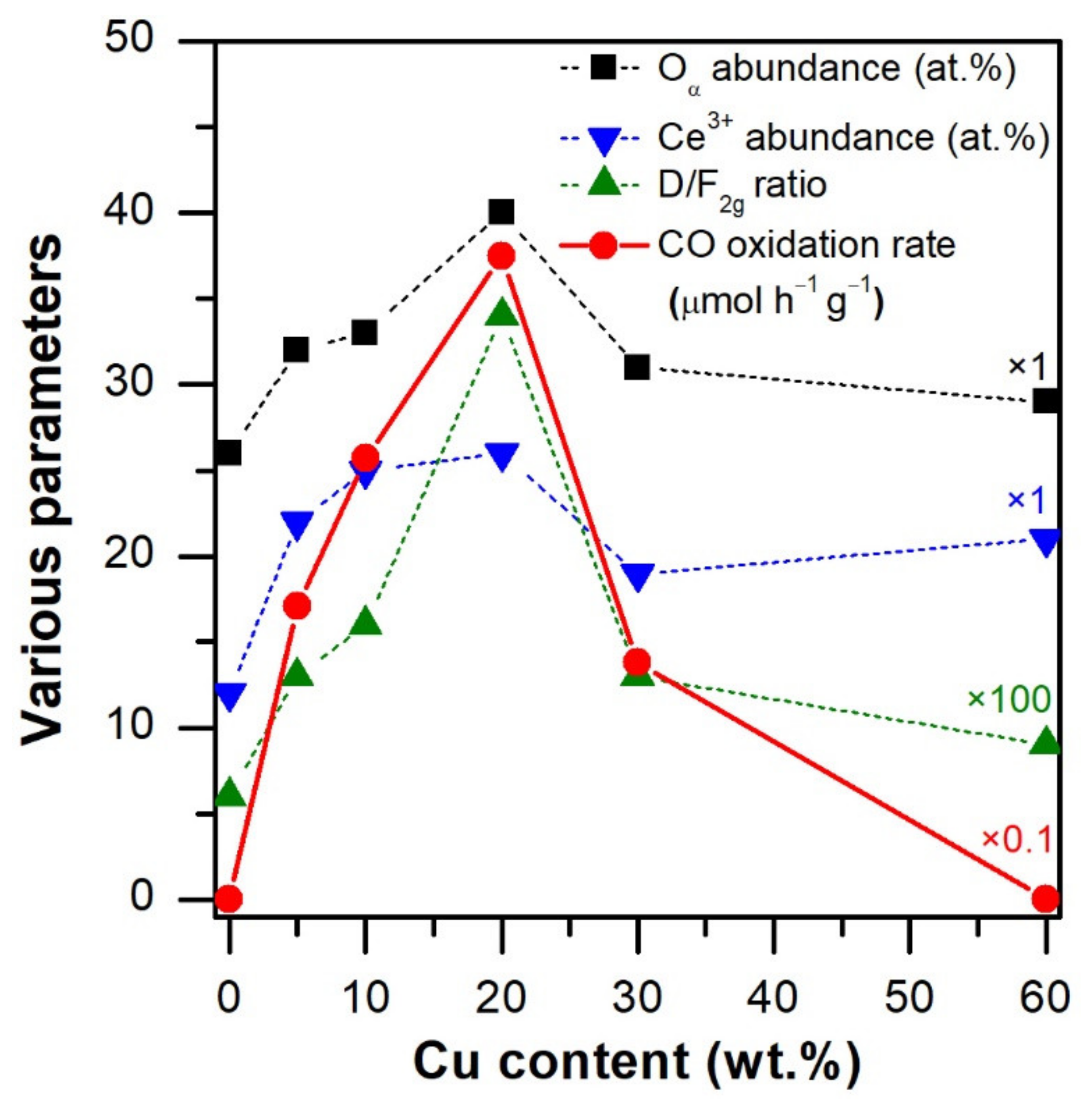
3.2.1. Surface Oxidation States
3.2.2. Reducibility Tests
3.3. Catalytic Activity
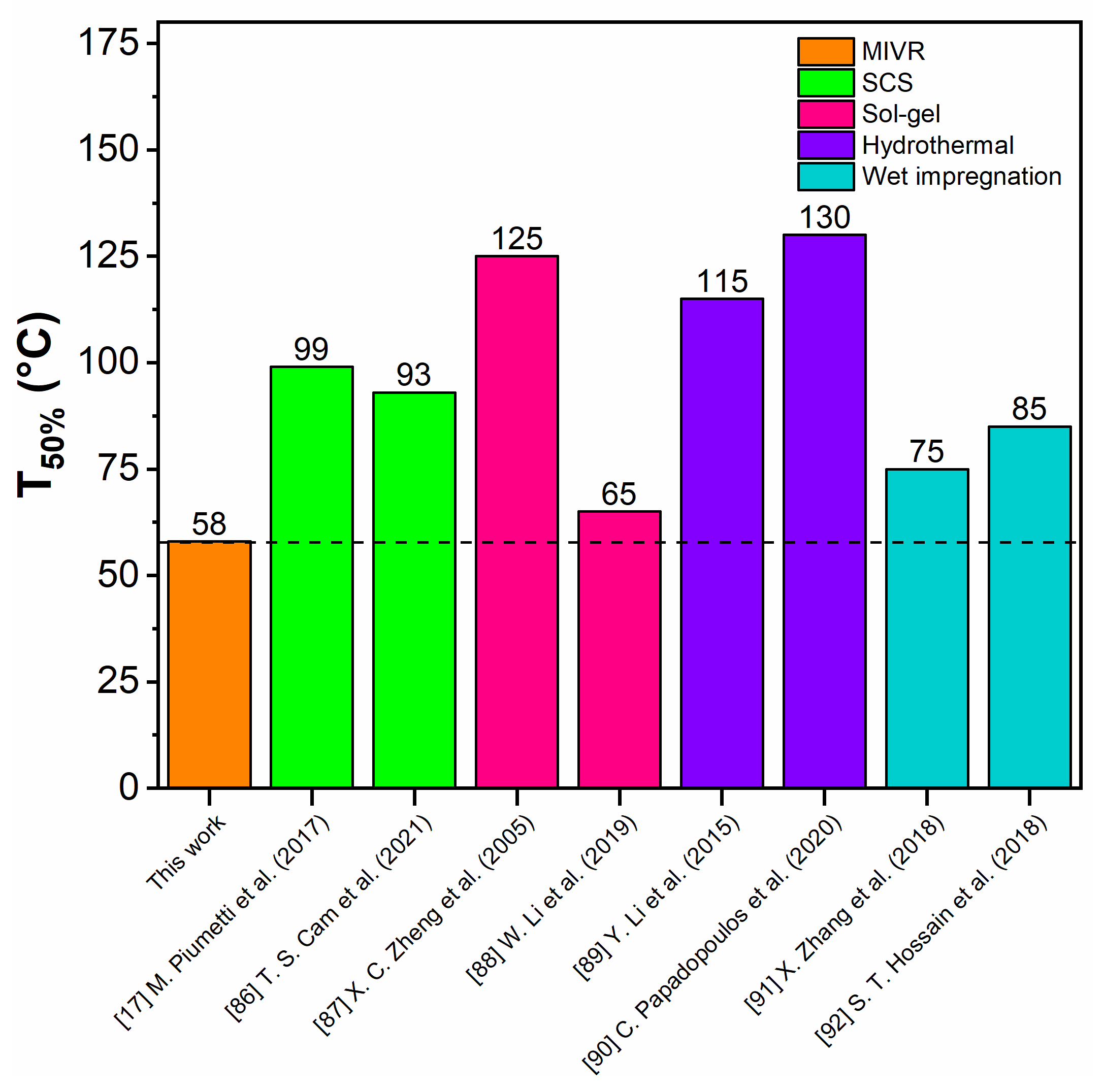
3.3.1. CO Oxidation Reaction
- First cycle: the starting temperature was fixed to 89 °C, at which the catalyst exhibits a CO conversion of about 95% and this temperature was kept constant for 2 h. Then, the catalytic system was cooled down until 58 °C (T50%) and it was kept constant for other 2 h. After that, the temperature was increased until 89 °C. Hence, the first cycle is characterized by a Time-on-Stream (TOS) of about 5 h.
- Second cycle: the same as the first one.
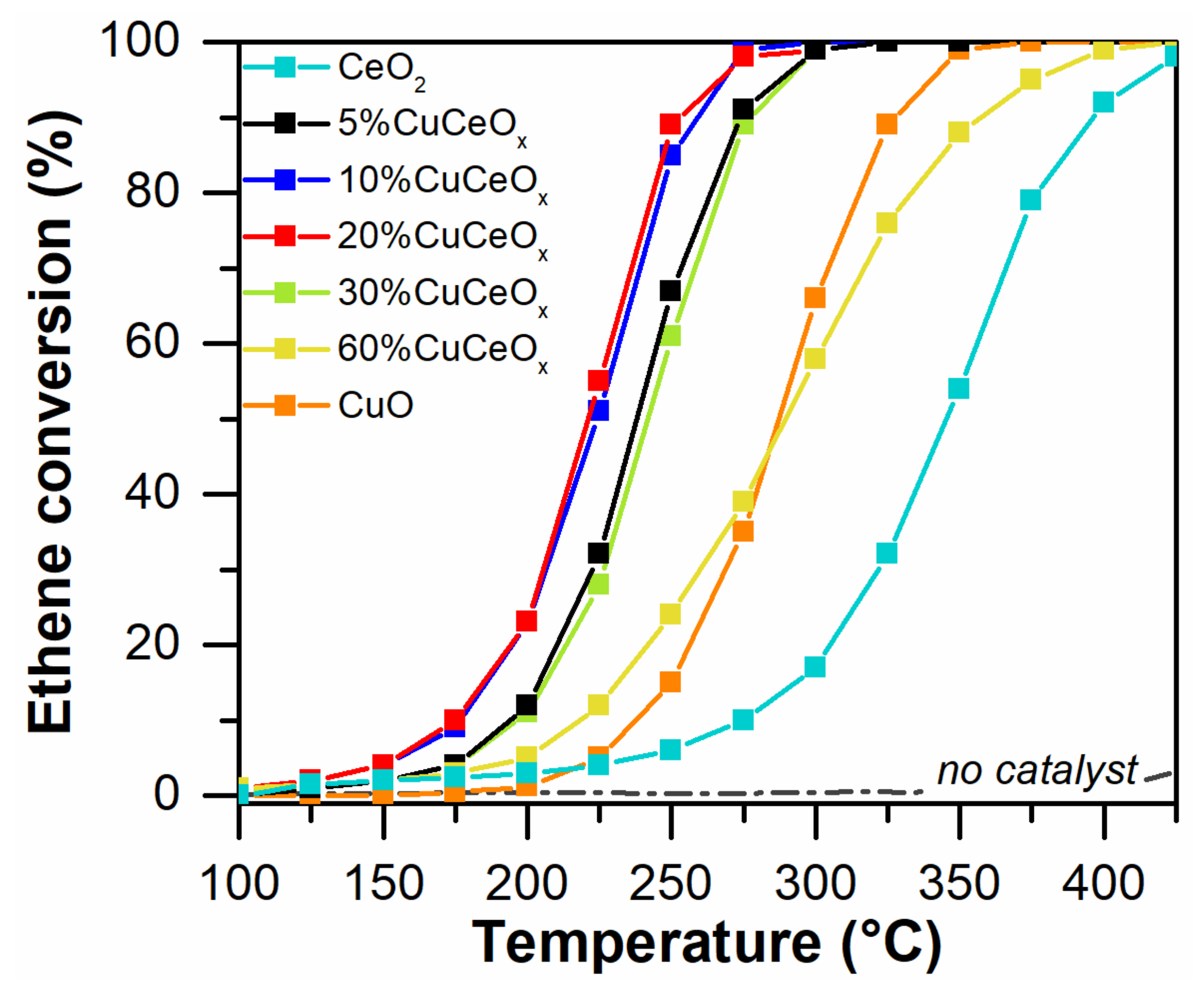
3.3.2. Ethene Oxidation Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trovarelli, A.; Fornasiero, P. Catalysis by Ceria and Related Materials; Catalytic Science Series; Imperial College Press: London, UK, 2013; Volume 12, ISBN 978-1-84816-963-0. [Google Scholar]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2008; Volume 1–5, ISBN 9783527619474. [Google Scholar]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control: Commercial Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-0-470-27503-0. [Google Scholar]
- Reşitoǧlu, I.A.; Altinişik, K.; Keskin, A. The pollutant emissions from diesel-engine vehicles and exhaust aftertreatment systems. Clean Technol. Environ. Policy 2015, 17, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.W.; Chen, R.H.; Pu, J.Y.; Lin, T.H. The influence of air-fuel ratio on engine performance and pollutant emission of an SI engine using ethanol-gasoline-blended fuels. In Atmospheric Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 2004; Volume 38, pp. 7093–7100. [Google Scholar]
- Faiz, A.; Weaver, C.S.; Walsh, M.P. Air Pollution from Motor Vehicles; The World Bank: Washington, DC, USA, 1996. [Google Scholar]
- Walsh, M.P. Mobile source related air pollution: Effects on health and the environment. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2019; pp. 436–442. ISBN 9780444639523. [Google Scholar]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.; Wasil, J.R.; Earnest, G.S. Carbon Monoxide Emissions from Marine Outboard Engines. SAE Trans. 2004, 113, 2127–2137. [Google Scholar]
- Koppmann, R. Volatile Organic Compounds in the Atmosphere; Blackwell: Hoboken, NJ, USA, 2007; ISBN 9780470988657. [Google Scholar]
- Burn, J.; Henk, J.; Bloemen, T. Chemistry and Analysis of Volatile Organic Compounds in the Environment; Blackie Academic & Professional: London, UK, 1993; ISBN 9789401049535. [Google Scholar]
- Finlayson-Pitts, B.J.; Pitts, J.N. Tropospheric air pollution: Ozone, airborne toxics, polycyclic aromatic hydrocarbons, and particles. Science 1997, 276, 1045–1051. [Google Scholar] [CrossRef] [Green Version]
- European Parliament; Council of the European Union. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; European Parliament: Strasbourg, France, 2008. [Google Scholar]
- Marin Figueredo, M.J.; Piumetti, M.; Bensaid, S.; Fino, D.; Nunzio, R. Catalytic Oxidation of Volatile Organic Compounds over Porous Manganese Oxides Prepared via Sol-Gel Method. In Nanostructured Catalysts for Environmental Applications; Springer: Cham, Switzerland, 2021; pp. 59–78. [Google Scholar]
- Njagi, E.C.; Genuino, H.C.; King’Ondu, C.K.; Dharmarathna, S.; Suib, S.L. Catalytic oxidation of ethylene at low temperatures using porous copper manganese oxides. Appl. Catal. A Gen. 2012, 421–422, 154–160. [Google Scholar] [CrossRef]
- Piumetti, M.; Fino, D.; Russo, N. Mesoporous manganese oxides prepared by solution combustion synthesis as catalysts for the total oxidation of VOCs. Appl. Catal. B Environ. 2015, 163, 277–287. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Russo, N.; Pirone, R.; Fino, D. Cerium-copper oxides prepared by solution combustion synthesis for total oxidation reactions: From powder catalysts to structured reactors. Appl. Catal. B Environ. 2017, 205, 455–468. [Google Scholar] [CrossRef]
- Marin Figueredo, M.J.; Andana, T.; Bensaid, S.; Dosa, M.; Fino, D.; Russo, N.; Piumetti, M. Cerium–Copper–Manganese Oxides Synthesized via Solution Combustion Synthesis (SCS) for Total Oxidation of VOCs. Catal. Lett. 2020, 150, 1821–1840. [Google Scholar] [CrossRef]
- Marin Figueredo, M.J.; Cocuzza, C.; Bensaid, S.; Fino, D.; Piumetti, M.; Russo, N. Catalytic Abatement of Volatile Organic Compounds and Soot over Manganese Oxide Catalysts. Materials 2021, 14, 4534. [Google Scholar] [CrossRef]
- Wu, X.; Han, R.; Liu, Q.; Su, Y.; Lu, S.; Yang, L.; Song, C.; Ji, N.; Ma, D.; Lu, X. A review of confined-structure catalysts in the catalytic oxidation of VOCs: Synthesis, characterization, and applications. Catal. Sci. Technol. 2021, 11, 5374–5387. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Veyre, L.; Thieuleux, C.; Russo, N.; Fino, D.; Quadrelli, E.A.; Pirone, R. CuO nanoparticles supported by ceria for NOx-assisted soot oxidation: Insight into catalytic activity and sintering. Appl. Catal. B Environ. 2017, 216, 41–58. [Google Scholar] [CrossRef]
- Choi, B.; Lee, K.S. LNT/CDPF catalysts for simultaneous removal of NOx and PM from diesel vehicle exhaust. Chem. Eng. J. 2014, 240, 476–486. [Google Scholar] [CrossRef]
- Jeong, J.W.; Choi, B.; Lim, M.T. Catalytic oxidation for carbon-black simulating diesel particulate matter over promoted Pt/Al2O3 catalysts. J. Ind. Eng. Chem. 2008, 14, 830–835. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Luo, H.; Weng, D.; Ran, R. Pt/Zeolite Catalysts for Soot Oxidation: Influence of Hydrothermal Aging. J. Phys. Chem. C 2015, 119, 17218–17227. [Google Scholar] [CrossRef]
- Liu, X.; Jia, S.; Yang, M.; Tang, Y.; Wen, Y.; Chu, S.; Wang, J.; Shan, B.; Chen, R. Activation of subnanometric Pt on Cu-modified CeO2 via redox-coupled atomic layer deposition for CO oxidation. Nat. Commun. 2020, 11, 4240. [Google Scholar] [CrossRef]
- Mergler, Y.J.; Van Aalst, A.; Van Delft, J.; Nieuwenhuys, B.E. CO oxidation over promoted Pt catalysts. Appl. Catal. B Environ. 1996, 10, 245–261. [Google Scholar] [CrossRef]
- Koop, J.; Deutschmann, O. Detailed surface reaction mechanism for Pt-catalyzed abatement of automotive exhaust gases. Appl. Catal. B Environ. 2009, 91, 47–58. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, N.; Cao, Y.; Gong, M.; Zhao, M.; Chen, Y. Effect of yttria in Pt/TiO2 on sulfur resistance diesel oxidation catalysts: Enhancement of low-temperature activity and stability. Catal. Sci. Technol. 2014, 4, 3032–3043. [Google Scholar] [CrossRef]
- Fino, D.; Bensaid, S.; Piumetti, M.; Russo, N. A review on the catalytic combustion of soot in Diesel particulate filters for automotive applications: From powder catalysts to structured reactors. Appl. Catal. A Gen. 2016, 509, 75–96. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. CO and Soot Oxidation over Ce-Zr-Pr Oxide Catalysts. Nanoscale Res. Lett. 2016, 11, 278. [Google Scholar] [CrossRef] [Green Version]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Andana, T.; Novara, C.; Giorgis, F.; Fino, D.; Russo, N. Novel Mn–Cu-Containing CeO2 Nanopolyhedra for the Oxidation of CO and Diesel Soot: Effect of Dopants on the Nanostructure and Catalytic Activity. Catal. Lett. 2018, 148, 298–311. [Google Scholar] [CrossRef]
- Dosa, M.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Novel Mn–Cu-Containing CeO2 Nanopolyhedra for the Oxidation of CO and Diesel Soot (Part II): Effect of Oxygen Concentration on the Catalytic Activity. Catal. Lett. 2019, 149, 107–118. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Andana, T.; Dosa, M.; Novara, C.; Giorgis, F.; Russo, N.; Fino, D. Nanostructured Ceria-Based Materials: Effect of the Hydrothermal Synthesis Conditions on the Structural Properties and Catalytic Activity. Catalysts 2017, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Delimaris, D.; Ioannides, T. VOC oxidation over CuO-CeO2 catalysts prepared by a combustion method. Appl. Catal. B Environ. 2009, 89, 295–302. [Google Scholar] [CrossRef]
- Li, C.; Xin, Q.; Guo, X.-X. Surface oxygen species and their reactivities in the mild oxidation of ethylene on cerium oxide studied by FT-IR spectroscopy. Catal. Lett. 1992, 12, 297–305. [Google Scholar] [CrossRef]
- Dole, H.A.E.; Baranova, E.A. Ethylene Oxidation in an Oxygen-Deficient Environment: Why Ceria is an Active Support? ChemCatChem 2016, 8, 1977–1986. [Google Scholar] [CrossRef]
- Andana, T.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Nanostructured ceria-praseodymia catalysts for diesel soot combustion. Appl. Catal. B Environ. 2016, 197, 125–137. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Investigations into nanostructured ceria-zirconia catalysts for soot combustion. Appl. Catal. B Environ. 2016, 180, 271–282. [Google Scholar] [CrossRef]
- Atribak, I.; López-Suárez, F.E.; Bueno-López, A.; García-García, A. New insights into the performance of ceria-zirconia mixed oxides as soot combustion catalysts. Identification of the role of “active oxygen” production. In Catalysis Today; Elsevier: Amsterdam, The Netherlands, 2011; Volume 176, pp. 404–408. [Google Scholar]
- Konsolakis, M. The role of Copper–Ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Rao, K.N.; Venkataswamy, P.; Reddy, B.M. Structural characterization and catalytic evaluation of supported copper-ceria catalysts for soot oxidation. Ind. Eng. Chem. Res. 2011, 50, 11960–11969. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno-López, A.; Makkee, M.; Moulijn, J.A. Potential rare earth modified CeO2 catalysts for soot oxidation. I. Characterisation and catalytic activity with O2. Appl. Catal. B Environ. 2007, 75, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Sartoretti, E.; Martini, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured equimolar ceria-praseodymia for total oxidations in low-O2 conditions. Catalysts 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Sartoretti, E.; Novara, C.; Chiodoni, A.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts doped with La and Nd: How acid-base sites and redox properties determine the oxidation mechanisms. Catal. Today 2021, 390–391, 117–134. [Google Scholar] [CrossRef]
- Dziembaj, R.; Molenda, M.; Chmielarz, L.; Zaitz, M.M.; Piwowarska, Z.; Rafalska-ŁAsocha, A. Optimization of Cu doped ceria nanoparticles as catalysts for low-temperature methanol and ethylene total oxidation. Catal. Today 2011, 169, 112–117. [Google Scholar] [CrossRef]
- Shan, W.; Shen, W.; Li, C. Structural Characteristics and Redox Behaviors of Ce1-xCuxOy Solid Solutions. Chem. Mater. 2003, 15, 4761–4767. [Google Scholar] [CrossRef]
- Jia, A.P.; Jiang, S.Y.; Lu, J.Q.; Luo, M.F. Study of catalytic activity at the CuO-CeO2 interface for CO oxidation. J. Phys. Chem. C 2010, 114, 21605–21610. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Navlani-García, M.; Lozano-Castelló, D.; Bueno-López, A.; Anderson, J.A. Role of Hydroxyl Groups in the Preferential Oxidation of CO over Copper Oxide-Cerium Oxide Catalysts. ACS Catal. 2016, 6, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Flytzani-Stephanopoulos, M. Total Oxidation of Carbon Monoxide and Methane over Transition Metal Fluorite Oxide Composite Catalysts I. Catalyst Composition and Activity. J. Catal. 1995, 153, 304–316. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Wang, X.; Wang, S.; Wu, S. Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl. Catal. A Gen. 2005, 295, 142–149. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. CuO/CeO2 catalysts: Redox features and catalytic behaviors. Appl. Catal. A Gen. 2005, 288, 116–125. [Google Scholar] [CrossRef]
- Bensaid, S.; Piumetti, M.; Novara, C.; Giorgis, F.; Chiodoni, A.; Russo, N.; Fino, D. Catalytic Oxidation of CO and Soot over Ce-Zr-Pr Mixed Oxides Synthesized in a Multi-Inlet Vortex Reactor: Effect of Structural Defects on the Catalytic Activity. Nanoscale Res. Lett. 2016, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bensaid, S.; Deorsola, F.A.; Marchisio, D.L.; Russo, N.; Fino, D. Flow field simulation and mixing efficiency assessment of the multi-inlet vortex mixer for molybdenum sulfide nanoparticle precipitation. Chem. Eng. J. 2014, 238, 66–77. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Piumetti, M.; Andana, T.; Bensaid, S.; Russo, N.; Fino, D.; Pirone, R. Study on the CO Oxidation over Ceria-Based Nanocatalysts. Nanoscale Res. Lett. 2016, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Etefagh, R.; Azhir, E.; Shahtahmasebi, N. Synthesis of CuO nanoparticles and fabrication of nanostructural layer biosensors for detecting Aspergillus niger fungi. Sci. Iran. 2013, 20, 1055–1058. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, H.; Sun, C.; Liu, P.; Li, L.; Yang, Z.; Feng, X.; Huo, F.; Lu, X. CuO/Cu2O porous composites: Shape and composition controllable fabrication inherited from metal organic frameworks and further application in CO oxidation. J. Mater. Chem. A 2015, 3, 5294–5298. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Lefferts, L.; Mojet, B.L. Defect chemistry of ceria nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defined surface planes by raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Ballauri, S.; Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Fino, D.; Russo, N.; Bensaid, S. Wide range temperature stability of palladium on ceria-praseodymia catalysts for complete methane oxidation. Catal. Today 2021, 390–391, 185–197. [Google Scholar] [CrossRef]
- Guillén-Hurtado, N.; Giménez-Mañogil, J.; Martínez-Munuera, J.C.; Bueno-López, A.; García-García, A. Study of Ce/Pr ratio in ceria-praseodymia catalysts for soot combustion under different atmospheres. Appl. Catal. A Gen. 2020, 590, 117339. [Google Scholar] [CrossRef] [Green Version]
- Andriopoulou, C.; Trimpalis, A.; Petallidou, K.C.; Sgoura, A.; Efstathiou, A.M.; Boghosian, S. Structural and Redox Properties of Ce1−xZrxO2−δ and Ce0.8Zr0.15RE0.05O2−δ (RE: La, Nd, Pr, Y) Solids Studied by High Temperature in Situ Raman Spectroscopy. J. Phys. Chem. C 2017, 121, 7931–7943. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalysts. Sci. Rep. 2019, 9, 3875. [Google Scholar] [CrossRef] [Green Version]
- Acharya, S.A.; Gaikwad, V.M.; D’Souza, S.W.; Barman, S.R. Gd/Sm dopant-modified oxidation state and defect generation in nano-ceria. Solid State Ionics 2014, 260, 21–29. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Fontana, M.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. New insights on the defect sites evolution during CO oxidation over doped ceria nanocatalysts probed by in situ Raman spectroscopy. Appl. Catal. A Gen. 2020, 596, 117517. [Google Scholar] [CrossRef]
- Vinodkumar, T.; Rao, B.G.; Reddy, B.M. Influence of isovalent and aliovalent dopants on the reactivity of cerium oxide for catalytic applications. Catal. Today 2015, 253, 57–64. [Google Scholar] [CrossRef]
- Reddy, K.R. Green synthesis, morphological and optical studies of CuO nanoparticles. J. Mol. Struct. 2017, 1150, 553–557. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Mojet, B.L.; Lefferts, L. Surface-Dependence of Defect Chemistry of Nanostructured Ceria. J. Phys. Chem. C 2015, 119, 12423–12433. [Google Scholar] [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.C. An FTIR study of surface ceria hydroxy groups during a redox process with H2. J. Chem. Soc. Faraday Trans. 1996, 92, 4669–4673. [Google Scholar] [CrossRef]
- Jacobs, G.; Keogh, R.A.; Davis, B.H. Steam reforming of ethanol over Pt/ceria with co-fed hydrogen. J. Catal. 2007, 245, 326–337. [Google Scholar] [CrossRef]
- Kettemann, F.; Witte, S.; Birnbaum, A.; Paul, B.; Clavel, G.; Pinna, N.; Rademann, K.; Kraehnert, R.; Polte, J. Unifying Concepts in Room-Temperature CO Oxidation with Gold Catalysts. ACS Catal. 2017, 7, 8247–8254. [Google Scholar] [CrossRef]
- Li, H.; Jiao, X.; Li, L.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y.; Zhang, B. Synthesis of glycerol carbonate by direct carbonylation of glycerol with CO2 over solid catalysts derived from Zn/Al/La and Zn/Al/La/M (M = Li, Mg and Zr) hydrotalcites. Catal. Sci. Technol. 2015, 5, 989–1005. [Google Scholar] [CrossRef]
- Yang, S.C.; Pang, S.H.; Sulmonetti, T.P.; Su, W.N.; Lee, J.F.; Hwang, B.J.; Jones, C.W. Synergy between Ceria Oxygen Vacancies and Cu Nanoparticles Facilitates the Catalytic Conversion of CO2 to CO under Mild Conditions. ACS Catal. 2018, 8, 12056–12066. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea-nitrate combustion method. Appl. Catal. A Gen. 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Mrabet, D.; Abassi, A.; Cherizol, R.; Do, T.O. One-pot solvothermal synthesis of mixed Cu-Ce-Ox nanocatalysts and their catalytic activity for low temperature CO oxidation. Appl. Catal. A Gen. 2012, 447–448, 60–66. [Google Scholar] [CrossRef]
- Tabakova, T.; Boccuzzi, F.; Manzoli, M.; Sobczak, J.W.; Idakiev, V.; Andreeva, D. A comparative study of nanosized IB/ceria catalysts for low-temperature water-gas shift reaction. Appl. Catal. A Gen. 2006, 298, 127–143. [Google Scholar] [CrossRef]
- Jernigan, G.G.; Somorjai, G.A. Carbon Monoxide Oxidation over Three Different Oxidation States of Copper: Metallic Copper, Copper (I) Oxide, and Copper (II) Oxide—A Surface Science and Kinetic Study. J. Catal. 1994, 147, 567–577. [Google Scholar] [CrossRef]
- Zhang, S.M.; Huang, W.P.; Qiu, X.H.; Li, B.Q.; Zheng, X.C.; Wu, S.H. Comparative study on catalytic properties for low-temperature CO oxidation of Cu/CeO2 and CuO/CeO2 prepared via solvated metal atom impregnation and conventional impregnation. Catal. Lett. 2002, 80, 41–46. [Google Scholar] [CrossRef]
- Lai, S.Y.; Qiu, Y.; Wang, S. Effects of the structure of ceria on the activity of gold/ceria catalysts for the oxidation of carbon monoxide and benzene. J. Catal. 2006, 237, 303–313. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, R. A new insight into the morphology effect of ceria on CuO/CeO2 catalysts for CO selective oxidation in hydrogen-rich gas. Catal. Sci. Technol. 2016, 6, 3862–3871. [Google Scholar] [CrossRef]
- Tang, X.; Li, Y.; Huang, X.; Xu, Y.; Zhu, H.; Wang, J.; Shen, W. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcination temperature. Appl. Catal. B Environ. 2006, 62, 265–273. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Caputo, T.; Lisi, L.; Pirone, R.; Russo, G. On the role of redox properties of CuO/CeO2 catalysts in the preferential oxidation of CO in H2-rich gases. Appl. Catal. A Gen. 2008, 348, 42–53. [Google Scholar] [CrossRef]
- Heynderickx, P.M.; Thybaut, J.W.; Poelman, H.; Poelman, D.; Marin, G.B. The total oxidation of propane over supported Cu and Ce oxides: A comparison of single and binary metal oxides. J. Catal. 2010, 272, 109–120. [Google Scholar] [CrossRef]
- Mars, P.; van Krevelen, D.W. Oxidations carried out by means of vanadium oxide catalysts. Chem. Eng. Sci. 1954, 3, 41–59. [Google Scholar] [CrossRef]
- Cam, T.S.; Omarov, S.O.; Chebanenko, M.I.; Sklyarova, A.S.; Nevedomskiy, V.N.; Popkov, V.I. One step closer to the low-temperature CO oxidation over non-noble CuO/CeO2 nanocatalyst: The effect of CuO loading. J. Environ. Chem. Eng. 2021, 9, 105373. [Google Scholar] [CrossRef]
- Zheng, X.C.; Wu, S.H.; Wang, S.P.; Wang, S.R.; Zhang, S.M.; Huang, W.P. The preparation and catalytic behavior of copper-cerium oxide catalysts for low-temperature carbon monoxide oxidation. Appl. Catal. A Gen. 2005, 283, 217–223. [Google Scholar] [CrossRef]
- Li, W.; Shen, X.; Zeng, R.; Chen, J.; Xiao, W.; Ding, S.; Chen, C.; Zhang, R.; Zhang, N. Constructing copper-ceria nanosheets with high concentration of interfacial active sites for enhanced performance in CO oxidation. Appl. Surf. Sci. 2019, 492, 818–825. [Google Scholar] [CrossRef]
- Li, Y.; Cai, Y.; Xing, X.; Chen, N.; Deng, D.; Wang, Y. Catalytic activity for CO oxidation of Cu-CeO2 composite nanoparticles synthesized by a hydrothermal method. Anal. Methods 2015, 7, 3238–3245. [Google Scholar] [CrossRef]
- Papadopoulos, C.; Kappis, K.; Papavasiliou, J.; Vakros, J.; Kuśmierz, M.; Gac, W.; Georgiou, Y.; Deligiannakis, Y.; Avgouropoulos, G. Copper-promoted ceria catalysts for CO oxidation reaction. Catal. Today 2020, 355, 647–653. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Song, L.; Hou, F.; Yang, Y.; Wang, Y.; Liu, N. Enhanced catalytic performance for CO oxidation and preferential CO oxidation over CuO/CeO2 catalysts synthesized from metal organic framework: Effects of preparation methods. Int. J. Hydrogen Energy 2018, 43, 18279–18288. [Google Scholar] [CrossRef]
- Hossain, S.T.; Azeeva, E.; Zhang, K.; Zell, E.T.; Bernard, D.T.; Balaz, S.; Wang, R. A comparative study of CO oxidation over Cu-O-Ce solid solutions and CuO/CeO2 nanorods catalysts. Appl. Surf. Sci. 2018, 455, 132–143. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [Green Version]
- Bera, P.; Gayen, A.; Hegde, M.S.; Lalla, N.P.; Spadaro, L.; Frusteri, F.; Arena, F. Promoting effect of CeO2 in combustion synthesized Pt/CeO2 catalyst for CO oxidation. J. Phys. Chem. B 2003, 107, 6122–6130. [Google Scholar] [CrossRef]
- Liu, H.H.; Wang, Y.; Jia, A.P.; Wang, S.Y.; Luo, M.F.; Lu, J.Q. Oxygen vacancy promoted CO oxidation over Pt/CeO2 catalysts: A reaction at Pt-CeO2 interface. Appl. Surf. Sci. 2014, 314, 725–734. [Google Scholar] [CrossRef]
- Piumetti, M. Molecular Dynamics and Complexity in Catalysis and Biocatalysis; Springer International Publishing: Cham, Switzerland, 2022. [Google Scholar]
- Piumetti, M.; Russo, N. Notes on Catalysis for Environment and Energy; CLUT—Politecnico di Torino: Turin, Italy, 2017; ISBN 9788879924085. [Google Scholar]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Zawadzki, M.; Trawczyński, J. Synthesis, characterization and catalytic performance of LSCF perovskite for VOC combustion. Catal. Today 2011, 176, 449–452. [Google Scholar] [CrossRef]
- Kamal, M.S.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Yang, C.; Miao, G.; Pi, Y.; Xia, Q.; Wu, J.; Li, Z.; Xiao, J. Abatement of various types of VOCs by adsorption/catalytic oxidation: A review. Chem. Eng. J. 2019, 370, 1128–1153. [Google Scholar] [CrossRef]
- Morales, M.R.; Yeste, M.P.; Vidal, H.; Gatica, J.M.; Cadus, L.E. Insights on the combustion mechanism of ethanol and n-hexane in honeycomb monolithic type catalysts: Influence of the amount and nature of Mn-Cu mixed oxide. Fuel 2017, 208, 637–646. [Google Scholar] [CrossRef]
- Wang, Q.; Yeung, K.L.; Bañares, M.A. Ceria and its related materials for VOC catalytic combustion: A review. Catal. Today 2020, 356, 141–154. [Google Scholar] [CrossRef]

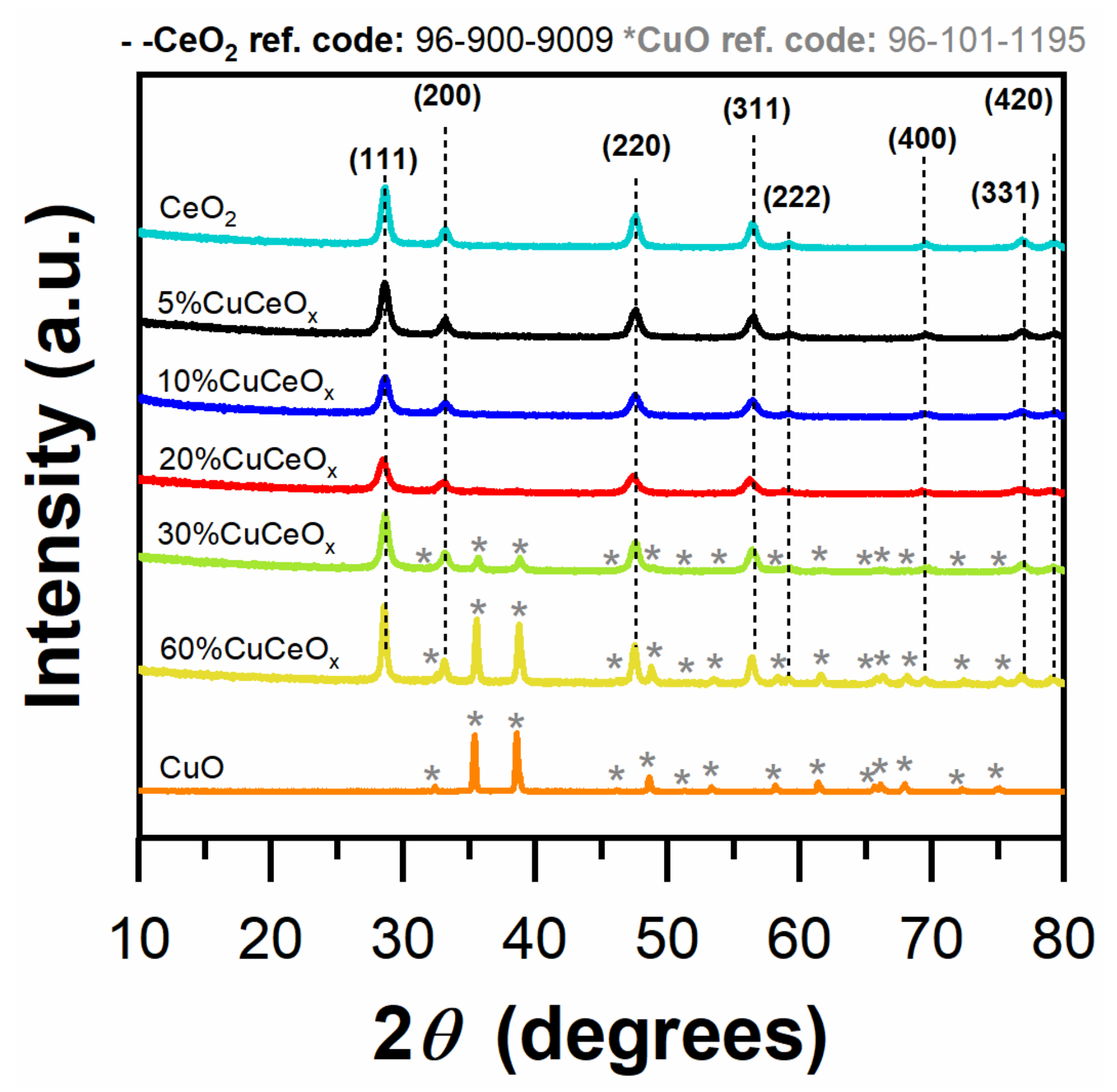
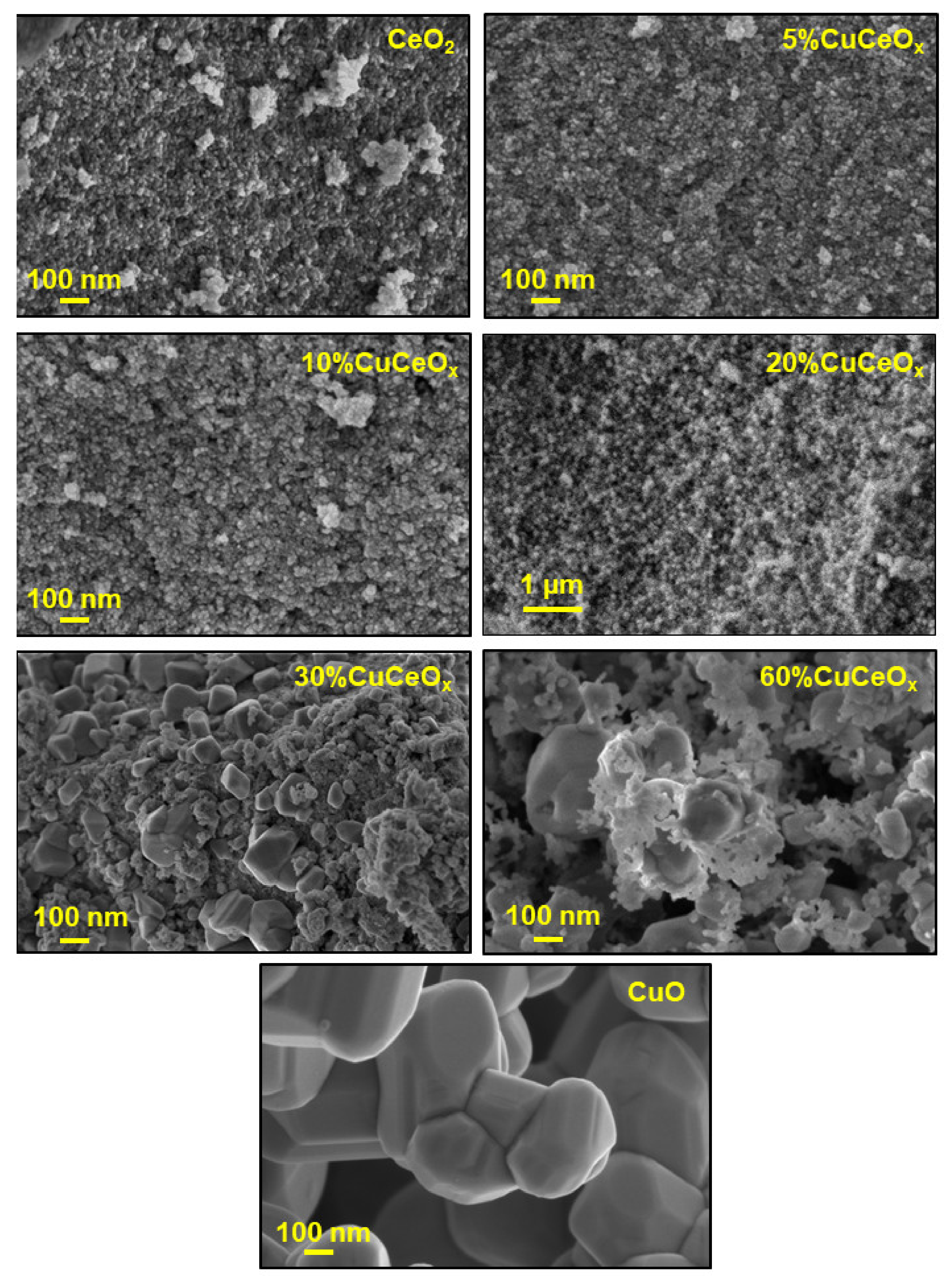

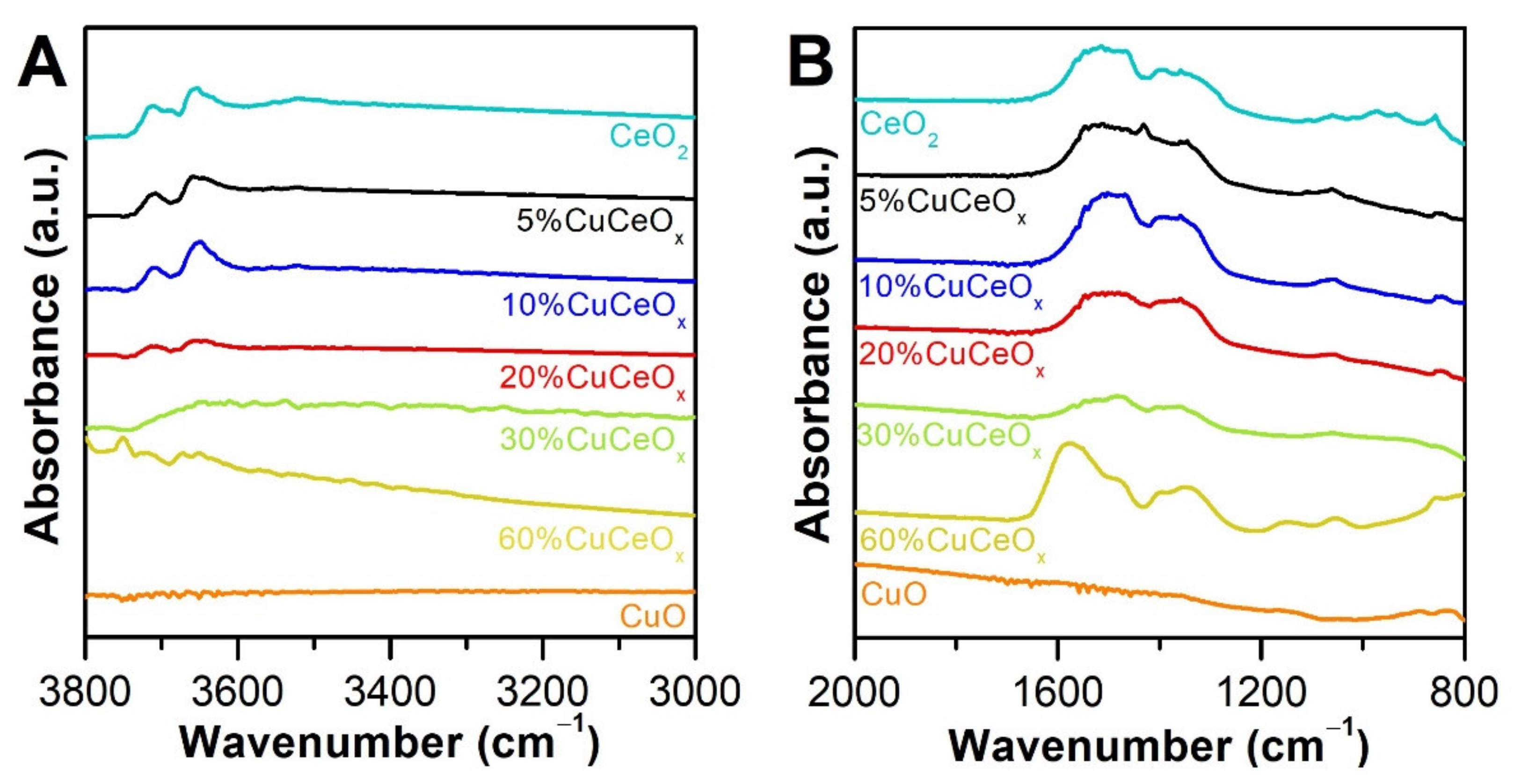






| Catalyst | Dc a (nm) | SSA b (m2 g−1) | Vp c (cm3 g−1) | D/F2g d | |
|---|---|---|---|---|---|
| CeO2 | CuO | ||||
| CeO2 | 15 | - | 45 | 0.07 | 0.06 |
| 5%CuCeOx | 13 | - | 50 | 0.08 | 0.13 |
| 10%CuCeOx | 11 | 7 | 55 | 0.10 | 0.16 |
| 20%CuCeOx | 10 | 9 | 50 | 0.09 | 0.34 |
| 30%CuCeOx | 14 | 26 | 22 | 0.03 | 0.13 |
| 60%CuCeOx | 16 | 39 | 11 | 0.06 | 0.09 |
| CuO | - | 73 | 2 | 0.01 | - |
| Catalyst | Elemental Composition (wt.%) | |||||
|---|---|---|---|---|---|---|
| EDX * | ICP | |||||
| Ce | Cu | Tot. | Ce | Cu | Tot. | |
| 5%CuCeOx | 97 | 3 | 100 | 97 | 3 | 100 |
| 10%CuCeOx | 92 | 8 | 100 | 90 | 10 | 100 |
| 20%CuCeOx | 82 | 18 | 100 | 79 | 21 | 100 |
| 30%CuCeOx | 67 | 33 | 100 | 67 | 33 | 100 |
| 60%CuCeOx | 32 | 68 | 100 | 37 | 63 | 100 |
| Catalyst | Ce3+ (at.%) | Ce4+ (at.%) |
|---|---|---|
| CeO2 | 12 | 88 |
| 5%CuCeOx | 22 | 78 |
| 10%CuCeOx | 25 | 75 |
| 20%CuCeOx | 26 | 74 |
| 30%CuCeOx | 19 | 81 |
| 60%CuCeOx | 21 | 79 |
| Catalyst | Oα | Oβ (Cu-O) | Oβ (Ce-O) | Oα/Oβ | |||
|---|---|---|---|---|---|---|---|
| BE (eV) | %-Atom | BE (eV) | %-Atom | BE (eV) | %-Atom | ||
| CeO2 | 531.2 | 26 | - | - | 529.0 | 74 | 0.35 |
| 5%CuCeOx | 530.9 | 32 | - | - | 529.1 | 68 | 0.47 |
| 10%CuCeOx | 531.4 | 33 | - | - | 529.4 | 67 | 0.49 |
| 20%CuCeOx | 531.3 | 40 | - | - | 529.3 | 60 | 0.67 |
| 30%CuCeOx | 531.4 | 31 | 529.9 | 36 | 528.8 | 33 | 0.45 |
| 60%CuCeOx | 531.3 | 29 | 529.6 | 45 | 528.7 | 26 | 0.41 |
| CuO | 531.4 | 43 | 529.8 | 57 | - | - | 0.75 |
| H2-Uptake Total | H2/Cu | H2-Uptake <200 °C | H2-Uptake 200–400 °C | H2-Uptake 400–500 °C | H2-Uptake >500 °C | |
|---|---|---|---|---|---|---|
| CeO2 | 1.41 | - | 0.01 | 0.11 | 0.16 | 1.13 |
| 5%CuCeOx | 1.33 | 3.46 | 0.16 | 0.52 | 0.05 | 0.60 |
| 10%CuCeOx | 1.91 | 1.50 | 0.15 | 1.21 | 0.06 | 0.49 |
| 20%CuCeOx | 3.59 | 1.34 | 0.09 | 2.52 | 0.30 | 0.68 |
| 30%CuCeOx | 5.35 | 1.27 | 0.05 | 4.07 | 0.43 | 0.79 |
| 60%CuCeOx | 8.83 | 1.11 | 0.02 | 5.86 | 1.99 | 0.96 |
| CuO | 12.57 | 1.00 | 0.00 | 4.37 | 5.92 | 2.28 |
| Catalyst | CO Oxidation Rate a (µmol h−1 g−1) | Tparameter (°C) | ||
|---|---|---|---|---|
| T10% | T50% | T90% | ||
| CeO2 | 2.6 × 10−2 | 258 | 314 | 372 |
| 5%CuCeOx | 171 | 46 | 69 | 98 |
| 10%CuCeOx | 257 | 35 | 62 | 90 |
| 20%CuCeOx | 375 | 30 | 58 | 75 |
| 30%CuCeOx | 138 | 46 | 73 | 105 |
| 60%CuCeOx | 2.7 × 10−4 | 104 | 139 | 171 |
| CuO | 3.7 × 10−4 | 151 | 179 | 197 |
| Catalyst | Ethene Oxidation Rate a (µmol h−1 g−1) | Tparameter (°C) | ||
|---|---|---|---|---|
| T10% | T50% | T90% | ||
| CeO2 | 15 | 275 | 345 | 396 |
| 5%CuCeOx | 25 | 194 | 238 | 273 |
| 10%CuCeOx | 56 | 177 | 223 | 259 |
| 20%CuCeOx | 62 | 175 | 221 | 252 |
| 30%CuCeOx | 26 | 197 | 242 | 277 |
| 60%CuCeOx | 21 | 217 | 290 | 357 |
| CuO | 3 | 237 | 287 | 328 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosa, M.; Marin-Figueredo, M.J.; Sartoretti, E.; Novara, C.; Giorgis, F.; Bensaid, S.; Fino, D.; Russo, N.; Piumetti, M. Cerium-Copper Oxides Synthesized in a Multi-Inlet Vortex Reactor as Effective Nanocatalysts for CO and Ethene Oxidation Reactions. Catalysts 2022, 12, 364. https://doi.org/10.3390/catal12040364
Dosa M, Marin-Figueredo MJ, Sartoretti E, Novara C, Giorgis F, Bensaid S, Fino D, Russo N, Piumetti M. Cerium-Copper Oxides Synthesized in a Multi-Inlet Vortex Reactor as Effective Nanocatalysts for CO and Ethene Oxidation Reactions. Catalysts. 2022; 12(4):364. https://doi.org/10.3390/catal12040364
Chicago/Turabian StyleDosa, Melodj, Miguel Jose Marin-Figueredo, Enrico Sartoretti, Chiara Novara, Fabrizio Giorgis, Samir Bensaid, Debora Fino, Nunzio Russo, and Marco Piumetti. 2022. "Cerium-Copper Oxides Synthesized in a Multi-Inlet Vortex Reactor as Effective Nanocatalysts for CO and Ethene Oxidation Reactions" Catalysts 12, no. 4: 364. https://doi.org/10.3390/catal12040364
APA StyleDosa, M., Marin-Figueredo, M. J., Sartoretti, E., Novara, C., Giorgis, F., Bensaid, S., Fino, D., Russo, N., & Piumetti, M. (2022). Cerium-Copper Oxides Synthesized in a Multi-Inlet Vortex Reactor as Effective Nanocatalysts for CO and Ethene Oxidation Reactions. Catalysts, 12(4), 364. https://doi.org/10.3390/catal12040364








