Quo Vadis Dry Reforming of Methane?—A Review on Its Chemical, Environmental, and Industrial Prospects
Abstract
:1. Introduction
2. The Educts and C1 Chemistry
2.1. CO2
2.2. CH4
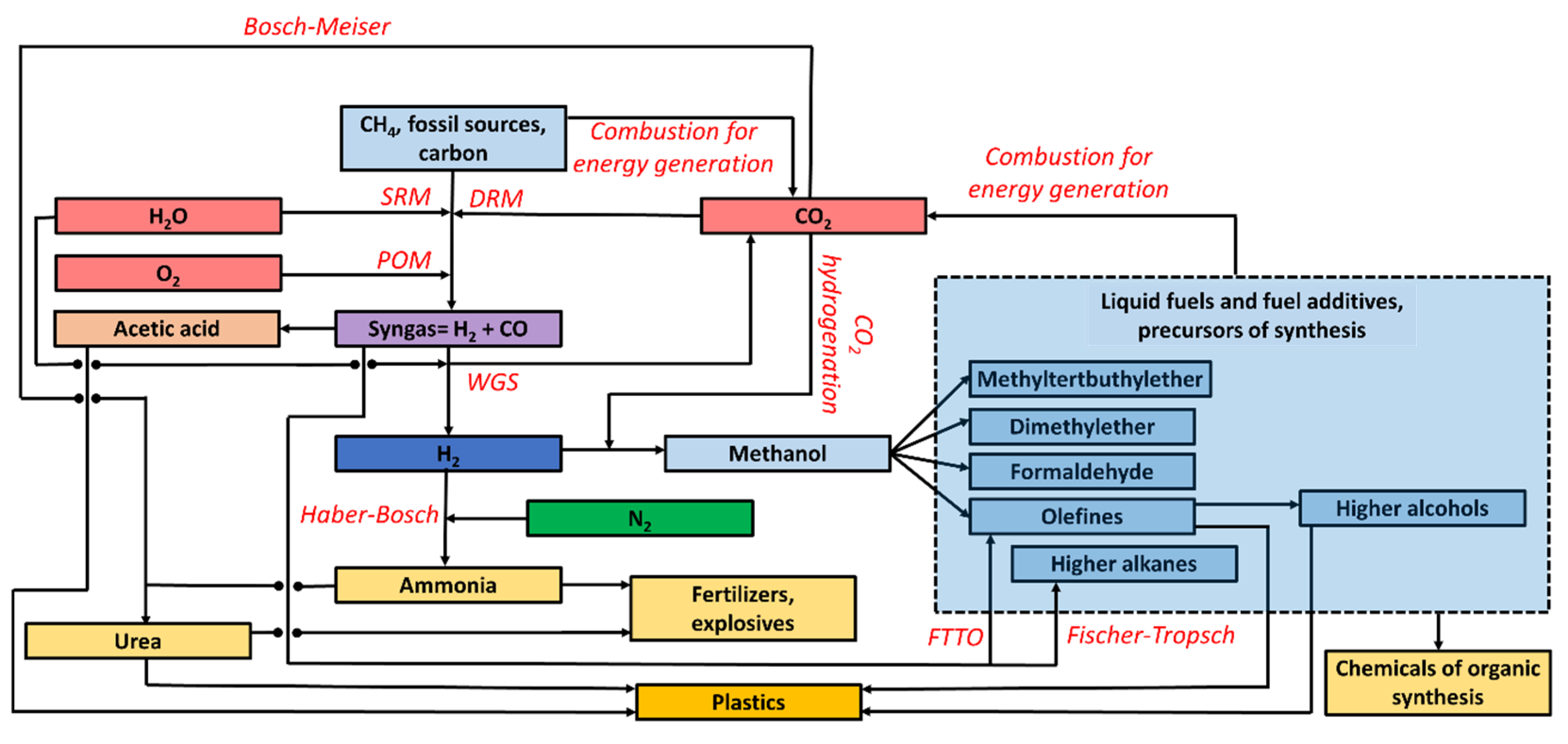
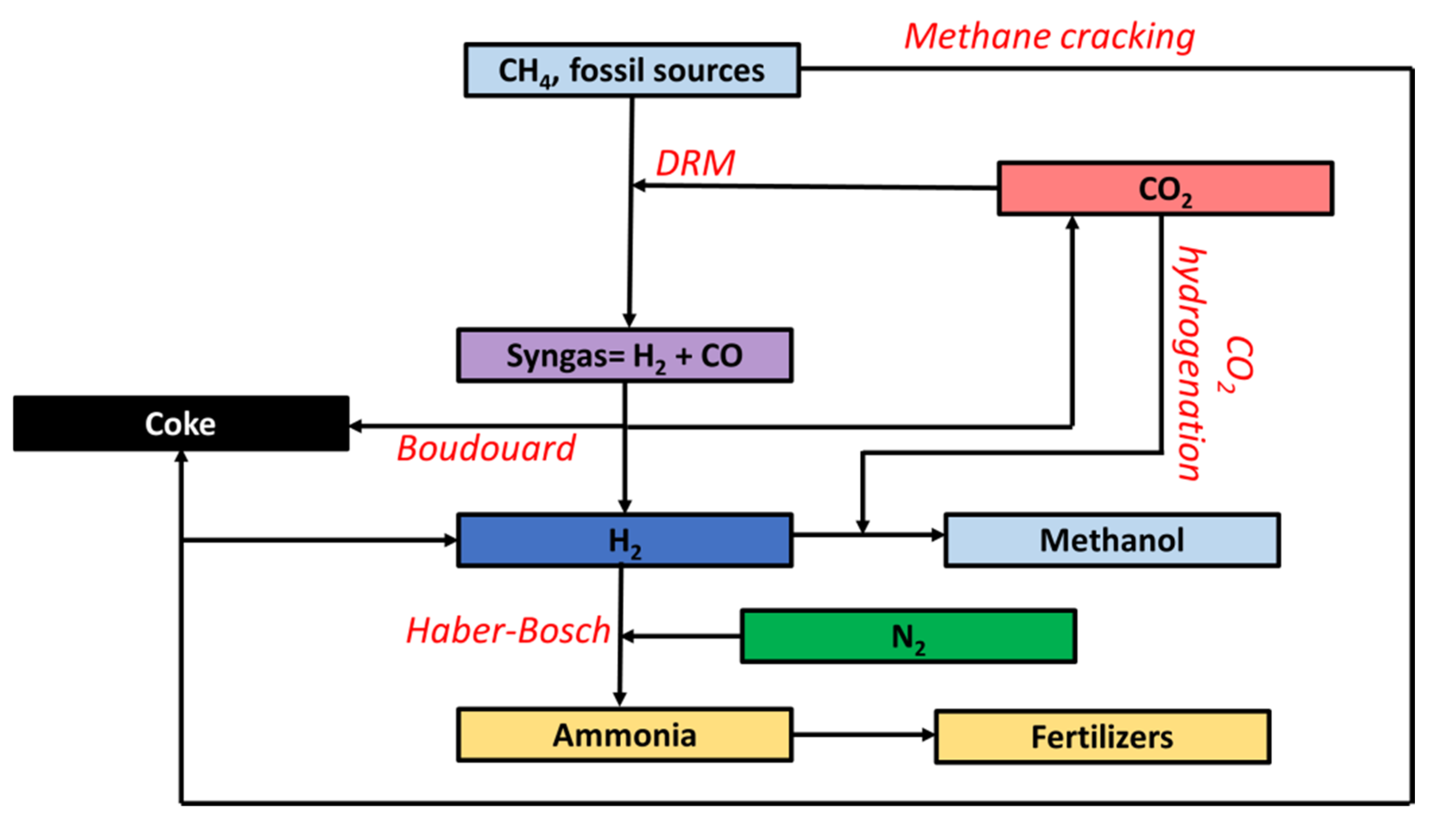
2.3. Industrial C1 Chemistry
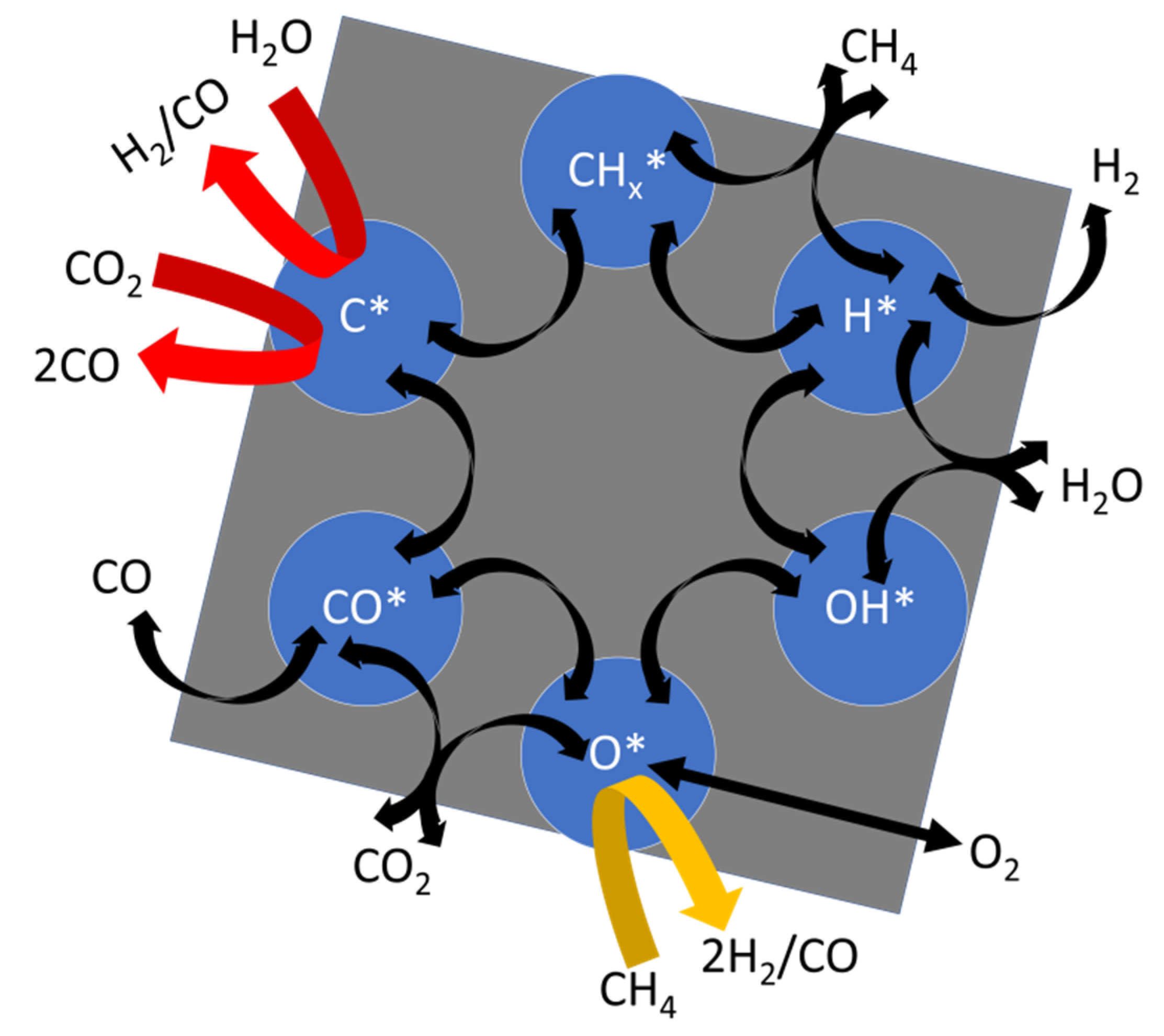
2.3.1. CH4 Activation
2.3.2. CO2 Activation
3. The Reaction Network of C1
3.1. Reforming of Methane
3.2. Thermodynamics of DRM
3.3. Kinetics of Dry Reforming
4. Deactivation of DRM
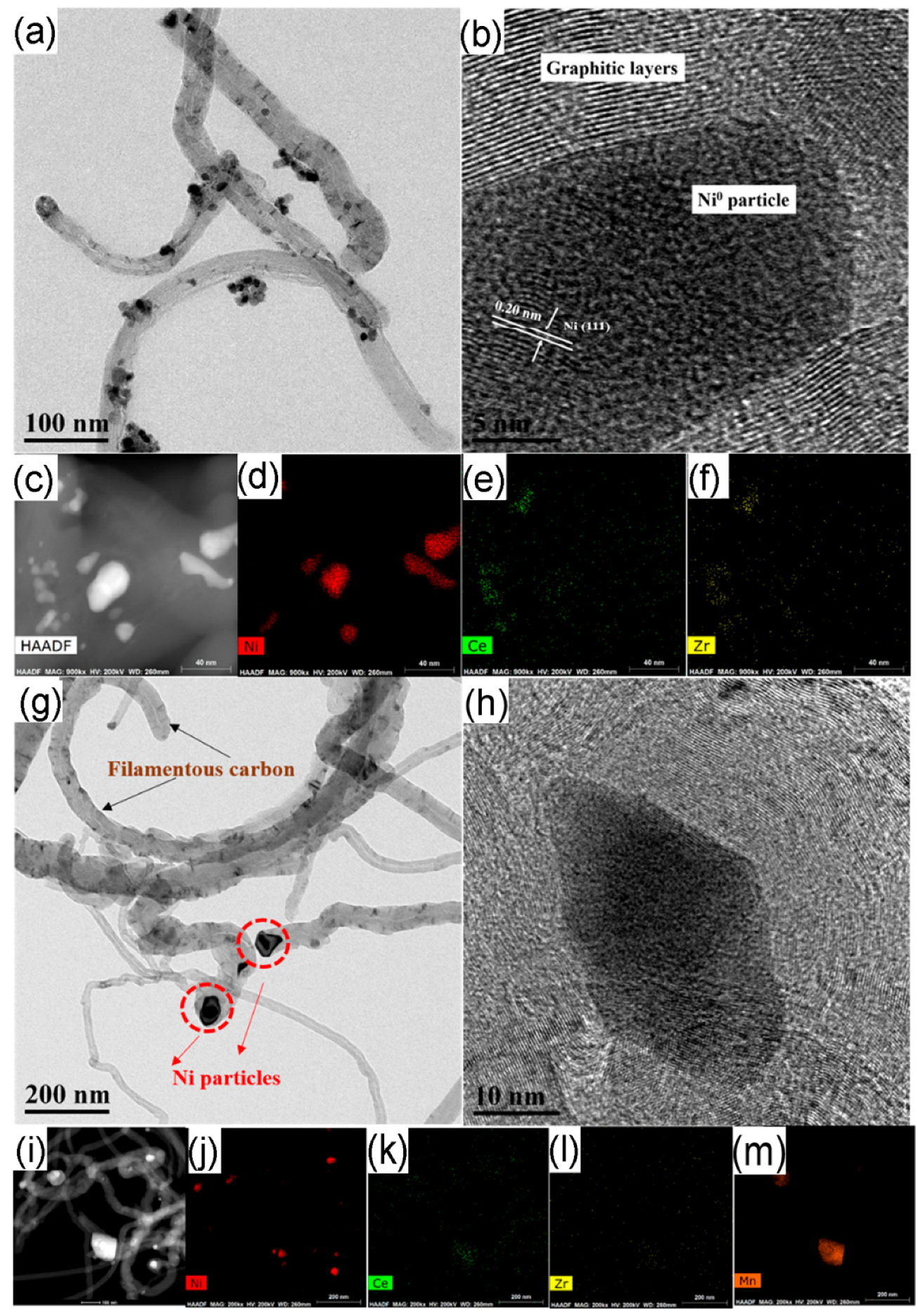
4.1. Coking
4.2. Sintering and Poisoning
5. Reaction Systems for DRM
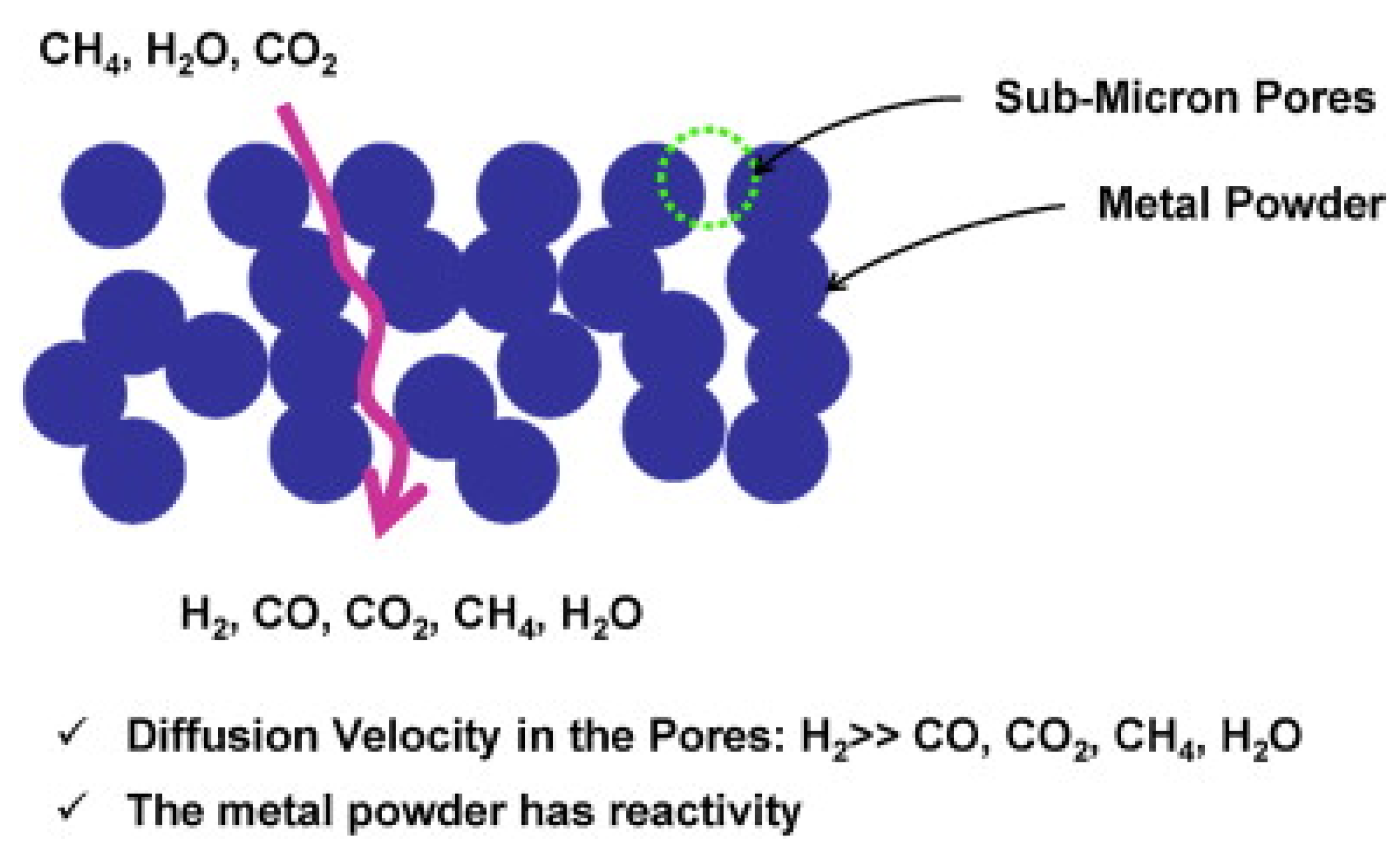
5.1. Thermocatalytic Dry Reforming
5.1.1. The Metallic Phase
- may stabilize the reduced state of active Ni0 by electronic effects,
- may improve the dispersion of Ni inside the metallic nanoparticle, thus preventing the occurrence of large ensembles of adjacent Ni atoms favorable to coking,
- may form in situ oxide phases highly reactive to oxidation of poisonous carbon,
- may tune the strength of the metal–support interaction, or
- may induce synergistic reactions by intrinsic catalysis or co-catalysis.
5.1.2. Promotion Effects
5.1.3. Support Chemistry
Silica Supports
Alumina Supports
Magnesia and Zirconia Supports
Rare Earth Oxide Supports
Carbide Supports
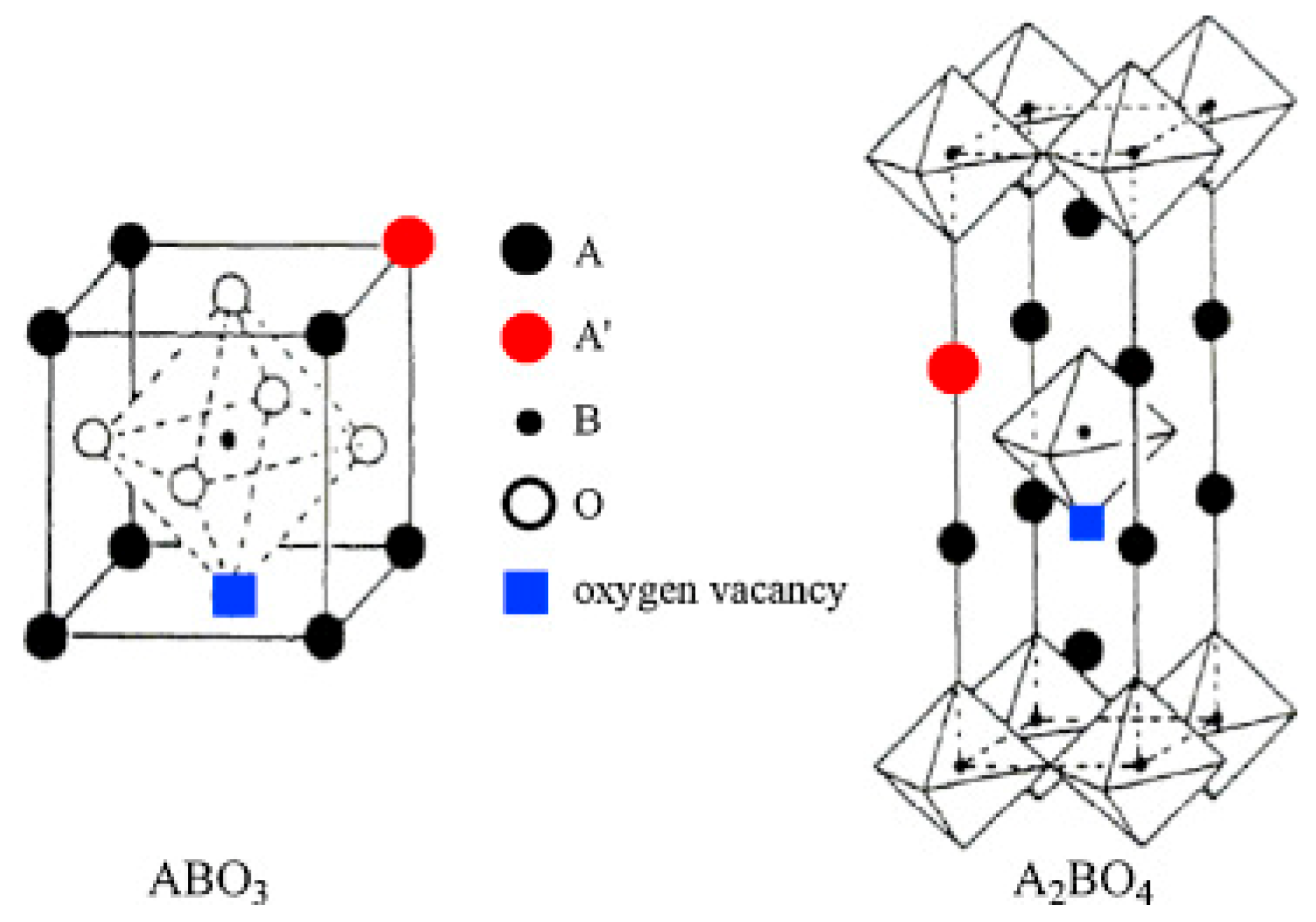
Perovskite Precursors
5.1.4. Non-Traditional Catalytic DRM
5.2. The Learning Curve of Catalytic DRM
5.3. Non-Catalytic DRM: Plasma and Photochemistry
- (i)
- Operating non-thermal plasma or photochemical process as catalyst pre-treatment. Previous studies have shown that non-thermal plasma can change some of the properties of the catalyst. Since non-thermal plasma and photochemistry generate many species, e.g., electrons with high kinetic energy, oxygen atoms, and hydrogen atoms, the collision between particles and solid catalysts may induce various reactions to alter the structure of the catalyst, e.g., reducing or oxidizing the metals on the catalyst surface. Hence, the catalyst pretreated with these strategies can be improved to have higher activity, better durability, or higher selectivity [226].
- (ii)
- Sequential plasma-catalysis. Placing the catalyst after the discharge region of the plasma is a possible way to induce synergistic effects between plasma and catalyst, which is also called post plasma catalysis (PPC). Both thermal plasma and non-thermal plasma can be adopted before the catalyst bed to form a two-stage hybrid system. In a non-thermal plasma catalysis system, the long-lived reactive species produced by non-thermal plasma, e.g., radicals, vibration excited species, and ionized molecules can react with the catalyst to induce catalytic reactions by several surface mechanisms [226]. Thus, the conversion and selectivity of syngas generation can be enhanced by combining two reforming techniques.
- (iii)
- Hybrid single reactor systems that combine plasma or photochemistry and catalysis. The system is composed of a catalyst inserted in the discharge region of plasma or directly uses a photocatalyst [238]. The catalyst can be further modified in situ with the help of the plasma. Moreover, two kinds of interaction can be induced and may influence each other. The first is the effect of plasma/light on the catalyst, such as the modification of physicochemical characteristics and the work function of the catalyst. The second is the effect of the catalyst on the plasma, such as changes in the electric field distribution and the distribution of species in the plasma. It is noted that thermal plasma is not suitable in this system due to the deactivation of the catalyst at the high operating temperature (103–104 °C) that the gas may reach [226].
5.4. The Requirement of Operando Studies for DRM
6. On the Maturity of DRM in Industry
7. Concluding Critical Remarks
7.1. General Considerations
7.2. Energy Storage
7.3. What to Do with the Carbon?
CH4 + CO2 → 2 CO + 2 H2 DRM
2 CO + 2 H2O → 2 CO2 + 2 H2 WGS
2 CO2 + 6 H2 → 2 CH3OH + 2 H2O Methanol synthesis
2 CH3OH + 3 O2 → 2 CO2 + 4 H2O energy release
2 CH4 + 2 CO2 → 4 CO + 4 H2 DRM
4 CO + 4 H2O → 4 CO2 + 4 H2 WGS
4 CO2 + 12 H2 → 4 CH3OH + 4 H2O Methanol synthesis
4 CH3OH + 6 O2 → 4 CO2 + 8 H2O energy release
4 CH4 + 4 CO2 → 8 CO + 8 H2 DRM
8 CO + 8 H2O → 8 CO2 + 8 H2 WGS
8 CO2 + 24 H2 → 8 CH3OH + 8 H2O Methanol synthesis
8 CH3OH + 12 O2 → 8 CO2 + 16 H2O energy release
7.4. Quo Vadis DRM?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostrup-Nielsen, J. Sulfur-passivated nickel catalysts for carbon-free steam reforming of methane. J. Catal. 1984, 85, 31–43. [Google Scholar] [CrossRef]
- Olah, G.A. Beyond Oil and Gas: The Methanol Economy. Angew. Chem. Int. Ed. 2005, 44, 2636–2639. [Google Scholar] [CrossRef] [PubMed]
- Wittich, K.; Krämer, M.; Bottke, N.; Schunk, S.A. Catalytic Dry Reforming of Methane: Insights from Model Systems. ChemCatChem 2020, 12, 2130–2147. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Chatterjee, S.; Huang, K.-W. The Insignificant Role of Dry Reforming of Methane in CO2 Emission Relief. ACS Energy Lett. 2020, 5, 2881–2885. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Blunt, M.J.; Anthony, E.J.; Park, A.-H.A.; Hughes, R.W.; Webley, P.A.; Yan, J. Advances in carbon capture, utilization and storage. Appl. Energy 2020, 278, 115627. [Google Scholar] [CrossRef]
- Fujikawa, S.; Selyanchyn, R.; Kunitake, T. A new strategy for membrane-based direct air capture. Polym. J. 2021, 53, 111–119. [Google Scholar] [CrossRef]
- Smit, B.; Park, A.-H.A.; Gadikota, G. The Grand Challenges in Carbon Capture, Utilization, and Storage. Front. Energy Res. 2014, 2, 2. [Google Scholar] [CrossRef] [Green Version]
- Eastman, E.D. Equilibria in the Systems Iron: Carbon Oxygen and Iron Hydrogen Oxygen, and the Free Energies of the Oxides of Iron1. J. Am. Chem. Soc. 1922, 44, 975–998. [Google Scholar] [CrossRef]
- Bazzanella, A.M.; Ausfelder, F. Low Carbon Energy and Feedstock for the European Chemical Industry. 2017, DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V. Available online: https://dechema.de/dechema_media/Downloads/Positionspapiere/Technology_study_Low_carbon_energy_and_feedstock_for_the_European_chemical_industry.pdf (accessed on 7 April 2022).
- Hannah Ritchie, M.R. CO2 and Greenhouse Gas Emissions. 2017. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 9 February 2020).
- Liu, Z.; Guan, D.; Wei, W.; Davis, S.J.; Ciais, P.; Bai, J.; Peng, S.; Zhang, Q.; Hubacek, K.; Marland, G.; et al. Reduced carbon emission estimates from fossil fuel combustion and cement production in China. Nature 2015, 524, 335–338. [Google Scholar] [CrossRef] [Green Version]
- Kuramochi, T.; Elzen, M.D.; Peters, G.; Global Emissions Trends and G20 Status and Outlook in Emissions GAP Report 2020. United Nations. 2021, pp. 3–24. Available online: https://wedocs.unep.org/xmlui/bitstream/handle/20.500.11822/34428/EGR20ch2.pdf?sequence=3 (accessed on 6 April 2022).
- USEIA. Hydrogen Explained Production of Hydrogen. 2022. Available online: https://www.eia.gov/energyexplained/hydrogen/production-of-hydrogen.php. (accessed on 27 January 2020).
- Lavoie, J.-M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [Green Version]
- Bhattar, S.; Abedin, A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Edenhofer, O.; Kadner, S.; Pichs-Madruga, R.; Sokona, Y.; Farahani, E.; Kadner, S.; Seyboth, A.A.; Baum, I.; Brunner, S.; Eickemeier, P.; et al. IPCC, 2014: Climate Change 2014: Mitigation of Climate Change. Contribution of Working Group III to the Fifth Assessment. In Report of the Intergovernmental Panel on Climate Change; Savolainen, J., Schlömer, S., von Stechow, C., Zwickel, T.C.J., Eds.; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- United States Environmental Protection Agency. Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 20 January 2022).
- Ritchie, H.; Roser, M.; CO2 and Greenhouse Gas Emissions. OurWorldInData.org. Available online: https://ourworldindata.org/co2-emissions#citation (accessed on 7 April 2022).
- Lyu, L.; Zeng, X.; Yun, J.; Wei, F.; Jin, F. No Catalyst Addition and Highly Efficient Dissociation of H2O for the Reduction of CO2 to Formic Acid with Mn. Environ. Sci. Technol. 2014, 48, 6003–6009. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO 2 Emissions and Climate Change from Existing Energy Infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [Green Version]
- Jin, F.; Gao, Y.; Jin, Y.; Zhang, Y.; Cao, J.; Wei, Z.; Smith, R.L., Jr. High-yield reduction of carbon dioxide into formic acid by zero-valent metal/metal oxide redox cycles. Energy Environ. Sci. 2011, 4, 881–884. [Google Scholar] [CrossRef]
- Vansant, J.; Koziel, P.-W. Technical and Industrial Applications of CO2 in An Economy Based on Carbon Dioxide and Water: Potential of Large Scale Carbon Dioxide Utilization; Aresta, M., Karimi, I., Kaw, S.i., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 73–103. [Google Scholar]
- Romano, M.C.; Anantharaman, R.; Arasto, A.; Ozcan, D.C.; Ahn, H.; Dijkstra, J.W.; Carbo, M.; Boavida, D. Application of Advanced Technologies for CO2 Capture From Industrial Sources. Energy Procedia 2013, 37, 7176–7185. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhao, Z.-J.; Cheng, D.; Li, H.; Yu, J.; Wang, Q.; Gao, H.; Guo, J.; Wang, H.; Ozin, G.A.; et al. Efficient CO2 electroreduction on facet-selective copper films with high conversion rate. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef]
- Cauwenbergh, R.; Das, S. Photochemical reduction of carbon dioxide to formic acid. Green Chem. 2021, 23, 2553–2574. [Google Scholar] [CrossRef]
- Wareing, P.F.; Khalifa, M.M.; Treharne, K.J. Rate-limiting Processes in Photosynthesis at Saturating Light Intensities. Nature 1968, 220, 453–457. [Google Scholar] [CrossRef]
- Administration U.S.E.I. EIA Expects U.S. Fossil Fuel Production to Reach New Highs in 2023. Today in Energy 2022. Available online: https://www.eia.gov/todayinenergy/detail.php?id=50978 (accessed on 24 January 2022).
- Liu, Y.; Deng, D.; Bao, X. Catalysis for Selected C1 Chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Fierro, J.L.G. Catalysis in C1 chemistry: Future and prospect. Catal. Lett. 1993, 22, 67–91. [Google Scholar] [CrossRef]
- Mesters, C. A Selection of Recent Advances in C1 Chemistry. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 223–238. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, G.B. Bond Dissociation Energies of Organic Molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Tomkins, P.; Ranocchiari, M.; van Bokhoven, J.A. Direct Conversion of Methane to Methanol under Mild Conditions over Cu-Zeolites and beyond. Acc. Chem. Res. 2017, 50, 418–425. [Google Scholar] [CrossRef]
- Horn, R.; Williams, K.; Degenstein, N.; Schmidt, L. Syngas by catalytic partial oxidation of methane on rhodium: Mechanistic conclusions from spatially resolved measurements and numerical simulations. J. Catal. 2006, 242, 92–102. [Google Scholar] [CrossRef]
- Zavyalova, U.; Holena, M.; Schlögl, R.; Baerns, M. Statistical Analysis of Past Catalytic Data on Oxidative Methane Coupling for New Insights into the Composition of High-Performance Catalysts. ChemCatChem 2011, 3, 1935–1947. [Google Scholar] [CrossRef]
- Guo, X.; Fang, G.; Li, G.; Ma, H.; Fan, H.; Yu, L.; Wu, X.; Deng, D.; Wei, M.; Tan, D.; et al. Direct, Nonoxidative Conversion of Methane to Ethylene, Aromatics, and Hydrogen. Science 2014, 344, 616–619. [Google Scholar] [CrossRef]
- Wang, L.; Tao, L.; Xie, M.; Xu, G.; Huang, J.; Xu, Y. Dehydrogenation and aromatization of methane under non-oxidizing conditions. Catal. Lett. 1993, 21, 35–41. [Google Scholar] [CrossRef]
- Pham, H.N.; Sattler, J.J.H.B.; Weckhuysen, B.M.; Datye, A. Role of Sn in the Regeneration of Pt/γ-Al2O3 Light Alkane Dehydrogenation Catalysts. ACS Catal. 2016, 6, 2257–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belgued, M.; Pareja, P.; Amariglio, A. Conversion of methane into higher hydrocarbons on platinum. Nature 1991, 352, 789–790. [Google Scholar] [CrossRef]
- Schwab, E.; Milanov, A.; Schunk, S.A.; Behrens, A.; Schödel, N. Dry Reforming and Reverse Water Gas Shift: Alternatives for Syngas Production? Chem. Ing. Tech. 2015, 87, 347–353. [Google Scholar] [CrossRef]
- Zhang, X.; Vajglova, Z.; Mäki-Arvela, P.; Peurla, M.; Palonen, H. Mono- and Bimetallic Ni−Co Catalysts in Dry Reforming of Methane. Chem. Sel. 2021, 6, 3424–3434. [Google Scholar] [CrossRef]
- Bown, R.M.; Joyce, M.; Zhang, Q.; Reina, T.R.; Duyar, M.S. Identifying Commercial Opportunities for the Reverse Water Gas Shift Reaction. Energy Technol. 2021, 9, 2100554. [Google Scholar] [CrossRef]
- Ramirez, A.; Lee, K.; Harale, A.; Gevers, L.; Telalovic, S.; Solami, B.A.; Gascon, J. Stable High-Pressure Methane Dry Reforming Under Excess of CO2. ChemCatChem 2020, 12, 5919–5925. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. New aspects of syngas production and use. Catal. Today 2000, 63, 159–164. [Google Scholar] [CrossRef]
- Behrens, M. Promoting the Synthesis of Methanol: Understanding the Requirements for an Industrial Catalyst for the Conversion of CO2. Angew. Chem. Int. Ed. 2016, 55, 14906–14908. [Google Scholar] [CrossRef]
- Zhang, B.; Fuentes, D.P.; Börner, A. Hydroformylation. ChemTexts 2021, 8, 2. [Google Scholar]
- Rostrup-Nielsen, R.J. Production of synthesis gas. Catal. Today 1993, 18, 305–324. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 Hydrogenation to Methanol over a Highly Active Cu–Ni/CeO2–Nanotube Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Mukainakano, Y.; Kado, S.; Miyazawa, T.; Okumura, K.; Miyao, T.; Naito, S.; Suzuki, K.; Fujimoto, K.-I.; Kunimori, K.; et al. Oxidative steam reforming of methane under atmospheric and pressurized conditions over Pd/NiO–MgO solid solution catalysts. Appl. Catal. A Gen. 2006, 308, 1–12. [Google Scholar] [CrossRef]
- Armor, J. The multiple roles for catalysis in the production of H2. Appl. Catal. A Gen. 1999, 176, 159–176. [Google Scholar] [CrossRef]
- Alves, H.J.; Junior, C.B.; Niklevicz, R.R.; Frigo, E.P.; Frigo, M.; Coimbra-Araújo, C.H. Overview of hydrogen production technologies from biogas and the applications in fuel cells. Int. J. Hydrog. Energy 2013, 38, 5215–5225. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H.; Claridge, J. Methane Oxyforming for Synthesis Gas Production. Catal. Rev. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Kahle, L.C.S.; Roussière, T.; Maier, L.; Delgado, K.H.; Wasserschaff, G.; Schunk, S.A.; Deutschmann, O. Methane Dry Reforming at High Temperature and Elevated Pressure: Impact of Gas-Phase Reactions. Ind. Eng. Chem. Res. 2013, 52, 11920–11930. [Google Scholar] [CrossRef]
- Kikuchi, R.; Iwasa, Y.; Takeguchi, T.; Eguchi, K. Partial oxidation of CH4 and C3H8 over hexaaluminate-type oxides. Appl. Catal. A Gen. 2005, 281, 61–67. [Google Scholar] [CrossRef]
- Sheldon, D. Methanol Production—A Technical History. Johns. Matthey Technol. Rev. 2017, 61, 172–182. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Qi, M.; Lee, I.; Moon, I. Process Integration of an Autothermal Reforming Hydrogen Production System with Cryogenic Air Separation and Carbon Dioxide Capture Using Liquefied Natural Gas Cold Energy. Ind. Eng. Chem. Res. 2021, 60, 7257–7274. [Google Scholar] [CrossRef]
- Zahedi nezhad, M.; Rowshanzamir, S.; Eikani, M.H. Autothermal reforming of methane to synthesis gas: Modeling and simulation. Int. J. Hydrog. Energy 2009, 34, 1292–1300. [Google Scholar] [CrossRef]
- Ghani, A.A.; Torabi, F.; Ibrahim, H. Autothermal reforming process for efficient hydrogen production from crude glycerol using nickel supported catalyst: Parametric and statistical analyses. Energy 2018, 144, 129–145. [Google Scholar] [CrossRef]
- Alyea, E.C.; He, D.; Wang, J. Alcohol synthesis from syngas: I. Performance of alkali-promoted Ni-Mo(MOVS) catalysts. Appl. Catal. A Gen. 1993, 104, 77–85. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Steam Reforming of Hydrocarbons. A Historical Perspective in Studies in Surface Science and Catalysis; Bao, X., Xu, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 121–126. [Google Scholar]
- Fischer, F.T.H. Conversion of methane into hydrogen and carbon monoxide. Brennst.Chem. 1928, 9, 23–27. [Google Scholar]
- Wei, J.; Iglesia, E. Mechanism and Site Requirements for Activation and Chemical Conversion of Methane on Supported Pt Clusters and Turnover Rate Comparisons among Noble Metals. J. Phys. Chem. B 2004, 108, 4094–4103. [Google Scholar] [CrossRef]
- Xiong, K.; Yin, Y.-L.; Cao, Y.; Liu, X.-T. Exergy Efficiency Promotion for the System of CO2 Hydrogenation to Methanol in Habitable Confined Space. Front. Energy Res. 2021, 9, 9. [Google Scholar] [CrossRef]
- Hank, C.; Gelpke, S.; Schnabl, A.; White, R.J.; Full, J.; Wiebe, N.; Smolinka, T.; Schaadt, A.; Henning, H.-M.; Hebling, C. Economics & carbon dioxide avoidance cost of methanol production based on renewable hydrogen and recycled carbon dioxide—Power-to-methanol. Sustain. Energy Fuels 2018, 2, 1244–1261. [Google Scholar]
- Wiesberg, I.L.; de Medeiros, J.L.; Alves, R.M.; Coutinho, P.L.; Araújo, O.Q. Carbon dioxide management by chemical conversion to methanol: Hydrogenation and Bi-Reforming. Energy Convers. Manag. 2016, 125, 320–335. [Google Scholar] [CrossRef]
- Borisut, P.; Nuchitprasittichai, A. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization. Front. Energy Res. 2019, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Hyeon, S.; Lee, J.; Kim, W.D.; Lee, D.C.; Kim, J.; Lee, H. Energy-efficient CO2 hydrogenation with fast response using photoexcitation of CO2 adsorbed on metal catalysts. Nat. Commun. 2018, 9, 2425–2434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamu, S.; Bawah, A.; Muraza, O.; Malaibari, Z.; Hossain, M.M. Effects of metal support interaction on dry reforming of methane over Ni/ Ce-Al2O3 catalysts. Can. J. Chem. Eng. 2020, 98, 2425–2434. [Google Scholar] [CrossRef]
- le Saché, E.; Moreno, A.A.; Reina, T.R. Biogas Conversion to Syngas Using Advanced Ni-Promoted Pyrochlore Catalysts: Effect of the CH4/CO2 Ratio. Front. Chem. 2021, 9, 672419. [Google Scholar] [CrossRef]
- Chein, R.; Chen, Y.; Yu, C.; Chung, J. Thermodynamic analysis of dry reforming of CH4 with CO2 at high pressures. J. Nat. Gas Sci. Eng. 2015, 26, 617–629. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, X.; Mi, Z. Thermodynamic analysis of autothermal steam and CO2 reforming of methane. Int. J. Hydrog. Energy 2008, 33, 2507–2514. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef] [Green Version]
- Schulz, L.A.; Kahle, L.C.; Delgado, K.H.; Schunk, S.A.; Jentys, A.; Deutschmann, O.; Lercher, J.A. On the coke deposition in dry reforming of methane at elevated pressures. Appl. Catal. A Gen. 2015, 504, 599–607. [Google Scholar] [CrossRef]
- Roussière, T.; Schulz, L.; Schelkle, K.M.; Wasserschaff, G.; Milanov, A.; Schwab, E.; Deutschmann, O.; Jentys, A.; Lercher, J.; Schunk, S.A. Structure-Activity Relationships of Nickel-Hexaaluminates in Reforming Reactions Part II: Activity and Stability of Nanostructured Nickel-Hexaaluminate-Based Catalysts in the Dry Reforming of Methane. ChemCatChem 2014, 6, 1447–1452. [Google Scholar] [CrossRef]
- Sandoval-Diaz, L.; Plodinec, M.; Ivanov, D.; Poitel, S.; Hammud, A.; Nerl, H.C.; Schlögl, R.; Lunkenbein, T. Visualizing the importance of oxide-metal phase transitions in the production of synthesis gas over Ni catalysts. J. Energy Chem. 2020, 50, 178–186. [Google Scholar] [CrossRef]
- AlSabban, B.; Falivene, L.; Kozlov, S.M.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.-L.; Cavallo, L.; Basset, J.-M.; Takanabe, M. In-operando elucidation of bimetallic CoNi nanoparticles during high-temperature CH4/CO2 reaction. Appl. Catal. B Environ. 2017, 213, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Tapia, A.A.; Ould-Chikh, S.; Lahera, E.; Prat, A.; Delnet, W.; Proux, O.; Kieffer, I.; Basset, J.-M.; Takanabe, K.; Hazemann, J.-L. A new high temperature reactor for operando XAS: Application for the dry reforming of methane over Ni/ZrO2 catalyst. Rev. Sci. Instrum. 2018, 89, 035109. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, M.A.; Bobadilla, L.; Garcilaso, V.; Centeno, M.A.; Odriozola, J.A. CO2 reforming of methane over Ni-Ru supported catalysts: On the nature of active sites by operando DRIFTS study. J. CO2 Util. 2018, 24, 509–515. [Google Scholar] [CrossRef]
- Aramouni, N.A.K.; Zeaiter, J.; Kwapinski, W.; Ahmad, M.N. Thermodynamic analysis of methane dry reforming: Effect of the catalyst particle size on carbon formation. Energy Convers. Manag. 2017, 150, 614–622. [Google Scholar] [CrossRef]
- Xu, J.; Froment, G.F. Methane steam reforming, methanation and water-gas shift: I. Intrinsic kinetics. AIChE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Gokon, N.; Osawa, Y.; Nakazawa, D.; Kodama, T. Kinetics of CO2 reforming of methane by catalytically activated metallic foam absorber for solar receiver-reactors. Int. J. Hydrog. Energy 2009, 34, 1787–1800. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Piña, J.; El Solh, T.; de Lasa, H.I. Coke Formation over a Nickel Catalyst under Methane Dry Reforming Conditions: Thermodynamic and Kinetic Models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X. Carbon dioxide reforming of methane to synthesis gas over supported Ni catalysts. Catal. Today 1994, 21, 589–595. [Google Scholar] [CrossRef]
- Yuan, K.; Zhong, J.-Q.; Zhou, X.; Xu, L.; Bergman, S.L.; Wu, K.; Xu, G.Q.; Bernasek, S.L.; Li, H.X.; Chen, W. Dynamic Oxygen on Surface: Catalytic Intermediate and Coking Barrier in the Modeled CO2 Reforming of CH4 on Ni (111). ACS Catal. 2016, 6, 4330–4339. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO2 or H2O to form synthesis gas and carbon on nickel catalysts. J. Catal. 2004, 224, 370–383. [Google Scholar] [CrossRef]
- Giehr, A.; Maier, L.; Angeli, S.; Schunk, S.A.; Deutschmann, O. Dry and Steam Reforming of CH4 on Co-Hexaaluminate: On the Formation of Metallic Co and Its Influence on Catalyst Activity. Ind. Eng. Chem. Res. 2020, 59, 18790–18797. [Google Scholar] [CrossRef]
- Keller, K.; Lott, P.; Stotz, H.; Maier, L.; Deutschmann, O. Microkinetic Modeling of the Oxidation of Methane Over PdO Catalysts—Towards a Better Understanding of the Water Inhibition Effect. Catalysts 2020, 10, 922. [Google Scholar] [CrossRef]
- Schmider, D.; Maier, L.; Deutschmann, O. Reaction Kinetics of CO and CO2 Methanation over Nickel. Ind. Eng. Chem. Res. 2021, 60, 5792–5805. [Google Scholar] [CrossRef]
- Akpan, E.; Sun, Y.; Kumar, P.; Ibrahim, H.; Aboudheir, A.; Idem, R. Kinetics, experimental and reactor modeling studies of the carbon dioxide reforming of methane (CDRM) over a new Ni/CeO2–ZrO2 catalyst in a packed bed tubular reactor. Chem. Eng. Sci. 2007, 62, 4012–4024. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Kroll, V.; Swaan, H.; Lacombe, S.; Mirodatos, C. Methane Reforming Reaction with Carbon Dioxide over Ni/SiO2 Catalyst: II. A Mechanistic Study. J. Catal. 1996, 164, 387–398. [Google Scholar] [CrossRef]
- Aparicio, L. Transient Isotopic Studies and Microkinetic Modeling of Methane Reforming over Nickel Catalysts. J. Catal. 1997, 165, 262–274. [Google Scholar] [CrossRef]
- Michaelides, A.; Hu, P. Methyl chemisorption on Ni(111) and CHM multicentre bonding: A density functional theory study. Surf. Sci. 1999, 437, 362–376. [Google Scholar] [CrossRef]
- Wang, S.-G.; Liao, X.-Y.; Hu, J.; Cao, D.-B.; Li, Y.-W.; Wang, J.; Jiao, H. Kinetic aspect of CO2 reforming of CH4 on Ni(111): A density functional theory calculation. Surf. Sci. 2007, 601, 1271–1284. [Google Scholar] [CrossRef]
- Muraza, O.; Galadima, A. A review on coke management during dry reforming of methane. Int. J. Energy Res. 2015, 39, 1196–1216. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Ashok, J.; Kawi, S. A comprehensive review of anti-coking, anti-poisoning and anti-sintering catalysts for biomass tar reforming reaction. Chem. Eng. Sci. X 2020, 7, 100065. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Kawi, S. Reforming of tar from biomass gasification in a hybrid catalysis-plasma system: A review. Appl. Catal. B Environ. 2019, 250, 250–272. [Google Scholar] [CrossRef]
- Ochoa, A.; Arregi, A.; Amutio, M.; Gayubo, A.G.; Olazar, M.; Bilbao, J.; Castaño, P. Coking and sintering progress of a Ni supported catalyst in the steam reforming of biomass pyrolysis volatiles. Appl. Catal. B Environ. 2018, 233, 289–300. [Google Scholar] [CrossRef]
- Yao, L.; Galvez, M.E.; Hu, C.; Da Costa, P. Synthesis Gas Production via Dry Reforming of Methane over Manganese Promoted Nickel/Cerium–Zirconium Oxide Catalyst. Ind. Eng. Chem. Res. 2018, 57, 16645–16656. [Google Scholar] [CrossRef]
- Bartholomew, C.H. Mechanisms of catalyst deactivation. Appl. Catal. A Gen. 2001, 212, 17–60. [Google Scholar] [CrossRef]
- Margossian, T.; Larmier, K.; Kim, S.M.; Krumeich, F.; Müller, C.; Copéret, C. Supported Bimetallic NiFe Nanoparticles through Colloid Synthesis for Improved Dry Reforming Performance. ACS Catal. 2017, 7, 6942–6948. [Google Scholar] [CrossRef]
- Goula, M.; Lemonidou, A.; Efstathiou, A. Characterization of Carbonaceous Species Formed during Reforming of CH4 with CO2 over Ni/CaO–Al2O3Catalysts Studied by Various Transient Techniques. J. Catal. 1996, 161, 626–640. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, X.; Zhu, J.; Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: A deactivation scheme from density functional theory. J. Catal. 2014, 311, 469–480. [Google Scholar] [CrossRef]
- Bermúdez, J.; Fidalgo, B.; Arenillas, A.; Menéndez, J. Dry reforming of coke oven gases over activated carbon to produce syngas for methanol synthesis. Fuel 2010, 89, 2897–2902. [Google Scholar] [CrossRef] [Green Version]
- Solh, T.E.; Jarosch, K.; de Lasa, H. Catalytic Dry Reforming of Methane in a CREC Riser Simulator Kinetic Modeling and Model Discrimination. Ind. Eng. Chem. Res. 2003, 42, 2507–2515. [Google Scholar] [CrossRef]
- Pekediz, A.; de Lasa, H.I. I. Methane oxidative coupling in a novel riser simulator reactor. Chem. Eng. Sci. 1994, 49, 4759–4770. [Google Scholar] [CrossRef]
- Becker, A.; Hüttinger, K. Chemistry and kinetics of chemical vapor deposition of pyrocarbon—IV pyrocarbon deposition from methane in the low temperature regime. Carbon 1998, 36, 213–224. [Google Scholar] [CrossRef]
- Li, A.; Deutschmann, O. Transient modeling of chemical vapor infiltration of methane using multi-step reaction and deposition models. Chem. Eng. Sci. 2007, 62, 4976–4982. [Google Scholar] [CrossRef]
- Li, A.; Norinaga, K.; Zhang, W.; Deutschmann, O. Modeling and simulation of materials synthesis: Chemical vapor deposition and infiltration of pyrolytic carbon. Compos. Sci. Technol. 2008, 68, 1097–1104. [Google Scholar] [CrossRef] [Green Version]
- Norinaga, K.; Deutschmann, O.; Saegusa, N.; Hayashi, J.-I. Analysis of pyrolysis products from light hydrocarbons and kinetic modeling for growth of polycyclic aromatic hydrocarbons with detailed chemistry. J. Anal. Appl. Pyrolysis 2009, 86, 148–160. [Google Scholar] [CrossRef]
- Dagaut, P.; Pengloan, G.; Ristori, A. Oxidation, ignition and combustion of toluene: Experimental and detailed chemical kinetic modelingElectronic supplementary information. Phys. Chem. Chem. Phys. 2002, 4, 1846–1854. [Google Scholar] [CrossRef]
- Becker, A.; Hu, Z.; Hüttinger, K. A hydrogen inhibition model of carbon deposition from light hydrocarbons. Fuel 2000, 79, 1573–1580. [Google Scholar] [CrossRef]
- Teuner, S.C.N.; Neumann, P.; von Linde, F. CO through CO2 Reforming. The Calcor Standard and Calcor Economy Processes. OIL GAS Eur. Mag. 2001, 3, 44–46. [Google Scholar]
- Tang, S.-B.; Qiu, F.-L.; Lu, S.-J. Effect of supports on the carbon deposition of nickel catalysts for methane reforming with CO2. Catal. Today 1995, 24, 253–255. [Google Scholar] [CrossRef]
- Kim, G.J.; Cho, D.-S.; Kim, K.-H.; Kim, J.-H. The reaction of CO2 with CH4 to synthesize H2 and CO over nickel-loaded Y-zeolites. Catal. Lett. 1994, 28, 41–52. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Zhu, Z.; Melaet, G.; Axnanda, S.; Alayoglu, S.; Liu, Z.; Salmeron, M.; A Somorjai, G. Structure and Chemical State of the Pt(557) Surface during Hydrogen Oxidation Reaction Studied by in Situ Scanning Tunneling Microscopy and X-ray Photoelectron Spectroscopy. J. Am. Chem. Soc. 2013, 135, 12560–12563. [Google Scholar] [CrossRef]
- Gabasch, H.; Hayek, K.; Klötzer, B.; Unterberger, W.; Kleimenov, E.; Teschner, D.; Zafeiratos, S.; Hävecker, M.; Knop-Gericke, A.; Schlögl, R.; et al. Methane Oxidation on Pd(111): In Situ XPS Identification of Active Phase. J. Phys. Chem. C 2007, 111, 7957–7962. [Google Scholar] [CrossRef]
- Teschner, D.; Pestryakov, A.; Kleimenov, E.; Havecker, M.; Bluhm, H.; Sauer, H.; Knopgericke, A.; Schlogl, R. High-pressure X-ray photoelectron spectroscopy of palladium model hydrogenation catalysts.: Part 1: Effect of gas ambient and temperature. J. Catal. 2005, 230, 186–194. [Google Scholar] [CrossRef]
- Mu, R.; Fu, Q.; Xu, H.; Zhang, H.; Huang, Y.; Jiang, Z.; Zhang, S.; Tan, D.; Bao, X. Synergetic Effect of Surface and Subsurface Ni Species at Pt−Ni Bimetallic Catalysts for CO Oxidation. J. Am. Chem. Soc. 2011, 133, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Merte, L.R.; Knudsen, J.; Eichhorn, F.M.; Porsgaard, S.; Zeuthen, H.; Grabow, L.C.; Lægsgaard, E.; Bluhm, H.; Salmeron, M.; Mavrikakis, M.; et al. CO-Induced Embedding of Pt Adatoms in a Partially Reduced FeOx Film on Pt(111). J. Am. Chem. Soc. 2011, 133, 10692–10695. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Reactivity of Chemisorbed Oxygen Atoms and Their Catalytic Consequences during CH4–O2 Catalysis on Supported Pt Clusters. J. Am. Chem. Soc. 2011, 133, 15958–15978. [Google Scholar] [CrossRef]
- Chen, C.S.; Lin, J.H.; You, J.H.; Yang, K.H. Effects of Potassium on NiAl2O3−K/ Catalysts in the Synthesis of Carbon Nanofibers by Catalytic Hydrogenation of CO2. J. Phys. Chem. A 2009, 114, 3773–3781. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, M.L.; Chin, Y.C.; Hidajat, K.; Kawi, S. Role of lattice oxygen in oxidative steam reforming of toluene as a tar model compound over Ni/La0.8Sr0.2AlO3 catalyst. Catal. Sci. Technol. 2015, 5, 3585–3597. [Google Scholar] [CrossRef]
- Li, C.; Hirabayashi, D.; Suzuki, K. A crucial role of O2− and O22− on mayenite structure for biomass tar steam reforming over Ni/Ca12Al14O33. Appl. Catal. B Environ. 2009, 88, 351–360. [Google Scholar] [CrossRef]
- Adnan, M.A.; Hidayat, A.; Ajumobi, O.O.; Adamu, S.; Muraza, O.; Hossain, M.M. Fluidizable Fe–Co/Ce–ZrO2 Catalysts for Steam Reforming of Toluene as a Tar Surrogate in Biomass Gasification. Energy Fuels 2018, 32, 12833–12842. [Google Scholar] [CrossRef]
- Alemán, J.V.; Chadwick, A.V.; He, J.; Hess, M.; Horie, K.; Jones, R.G.; Kratochvíl, P.; Meisel, I.; Mita, I.; Moad, G.; et al. Definitions of terms relating to the structure and processing of sols, gels, networks, and inorganic-organic hybrid materials (IUPAC Recommendations 2007). Pure Appl. Chem. 2007, 79, 1801–1829. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Muzart, J.; Sahebdelfar, S. NiO–MgO Solid Solution Prepared by Sol–Gel Method as Precursor for Ni/MgO Methane Dry Reforming Catalyst: Effect of Calcination Temperature on Catalytic Performance. Catal. Lett. 2016, 146, 238–248. [Google Scholar] [CrossRef]
- Mazumder, J.; de Lasa, H.I. Fluidizable La2O3 promoted Ni/γ-Al2O3 catalyst for steam gasification of biomass: Effect of catalyst preparation conditions. Appl. Catal. B Environ. 2015, 168-169, 250–265. [Google Scholar] [CrossRef]
- Ammendola, P.; Cammisa, E.; Chirone, R.; Lisi, L.; Ruoppolo, G. Effect of sulphur on the performance of Rh–LaCoO3 based catalyst for tar conversion to syngas. Appl. Catal. B Environ. 2012, 113-114, 11–18. [Google Scholar] [CrossRef]
- Yeo, T.Y.; Ashok, J.; Kawi, S. Recent developments in sulphur-resilient catalytic systems for syngas production. Renew. Sustain. Energy Rev. 2019, 100, 52–70. [Google Scholar] [CrossRef]
- Zuber, C.; Husmann, M.; Schroettner, H.; Hochenauer, C.; Kienberger, T. Investigation of sulfidation and regeneration of a ZnO-adsorbent used in a biomass tar removal process based on catalytic steam reforming. Fuel 2015, 153, 143–153. [Google Scholar] [CrossRef]
- Rangan, M.; Yung, M.M.; Medlin, J.W. Experimental and computational investigations of sulfur-resistant bimetallic catalysts for reforming of biomass gasification products. J. Catal. 2011, 282, 249–257. [Google Scholar] [CrossRef]
- Rangan, M.; Yung, M.M.; Medlin, J.W. NiW and NiRu Bimetallic Catalysts for Ethylene Steam Reforming: Alternative Mechanisms for Sulfur Resistance. Catal. Lett. 2012, 142, 718–727. [Google Scholar] [CrossRef]
- Sato, K.; Fujimoto, K. Development of new nickel based catalyst for tar reforming with superior resistance to sulfur poisoning and coking in biomass gasification. Catal. Commun. 2007, 8, 1697–1701. [Google Scholar] [CrossRef]
- Veksha, A.; Giannis, A.; Oh, W.-D.; Chang, V.W.-C.; Lisak, G.; Lim, T.-T. Catalytic activities and resistance to HCl poisoning of Ni-based catalysts during steam reforming of naphthalene. Appl. Catal. A Gen. 2018, 557, 25–38. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, C.; Park, J.S.; Lee, H. Highly Coke-Resistant Ni Nanoparticle Catalysts with Minimal Sintering in Dry Reforming of Methane. ChemSusChem 2014, 7, 451–456. [Google Scholar] [CrossRef]
- Akri, M.; Zhao, S.; Li, X.; Zang, K.; Lee, A.F.; Isaacs, M.A.; Xi, W.; Gangarajula, Y.; Luo, J.; Ren, Y.; et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 2019, 10, 5181. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Zuo, Z.; Liu, S.; Wang, Z.; Liu, C.; Huang, W.; Huang, J.; Liu, P. Dry Reforming of Methane on Single-Site Ni/MgO Catalysts: Importance of Site Confinement. ACS Catal. 2018, 8, 9821–9835. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Lee, S.-W.; Park, J.-W.; Oh, D.-K.; Park, J.-S.; Kim, S.S. Combined steam and CO2 reforming of methane using catalytic nickel membrane for gas to liquid (GTL) process. Catal. Today 2014, 236, 49–56. [Google Scholar] [CrossRef]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
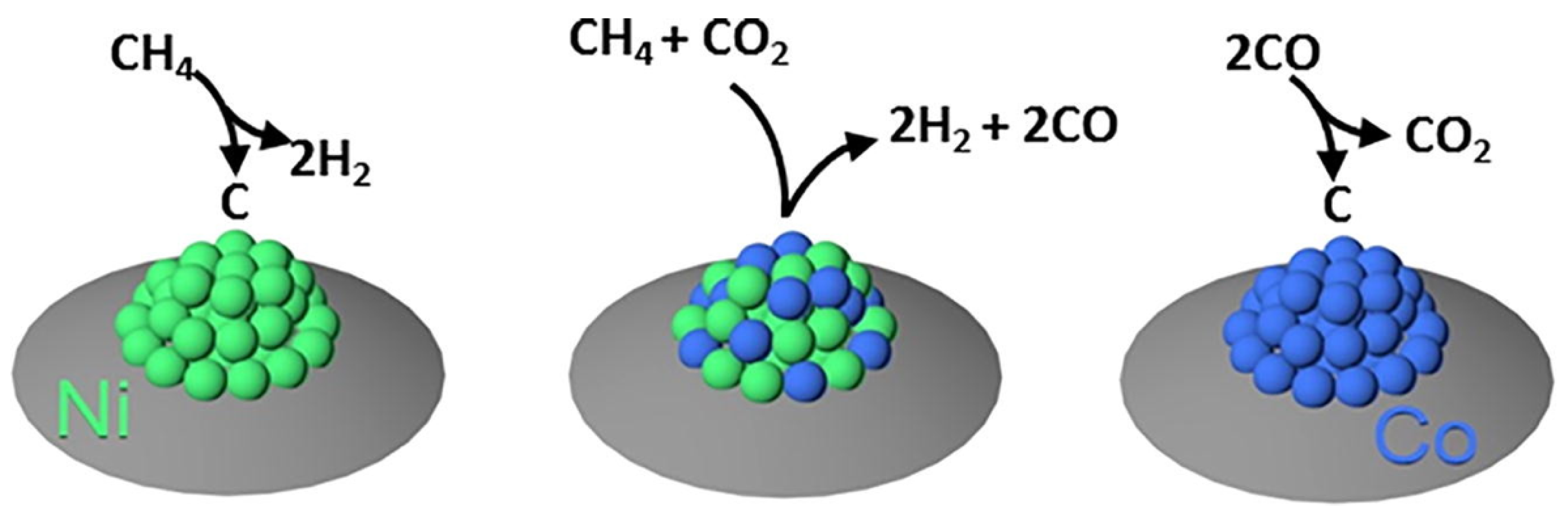
- San-José-Alonso, D.; Juan-Juan, J.; Illán-Gómez, M.; Román-Martínez, M. Ni, Co and bimetallic Ni–Co catalysts for the dry reforming of methane. Appl. Catal. A Gen. 2009, 371, 54–59. [Google Scholar] [CrossRef]
- Horlyck, J.; Lawrey, C.; Lovell, E.; Amal, R.; Scott, J. Elucidating the impact of Ni and Co loading on the selectivity of bimetallic NiCo catalysts for dry reforming of methane. Chem. Eng. J. 2018, 352, 572–580. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Highly carbon-resistant Ni–Co/SiO2 catalysts derived from phyllosilicates for dry reforming of methane. J. CO2 Util. 2017, 18, 345–352. [Google Scholar] [CrossRef]
- Benrabaa, R.; Löfberg, A.; Caballero, J.G.; Bordes-Richard, E.; Rubbens, A.; Vannier, R.-N.; Boukhlouf, H.; Barama, A. Sol–gel synthesis and characterization of silica supported nickel ferrite catalysts for dry reforming of methane. Catal. Commun. 2015, 58, 127–131. [Google Scholar] [CrossRef]
- Kim, S.M.; Abdala, P.M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and Dynamics Increase the Performance of NiFe Dry Reforming Catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949. [Google Scholar] [CrossRef]
- Chen, H.-W.; Wang, C.-Y.; Yu, C.-H.; Tseng, L.-T.; Liao, P.-H. Carbon dioxide reforming of methane reaction catalyzed by stable nickel copper catalysts. Catal. Today 2004, 97, 173–180. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Q.; Cai, W.; Zhang, P.; Song, X.; Sun, Z.; Gao, L. Phyllosilicate evolved hierarchical Ni- and Cu–Ni/SiO2 nanocomposites for methane dry reforming catalysis. Appl. Catal. A Gen. 2015, 503, 94–102. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.; Herrera, C.; Larrubia, M.; Alemany, L.J. Improved Pt-Ni nanocatalysts for dry reforming of methane. Appl. Catal. A Gen. 2010, 377, 191–199. [Google Scholar] [CrossRef]
- Károlyi, J.; Németh, M.; Evangelisti, C.; Sáfrán, G.; Schay, Z.; Horváth, A.; Somodi, F. Carbon dioxide reforming of methane over Ni–In/SiO2 catalyst without coke formation. J. Ind. Eng. Chem. 2018, 58, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, W.; Jin, M.; Jin, X. Strain-Induced Reverse Phase Transformation in Nanocrystalline Co-Ni Alloys. Mater. Res. Lett. 2014, 3, 107–113. [Google Scholar] [CrossRef]
- Shamsi, A. Partial Oxidation and Dry Reforming of Methane Over Ca/Ni/K(Na) Catalysts. Catal. Lett. 2006, 109, 189–193. [Google Scholar] [CrossRef]
- Luna, A.E.C.; Iriarte, M.E. Carbon dioxide reforming of methane over a metal modified Ni-Al2O3 catalyst. Appl. Catal. A Gen. 2008, 343, 10–15. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R. The effect of potassium on the activity and stability of Ni–MgO–ZrO2 catalysts for the dry reforming of methane to give synthesis gas. Catal. Today 2011, 178, 132–136. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Utilization of Greenhouse Gases through Dry Reforming: Screening of Nickel-Based Bimetallic Catalysts and Kinetic Studies. ChemSusChem 2011, 4, 1643–1653. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Huang, K.; Wu, Q.; Dai, S. Solvent-Free Self-Assembly to the Synthesis of Nitrogen-Doped Ordered Mesoporous Polymers for Highly Selective Capture and Conversion of CO2. Adv. Mater. 2017, 29, 1700445. [Google Scholar] [CrossRef]
- Ferreira-Aparicio, P.; Rodríguez-Ramos, I.; Anderson, J.A.; Guerrero-Ruiz, A. Mechanistic aspects of the dry reforming of methane over ruthenium catalysts. Appl. Catal. A Gen. 2000, 202, 183–196. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, M.; Patel, J.; Bordoloi, A. A study of the synergy between support surface properties and catalyst deactivation for CO 2 reforming over supported Ni nanoparticles. Appl. Catal. A Gen. 2017, 545, 113–126. [Google Scholar] [CrossRef]
- Múnera, J.; Faroldi, B.; Frutis, E.; Lombardo, E.; Cornaglia, L.; Carrazán, S.G. Supported Rh nanoparticles on CaO–SiO2 binary systems for the reforming of methane by carbon dioxide in membrane reactors. Appl. Catal. A Gen. 2014, 474, 114–124. [Google Scholar] [CrossRef]
- Steib, M.; Lou, Y.; Jentys, A.; Lercher, J.A. Enhanced Activity in Methane Dry Reforming by Carbon Dioxide Induced Metal-Oxide Interface Restructuring of Nickel/Zirconia. ChemCatChem 2017, 9, 3809–3813. [Google Scholar] [CrossRef]
- Odedairo, T.; Chen, J.; Zhu, Z. Metal–support interface of a novel Ni–CeO2 catalyst for dry reforming of methane. Catal. Commun. 2013, 31, 25–31. [Google Scholar] [CrossRef]
- Daoura, O.; Boutros, M.; Launay, F. 3 An overview of recent works on Ni silica-based catalysts for the dry reforming of methane. Hydrog. Prod. Energy Transit. 2021, 193–212. [Google Scholar] [CrossRef]
- Das, S.; Jangam, A.; Xi, S.; Borgna, A.; Hidajat, K.; Kawi, S. Highly Dispersed Ni/Silica by Carbonization–Calcination of a Chelated Precursor for Coke-Free Dry Reforming of Methane. ACS Appl. Energy Mater. 2020, 3, 7719–7735. [Google Scholar] [CrossRef]
- Zhang, J.; Li, F. Coke-resistant NiSiO2 catalyst for dry reforming of methane. Appl. Catal. B Environ. 2015, 176–177, 513–521. [Google Scholar] [CrossRef]
- Shen, D.; Huo, M.; Li, L.; Lyu, S.; Wang, J.; Wang, X.; Zhang, Y.; Li, J. Effects of alumina morphology on dry reforming of methane over Ni/Al2O3 catalysts. Catal. Sci. Technol. 2020, 10, 510–516. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, Y.; Li, H.; Li, H. CO2 conversion to synthesis gas via DRM on the durable Al2O3/Ni/Al2O3 sandwich catalyst with high activity and stability. Green Chem. 2018, 20, 2781–2787. [Google Scholar] [CrossRef]
- Jabbour, K.; El Hassan, N.; Davidson, A.; Casale, S.; Massiani, P. Factors affecting the long-term stability of mesoporous nickel-based catalysts in combined steam and dry reforming of methane. Catal. Sci. Technol. 2016, 6, 4616–4631. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.; Liao, X.; Zhao, Y. Comparative study of the dry reforming of methane on fluidised aerogel and xerogel Ni/Al2O3 catalysts. Appl. Petrochem. Res. 2013, 3, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Djebarri, B.; Gonzalez-Delacruz, V.M.; Halliche, D.; Bachari, K.; Saadi, A.; Caballero, A.; Holgado, J.P.; Cherifi, O. Promoting effect of Ce and Mg cations in Ni/Al catalysts prepared from hydrotalcites for the dry reforming of methane. React. Kinet. Mech. Catal. 2014, 111, 259–275. [Google Scholar] [CrossRef]
- Zhou, L.; Li, L.; Wei, N.; Li, J.; Basset, J.-M. Corrigendum: Effect of NiAl2O4Formation on Ni/Al2O3Stability during Dry Reforming of Methane. ChemCatChem 2015, 7, 2406. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Naeem, M.A.; Fakeeha, A.H.; Abasaeed, A.E. Role of La2O3 as Promoter and Support in Ni/γ-Al2O3 Catalysts for Dry Reforming of Methane. Chin. J. Chem. Eng. 2014, 22, 28–37. [Google Scholar] [CrossRef]
- Cao, Y.; Li, H.; Zhang, J.; Shi, L.; Zhang, D. Promotional effects of rare earth elements (Sc, Y, Ce, and Pr) on NiMgAl catalysts for dry reforming of methane. RSC Adv. 2016, 6, 112215–112225. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique Catalytic Structure for Stable CO2 Reforming of Methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, G.; Xue, Q.; Chen, L.; Lu, Y. High carbon-resistance Ni/CeAlO3-Al2O3 catalyst for CH4/CO2 reforming. Appl. Catal. B Environ. 2013, 136–137, 260–268. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-Temperature Catalytic CO2 Dry Reforming of Methane on Ni-Si/ZrO2 Catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Wang, Y.; Costa, P.D.; Hu, C. Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts 2019, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.A.; Al-Fatesh, A.S.; Kumar, N.S.; Abasaeed, A.E.; Kasim, S.O.; Fakeeha, A.H. Dry Reforming of Methane Using Ce-modified Ni Supported on 8%PO4 + ZrO2 Catalysts. Catalysts 2020, 10, 242. [Google Scholar] [CrossRef] [Green Version]
- Titus, J.; Goepel, M.; Schunk, S.; Wilde, N. The role of acid/base properties in Ni/MgO-ZrO2–based catalysts for dry reforming of methane. Catal. Commun. 2017, 100, 76–80. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef]
- Mette, K.; Kühl, S.; Tarasov, A.; Willinger, M.G.; Kröhnert, J.; Wrabetz, S.; Trunschke, A.; Scherzer, M.; Girgsdies, F.; Düdder, H.; et al. High-Temperature Stable Ni Nanoparticles for the Dry Reforming of Methane. ACS Catal. 2016, 6, 7238–7248. [Google Scholar] [CrossRef]
- Liu, H.; Wierzbicki, D.; Dębek, R.; Motak, M.; Grzybek, T.; Da Costa, P.; Galvez, M.E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16. [Google Scholar] [CrossRef]
- Gaur, S.; Haynes, D.J.; Spivey, J.J. Rh, Ni, and Ca substituted pyrochlore catalysts for dry reforming of methane. Appl. Catal. A Gen. 2011, 403, 142–151. [Google Scholar] [CrossRef]
- Liu, H.; Hadjltaief, H.B.; Benzina, M.; Galvez, M.E.; Da Costa, P. Natural clay based nickel catalysts for dry reforming of methane: On the effect of support promotion (La, Al, Mn). Int. J. Hydrog. Energy 2019, 44, 246–255. [Google Scholar] [CrossRef]
- Brungs, A.J.; York, A.P.; Green, M.L. Comparison of the group V and VI transition metal carbides for methane dry reforming and thermodynamic prediction of their relative stabilities. Catal. Lett. 1999, 57, 65–69. [Google Scholar] [CrossRef]
- Brungs, A.J.; York, A.P.E.; Claridge, J.; Marquez-Alvarez, C.; Green, M.L.H. Dry reforming of methane to synthesis gas over supported molybdenum carbide catalysts. Catal. Lett. 2000, 70, 117–122. [Google Scholar] [CrossRef]
- Zhu, J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Shiozaki, R.; Andersen, A.G.; Hayakawa, T.; Hamakawa, S.; Suzuki, K.; Shimizu, M.; Takehira, K. Sustainable Ni/BaTiO3 Catalysts for Partial Oxidation of Methane to Synthesis Gas in Studies in Surface Science and Catalysis; Grasselli, R.K., Oyama, S.T., Gaffney, A.M., Lyons, J.E., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 701–710. [Google Scholar]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses, A.; Martinez, F.; Barrault, J.; Tatibouët, J. Pulse study of CO2 reforming of methane over LaNiO3. Appl. Catal. A Gen. 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Gallego, G.A.S.; Mondragon, F.; Barrault, J.; Tatibouët, J.-M. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107-108, 474–480. [Google Scholar] [CrossRef]
- Zhang, Z.; Verykios, X.E.; MacDonald, S.M.; Affrossman, S. Comparative Study of Carbon Dioxide Reforming of Methane to Synthesis Gas over Ni/La2O3 and Conventional Nickel-Based Catalysts. J. Phys. Chem. 1996, 100, 744–754. [Google Scholar] [CrossRef]
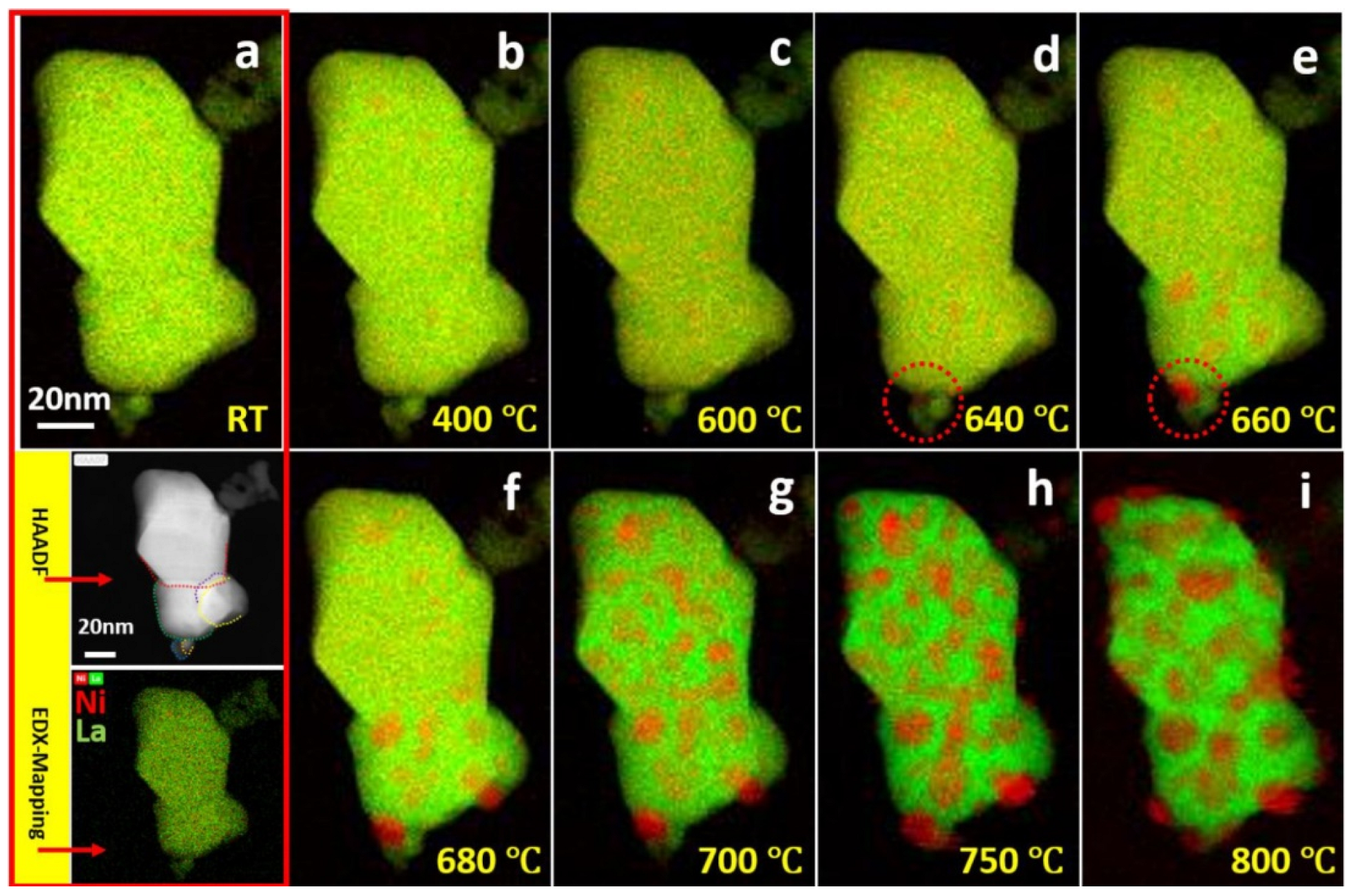
- Cao, P.; Tang, P.; Bekheet, M.F.; Du, H.; Yang, L.; Haug, L.; Gili, A.; Bischoff, B.; Gurlo, A.; Kunz, M.; et al. Atomic-Scale Insights into Nickel Exsolution on LaNiO3 Catalysts via In Situ Electron Microscopy. J. Phys. Chem. C 2021, 126, 786–796. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.; Saccoccio, M.; Lu, Z.; Ciucci, F. From material design to mechanism study: Nanoscale Ni exsolution on a highly active A-site deficient anode material for solid oxide fuel cells. Nano Energy 2016, 27, 499–508. [Google Scholar] [CrossRef]
- Malashevich, A.; Ismail-Beigi, S. First-principles study of oxygen-deficient LaNiO3 structures. Phys. Rev. B 2015, 92, 144102. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.S.; Liu, Y.; Rong, X.; Kolpak, A.M. Electronic Origin and Kinetic Feasibility of the Lattice Oxygen Participation During the Oxygen Evolution Reaction on Perovskites. J. Phys. Chem. Lett. 2018, 9, 1473–1479. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 dry-reforming of methane over La0.8Sr0.2Ni0.8M0.2O3 perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of lattice oxygen on C–H activation and carbon suppression. Int. J. Hydrog. Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Song, X.; Dong, X.; Yin, S.; Wang, M.; Li, M.; Wang, H. Effects of Fe partial substitution of La2NiO4/LaNiO3 catalyst precursors prepared by wet impregnation method for the dry reforming of methane. Appl. Catal. A Gen. 2016, 526, 132–138. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M. Dry reforming of CH4 over solid solutions of LaNi1−xCoxO3. Catal. Today 2008, 133–135, 142–148. [Google Scholar] [CrossRef]
- Mousavi, M.; Pour, A.N. Performance and structural features of LaNi0.5Co0.5O3 perovskite oxides for the dry reforming of methane: Influence of the preparation method. New J. Chem. 2019, 43, 10763–10773. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Gallego, G.S.; Batiot-Dupeyrat, C.; Barrault, J.; Florez, E.; Mondragóna, F. Dry reforming of methane over LaNi1−yByO3±δ (B = Mg, Co) perovskites used as catalyst precursor. Appl. Catal. A Gen. 2008, 334, 251–258. [Google Scholar] [CrossRef]
- Takenaka, S.; Kato, E.; Tomikubo, Y.; Otsuka, K. Structural change of Ni species during the methane decomposition and the subsequent gasification of deposited carbon with CO2 over supported Ni catalysts. J. Catal. 2003, 219, 176–185. [Google Scholar] [CrossRef]
- Steib, M.; Jentys, A.; A Lercher, J. Structural response of Ni/ZrO2 to feed modulations during CH4reforming reactions. J. Phys. Conf. Ser. 2016, 712, 12049. [Google Scholar] [CrossRef]
- Xu, B.-Q.; Wei, J.-M.; Yu, Y.-T.; Li, J.-L.; Zhu, Q.-M. Carbon Dioxide Reforming of Methane Over Nanocomposite Ni/ZrO2 Catalysts. Top. Catal. 2003, 22, 77–85. [Google Scholar] [CrossRef]
- Palmer, C.; Upham, D.C.; Smart, S.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 2020, 3, 83–89. [Google Scholar] [CrossRef]
- Onstot, W.J.; Minet, R.G.; Tsotsis, T.T. Design Aspects of Membrane Reactors for Dry Reforming of Methane for the Production of Hydrogen. Ind. Eng. Chem. Res. 2000, 40, 242–251. [Google Scholar] [CrossRef]
- Chai, M.; Machida, M.; Eguchi, K.; Arai, H. Promotion of hydrogen permeation on metal-dispersed alumina membranes and its application to a membrane reactor for methane steam reforming. Appl. Catal. A Gen. 1994, 110, 239–250. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Gade, S.K.; Keeling, M.K.; Thoen, P.M.; Davidson, A.; Way, J.D. Palladium and palladium alloy membranes for hydrogen separation and production: History, fabrication strategies, and current performance. Sep. Purif. Technol. 2010, 73, 59–64. [Google Scholar] [CrossRef]
- Nishimura, A.; Takada, T.; Ohata, S.; Kolhe, M. Biogas Dry Reforming for Hydrogen through Membrane Reactor Utilizing Negative Pressure. Fuels 2021, 2, 194–209. [Google Scholar] [CrossRef]
- Anzelmo, B.; Wilcox, J.; Liguori, S. Natural gas steam reforming reaction at low temperature and pressure conditions for hydrogen production via Pd/PSS membrane reactor. J. Membr. Sci. 2017, 522, 343–350. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Tantayanon, S.; Kiatgamolchai, S.; Sukonket, T. Improved hydrogen production from dry reforming reaction using a catalytic packed-bed membrane reactor with Ni-based catalyst and dense PdAgCu alloy membrane. Int. J. Hydrog. Energy 2016, 41, 2621–2630. [Google Scholar] [CrossRef]
- Trunschke, A.; Bellini, G.; Boniface, M.; Carey, S.J.; Dong, J.; Erdem, E.; Foppa, L.; Frandsen, W.; Geske, M.; Ghiringhelli, L.M.; et al. Towards Experimental Handbooks in Catalysis. Top. Catal. 2020, 63, 1683–1699. [Google Scholar] [CrossRef]
- Tavasoli, A.; Ozin, G. Green Syngas by Solar Dry Reforming. Joule 2018, 2, 571–575. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Mao, M.; Zhang, Q.; Li, Y.; Bai, J.; Yang, Y.; Zeng, M.; Zhao, X. Solar-Light-Driven CO2 Reduction by CH4 on Silica-Cluster-Modified Ni Nanocrystals with a High Solar-to-Fuel Efficiency and Excellent Durability. Adv. Energy Mater. 2018, 8, 1702472. [Google Scholar] [CrossRef]
- Simakov, D.S.A.; Wright, M.M.; Ahmed, S.; Mokheimer, E.M.A.; Román-Leshkov, Y. Solar thermal catalytic reforming of natural gas: A review on chemistry, catalysis and system design. Catal. Sci. Technol. 2015, 5, 1991–2016. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 118195. [Google Scholar] [CrossRef]
- Puliyalil, H.; Jurković, D.L.; Dasireddy, V.D.B.C.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar] [CrossRef] [Green Version]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Tao, X.; Bai, M.; Li, X.; Long, H.; Shang, S.; Yin, Y.; Dai, X. CH4–CO2 reforming by plasma—challenges and opportunities. Prog. Energy Combust. Sci. 2011, 37, 113–124. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chao, Y.; Chang, M.B. Review of plasma catalysis on hydrocarbon reforming for hydrogen production—Interaction, integration, and prospects. Appl. Catal. B Environ. 2008, 85, 1–9. [Google Scholar] [CrossRef]
- Chen, H.L.; Lee, H.M.; Chen, S.H.; Chang, M.B.; Yu, S.J.; Li, S.N. Removal of Volatile Organic Compounds by Single-Stage and Two-Stage Plasma Catalysis Systems: A Review of the Performance Enhancement Mechanisms, Current Status, and Suitable Applications. Environ. Sci. Technol. 2009, 43, 2216–2227. [Google Scholar] [CrossRef]
- Gesser, H.D.; Hunter, N.R.; Probawono, D. The CO2 Reforming of Natural Gas in a Silent Discharge Reactor. Plasma Chem. Plasma Process. 1998, 18, 241–245. [Google Scholar] [CrossRef]
- Zhou, L.M.; Xue, B.; Kogelschatz, U.; Eliasson, B. Nonequilibrium Plasma Reforming of Greenhouse Gases to Synthesis Gas. Energy Fuels 1998, 12, 1191–1199. [Google Scholar] [CrossRef]
- Sobolewski, M.A.; Langan, J.G.; Felke, B.S. Electrical optimization of plasma-enhanced chemical vapor deposition chamber cleaning plasmas. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 1998, 16, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Okumura, T. Inductively Coupled Plasma Sources and Applications. Phys. Res. Int. 2010, 2010, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kroesen, G.M.W.; Schram, D.C.; de Haas, J.C.M. Description of a flowing cascade arc plasma. Plasma Chem. Plasma Process. 1990, 10, 531–551. [Google Scholar] [CrossRef]
- Fridman, A.; Gutsol, A.; Cho, Y.I. Non-Thermal Atmospheric Pressure Plasma, in Advances in Heat Transfer; Fridman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 1–142. [Google Scholar]
- Lu, X.; Naidis, G.; Laroussi, M.; Ostrikov, K. Guided ionization waves: Theory and experiments. Phys. Rep. 2014, 540, 123–166. [Google Scholar] [CrossRef]
- Li, M.-W.; Tian, Y.-L.; Xu, G.-H. Characteristics of Carbon Dioxide Reforming of Methane via Alternating Current (AC) Corona Plasma Reactions. Energy Fuels 2007, 21, 2335–2339. [Google Scholar] [CrossRef]
- Drost, H.; Rutkowsky, J.; Mach, R.; Klotz, H.-D.; Schulz, G. Plasma-chemical methane conversion under nonthermal and thermal conditions: An attempt toward uniform kinetic modeling. Plasma Chem. Plasma Process. 1985, 5, 283–291. [Google Scholar] [CrossRef]
- Tao, X.; Qi, F.; Yin, Y.; Dai, X. CO2 reforming of CH4 by combination of thermal plasma and catalyst. Int. J. Hydrog. Energy 2008, 33, 1262–1265. [Google Scholar] [CrossRef]
- Rao, Z.; Cao, Y.; Huang, Z.; Yin, Z.; Wan, W.; Ma, M.; Wu, Y.; Wang, J.; Yang, G.; Cui, Y.; et al. Insights into the Nonthermal Effects of Light in Dry Reforming of Methane to Enhance the H2/CO Ratio Near Unity over Ni/Ga2O3. ACS Catal. 2021, 11, 4730–4738. [Google Scholar] [CrossRef]
- Schlögl, R. Heterogeneous Catalysis. Angew. Chem. Int. Ed. 2015, 54, 3465–3520. [Google Scholar] [CrossRef] [Green Version]
- Greiner, M.T.; JonesOrcid, T.E.; Klyushin, A.; Knop-Gericke, A.; Schlögl, R. Ethylene Epoxidation at the Phase Transition of Copper Oxides. J. Am. Chem. Soc. 2017, 139, 11825–11832. [Google Scholar] [CrossRef]
- Linde-Engineering. Technologies That Do More with Less. Available online: https://www.linde-engineering.com/en/about-linde-engineering/success-stories/technologies-more-with-less.html (accessed on 5 April 2022).
- BASF-Catalysts. SynspireTM Catalysts for Dry Reforming. 2019. Available online: https://catalysts.basf.com/industries/chemical/syngas-catalysts/synspire-dry-reforming-catalysts (accessed on 4 April 2022).
- Tullo, A.H. Dry reforming puts CO2 to work. C&EN Glob. Enterp. 2016, 94, 30. [Google Scholar]
- Fujita, T.; Peng, X.; Yamaguchi, A.; Cho, Y.; Zhang, Y.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Miyauchi, M.; et al. Nanoporous Nickel Composite Catalyst for the Dry Reforming of Methane. ACS Omega 2018, 3, 16651–16657. [Google Scholar] [CrossRef]
- Shoji, S.; Peng, X.; Imai, T.; Kumar, P.S.M.; Higuchi, K.; Yamamoto, Y.; Tokunaga, T.; Arai, S.; Ueda, S.; Hashimoto, A.; et al. Topologically immobilized catalysis centre for long-term stable carbon dioxide reforming of methane. Chem. Sci. 2019, 10, 3701–3705. [Google Scholar] [CrossRef] [Green Version]
- Kappes, H.B.; Energy Transition in the European Steel Industry—Reality Not Exception, in Direct from MIDREX. 2021, MIDREX. Available online: https://www.midrex.com/tech-article/energy-transition-in-the-european-steel-industry-reality-not-exception/ (accessed on 5 April 2022).
- Chevrier, V.; Lauren, L.; Michishita, H. MIDREX® Process: Bridge to Ultra-low CO2 Ironmaking. Kobelco Technol. Rev. 2021, 39, 33–40. [Google Scholar]
- Atsushi, M.; Hiroshi, U.; Sakaguchi, T. MIDREX Processes. Kobelco Technol. Rev. 2010, 29, 50–57. [Google Scholar]
- ArcelorMittal. Carbon2Value. Available online: https://www.carbon2value.be/en/ (accessed on 4 April 2022).
- Group, S. Paul Wurth and the Steel Partners Dillinger and Saarstahl Join Forces on Development of Dry Reforming Technology. 2021. Available online: https://www.sms-group.com/press-media/press-releases/press-detail/paul-wurth-and-the-steel-partners-dillinger-and-saarstahl-join-forces-on-development-of-dry-reforming-technology (accessed on 5 April 2022).
- Buelens Lukas, C.; Galvita, V.V.; Poelman, H.; Detavernier, C.; Marin, G.B. Super-dry reforming of methane intensifies CO2 utilization via Le Chatelier’s principle. Science 2016, 354, 449–452. [Google Scholar] [CrossRef]
- Galvita, V.V.; Poelman, H.; Marin, G.B. Combined chemical looping for energy storage and conversion. J. Power Sources 2015, 286, 362–370. [Google Scholar] [CrossRef]
- Catalisti. Flanders Industry Innovation Moonshot SDR. Available online: https://moonshotflanders.be/mot3-sdr/ (accessed on 4 April 2022).
- Wismann Sebastian, T. Electrified methane reforming: A compact approach to greener industrial hydrogen production. Science 2019, 364, 756–759. [Google Scholar] [CrossRef] [Green Version]
- News, B.-B.F. BASF, SABIC and Linde Join Forces to Realize the World’s First Electrically Heated Steam Cracker Furnace 2021. Available online: https://www.basf.com/global/en/who-we-are/sustainability/whats-new/sustainability-news/2021/basf-sabic-and-linde-join-forces-to-realize-wolds-first-electrically-heated-steam-cracker-furnace.html (accessed on 5 April 2022).
- Labrecque, R.; Lavoie, J.-M. Dry reforming of methane with CO2 on an electron-activated iron catalytic bed. Bioresour. Technol. 2011, 102, 11244–11248. [Google Scholar] [CrossRef]
- Banville, M.L.; Lavoie, J.-M.R. Dry Reforming of Methane Under An Electro-catalytic Bed: Effect of Electrical Current And Catalyst Composition. Energy Sustain. 2015, 186, 9. [Google Scholar]
- Vasconcelos, B.R.L. Is dry reforming the solution to reduce natural gas carbon footprint? Int. J. Energy Prod. Manag. 2018, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Dokania, A.; Ramirez, A.; Bavykina, A.; Gascon, J. Heterogeneous Catalysis for the Valorization of CO2: Role of Bifunctional Processes in the Production of Chemicals. ACS Energy Lett. 2019, 4, 167–176. [Google Scholar] [CrossRef]
- Parker, L. The World’s Plastic Pollution Crisis Explained 2019. Available online: https://www.nationalgeographic.com/environment/article/plastic-pollution (accessed on 28 January 2022).
- Han, B.; Wei, W.; Chang, L.; Cheng, P.; Hu, Y.H. Efficient Visible Light Photocatalytic CO2 Reforming of CH4. ACS Catal. 2016, 6, 494–497. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Dao, T.D.; Zhang, H.; Li, P.; Chang, K.; Wang, T.; Li, M.; Nagao, T.; Ye, J. Conversion of Carbon Dioxide by Methane Reforming under Visible-Light Irradiation: Surface-Plasmon-Mediated Nonpolar Molecule Activation. Angew. Chem. Int. Ed. 2015, 54, 11545–11549. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Choudhury, H.A.; Wang, D.; El-Halwagi, M.M.; Weitz, E.; Elbashir, N.O. A novel CO2 utilization technology for the synergistic co-production of multi-walled carbon nanotubes and syngas. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]













| Source | Fraction (%) | Absolute (gt) | Sub-Source | Fraction (%) | Absolute (gt) |
|---|---|---|---|---|---|
| Energy | 73.2 | 36.2 | Industry * | 24.2 | 11.95 |
| Mobility ** | 16.2 | 8.00 | |||
| Heating | 17.5 | 8.54 | |||
| Other combustion | 7.8 | 3.85 | |||
| Leaks | 5.8 | 2.87 | |||
| Energy in agriculture | 1.7 | 0.84 | |||
| Industrial products | 5.2 | 2.57 | Cement | 3.0 | 1.48 |
| Chemicals | 2.2 | 1.09 | |||
| Waste | 3.2 | 1.58 | Sewage | 1.3 | 0.64 |
| Landfill | 1.9 | 0.94 | |||
| Agriculture | 18.4 | 9.09 | Animals | 5.8 | 2.86 |
| Cropland | 6.8 | 3.36 | |||
| Combustion of crop waste | 3.5 | 1.73 |
| CO2 | CH4 | ||||
|---|---|---|---|---|---|
| Partner | Products | Waste | Partner | Products | Waste |
| H2 | Methanol Dimethylether CO Olefins | H2O H2O H2O H2O | O2 | Syngas Energy | -- CO2, H2O |
| CH4 | Syngas | -- | CO2 | Syngas | -- |
| NH3 | Urea | H2O | H2O | Syngas | H2O |
| Bond | Dissociation Energy [kJmol−1] | Molecule |
|---|---|---|
| H3C-H | 439 | Methane |
| OC=O | 532 | carbon dioxide |
| C≡O | 1077 | carbon monoxide |
| H3CO-H | 440 | Methanol |
| H-H | 436 | Hydrogen |
| O=O | 498 | Oxygen |
| N≡N | 945 | Nitrogen |
| External Parameter | Pressure | Temperature | CO2/CH4 |
|---|---|---|---|
| Effect of increasing the value of the external parameter while the other ones are constant | H2/CO increases | H2/CO decreases and stabilizes at 1.00 | H2/CO decreases |
| H2O increases | H2O decreases | H2O increases | |
| Coke increases | Coke decreases | Coke decreases | |
| Conversion decreases | Conversion increases | Conversion of CH4 increases |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Diaz, L.E.; Schlögl, R.; Lunkenbein, T. Quo Vadis Dry Reforming of Methane?—A Review on Its Chemical, Environmental, and Industrial Prospects. Catalysts 2022, 12, 465. https://doi.org/10.3390/catal12050465
Sandoval-Diaz LE, Schlögl R, Lunkenbein T. Quo Vadis Dry Reforming of Methane?—A Review on Its Chemical, Environmental, and Industrial Prospects. Catalysts. 2022; 12(5):465. https://doi.org/10.3390/catal12050465
Chicago/Turabian StyleSandoval-Diaz, Luis E., Robert Schlögl, and Thomas Lunkenbein. 2022. "Quo Vadis Dry Reforming of Methane?—A Review on Its Chemical, Environmental, and Industrial Prospects" Catalysts 12, no. 5: 465. https://doi.org/10.3390/catal12050465
APA StyleSandoval-Diaz, L. E., Schlögl, R., & Lunkenbein, T. (2022). Quo Vadis Dry Reforming of Methane?—A Review on Its Chemical, Environmental, and Industrial Prospects. Catalysts, 12(5), 465. https://doi.org/10.3390/catal12050465






