Study of the Relationship between Metal–Support Interactions and the Electrocatalytic Performance of Pt/Ti4O7 with Different Loadings
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Ti4O7
2.2. Synthesized Pt/Ti4O7 Catalysts
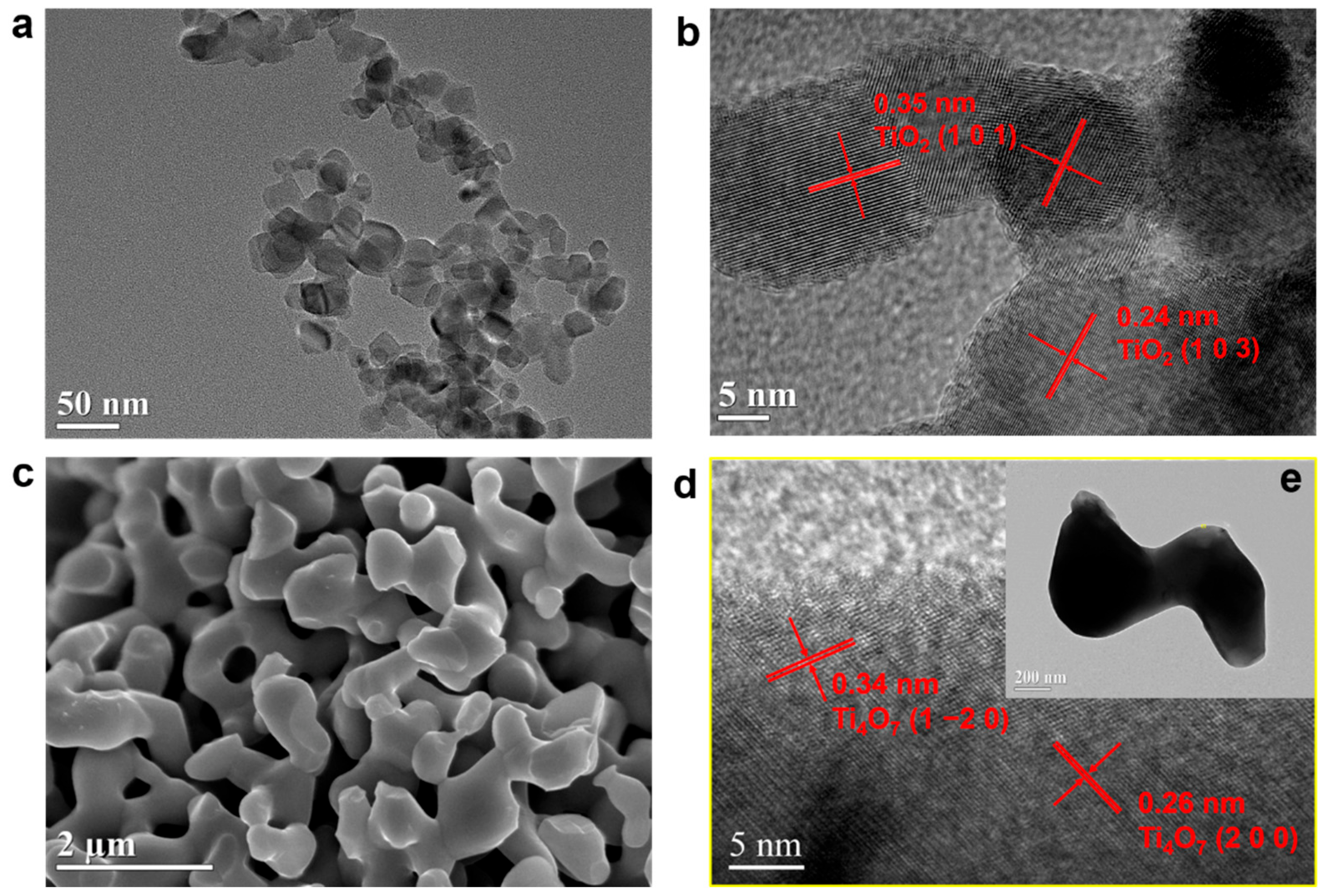
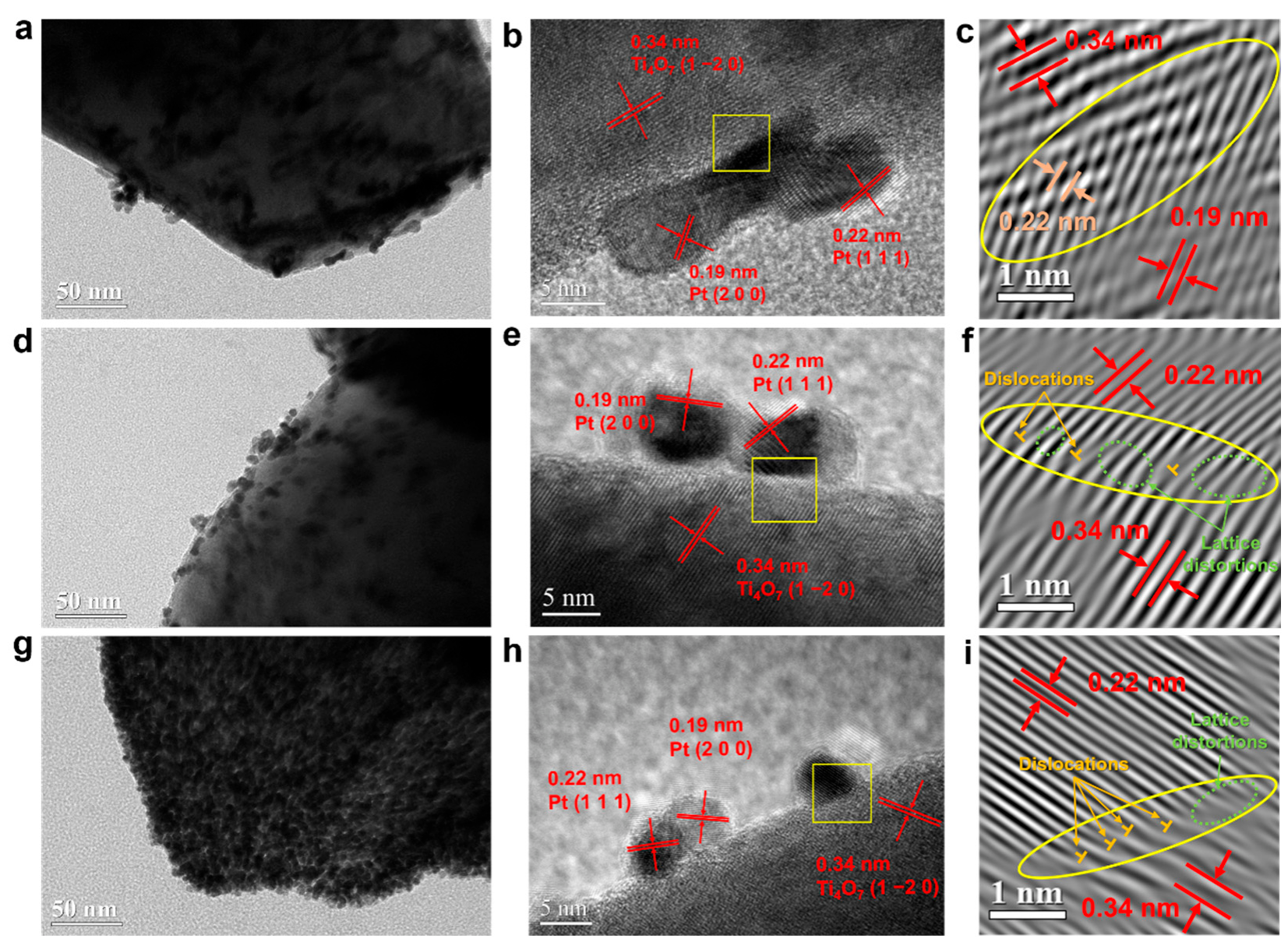
2.2.1. Morphologies and Crystalline Structures
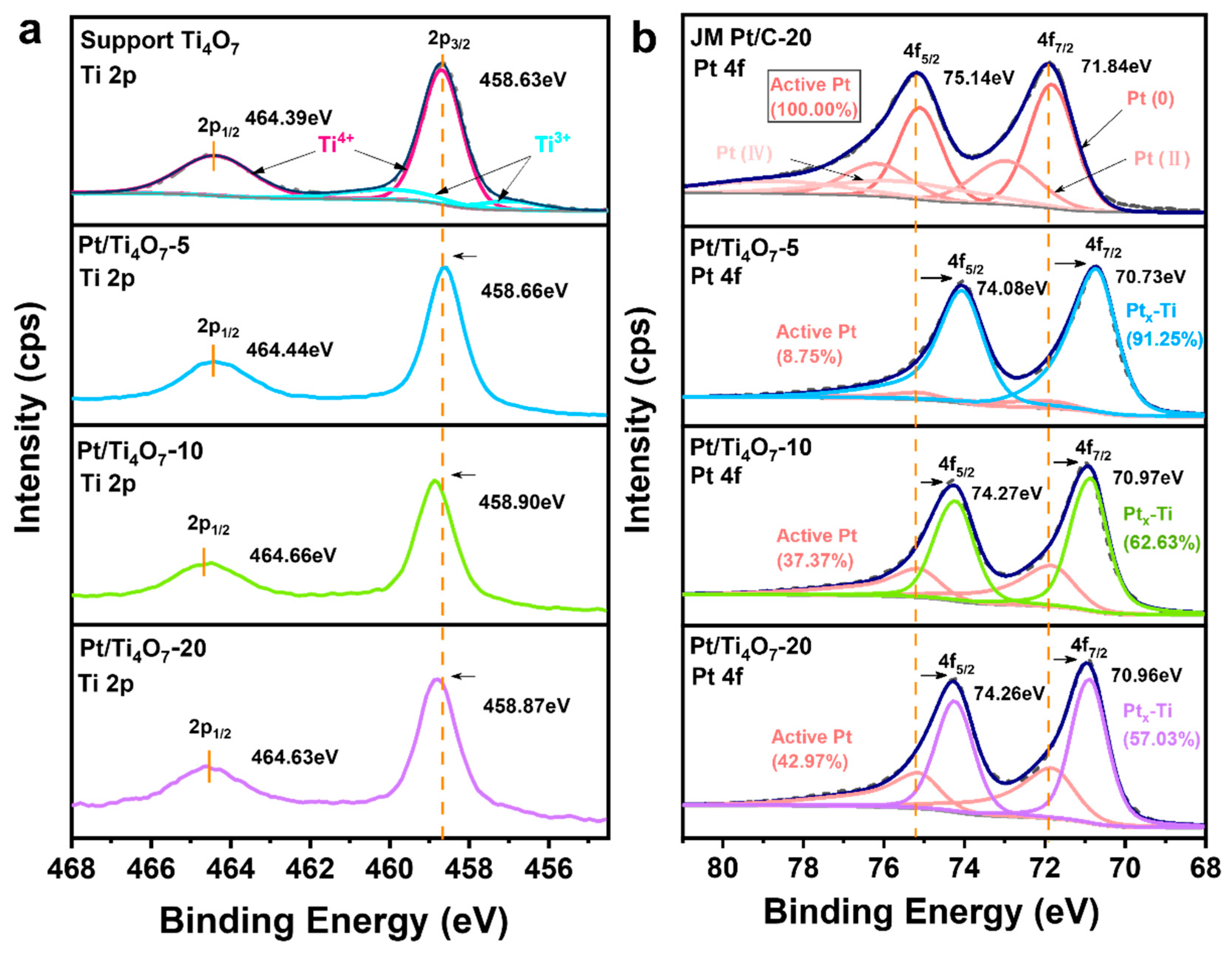
2.2.2. Electrocatalytic Performances of Pt/Ti4O7 Samples
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Catalysts
3.3. Characterization
3.4. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shao, M.; Chang, Q.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-group-metal catalysts for proton exchange membrane fuel cells: From catalyst design to electrode structure optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J.C.; Chen, S.; Yu, Y.; Zhang, J. Advanced Noncarbon Materials as Catalyst Supports and Non-noble Electrocatalysts for Fuel Cells and Metal–Air Batteries. Electrochem. Energy Rev. 2021, 4, 336–381. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wilkinson, D.P.; Zhang, J. Noncarbon Support Materials for Polymer Electrolyte Membrane Fuel Cell Electrocatalysts. Chem. Rev. 2011, 111, 7625–7651. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Park, S.; Popov, B.N. Development of a Titanium Dioxide-Supported Platinum Catalyst with Ultrahigh Stability for Polymer Electrolyte Membrane Fuel Cell Applications. J. Am. Chem. Soc. 2009, 131, 13898–13899. [Google Scholar] [CrossRef]
- Huang, S.Y.; Ganesan, P.; Popov, B. Titania supported platinum catalyst with high electrocatalytic activity and stability for polymer electrolyte membrane fuel cell. Appl. Catal. B Environ. 2011, 102, 71–77. [Google Scholar] [CrossRef]
- Mirshekari, G.R.; Rice, C.A. Effects of support particle size and Pt content on catalytic activity and durability of Pt/TiO2 catalyst for oxygen reduction reaction in proton exchange membrane fuel cells environment. J. Power Sources 2018, 396, 606–614. [Google Scholar] [CrossRef]
- Gustavsson, M.; Ekström, H.; Hanarp, P.; Eurenius, L.; Lindbergh, G.; Olsson, E.; Kasemo, B. Thin film Pt/TiO2 catalysts for the polymer electrolyte fuel cell. J. Power Sources 2007, 163, 671–678. [Google Scholar] [CrossRef]
- Bauer, A.; Lee, K.; Song, C.; Xie, Y.; Zhang, J.; Hui, R. Pt nanoparticles deposited on TiO2 based nanofibers: Electrochemical stability and oxygen reduction activity. J. Power Sources 2010, 195, 3105–3110. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.J.; Wilkinson, D.P.; Zhang, J. Synthesis of conductive rutile-phased Nb0.06Ti0.94O2 and its supported Pt electrocatalysts (Pt/Nb0.06Ti0.94O2) for the oxygen re-duction reaction. Dalton Trans. 2012, 41, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sankarasubramanian, S.; Matanovic, I.; Atanassov, P.; Ramani, V. Understanding the Oxygen Reduction Reaction Activity and Oxidative Stability of Pt Supported on Nb-Doped TiO2. ChemSusChem 2019, 12, 3468–3480. [Google Scholar] [CrossRef] [PubMed]
- Arashi, T.; Seo, J.; Takanabe, K.; Kubota, J.; Domen, K. Nb-doped TiO2 cathode catalysts for oxygen reduction reaction of polymer electrolyte fuel cells. Catal. Today 2014, 233, 181–186. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Wang, L.; Chen, P.; Xie, A.; Shen, Y. Nanocomposite of N-Doped TiO2 Nanorods and Graphene as an Effective Electrocatalyst for the Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2014, 6, 21978–21985. [Google Scholar] [CrossRef]
- Hassen, D.; Shenashen, M.A.; El Safty, S.A.; Selim, M.M.; Isago, H.; Elmarakbi, A.; El Safty, A.; Yamaguchi, H. Nitrogen-doped carbon-embedded TiO2 nanofibers as promising oxygen reduction reaction electrocatalysts. J. Power Sources 2016, 330, 292–303. [Google Scholar] [CrossRef]
- Bing, Y.; Neburchilov, V.; Song, C.; Baker, R.; Guest, A.; Ghosh, D.; Ye, S.; Campbell, S.; Zhang, J. Effects of synthesis condition on formation of desired crystal structures of doped-TiO2/carbon composite supports for ORR electrocatalysts. Electrochim. Acta 2012, 77, 225–231. [Google Scholar] [CrossRef]
- Montero Ocampo, C.; Garcia, J.R.V.; Estrada, E.M.A. Comparison of TiO2 and TiO2-CNT as Cathode Catalyst Supports for ORR. Int. J. Electrochem. Sci. 2013, 8, 12780–12800. [Google Scholar]
- Walsh, F.; Wills, R. The continuing development of Magnéli phase titanium sub-oxides and Ebonex® electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Bartholomew, R.F.; Frankl, D.R. Electrical Properties of Some Titanium Oxides. Phys. Rev. 1969, 187, 828–833. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Fujiwara, N.; Yamazaki, S.-I.; Yasuda, K. Sub-stoichiometric titanium oxide-supported platinum electrocatalyst for polymer electrolyte fuel cells. Electrochem. Commun. 2005, 7, 183–188. [Google Scholar] [CrossRef]
- Ioroi, T.; Akita, T.; Yamazaki, S.-i.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Corrosion-Resistant PEMFC Cathode Catalysts Based on a Magnéli-Phase Titanium Oxide Support Synthesized by Pulsed UV Laser Irradiation. J. Electrochem. Soc. 2011, 158, C329. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of Corrosion-Resistant Magnéli-Phase Ti4O7-Supported PEMFC Catalysts at High Potentials. J. Electrochem. Soc. 2008, 155, B321. [Google Scholar] [CrossRef]
- Senevirathne, K.; Hui, R.; Campbell, S.; Ye, S.; Zhang, J. Electrocatalytic activity and durability of Pt/NbO2 and Pt/Ti4O7 nanofibers for PEM fuel cell oxygen reduction reaction. Electrochim. Acta 2012, 59, 538–547. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Li, F.; Li, X.; Xia, D. Fiber-like nanostructured Ti4O7 used as durable fuel cell catalyst support in oxygen reduction catalysis. J. Mater. Chem. 2012, 22, 16560–16565. [Google Scholar] [CrossRef]
- Zhao, E.W.; Zheng, H.; Ludden, K.; Xin, Y.; Hagelin-Weaver, H.E.; Bowers, C.R. Strong Metal–Support Interactions Enhance the Pairwise Selectivity of Parahydrogen Addition over Ir/TiO2. ACS Catal. 2016, 6, 974–978. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2–x-Modified Ni Nanocatalyst with Tunable Metal–Support Interaction for Water–Gas Shift Reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly Active and Stable Metal Single-Atom Catalysts Achieved by Strong Electronic Metal–Support Interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, X.; Li, L.; Qi, H.; Yang, C.; Liu, W.; Pan, X.; Liu, X.; Yang, X.; Huang, Y.; et al. Ru/TiO2 Catalysts with Size-Dependent Metal/Support Interaction for Tunable Reactivity in Fischer–Tropsch Synthesis. ACS Catal. 2020, 10, 12967–12975. [Google Scholar] [CrossRef]
- Nayak, S.; Chaplin, B.P. Fabrication and characterization of porous, conductive, monolithic Ti4O7 electrodes. Electrochim. Acta 2018, 263, 299–310. [Google Scholar] [CrossRef]
- Dogan, D.C.; Hwang, S.M.; Jang, E.H.; Yim, S.D.; Sohn, Y.J.; Kim, S.H.; Yang, T.H.; Park, G.G. Highly Platinum-Loaded Magneli Phase Titanium Oxides as a High Voltage Tolerant Electrocatalyst for Polymer Electrolyte Fuel Cells. J. Nanosci. Nanotechnol. 2015, 15, 6988–6994. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto Mahaney, O.O.; Li, D.; Abe, R. What is Degussa (Evonik) P25 Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Tauster, S.J.; Fung, S.C.; Baker, R.T.K.; Horsley, J.A. Strong Interactions in Supported-Metal Catalysts. Science 1981, 211, 1121–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kao, C.C.; Tsai, S.C.; Bahl, M.K.; Chung, Y.W.; Lo, W.J. Electronic properties, structure and temperature-dependent composition of nickel deposited on rutile titanium dioxide (110) surfaces. Surf. Sci. 1980, 95, 1–14. [Google Scholar] [CrossRef]
- Tominaka, S.; Tsujimoto, Y.; Matsushita, Y.; Yamaura, K. Synthesis of Nanostructured Reduced Titanium Oxide: Crystal Structure Transformation Maintaining Nanomorphology. Angew. Chem. Int. Ed. 2011, 50, 7418–7421. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Zhang, H.; Pan, S.P.; Song, Y.F.; Liu, X.; Liu, W.H. Effects of service environment and pre-deformation on the fatigue behaviour of 2524 aluminium alloy. Arch. Civ. Mech. Eng. 2019, 20, 5. [Google Scholar] [CrossRef]
- Bock, C.; Paquet, C.; Couillard, M.; Botton, G.A.; MacDougall, B.R. Size-Selected Synthesis of PtRu Nano-Catalysts: Reaction and Size Control Mechanism. J. Am. Chem. Soc. 2004, 126, 8028–8037. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.J.; Tsai, M.C.; Su, W.N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.Y.; Hwang, B.J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Bäumer, M.; Freund, H.J. Metal deposits on well-ordered oxide films. Prog. Surf. Sci. 1999, 61, 127–198. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez DelaCruz, V.M.; Holgado, J.P.; Pereñíguez, R.; Caballero, A. Morphology changes induced by strong metal–support interaction on a Ni–ceria catalytic system. J. Catal. 2008, 257, 307–314. [Google Scholar] [CrossRef]
- Weber, D.J.; Dosche, C.; Oezaslan, M. Tuning of Pt–Co nanoparticle motifs for enhancing the HOR performance in alkaline media. J. Mater. Chem. A 2021, 9, 15415–15431. [Google Scholar] [CrossRef]
- Geng, P.; Su, J.; Miles, C.; Comninellis, C.; Chen, G. Highly-Ordered Magnéli Ti4O7 Nanotube Arrays as Effective Anodic Material for Electro-oxidation. Electrochim. Acta 2015, 153, 316–324. [Google Scholar] [CrossRef]
- Geng, P.; Chen, G. Magnéli Ti4O7 modified ceramic membrane for electrically-assisted filtration with antifouling property. J. Membr. Sci. 2016, 498, 302–314. [Google Scholar] [CrossRef]
- Li, X.; Zhu, A.L.; Qu, W.; Wang, H.; Hui, R.; Zhang, L.; Zhang, J. Magneli phase Ti4O7 electrode for oxygen reduction reaction and its implication for zinc-air rechargeable batteries. Electrochim. Acta 2010, 55, 5891–5898. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, X.; Zhu, H. Surface modifications of Pt-based atomically ordered nanoparticles to improve catalytic performances for oxygen reduction reaction. Prog. Nat. Sci. Mater. Int. 2020, 30, 890–895. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Zhu, H. Synthesis of H2O2–CTAB dual-modified carbon black-supported Pt3Ni to improve catalytic activity for ORR. J. Mater. Sci. 2020, 55, 11241–11252. [Google Scholar] [CrossRef]
- Li, Z.; Yu, L.; Milligan, C.; Ma, T.; Zhou, L.; Cui, Y.; Qi, Z.; Libretto, N.; Xu, B.; Luo, J.; et al. Two-dimensional transition metal carbides as supports for tuning the chemistry of catalytic nanoparticles. Nat. Commun. 2018, 9, 5258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, D.; Han, S.; Li, Y.; Liu, J.; Zhou, Y.; Li, M.; Zhang, X.; Shen, W. Metal–Support Interaction in Pt/TiO2: Formation of Surface Pt–Ti Alloy. J. Phys. Chem. C 2021, 125, 10386–10396. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division, Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, Z.; Yan, W.; Zhou, C. Study of the Relationship between Metal–Support Interactions and the Electrocatalytic Performance of Pt/Ti4O7 with Different Loadings. Catalysts 2022, 12, 480. https://doi.org/10.3390/catal12050480
Sun X, Wang Z, Yan W, Zhou C. Study of the Relationship between Metal–Support Interactions and the Electrocatalytic Performance of Pt/Ti4O7 with Different Loadings. Catalysts. 2022; 12(5):480. https://doi.org/10.3390/catal12050480
Chicago/Turabian StyleSun, Xiuyu, Zhenwei Wang, Wei Yan, and Chuangan Zhou. 2022. "Study of the Relationship between Metal–Support Interactions and the Electrocatalytic Performance of Pt/Ti4O7 with Different Loadings" Catalysts 12, no. 5: 480. https://doi.org/10.3390/catal12050480




