Emissions Merit Function for Evaluating Multifunctional Catalyst Beds
Abstract
:1. Introduction
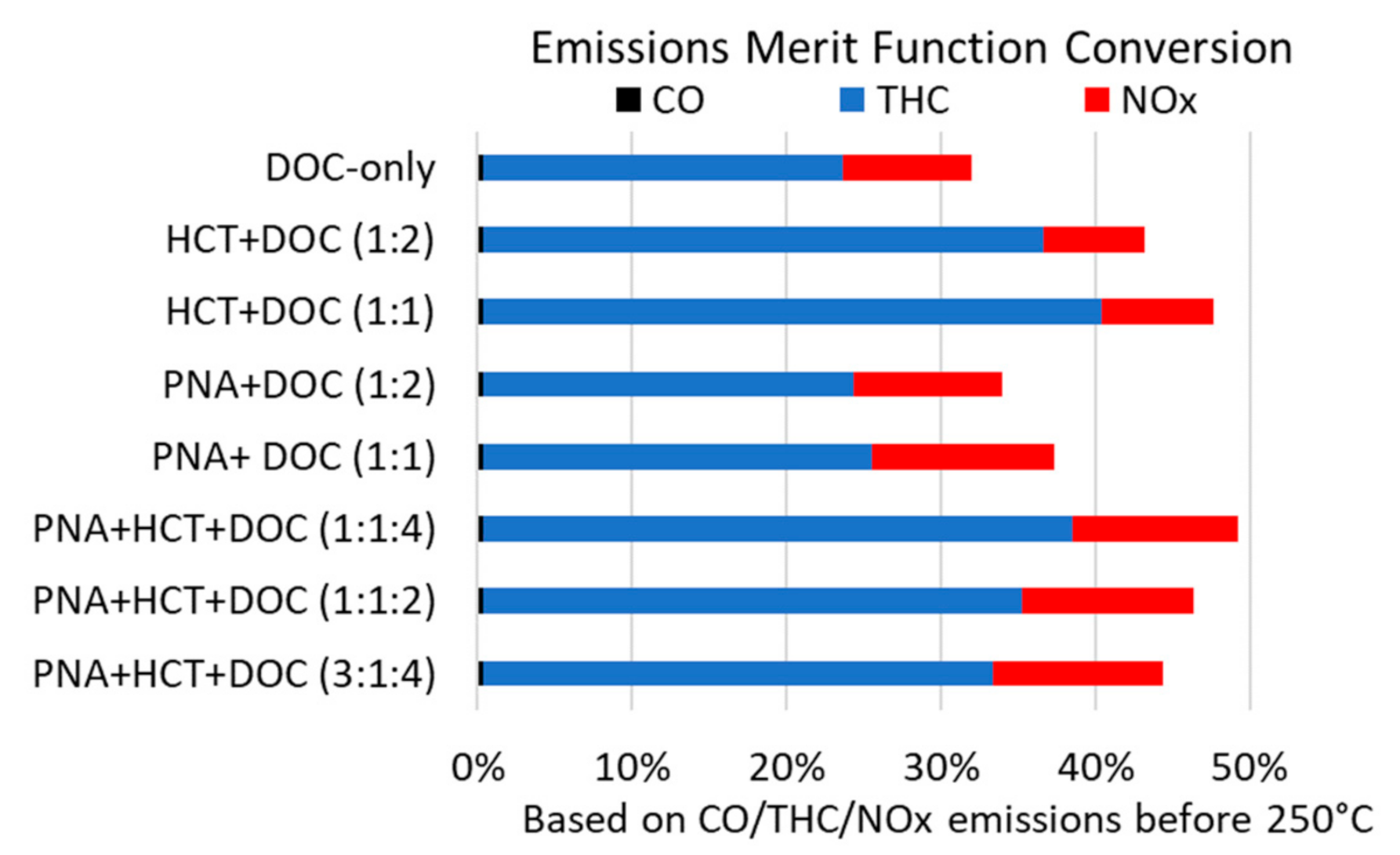
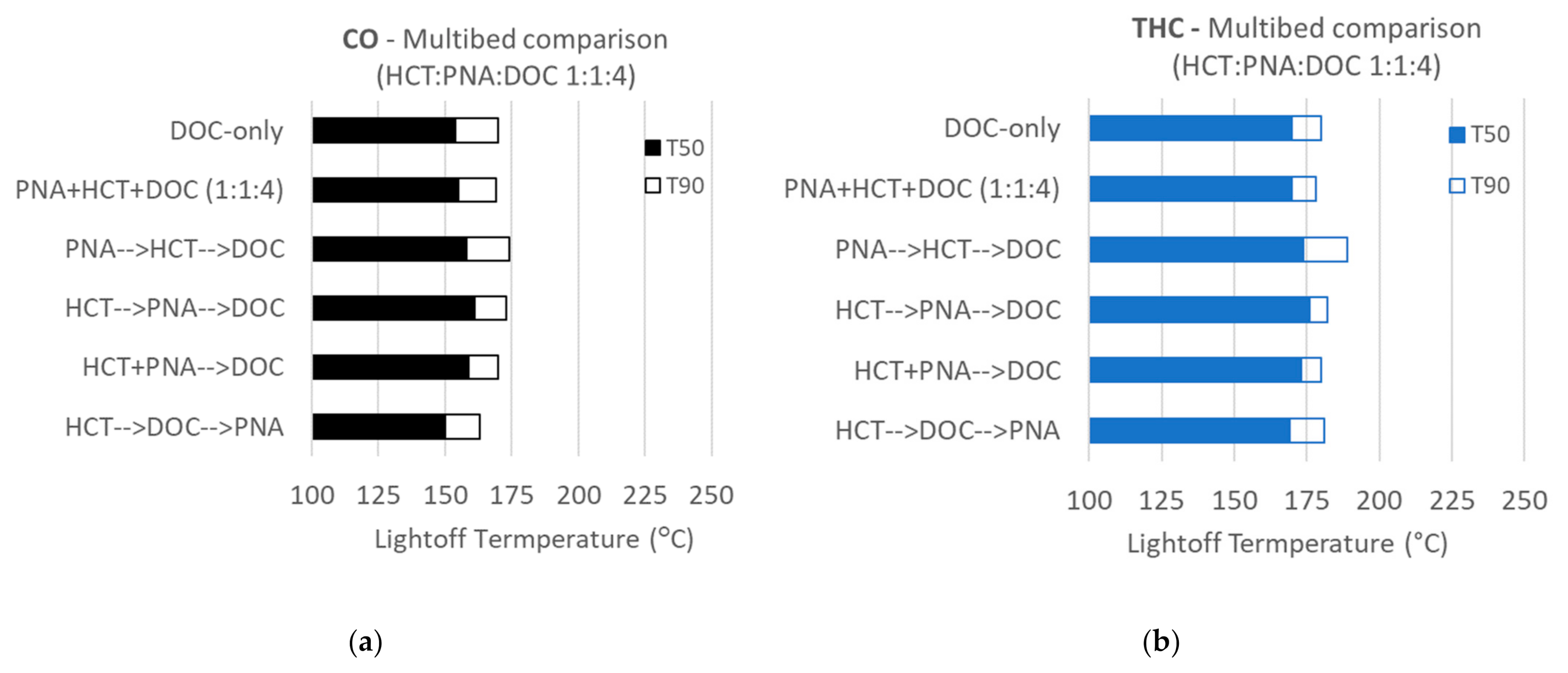
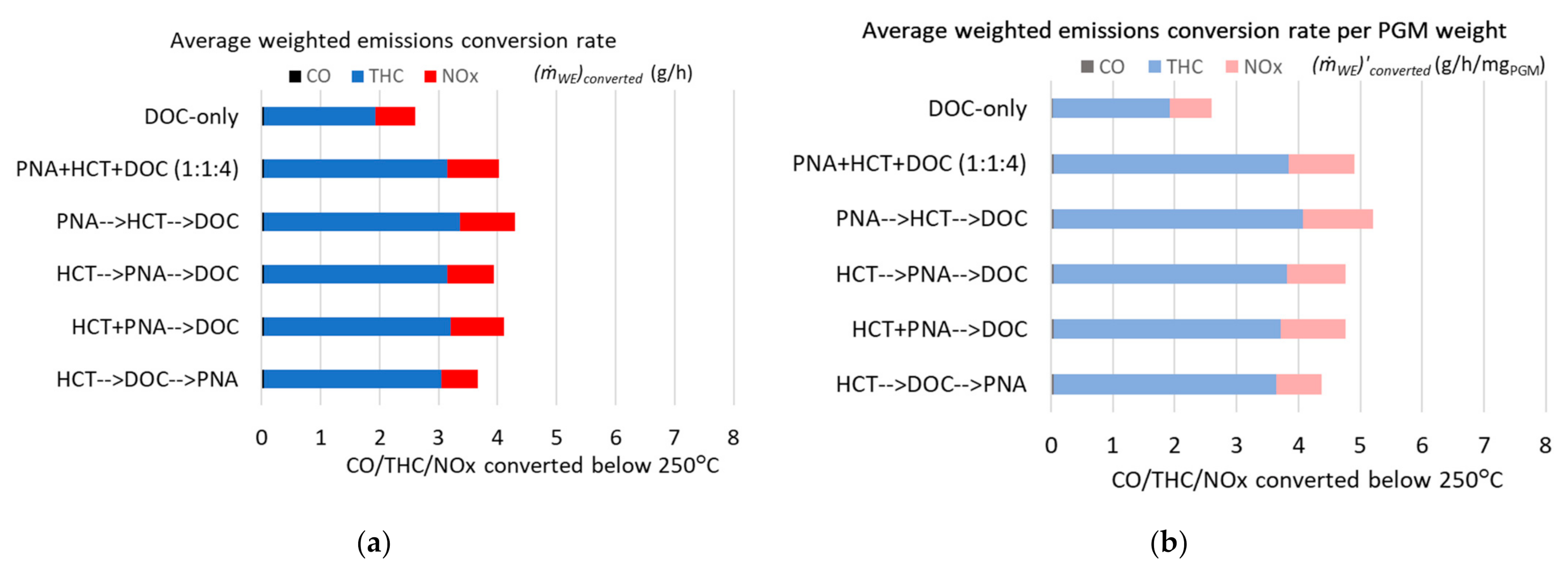
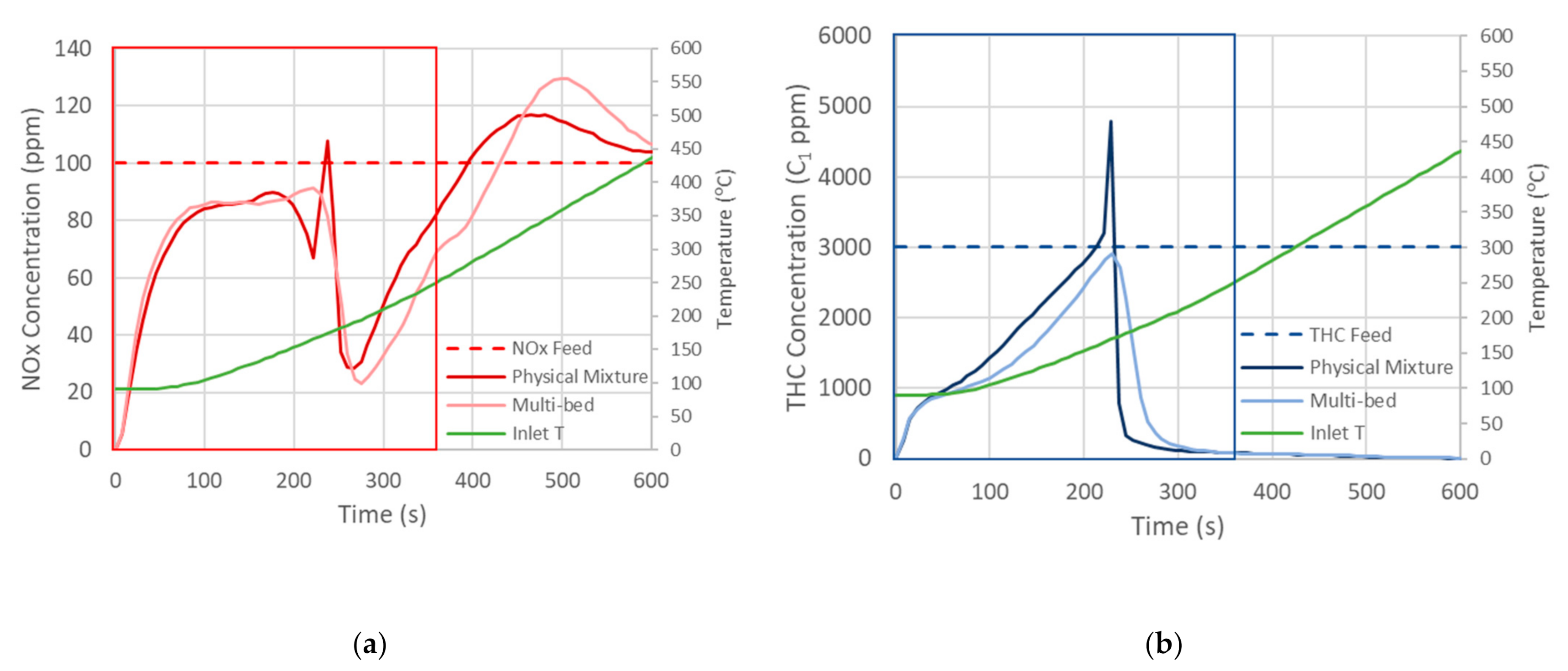
2. Results and Discussion

| CO | THC | NOx | ||
|---|---|---|---|---|
| (CO) | (C1H1.8) | (NO1.1) | ||
| Future heavy-duty regulation | 15.5 | 0.14 | 0.02 | g/bhp/hr |
| Tailpipe ratios | 775.0 | 7.0 | 1.0 | |
| Weighting Factor (WF) | 0.0013 | 0.14 | 1 | 1/tailpipe ratios |
| Molecular weight | 28.0 | 13.8 | 31.6 | g/mol |
| Feed Concentration (LTC-D) | 2000 | 3000 | 100 | ppm (vol) |
| Mass flowrate (at 333 s-cm3/min) | 50.0 | 37.0 | 2.8 | g/hr |
3. Experimental
3.1. Catalyst Preparation
3.2. Reactor
3.3. Catalyst Evaluation
- Heat reactor to 700 °C in H2O, CO2, and O2 at 20 °C/min, and hold for 4 h;
- Cool to 100 °C in H2O, CO2, and O2 as fast as possible, and stabilize for 2 h;
- Simultaneously introduce THC/CO/NOx to reactor and immediately begin ramp up to 600 °C at 40 °C/min, then hold for 1 h;
- Switch off THC/CO/NOx flows and cool to 100 °C for repeated measurements;
- Record a total of three 40 °C/min ramps to 600 °C.
4. Conclusions
- Emission standards for NOx, THC, and CO;
- Expected emission concentrations and mass flow rates;
- Platinum group metal (PGM) content (if desired).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zammit, M.; Di Maggio, C.; Kim, C.; Lambert, C.; Muntean, G.; Peden, C.; Parks, J.; Howden, K. Future Automotive Aftertreatment Solutions: The 150 °C Challenge Workshop Report. Available online: https://cleers.org/wp-content/uploads/2012_The_150C_Challenge_Workshop_Report.pdf (accessed on 4 April 2022).
- U.S. Environmental Protection Agency. EPA Proposes Tier 3 Motor Vehicle Emission and Fuel Standards; EPA-420-F-13-016a; U.S. Environmental Protection Agency: Washington, DC, USA, 2013; pp. 1–4. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi/P100G84Y.PDF?Dockey=P100G84Y.PDF (accessed on 4 April 2022).
- U.S. Environmental Protection Agency; U.S. Department of Transportation; National Highway Traffic Safety Administration. 77 FR 62623—2017 and Later Model Year Light-Duty Vehicle Greenhouse Gas Emissions and Corporate Average Fuel Economy Standards; Office of the Federal Register, National Archives and Records Administration: College Park, MD, USA, 2012; Volume 77, pp. 62623–63200.
- U.S. Environmental Protection Agency. Certification and Compliance. Available online: https://www.epa.gov/vehicles-and-engines (accessed on 10 November 2016).
- California Environmental Protection Agency Air Resources Board. Amendments to the Low-Emission Vehicle Program—LEV III. Available online: www.arb.ca.gov/msprog/levprog/leviii/leviii.htm (accessed on 28 July 2016).
- Chilumukuru, K.; Gupta, A.; Ruth, M.; Cunningham, M.; Kothandaraman, G.; Cumaranatunge, L.; Hess, H. Aftertreatment Architecture and Control Methodologies for Future Light Duty Diesel Emission Regulations. SAE Int. J. Engines 2017, 10, 1580–1587. [Google Scholar] [CrossRef]
- Lou, D.; Zhao, Y.; Zhang, Y.; Sun, Y. Modeling and Analysis on Emission Characteristics of Light-Duty Diesel Engine After-Treatment System Based on Neural Network; SAE Technical Paper, 2021-01-0595; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Dahodwala, M.; Joshi, S.; Koehler, E.; Franke, M.; Tomazic, D. Strategies for Meeting Phase 2 GHG and Ultra-Low NOx Emission Standards for Heavy-Duty Diesel Engines. SAE Int. J. Engines 2018, 11, 1109–1122. [Google Scholar] [CrossRef]
- DieselNet. United States: Heavy-Duty Onroad Engines. Available online: https://dieselnet.com/standards/us/hd.php (accessed on 28 October 2021).
- Joshi, A. Review of Vehicle Engine Efficiency and Emissions; SAE Technical Paper, 2021-01-0575; SAE International: Warrendale, PA, USA, 2021; Volume 1. [Google Scholar] [CrossRef]
- Park, J.; Park, S.J.; Nam, I.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. A fast and quantitative assay for developing zeolite-type hydrocarbon trap catalyst. Microporous Mesoporous Mater. 2007, 101, 264–270. [Google Scholar] [CrossRef]
- Park, J.; Park, S.J.; Ahn, H.A.; Nam, I.; Yeo, G.K.; Kil, J.K.; Youn, Y.K. Promising zeolite-type hydrocarbon trap catalyst by a knowledge-based combinatorial approach. Microporous Mesoporous Mater. 2009, 117, 178–184. [Google Scholar] [CrossRef]
- Toops, T.; Binder, A.; Kunal, P.; Kyriakidou, E.; Choi, J.-S. Analysis of Ion-Exchanged ZSM-5, BEA, and SSZ-13 Zeolite Trapping Materials under Realistic Exhaust Conditions. Catalysts 2021, 11, 449. [Google Scholar] [CrossRef]
- Endo, Y.; Nishikawa, J.; Iwakura, H.; Inamura, M.; Wakabayashi, T.; Nakahara, Y.; Ogasawara, M.; Sumio, K. Development of Highly Durable Zeolites as Hydrocarbon Trap Materials for Automotive Catalysts; SAE Technical Paper, 2018-01-0947; SAE International: Warrendale, PA, USA, 2018. [Google Scholar] [CrossRef]
- Luo, J.; McCabe, R.W.; Dearth, M.A.; Gorte, R.J. Transient adsorption studies of automotive hydrocarbon traps. AIChE J. 2014, 60, 2875–2881. [Google Scholar] [CrossRef]
- Moser, D.H.; Nipunage, S.; Nunan, J.; Day, R.; Alltizer, C. An Unconventional Application of a HC Trap to Meet SULEV20; SAE Technical Paper 2021-01-0574; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Malamis, S.A.; Harold, M.P. Optimizing the lean hydrocarbon NOx trap: Sequential and dual-layer configurations. Catal. Today 2021, 360, 388–400. [Google Scholar] [CrossRef]
- Malamis, S.A.; Harold, M.P.; Epling, W.S. Coupled NO and C3H6 Trapping, Release and Conversion on Pd/BEA: Evaluation of the Lean Hydrocarbon NOx Trap. Ind. Eng. Chem. Res. 2019, 58, 22912–22923. [Google Scholar] [CrossRef]
- Lee, J. Zeolite-Based Hydrocarbon Traps and Passive NOx Adsorbers for Vehicle Cold Start Applications (Order No. 28417752). Ph.D. Thesis, State University of New York at Buffalo, New York, NY, USA, 2021; p. 2555619491. [Google Scholar]
- Lee, J.; Theis, J.R.; Kyriakidou, E.A. Vehicle emissions trapping materials: Successes, challenges, and the path forward. Appl. Catal. B 2019, 243, 397–414. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hu, J.Z.; Gao, F.; Wang, Y.; Szanyi, J. Palladium/Zeolite Low Temperature Passive NOx Adsorbers (PNA): Structure-Adsorption Property Relationships for Hydrothermally Aged PNA Materials. Emiss. Control Sci. Technol. 2020, 6, 126–138. [Google Scholar] [CrossRef]
- Gu, Y.; Epling, W.S. Passive NOx adsorber: An overview of catalyst performance and reaction chemistry. Appl. Catal. A Gen. 2019, 570, 1–14. [Google Scholar] [CrossRef]
- Ji, Y.; Bai, S.; Crocker, M. Al2O3-based passive NOx adsorbers for low temperature applications. Appl. Catal. B Environ. 2015, 170, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Berndt, C.T. An Experimental Study of a Passive NOx Adsorber (PNA) for the Reduction of Cold Start Diesel Emissions (Order No. 27668392). Ph.D. Thesis, Michigan Technological University, Houghton, MI, USA, 2019; p. 2377950409. [Google Scholar]
- Kunal, P.; Toops, T.J.; Kidder, M.K.; Lance, M.J. Deactivation trends of Pd/SSZ-13 under the simultaneous presence of NO, CO, hydrocarbons and water for passive NOx adsorption. Appl. Catal. B Environ. 2021, 299, 120591. [Google Scholar] [CrossRef]
- HChen, Y.; Mulla, S.; Weigert, E.; Camm, K.; Ballinger, T.; Cox, J.; Blakeman, P. Cold Start Concept (CSCTM): A Novel Catalyst for Cold Start Emission Control; SAE Technal Paper; SAE International: Warrendale, PA, USA, 2013; Volume 2, pp. 372–381. [Google Scholar]
- Chen, H.Y.; Collier, J.E.; Liu, D.; Mantarosie, L.; Duran-Martín, D.; Novak, V.; Rajaram, R.R.; Thompsett, D. Low temperature NO storage of zeolite supported Pd for low temperature diesel engine emission control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Ballinger, T.; Manning, W.; Lafyatis, D. Hydrocarbon Trap Technology for the Reduction of Cold-Start Hydrocarbon Emissions; SAE Technical Paper 970741; SAE International: Warrendale, PA, USA, 1997. [Google Scholar] [CrossRef]
- Murakami, K.; Tominaga, S.; Hamada, I.; Nagayama, T.; Kijima, Y.; Katougi, K.; Nakagawa, S. Development of a High Performance Catalyzed Hydrocarbon Trap Using Ag-Zeolite; SAE Technical Paper 2004-01-1275; SAE International: Warrendale, PA, USA, 2004. [Google Scholar] [CrossRef]
- Murata, Y.; Morita, T.; Wada, K.; Ohno, H. NOx Trap Three-Way Catalyst (N-TWC) Concept: TWC with NOx Adsorption Properties at Low Temperatures for Cold-Start Emission Control. SAE Int. J. Fuels Lubr. 2015, 8, 454–459. [Google Scholar] [CrossRef]
- Addy Majewski, W. Urea Dosing and Injection Systems, DieselNet. Available online: https://dieselnet.com/tech/cat_scr_diesel_urea_dosing.php#:~:text=The%20highest%20risk%20of%20deposit,about%20125%2D175%C2%B0C (accessed on 28 October 2021).
- Lee, J.; Ryou, Y.S.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Wang, A.; Lindgren, K.; Di, M.; Bernin, D.; Carlsson, P.-A.; Thuvander, M.; Olsson, L. Insight into hydrothermal aging effect on Pd sites over Pd/LTA and Pd/SSZ-13 as PNA and CO oxidation monolith catalysts. Appl. Catal. B Environ. 2020, 278, 119315. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Hwang, S.; Lee, E.; Lee, H.; Kim, C.H.; Kim, D.H. Deactivation of Pd/Zeolites passive NOx adsorber induced by NO and H2O: Comparative study of Pd/ZSM-5 and Pd/SSZ-13. Catal. Today 2021, 360, 350–355. [Google Scholar] [CrossRef]
- Theis, J.R.; Ura, J.A. Assessment of zeolite-based Low temperature NOx adsorbers: Effect of reductants during multiple sequential cold starts. Catal. Today 2021, 360, 340–349. [Google Scholar] [CrossRef]
- Zhao, H.; Hill, A.J.; Ma, L.; Bhat, A.; Jing, G.; Schwank, J.W. Progress and future challenges in passive NO adsorption over Pd/zeolite catalysts. Catal. Sci. Technol. 2021, 11, 5986–6000. [Google Scholar] [CrossRef]
- Gu, Y.; Marino, S.; Cortés-Reyes, M.; Pieta, I.S.; Pihl, J.A.; Epling, W.S. Integration of an Oxidation Catalyst with Pd/Zeolite-Based Passive NOx Adsorbers: Impacts on Degradation Resistance and Desorption Characteristics. Ind. Eng. Chem. Res. 2021, 60, 6455–6464. [Google Scholar] [CrossRef]
- Gu, Y.; Zelinsky, R.P.; Chen, Y.; Epling, W.S. Investigation of an irreversible NOx storage degradation Mode on a Pd/BEA passive NOx adsorber. Appl. Catal. B Environ. 2019, 258, 118032. [Google Scholar] [CrossRef]
- Gu, Y.; Majumdar, S.S.; Pihl, J.A.; Epling, W.S. Investigation of NO adsorption and desorption phenomena on a Pd/ZSM-5 passive NOx adsorber. Appl. Catal. B Environ. 2021, 298, 120561. [Google Scholar] [CrossRef]
- Rappé, K.; DiMaggio, C.; Pihl, J.; Theis, J.R.; Oh, S.H.; Fisher, G.B.; Parks, J.; Easterling, V.G.; Yang, M.; Stewart, M.L.; et al. Aftertreatment Protocols for Catalyst Characterization and Performance Evaluation: Low-Temperature Oxidation, Storage, Three-Way, and NH3-SCR Catalyst Test Protocols. Emiss. Control. Sci. Technol. 2019, 5, 183. [Google Scholar] [CrossRef]
- Villamaina, R.; Iacobone, U.; Nova, I.; Tronconi, E.; Ruggeri, M.P.; Mantarosie, L.; Collier, J.; Thompsett, D. Mechanistic insight in NO trapping on Pd/Chabazite systems for the low-temperature NOx removal from Diesel exhausts. Appl. Catal. B Environ. 2021, 284, 119724. [Google Scholar] [CrossRef]
- Khivantsev, K.; Gao, F.; Kovarik, L.; Wang, Y.; Szanyi, J. Molecular Level Understanding of How Oxygen and Carbon Monoxide Improve NOx Storage in Palladium/SSZ-13 Passive NOx Adsorbers: The Role of NO+ and Pd(II)(CO)(NO) Species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Zelinsky, R.; Epling, W.S. Effects of CO and H2O Co-Feed on the Adsorption and Oxidation Properties of a Pd/BEA Hydrocarbon Trap. Catalysts 2021, 11, 348. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E., II. Overview of the Fundamental Reactions and Degradation Mechanisms of NOx Storage/Reduction Catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Medhekar, V.; Balakotaiah, V.; Harold, M.P. TAP study of NOx storage and reduction on Pt/Al2O3 and Pt/Ba/Al2O3. Catal. Today 2007, 121, 226–236. [Google Scholar] [CrossRef]
- Fridell, E.; Persson, H.; Westerberg, B.; Olsson, L.; Skoglundh, M. The mechanism for NOx storage. Catal. Lett. 2000, 66, 71–74. [Google Scholar] [CrossRef]
- Szailer, T.; Kwak, J.H.; Kim, D.H.; Hanson, J.C.; Peden, C.H.F.; Szanyi, J. Reduction of stored NOx on Pt/Al2O3 and Pt/BaO/Al2O3 catalysts with H2 and CO. J. Catal. 2006, 239, 51–64. [Google Scholar] [CrossRef]
- Olsson, L.; Fridell, E. The Influence of Pt Oxide Formation and Pt Dispersion on the Reactions NO2⇔NO+1/2 O2 over Pt/Al2O3 and Pt/BaO/Al2O3. J. Catal. 2002, 210, 340–353. [Google Scholar] [CrossRef]
- Olsson, L.; Jozsa, P.; Nilsson, M.; Jobson, E. Fundamental studies of NOx storage at low temperatures. Top. Catal. 2007, 42, 95–98. [Google Scholar] [CrossRef]
- Toops, T.J.; Smith, D.B.; Epling, W.S.; Parks, J.E.; Partridge, W.P. Quantified NOx adsorption on Pt/K/gamma-Al2O3 and the effects of CO2 and H2O. Appl. Catal. B Environ. 2007, 58, 255–264. [Google Scholar] [CrossRef]
- Burch, R.; Millington, P.J. Selective Reduction of NOx by Hydrocarbons in Excess Oxygen by Alumina and Silica-supported Catalysts. Catal. Today 1996, 29, 37–42. [Google Scholar] [CrossRef]
- Burch, R.; Watling, T.C. The Effect of Promoters on Pt/A12O3 Catalysts for The Reduction of NO by C3H6 under Lean-burn Conditions. Appl. Catal. B 1997, 11, 207–216. [Google Scholar] [CrossRef]
- IEA-AMF. Composition of Gasoline and Diesel. Available online: https://www.iea-amf.org/content/fuel_information/diesel_gasoline (accessed on 29 October 2021).
- DieselNet. Gaseous Emissions. Available online: https://dieselnet.com/tech/emi_gas.php (accessed on 29 October 2021).





| Physical Mixture | PNA | HCT | DOC | Total | Flow Rate | WHSV | PGM |
|---|---|---|---|---|---|---|---|
| Composition | (mg) | (mg) | (mg) | (mg) | (s-cm3/min) | (L/g/h) | (mg) |
| DOC-only | 0 | 0 | 100.6 | 100.6 | 333 | 199 | 1.01 |
| HCT + DOC (1:2) | 0 | 33.9 | 67.5 | 101.4 | 333 | 197 | 0.68 |
| HCT + DOC (1:1) | 0 | 51.6 | 53.1 | 104.7 | 333 | 191 | 0.53 |
| PNA + DOC (1:2) | 32.8 | 0 | 65.9 | 98.7 | 333 | 202 | 0.99 |
| PNA+ DOC (1:1) | 52.2 | 0 | 49.9 | 102.1 | 333 | 196 | 1.02 |
| PNA + HCT + DOC (1:1:4) | 16.9 | 18.4 | 65.1 | 100.4 | 333 | 199 | 0.82 |
| PNA + HCT + DOC (1:1:2) | 25.9 | 26.5 | 51.5 | 103.9 | 333 | 192 | 0.77 |
| PNA + HCT + DOC (3:1:4) | 38.6 | 13.8 | 49.1 | 101.5 | 333 | 197 | 0.88 |
| Physical Mixture | PNA | HCT | DOC | Total | Flow Rate | WHSV | PGM |
|---|---|---|---|---|---|---|---|
| Composition | (mg) | (mg) | (mg) | (mg) | (s-cm3/min) | (L/g/h) | (mg) |
| PNA→HCT→DOC | 16.7 | 16.9 | 65.7 | 99.3 | 333 | 201 | 0.82 |
| HCT→PNA→DOC | 17.9 | 18.0 | 64.7 | 100.6 | 333 | 199 | 0.83 |
| HCT + PNA→DOC | 18.4 | 18.2 | 67.7 | 104.3 | 333 | 192 | 0.86 |
| HCT→DOC→PNA | 17.9 | 18.6 | 65.8 | 102.3 | 333 | 195 | 0.84 |
| Gas Component | Concentration (Volume) |
|---|---|
| THC: Total Hydrocarbons (C1-basis) | 3000 ppm |
| C2H4 | 500 ppm |
| C3H6 | 300 ppm |
| C3H8 | 100 ppm |
| C10H22 | 2100 ppm |
| CO | 2000 ppm |
| NO | 100 ppm |
| H2O | 6% |
| CO2 | 6% |
| O2 | 12% |
| Ar | Balance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toops, T.J.; Kunal, P. Emissions Merit Function for Evaluating Multifunctional Catalyst Beds. Catalysts 2022, 12, 419. https://doi.org/10.3390/catal12040419
Toops TJ, Kunal P. Emissions Merit Function for Evaluating Multifunctional Catalyst Beds. Catalysts. 2022; 12(4):419. https://doi.org/10.3390/catal12040419
Chicago/Turabian StyleToops, Todd J., and Pranaw Kunal. 2022. "Emissions Merit Function for Evaluating Multifunctional Catalyst Beds" Catalysts 12, no. 4: 419. https://doi.org/10.3390/catal12040419
APA StyleToops, T. J., & Kunal, P. (2022). Emissions Merit Function for Evaluating Multifunctional Catalyst Beds. Catalysts, 12(4), 419. https://doi.org/10.3390/catal12040419








