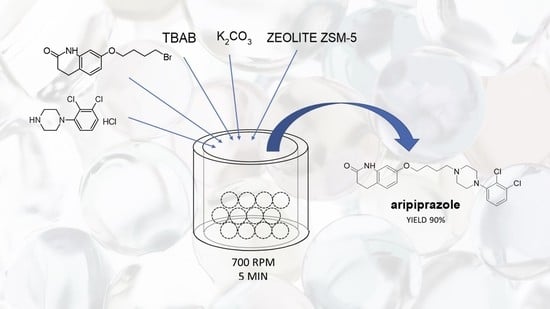
Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions
Abstract
:1. Introduction
2. Results
3. Conclusions
4. Materials and Methods
5. Synthesis
5.1. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) in a Mortar
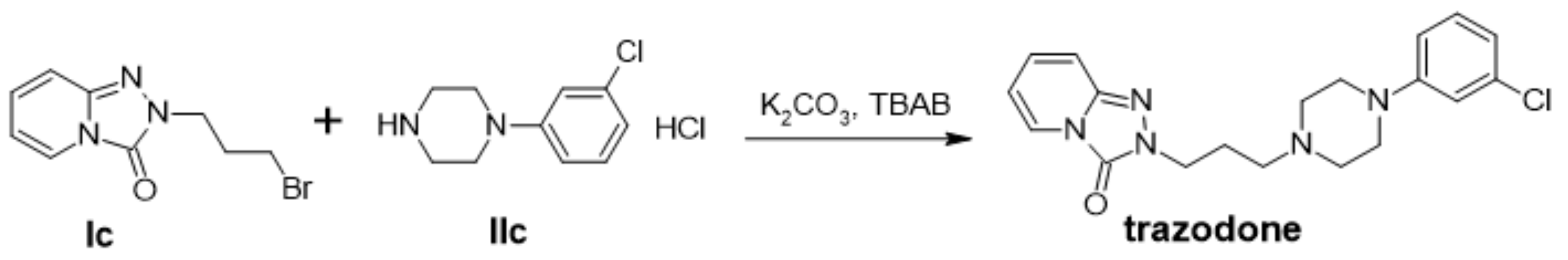
5.2. Synthesis of Trazodone (2-{3-[4-(3-Chlorophenyl)piperazin-1-yl]propyl}-[1,2,4]triazolo [4,3-a]pyridin-3-one) (IIIb) in a Mortar
5.3. Synthesis of Flibanserin (1-(2-{4-[3-(Trifluoromethyl)phenyl]piperazin-1-yl}ethyl)-1,3-dihydro-2H-benzimidazol-2-one) (IIIc) in a Mortar
5.4. Synthesis of Ipsapirone (9,9-Dioxo-8-[4-(4-pyrimidin-2-yl-piperazin-1-yl)butyl]o-9λ6-thia-8-azabicyclo [4.3.0]nona-1,3,5-trien-7-one) (IIId) in a Mortar
5.5. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl) piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.6. Synthesis of Trazodone (2-{3-[4-(3-Chlorophenyl)piperazin-1-yl]propyl}-[1,2,4]triazolo [4,3-a]pyridin-3-one) (IIIb) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.7. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Starch (23% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.8. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Starch (32% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.9. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite A (3% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.10. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite A (5.5% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.11. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite A (8% by weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.12. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite A (15% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.13. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite Y (3% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.14. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite Y (5.5% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.15. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite Y (8% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.16. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite Y (15% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.17. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite Y (Sodium Powder 5:1:1 SiO2:Al2O3, ALFA AESAR) (8% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.18. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeolite ZSM-5 (ACROS ORGANICS) (8% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.19. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Zeosil 1165 (Solvay) (8% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.20. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Boehmite Alumina Powder (Disperal, SASOL) (8% by Weight) in a Ball Mill (Planetary Ball Mill PM 100-RETSCH)
5.21. Synthesis of Aripiprazole (7-[4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy]-3,4-dihydro-1H-quinolin-2-one) (IIIa) with the Addition of Additives Improving the Insulation of the Product (8% by Weight) in a Ball Mill (Planetary Ball Mill Pulverisette 7 Premium Line, Fritsch GmbH)
6. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makosza, M. Phase-transfer catalysis. A general green methodology in organic synthesis. Pure Appl. Chem. 2000, 72, 1399–1403. [Google Scholar] [CrossRef] [Green Version]
- Mąkosza, M.; Fedoryński, M. Phase Transfer Catalysis. Catal. Rev. 2003, 45, 321–367. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, Reactions, Mechanisms and Structure, 7th ed.; WILEY: Hoboken, NJ, USA, 2018; pp. 442–445. [Google Scholar]
- Starks, C.M.; Liotta, C. Phase Transfer Catalysis; Academic Press: New York, NY, USA, 1978; Volume 326, p. 2. [Google Scholar]
- Starks, C.M.; Liotta, C.L.; Halpern, M. Phase-Transfer Catalysis. Fundamentals, Applications and Industrial Perspectives; Chapman & Hall: New York, NY, USA, 1994. [Google Scholar]
- Albanese, D. Liquid–Liquid Phase Transfer Catalysis: Basic Principles and Synthetic Applications. Catal. Rev. 2003, 45, 369–395. [Google Scholar] [CrossRef]
- Starks, C.M. Phase-transfer catalysis. I. Heterogeneous reactions involving anion transfer by quaternary ammonium and phosphonium salts. J. Am. Chem. Soc. 1971, 93, 195–199. [Google Scholar] [CrossRef]
- Mąkosza, M.; Serafinowa, B. Reactions of organic anions. I. Catalytic ethylation of phenylacetonitrile in aqueous medium. Rocz. Chem. 1965, 39, 1223–1231. [Google Scholar]
- Mąkosza, M. Reactions of organic anions. XI. Catalytic alkylation of indene. Tetrahedron Lett. 1966, 7, 4621–4624. [Google Scholar] [CrossRef]
- Mąkosza, M. Reactions of organic anions XVI. Catalytic nitroarylation of phenylacetonitrile derivatives in aqueous medium. Tetrahedron Lett. 1969, 10, 673–676. [Google Scholar] [CrossRef]
- Mąkosza, M. Reactions of organic anions. XVII. Catalytic alkylation of reissert compound in aqueous medium. Tetrahedron Lett. 1969, 10, 677–678. [Google Scholar] [CrossRef]
- Mąkosza, M.; Wawrzyniewicz, M. Reactions of organic anions. XXIV. Catalytic method for preparation of dichlorocyclopropane derivatives in aqueous medium. Tetrahedron Lett. 1969, 10, 4659–4662. [Google Scholar] [CrossRef]
- Mąkosza, M.; Fedoryński, M. Interfacial Processes—The Key Steps of Phase Transfer Catalyzed Reactions. Catalysts 2020, 10, 1436. [Google Scholar] [CrossRef]
- Siewniak, A.; Chrobok, A. Phase-transfer catalysis as a modern technique in organic synthesis. Wiadomości Chem. 2021, 75, 9–10. [Google Scholar]
- Yadav, G. Insight into Green Phase Transfer Catalysis. Top. Catal. 2004, 29, 145–161. [Google Scholar] [CrossRef]
- Santos, G.R.; Chiari, L.P.A.; da Silva, A.P.; Lipinski, C.F.; Oliveira, A.A.; Honorio, K.M.; de Sousa, A.G.; da Silva, A.B.F. A partial least squares and artificial neural network study for a series of arylpiperazines as antidepressant agents. J. Mol. Model. 2021, 27, 297. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Li, F.; Wang, C.; Wang, J.; Yang, Y.; Yang, L.; Li, Y. Mechanism Exploration of Arylpiperazine Derivatives Targeting the 5-HT2A Receptor by in Silico Methods. Molecules 2017, 22, 1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrone, R.; Berardi, F.; Colabufo, N.A.; Lacivita, E.; Larizza, C.; Leopoldo, M.; Tortorella, V. Design and synthesis of long-chain arylpiperazines with mixed affinity for serotonin transporter (SERT) and 5-HT(1A) receptor. J. Pharm. Pharmacol. 2005, 57, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, M.; Niso, M.; Abate, C.; Proschak, E.; Dubiel, M.; Stark, H.; Castro, M.; Lacivita, E.; Leopoldo, M. Design and Synthesis of Arylpiperazine Serotonergic/Dopaminergic Ligands with Neuroprotective Properties. Molecules 2022, 27, 1297. [Google Scholar] [CrossRef] [PubMed]
- Preda, A.; Shapiro, B.B. A safety evaluation of aripiprazole in the treatment of schizophrenia. Expert Opin. Drug Saf. 2020, 19, 1529–1538. [Google Scholar] [CrossRef]
- Cuomo, A.; Ballerini, A.; Bruni, A.C.; Decina, P.; Di Sciascio, G.; Fiorentini, A.; Scaglione, F.; Vampini, C.; Fagiolini, A. Clinical guidance for the use of trazodone in major depressive disorder and concomitant conditions: Pharmacology and clinical practice. Riv. Di Psichiatr. 2019, 54, 137–149. [Google Scholar]
- Fanelli, R.J.; Schuurman, T.; Glaser, T.; Traber, J. Ipsapirone: A novel anxiolytic and selective 5-HT1A receptor ligand. Prog. Clin. Biol. Res. 1990, 361, 461–467. [Google Scholar]
- Rittenhouse, P.A.; Bakkum, E.A.; O’Connor, P.A.; Carnes, M.; Bethea, C.L.; van de Kar, L.D. Comparison of neuroendocrine and behavioral effects of ipsapirone, a 5-HT1A agonist, in three stress paradigms: Immobilization, forced swim and conditioned fear. Brain Res. 1992, 580, 205–214. [Google Scholar] [CrossRef]
- Dean, L. Flibanserin Therapy and CYP2C19 Genotype. Medical Genetics Summaries [Internet] 2019; National Center for Biotechnology Information: Bethesda, MD, USA, 2012.
- Zajdel, P.; Marciniec, K.; Maślankiewicz, A.; Grychowska, K.; Satała, G.; Duszyńska, B.; Lenda, T.; Siwek, A.; Nowak, G.; Partyka, A.; et al. Antidepressant and antipsychotic activity of new quinoline- and isoquinoline-sulfonamide analogs of aripiprazole targeting serotonin 5-HT₁A/5-HT₂A/5-HT₇ and dopamine D₂/D₃ receptors. Eur. J. Med. Chem. 2013, 60, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, H.; Mishra, A.; Mishra, A.K. Aripiprazole: An FDA Approved Bioactive Compound to Treat Schizophrenia- A Mini Review. Curr. Drug Discov. Technol. 2020, 17, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Jaśkowska, J.; Drabczyk, A.K.; Kułaga, D.; Przemysław, Z.; Majka, Z. Solvent-free microwave-assisted synthesis of aripiprazole. Curr. Chem. Lett. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Ramakrishnan, A.; Subhash, V.D.G.; Panchal, D. A Novel Process for Preparation of Aripiprazole and Its Intermediates. Patent WO2007094009, 23 August 2007. [Google Scholar]
- Koftis, T.V.; Soni, R.R.; Acharya, H.H.; Patel, K.H.; Ahirrao, M.D. Process for the Preparation of Aripiprazole. Patent WO2013020672, 14 February 2013. [Google Scholar]
- Shi, H.; Babinski, D.J.; Ritter, T. Modular C–H Functionalization Cascade of Aryl Iodides. J. Am. Chem. Soc. 2015, 137, 3775–3778. [Google Scholar] [CrossRef]
- Nagarimadugu, M.; Kaushik, K.V.; Dandala, R.; Meenakshisunderam, S. Process for the Preparation of Aripiprazole. U.S. Patent 2010130744, 27 May 2010. [Google Scholar]
- Oshiro, Y.; Sato, S.; Kurahashi, N. Carbostyril Derivatives. U.S. Patent 5.006.528, 31 October 1988. [Google Scholar]
- Tsujimori, H.; Yamaguchi, T. Process for Preparing Aripiprazole. JP Patent WO2004063162, 29 July 2004. [Google Scholar]
- Kikuchi, T.; Iwamoto, T.; Hirose, T. Carbostyril Derivatives and Mood Stabilizers for Treating Mood Disorders. JP Patent WO2004105682, 9 December 2004. [Google Scholar]
- Leś, A.; Badowska-Rosłonek, K.; Łaszcz, M.; Kamieńska-Duda, A.; Baran, P.; Kaczmarek, Ł. Optimization of aripiprazole synthesis. Acta Pol. Pharm. 2010, 67, 151–157. [Google Scholar]
- Gant, T.G.; Sarshar, S.; Zhang, C. Arylpiperazine Modulators of D2 Receptors, 5-HT1A Receptors, and/or 5-HT2A. U.S. Patent 20100069399, 18 March 2008. [Google Scholar]
- Gupta, V.S.; Kumar, P.; Vir, D. Process for Producing Aripiprazole in Anhydrous Type I Crystals. Patent WO2012131451, 4 October 2011. [Google Scholar]
- Deshpande, P.B.; Luthra, P.K.; Shanishchara, A.P.; Manepalli, R.; Mistry, D.B. A Process for the Preparation of Aripiprazole. Patent WO2007113846, 11 October 2007. [Google Scholar]
- Palazzo, G.; Silvestrini, B. Triazole-(4,3-a)-pyridines. Patent US3381009, 30 April 1968. [Google Scholar]
- Pai, N.R.; Pusalkar, D.A. An efficient synthesis of neuroleptic drugs under microwave irradiation. J. Chem. Pharm. Res. 2010, 2, 506–517. [Google Scholar]
- Gant, T.G.; Sarshar, S. Substituted Triazolopyridines. Patent US20090209550, 20 August 2009. [Google Scholar]
- Jaśkowska, J. Method for Obtaining Trazodone. Patent P.420845, 24 September 2018. [Google Scholar]
- Seidel, P.R.; Horstmann, H.; Traber, J.; Dompert, W.; Glaser, T.; Schuurman, T. 2-pyrimidinyl-1-piperazine Derivatives, Processes for Their Preparation and Medicaments Containing Them. Patent CA1300624, 12 May 1992. [Google Scholar]
- Kułaga, D.; Jaśkowska, J.; Jasiński, R. Microwave-Assisted Solvent-Free Synthesis of Ipsapirone. J. Heterocycl. Chem. 2019, 56, 1498–1504. [Google Scholar] [CrossRef]
- Yang, F.; Wu, C.; Li, Z.; Tian, G.; Wu, J.; Zhu, F.; Zhang, J.; He, Y.; Shen, J. A Facile Route of Synthesis for Making Flibanserin. Org. Process Res. Dev. 2016, 20, 1576–1580. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Zhao, Y.; Chen, W.; Zhao, Z.; Lu, Z. Preparation method of Flibanserin intermediate. Patent CN109384680, 26 February 2019. [Google Scholar]
- Turconi, M.; Bietti, G.; Giraldo, E.; Borsini, F.; Bignotti, M. Benzimidazolone Derivatives As 5-HT1A and 5-HT2 Antagonists. Patent EP0526434, 3 February 1993. [Google Scholar]
- Margetić, D.; Štrukil, V. Mechanochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [Green Version]
- Margetić, D. Mechanic-chemical organic reactions without the use of solvents. Kem. U Ind. 2005, 54, 351–358. [Google Scholar]
- Waddell, D.C.; Thiel, I.; Bunger, A.; Nkata, D.; Maloney, A.; Clark, T.; Smith, B.; Mack, J. Investigating the formation of dialkyl carbonates using high speed ball milling. Green Chem. 2011, 13, 3156–3161. [Google Scholar] [CrossRef]
- Nun, P.; Martin, C.; Martinez, J.; Lamaty, F. Solvent-free synthesis of hydrazones and their subsequent N-alkylation in a Ball-mill. Tetrahedron 2011, 67, 8187–8194. [Google Scholar] [CrossRef]
- Kaupp, G.; Schmeyers, J.; Boy, J. Iminium Salts in Solid-State Syntheses Giving 100% Yield. J. Für Prakt. Chem. 2000, 342, 269–280. [Google Scholar] [CrossRef]
- Nun, P.; Pérez, V.; Calmés, M.; Martinez, J.; Lamaty, F. Preparation of Chiral Amino Esters by Asymmetric Phase-Transfer Catalyzed Alkylations of Schiff Bases in a Ball Mill. Chem. A Eur. J. 2012, 18, 3773–3779. [Google Scholar] [CrossRef] [PubMed]
- Briš, A.; Đud, M.; Margetić, D. Mechanochemical N-alkylation of imides. Beilstein J. Org. Chem. 2017, 13, 1745–1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swinburne, A.N.; Steed, J.W. The mechanochemical synthesis of podand anion receptors. CrystEngComm 2009, 11, 433–438. [Google Scholar] [CrossRef]
- Im, J.; Kim, J.; Kim, S.; Hahn, B.; Toda, F. N-Glycosylation reactions in the solid to solid state. Tetrahedron Lett. 1997, 38, 451–452. [Google Scholar] [CrossRef]
- Beillard, A.; Golliard, E.; Gillet, V.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Expedient Mechanosynthesis of N,N-Dialkyl Imidazoliums and Silver(I)–Carbene Complexes in a Ball-Mill. Chem. A Eur. J. 2015, 21, 17614–17617. [Google Scholar] [CrossRef]
- Métro, T.-X.; Salom-Roig, X.J.; Reverte, M.; Martinez, J.; Lamaty, F. Faster and cleaner dynamic kinetic resolution via mechanochemistry. Green Chem. 2015, 17, 204–208. [Google Scholar] [CrossRef]
- Shi, J.; Anderson, M.W.; Carr, S.W. Direct Observation of Zeolite A Synthesis by Situ Solid-State NMR. Chem. Mater. 1996, 8, 369–375. [Google Scholar] [CrossRef]
- Jodłowski, P.J.; Kuterasiński, Ł.; Jędrzejczyk, R.J.; Chlebda, D.; Gancarczyk, A.; Basąg, S.; Chmielarz, L. DeNOx Abatement Modelling over Sonically Prepared Copper USY and ZSM5 Structured Catalysts. Catalysts 2017, 7, 205. [Google Scholar] [CrossRef]
- Ding, J.; Han, B.; Wu, E. Synthesis Method of High-Purity Aripiprazole and Preparation Method of Hydrate Particles of Aripiprazole. CN Patent 113214150, 6 June 2021. [Google Scholar]
- Zhang, W.; LU, X.-Y. Use of Leptin for the Treatment or Prevention of Parkinson’s Disease. Patent WO200801188, 25 September 2008. [Google Scholar]
- Laitinen, I. A Process for the Preparation of Aripiprazole and Intermediates Thereof. Patent WO2007118923A1, 25 October 2007. [Google Scholar]
- Wang, D.; Gao, D.; Zhang, Y. Preparation Method of Aripiprazole. Patent CN103172564, 13 April 2016. [Google Scholar]
- Cai, H.; Gong, W.; Wang, B.; Liu, Y.; Li, B. Preparation Method of Aripiprazole. Patent CN109180577, 11 January 2019. [Google Scholar]
- Tetsuro, K.; Taro, I.; Tsuyoshi, H. Carbostyril Derivatives and Mood Stabilizers for Treating Mood Disorders. Patent US9125939, 12 June 2013. [Google Scholar]






| Symbol | R1 | n | X | R2 | Name | Reaction Conditions | Yield [%] |
|---|---|---|---|---|---|---|---|
| a |  | 4 | Br |  | aripiprazole | K2CO3, TBAB mortar, 30 min | 37 |
| b |  | 3 | Br, Cl |  | trazodone | 46 | |
| c |  | 2 | Br |  | flibanserin | 22 | |
| d |  | 4 | Br |  | ipsapirone | 25 |
| Name | Reaction Conditions | Yield [%] | |
|---|---|---|---|
| IIIa | aripiprazole | K2CO3, TBAB ball mill, 30 min | 86 |
| IIIb | trazodone | 64 |
| Entry | Reaction Conditions | |||
|---|---|---|---|---|
| Additives Improving the Insulation of the Product | % by Mass of the Additive | Yield [%] | ||
| 1 | Starch | 23 | K2CO3, TBAB ball mill, 300 rpm, 30 min | 62 |
| 2 | 32 | 62 | ||
| 3 | Zeolite A 1 | 3 | 44 | |
| 4 | 5.5 | 48 | ||
| 5 | 8 | 70 | ||
| 6 | 15 | 57 | ||
| 7 | Zeolite Y 2 | 3 | 43 | |
| 8 | 5.5 | 50 | ||
| 9 | 8 | 63 | ||
| 10 | 15 | 50 | ||
| 11 | Zeolite Y 3 | 8 | 77 | |
| 12 | Zeolite ZSM-5 4 | 8 | 79 | |
| 13 | Zeosil 1165 5 | 8 | 84 | |
| 14 | Disperal 6 | 8 | 81 | |
| Entry | Reaction Conditions | Time [min] | Yield [%] | ||
|---|---|---|---|---|---|
| Additives Improving the Insulation of the Product (8% Mass) | rpm | Cat. PT | |||
| 1 | Zeosil 1165 1 | 500 | TBAB | 10 | 58 |
| 2 | Zeosil 1165 1 | 500 * | TBAB | 10 | 63 |
| 3 | Zeosil 1165 1 | 700 | TBAB | 5 | 68 |
| 4 | Zeosil 1165 1 | 700 | TBAB | 7 | 72 |
| 5 | Zeosil 1165 1 | 700 | TBAB | 10 | 69 |
| 6 | Zeosil 1165 1 | 700 | TBAB | 15 | 74 |
| 7 | H2O | 500 | TBAB | 5 | 62 |
| 8 | H2O | 700 | TBAB | 5 | 66 |
| 9 | α-Al2O3 | 700 | TBAB | 10 | 71 |
| 10 | Silica sand (1) 2 | 500 | TBAB | 5 | 70 |
| 11 | Silica sand (2) 3 | 500 | TBAB | 5 | 90 |
| 12 | Zeosil 1165 1 | 700 | - | 5 | 65 |
| 13 | Zeolite ZSM-5 4 | 700 | - | 5 | 67 |
| 14 | Zeolite ZSM-5 4 | 300 | TBAB | 5 | 86 |
| 15 | Zeolite ZSM-5 4 | 500 | TBAB | 5 | 85 |
| 16 | Zeolite ZSM-5 4 | 700 | TBAB | 5 | 90 |
| 17 | Zeolite ZSM-5 4 | 500 | TEBA 5 | 5 | 83 |
| 18 | Zeolite ZSM-5 4 | 500 | TMAB 6 | 5 | 82 |
| 19 | Zeolite ZSM-5 4 | 500 | TEAC 7 | 5 | 78 |
| 20 | Zeolite ZSM-5 4 | 500 | BTBAC 8 | 5 | 71 |
| 21 | Zeolite ZSM-5 4 | 500 | CTAB 9 | 5 | 46 |
| 22 | Zeolite ZSM-5 4 | 500 | DABCO 10* | 5 | 88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaśkowska, J.; Drabczyk, A.K.; Michorczyk, P.; Kułaga, D.; Zaręba, P.; Jodłowski, P.; Majka, Z.; Jakubski, J.; Pindelska, E. Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions. Catalysts 2022, 12, 464. https://doi.org/10.3390/catal12050464
Jaśkowska J, Drabczyk AK, Michorczyk P, Kułaga D, Zaręba P, Jodłowski P, Majka Z, Jakubski J, Pindelska E. Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions. Catalysts. 2022; 12(5):464. https://doi.org/10.3390/catal12050464
Chicago/Turabian StyleJaśkowska, Jolanta, Anna Karolina Drabczyk, Piotr Michorczyk, Damian Kułaga, Przemysław Zaręba, Przemysław Jodłowski, Zbigniew Majka, Jarosław Jakubski, and Edyta Pindelska. 2022. "Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions" Catalysts 12, no. 5: 464. https://doi.org/10.3390/catal12050464
APA StyleJaśkowska, J., Drabczyk, A. K., Michorczyk, P., Kułaga, D., Zaręba, P., Jodłowski, P., Majka, Z., Jakubski, J., & Pindelska, E. (2022). Mechanochemical Synthesis Method for Drugs Used in the Treatment of CNS Diseases under PTC Conditions. Catalysts, 12(5), 464. https://doi.org/10.3390/catal12050464