Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Chemicals and Analysis
2.2. Biochar Preparation
2.3. Experimental Procedure
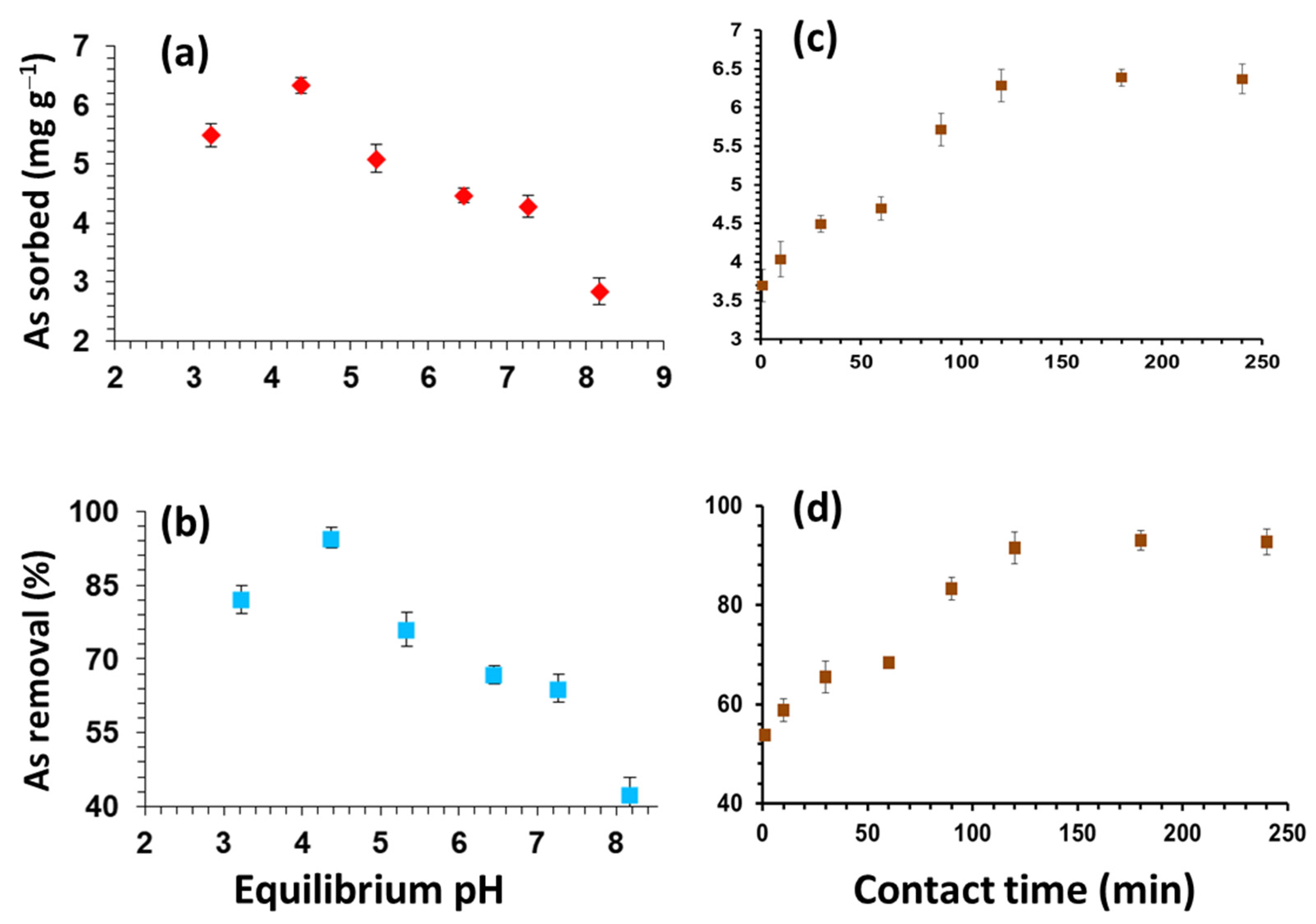
2.4. pH
2.5. Contact Time and Kinetic Modeling
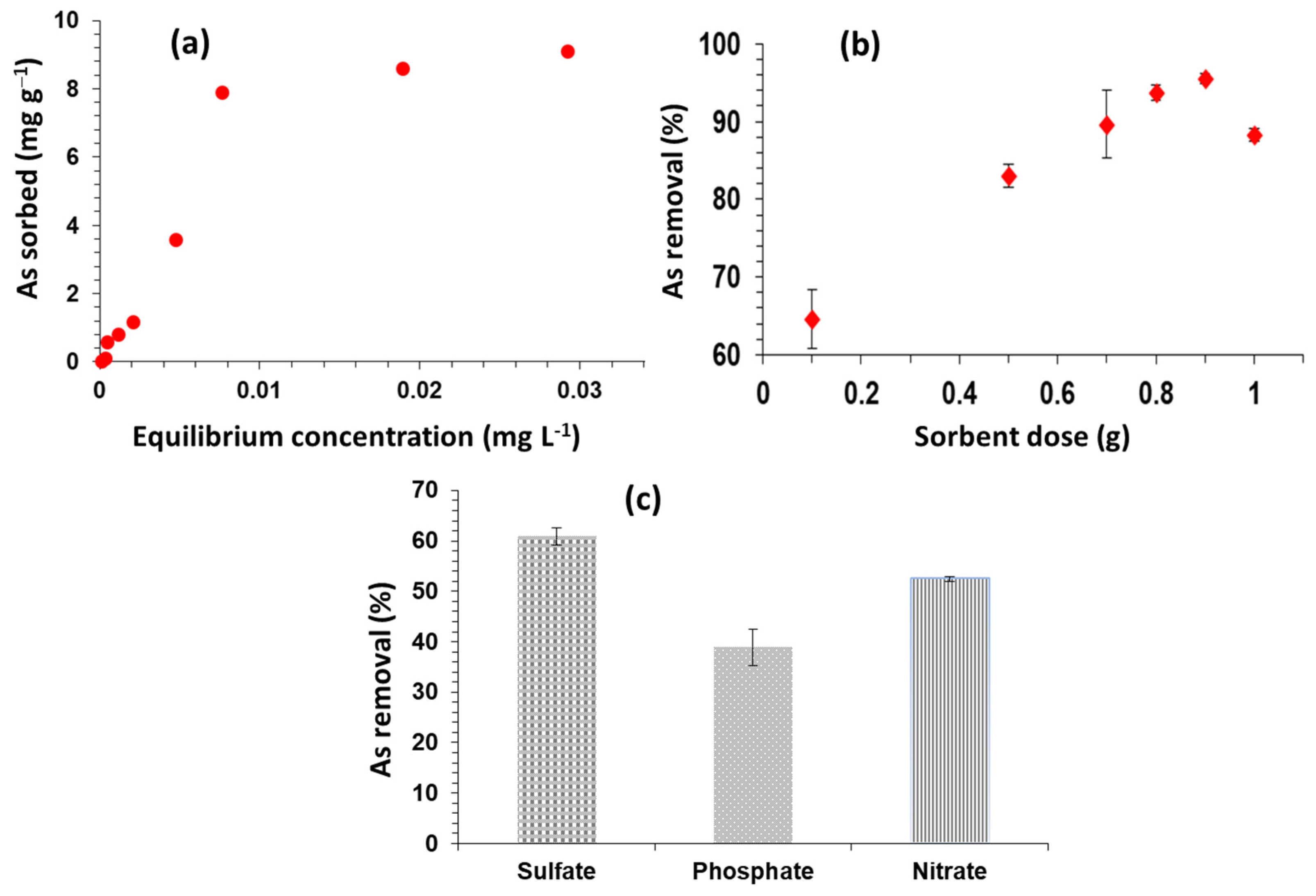
2.6. Initial Concentration of As
2.7. Biochar Dose
2.8. Effect of Anions
2.9. Molybdenum Blue Method for As Analysis
2.10. Analyses of Surface Properties
3. Results and Discussion
3.1. Solution pH
3.2. Contact Time and Kinetic Modelling
3.3. Initial As(V) Concentration
3.4. Sorbent Dose
3.5. Effects of Anions
3.6. FTIR
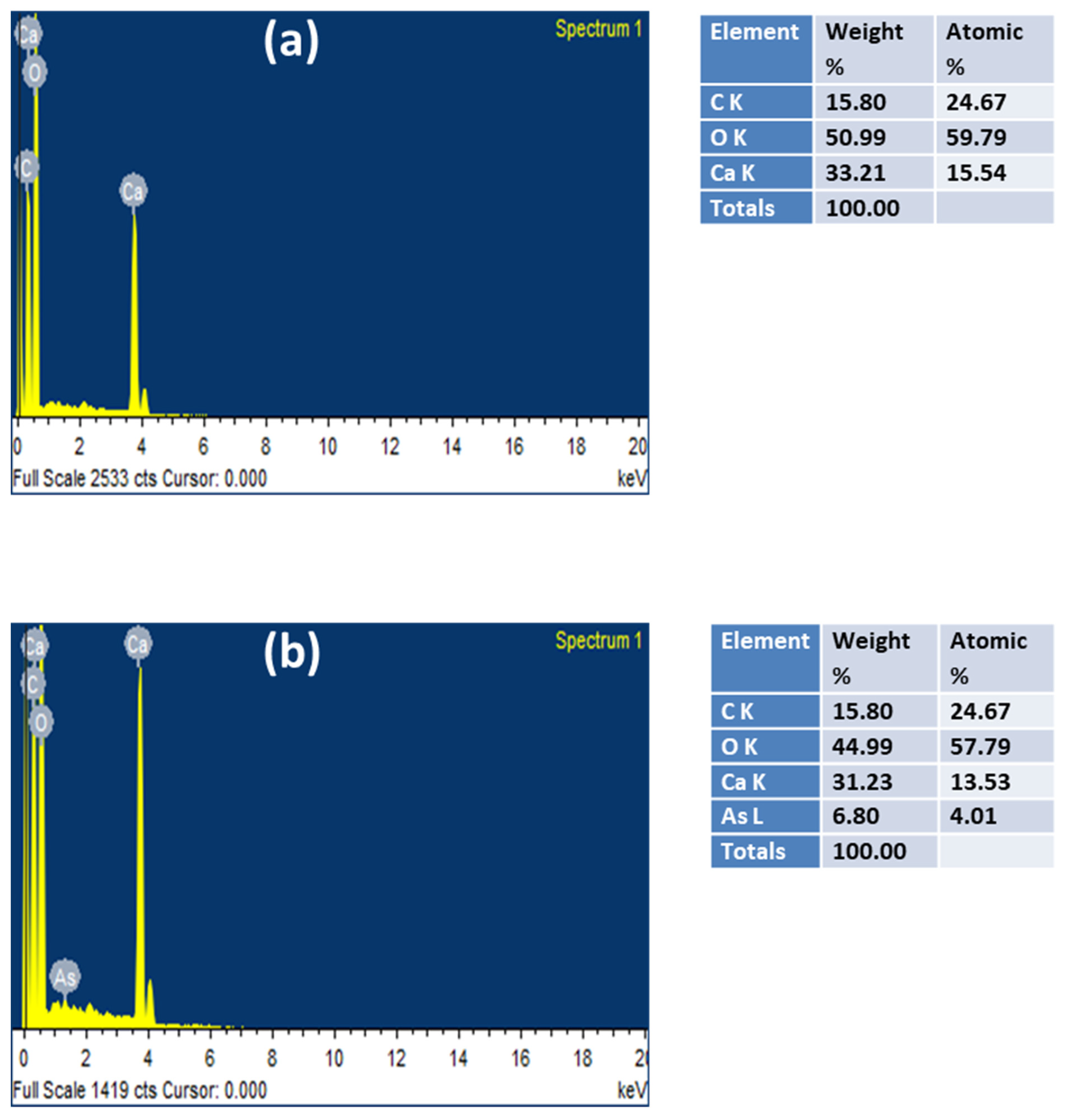
3.7. SEM-EDS
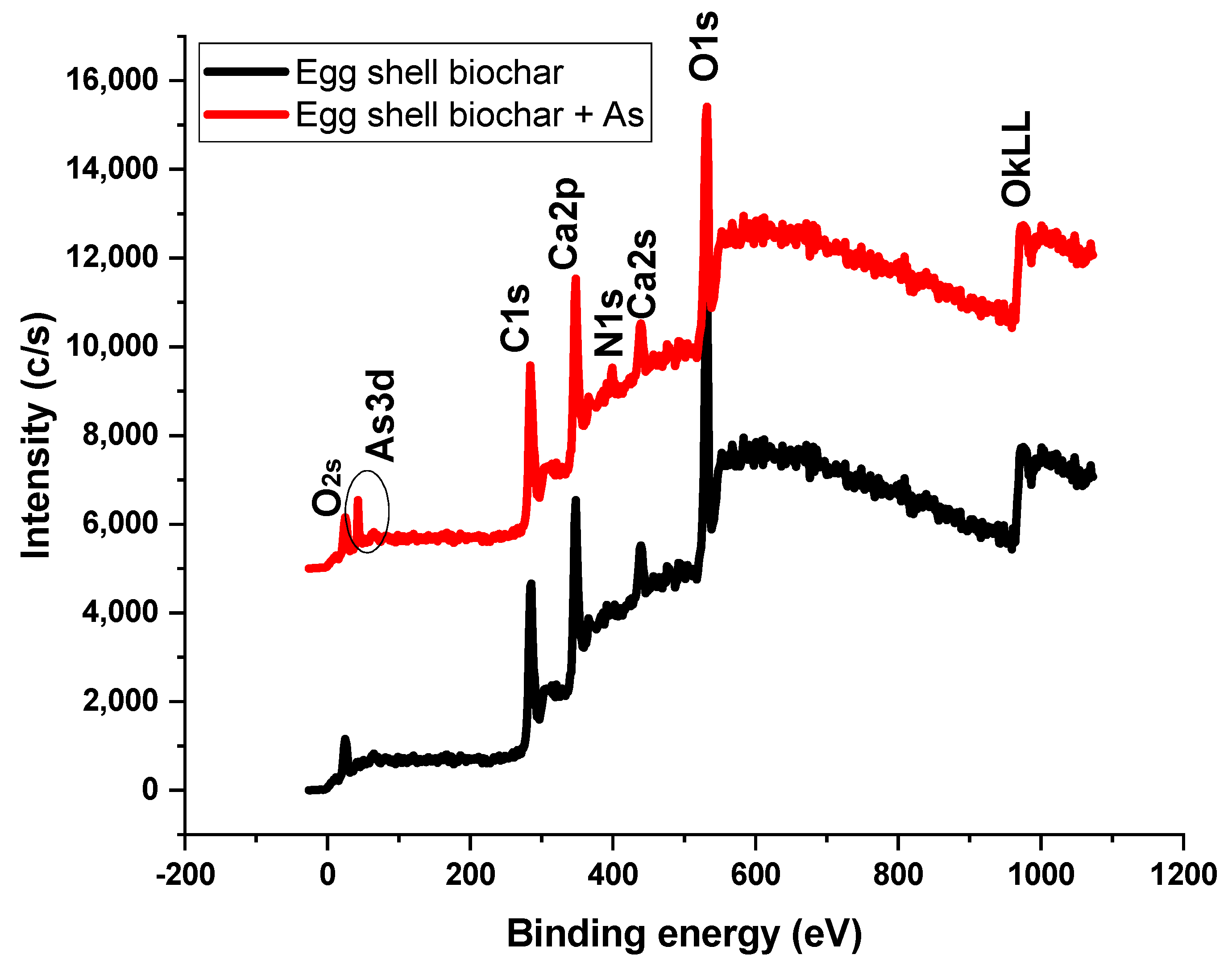
3.8. XPS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muthusaravanan, S.; Sivarajasekar, N.; Vivek, J.S.; Paramasivan, T.; Naushad, M.; Prakashmaran, J.; Gayathri, V.; Al-Duaij, O.K. Phytoremediation of Heavy Metals: Mechanisms, Methods and Enhancements. Environ. Chem. Lett. 2018, 16, 1339–1359. [Google Scholar] [CrossRef]
- Nadeem, N.; Zahid, M.; Tabasum, A.; Mansha, A.; Jilani, A.; Bhatti, I.A.; Bhatti, H.N. Degradation of reactive dye using heterogeneous photo-Fenton catalysts: ZnFe2O4 and GO-ZnFe2O4 composite. Mater. Res. Express 2020, 7, 015519. [Google Scholar] [CrossRef]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative Efficiency of Peanut Shell and Peanut Shell Biochar for Removal of Arsenic from Water. Environ. Sci. Pollut. Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef] [PubMed]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic Pollution Sources. Rev. Environ. Contam. Vol. 2009, 197, 17–60. [Google Scholar]
- Welch, A.H.; Westjhon, D.B.; Helsel, D.R.; Wanty, R.B. Arsenic in Ground Water of the United States: Occurrence and Geochemistry. Groundwater 2000, 38, 589–604. [Google Scholar] [CrossRef]
- Rai, A.; Tripathi, P.; Dwivedi, S.; Dubey, S.; Shri, M.; Kumar, S.; Tripathi, P.K.; Dave, R.; Kumar, A.; Singh, R. Arsenic Tolerances in Rice (Oryza Sativa) Have a Predominant Role in Transcriptional Regulation of Set of Genes Including Sulphur Assimilation Pathway and Antioxidant System. Chemosphere 2011, 82, 986–995. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Das, B.; Murrill, M.; Dey, S.; Mukherjee, S.C.; Dhar, R.K.; Biswas, B.K.; Chowdhury, U.K.; Roy, S.; et al. Status of Groundwater Arsenic Contamination in Bangladesh: A 14-Year Study Report. Water Res. 2010, 44, 5789–5802. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011; Volume 4.
- Siddiqui, S.I.; Naushad, M.; Chaudhry, S.A. Promising Prospects of Nanomaterials for Arsenic Water Remediation: A Comprehensive Review. Process Saf. Environ. Prot. 2019, 126, 60–97. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2016, 13, 62. [Google Scholar] [CrossRef]
- Jilani, A.; Othman, M.H.D.; Ansari, M.O.; Hussain, S.Z.; Ismail, A.F.; Khan, I.U. Graphene and its derivatives: Synthesis, modifications, and applications in wastewater treatment. Environ. Chem. Lett. 2018, 16, 1301–1323. [Google Scholar] [CrossRef]
- Al-Othman, Z.A.; Ali, R.; Naushad, M. Hexavalent Chromium Removal from Aqueous Medium by Activated Carbon Prepared from Peanut Shell: Adsorption Kinetics, Equilibrium and Thermodynamic Studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Mathew, B.B.; Jaishankar, M.; Biju, V.G.; Beeregowda, K.N. Role of Bioadsorbents in Reducing Toxic Metals. J. Toxicol. 2016, 2016, 4369604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Shahid, M.; Saqib, Z.A.; Nawaz, M.F.; Shaheen, S.M.; Wang, H.; Tsang, D.C.W.; Bundschuh, J.; et al. Exploring the Arsenic Removal Potential of Various Biosorbents from Water. Environ. Int. 2019, 123, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Ali, S.; Rizwan, M.; Abbas, F.; Bibi, I.; Riaz, M.; Khalil, U.; Niazi, N.K.; Rinklebe, J. A review of biochar-based sorbents for separation of heavy metals from water. Int. J. Phytoremediation 2020, 22, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent Advances in Biochar Application for Water and Wastewater Treatment: A Review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef] [PubMed]
- Kokab, T.; Ashraf, H.S.; Shakoor, M.B.; Jilani, A.; Ahmad, S.R.; Majid, M.; Ali, S.; Farid, N.; Alghamdi, R.A.; Al-Quwaie, D.A.H.; et al. Effective Removal of Cr(VI) from Wastewater Using Biochar Derived from Walnut Shell. Int. J. Environ. Res. Public Health 2021, 18, 9670. [Google Scholar] [CrossRef]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A Systematic Review of Biochar Research, with a Focus on Its Stability in situ and Its Promise as a Climate Mitigation Strategy. PLoS ONE 2013, 8, e75932. [Google Scholar] [CrossRef] [Green Version]
- Makuchowska-Fryc, J. Use of the Eggshells in Removing Heavy Metals from Waste Water—The Process Kinetics and Efficiency. Ecol. Chem. Eng. S 2019, 26, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Shakoor, M.B.; Bibi, I.; Niazi, N.K.; Shahid, M.; Nawaz, M.F.; Farooqi, A.; Naidu, R.; Rahman, M.M.; Murtaza, G.; Luttge, A. The Evaluation of Arsenic Contamination Potential, Speciation and Hydrogeochemical Behaviour in Aquifers of Punjab, Pakistan. Chemosphere 2018, 199, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, M.F.; Abid, M.; Danish, S.; Saeed, M.K.; Ali, M.A. Effects of Various Biochars on Seed Germination and Carbon Mineralization in an Alkaline Soil. Pak. J. Agric. Sci. 2015, 51, 977–982. [Google Scholar]
- Prasad, K.S.; Ramanathan, A.L.; Paul, J.; Subramanian, V.; Prasad, R. Biosorption of Arsenate (As+3) and Arsenate (As+5) from Aqueous Solution by Arthrobacter sp. Biomass. Environ. Technol. 2013, 34, 2701–2708. [Google Scholar] [CrossRef]
- Lenoble, V.; Deluchat, V.; Serpaud, B.; Bollinger, J.C. Arsenite Oxidation and Arsenate Determination by the Molybdene Blue Method. Talanta 2003, 61, 267–276. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Nawaz, R.; Hussain, F.; Raza, M.; Ali, S.; Rizwan, M.; Oh, S.-E.; Ahmad, S. Human Health Implications, Risk Assessment and Remediation of As-Contaminated Water: A Critical Review. Sci. Total Environ. 2017, 601, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the Adsorption-Reduction Mechanism of Hexavalent Chromium by Ramie Biochars of Different Pyrolytic Temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S.; et al. Remediation of Arsenic-Contaminated Water Using Agricultural Wastes as Biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Marin-Rangel, V.M.; Cortes-Martinez, R.; Cuevas Villanueva, R.A.; Garnica-Romo, M.G.; Martinez-Flores, H.E. As (V) Biosorption in an Aqeous Solution Using Chemically Treated Lemon (Citrus Aurantifoliaswingle) Residues. J. Food Sci. 2012, 77, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M. Tea Waste as a Potential Biowaste for Removal of Hexavalent Chromium from Wastewater: Equilibrium and Kinetics Studies. Arab. J. Geosci. 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Shakoor, M.B.; Jilani, A.; Anjum, R. High sorption efficiency for As (III) and As (V) from aqueous solutions using novel almond shell biochar. Chemosphere 2020, 243, 125330. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jean, J.S.; Kar, S. Bioaccessibility and Health Risk Assessment of Arsenic in Arsenic-Enriched Soils, Central India. Ecotoxicol. Environ. Saf. 2013, 92, 252–257. [Google Scholar] [CrossRef]
- Feng, N.; Guo, X.; Liang, S.; Zhu, Y.; Liu, J. Biosorption of Heavy Metals from Aqueous Solutions by Chemically Modified Orange Peel. J. Hazard. Mater. 2011, 185, 49–54. [Google Scholar] [CrossRef]
- Sahmoune, M.N.; Louhab, K.; Boukhiar, A. Advanced Biosorbents Materials for Removal of Chromium from Water and Wastewaters. Environ. Prog. Sustain. Energy 2011, 30, 284–293. [Google Scholar] [CrossRef]
- Pehlivan, E.; Tran, H.T.; Ouédraogo, W.K.I.; Schmidt, C.; Zachmann, D.; Bahadir, M. Sugarcane Bagasse Treated with Hydrous Ferric Oxide as a Potential Adsorbent for the Removal of As (V) From Aqueous Solutions. Food Chem. 2013, 138, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, H.T.; Pehlivan, E. Cr6+ removal using oleaster (Elaeagnus) seed and cherry (Prunus avium) stone biochar. Powder Technol. 2017, 306, 61–67. [Google Scholar] [CrossRef]
- Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and removal of chromium VI in water by powdered activated carbon. Materials 2018, 11, 269. [Google Scholar] [CrossRef] [Green Version]



| PFO | PSO | ||||
|---|---|---|---|---|---|
| qe (mg g−1) | k1 (min−1) | R2 | qe (mg g−1) | k2 (g mg−1 min−1) | R2 |
| 3.89 | 0.01 | 0.97 | 6.53 | 0.15 | 0.99 |
| Isotherm Models | Parameters | |
|---|---|---|
| Langmuir | QL | 0.89 (mg g−1) |
| R2 | 0.89 | |
| KL | 5.55 (L g−1) | |
| Freundlich | QF | 1.83 (mg1−n g−1 Ln) |
| R2 | 0.97 | |
| N | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akram, A.; Muzammal, S.; Shakoor, M.B.; Ahmad, S.R.; Jilani, A.; Iqbal, J.; Al-Sehemi, A.G.; Kalam, A.; Aboushoushah, S.F.O. Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions. Catalysts 2022, 12, 431. https://doi.org/10.3390/catal12040431
Akram A, Muzammal S, Shakoor MB, Ahmad SR, Jilani A, Iqbal J, Al-Sehemi AG, Kalam A, Aboushoushah SFO. Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions. Catalysts. 2022; 12(4):431. https://doi.org/10.3390/catal12040431
Chicago/Turabian StyleAkram, Asma, Shazma Muzammal, Muhammad Bilal Shakoor, Sajid Rashid Ahmad, Asim Jilani, Javed Iqbal, Abdullah G. Al-Sehemi, Abul Kalam, and Samia Faisal O. Aboushoushah. 2022. "Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions" Catalysts 12, no. 4: 431. https://doi.org/10.3390/catal12040431
APA StyleAkram, A., Muzammal, S., Shakoor, M. B., Ahmad, S. R., Jilani, A., Iqbal, J., Al-Sehemi, A. G., Kalam, A., & Aboushoushah, S. F. O. (2022). Synthesis and Application of Egg Shell Biochar for As(V) Removal from Aqueous Solutions. Catalysts, 12(4), 431. https://doi.org/10.3390/catal12040431








